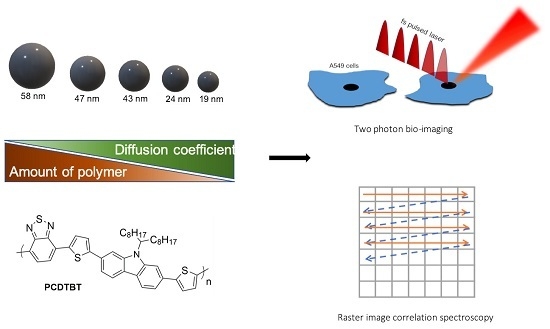
Fluorescent PCDTBT Nanoparticles with Tunable Size for Versatile Bioimaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
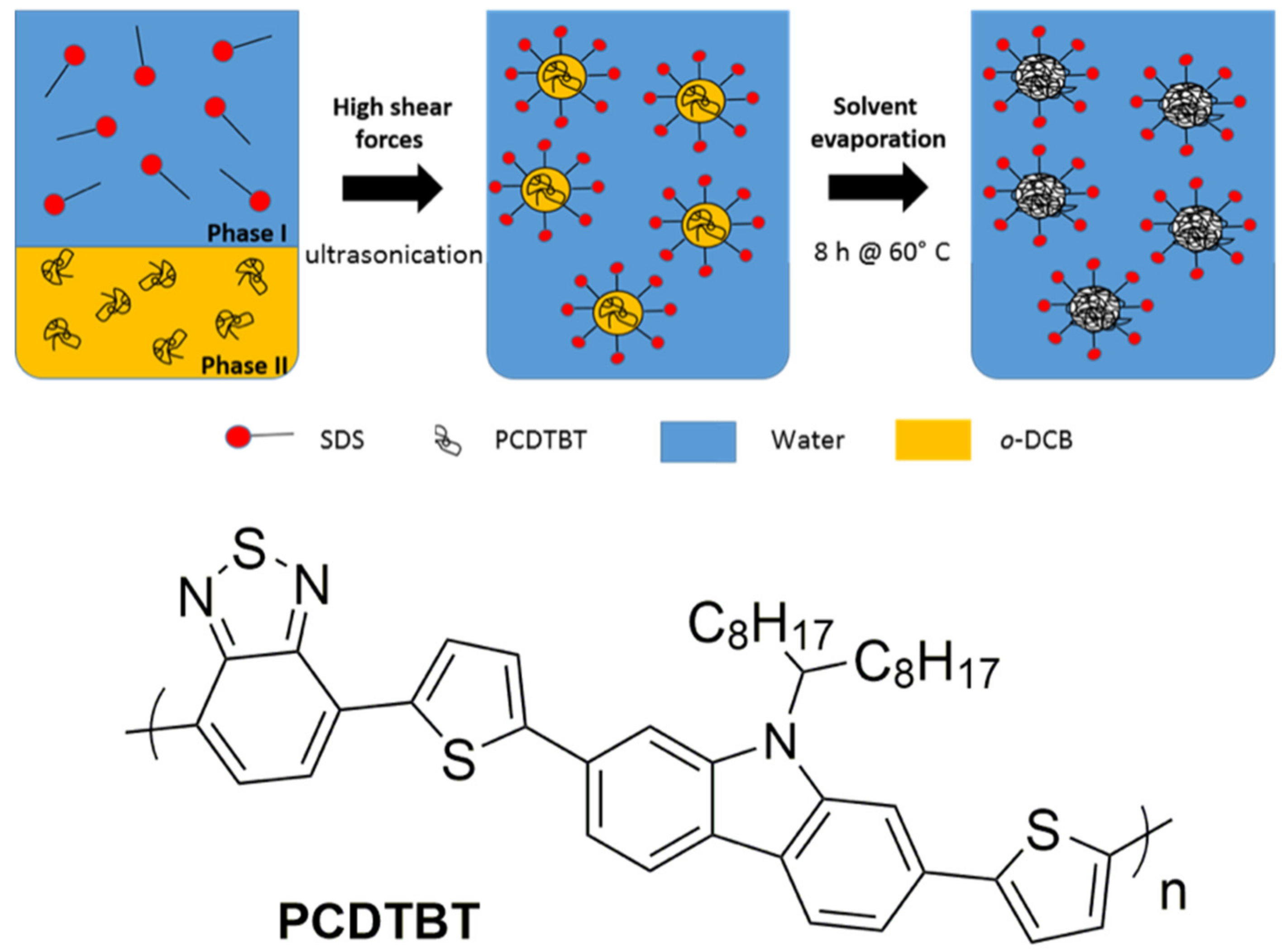
2.2. Nanoparticle Synthesis
2.3. Transmission Electron Microscopy (TEM)
2.4. Dynamic Light Scattering (DLS)
2.5. Stationary UV-Vis Absorption and Fluorescence Spectroscopy
2.6. Cell Cultures
2.7. Cytotoxicity Studies
2.8. In Vitro Imaging
2.9. Raster Image Correlation Spectroscopy
3. Results and Discussion
3.1. Nanoparticle Synthesis and Characterization
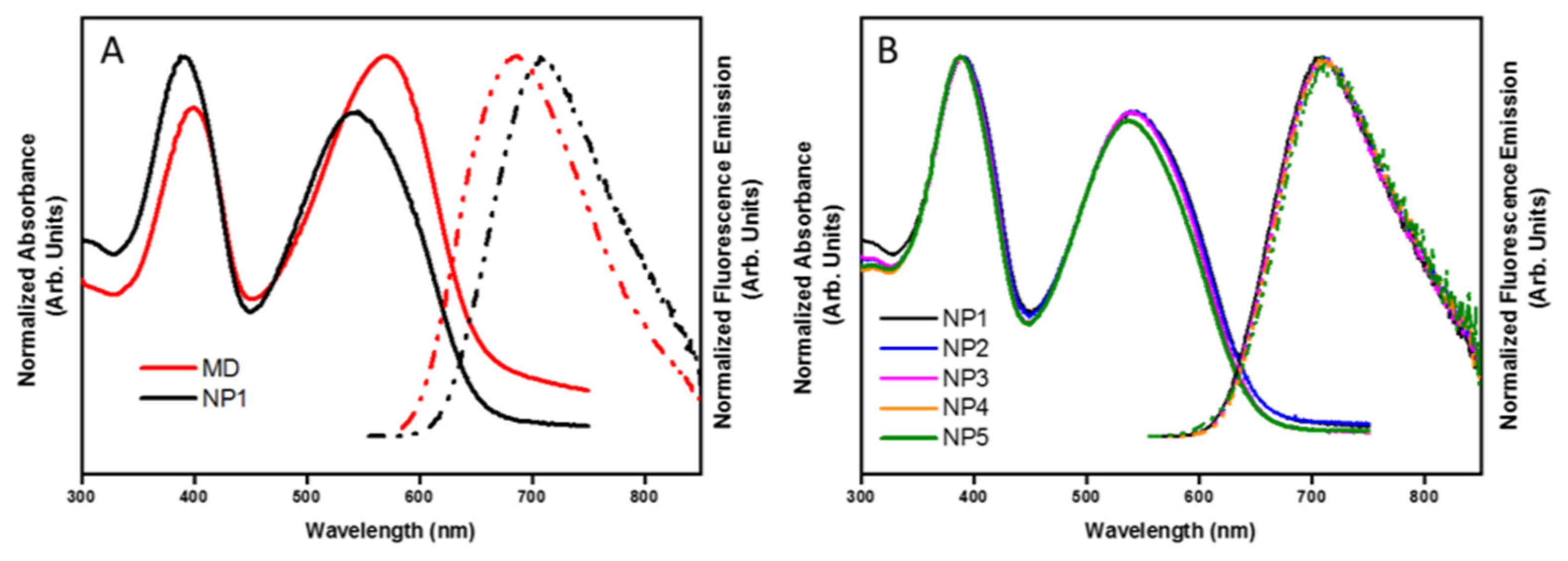
3.2. Size-Dependent Optical Properties
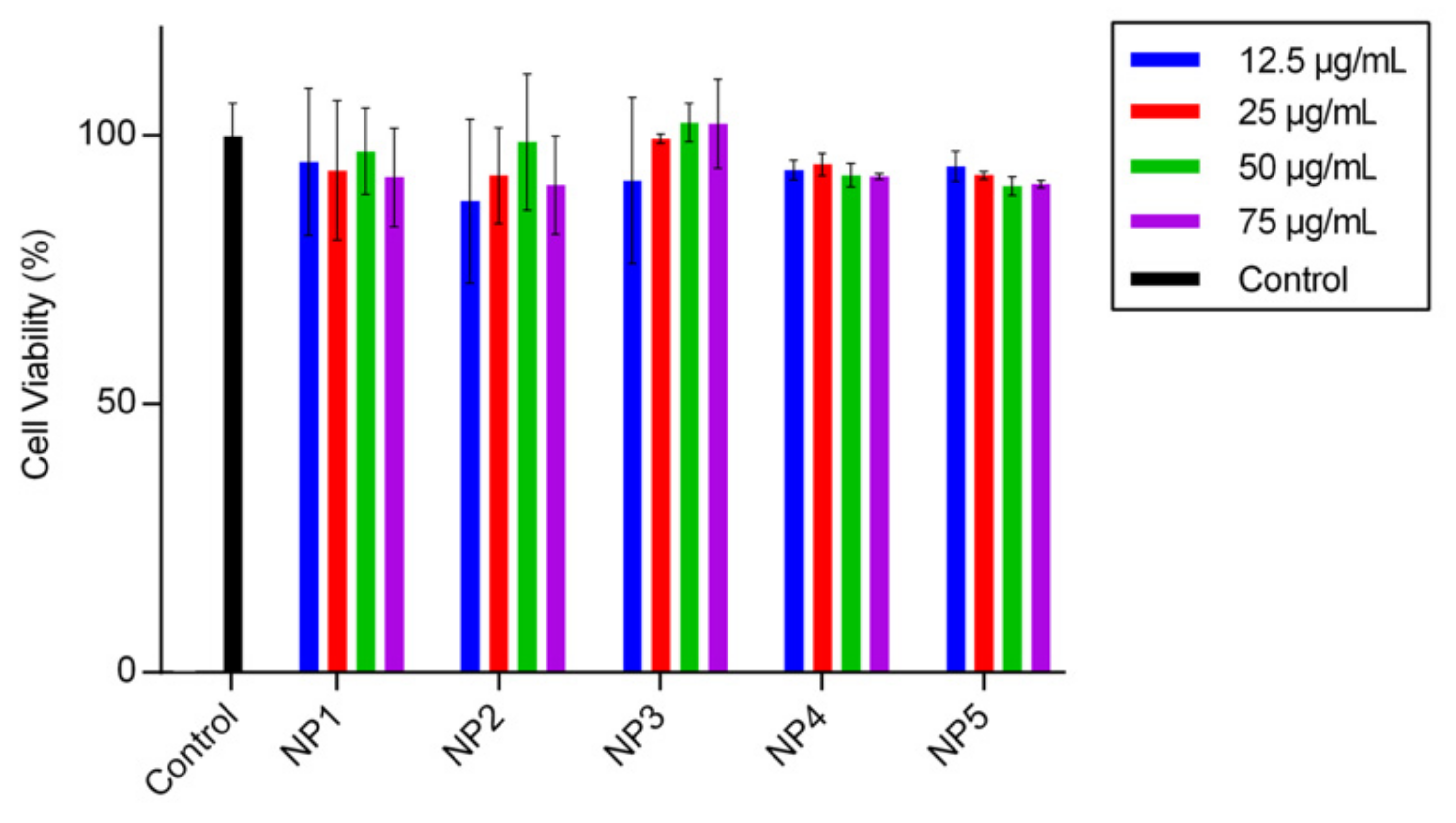
3.3. Biocompatibility Studies
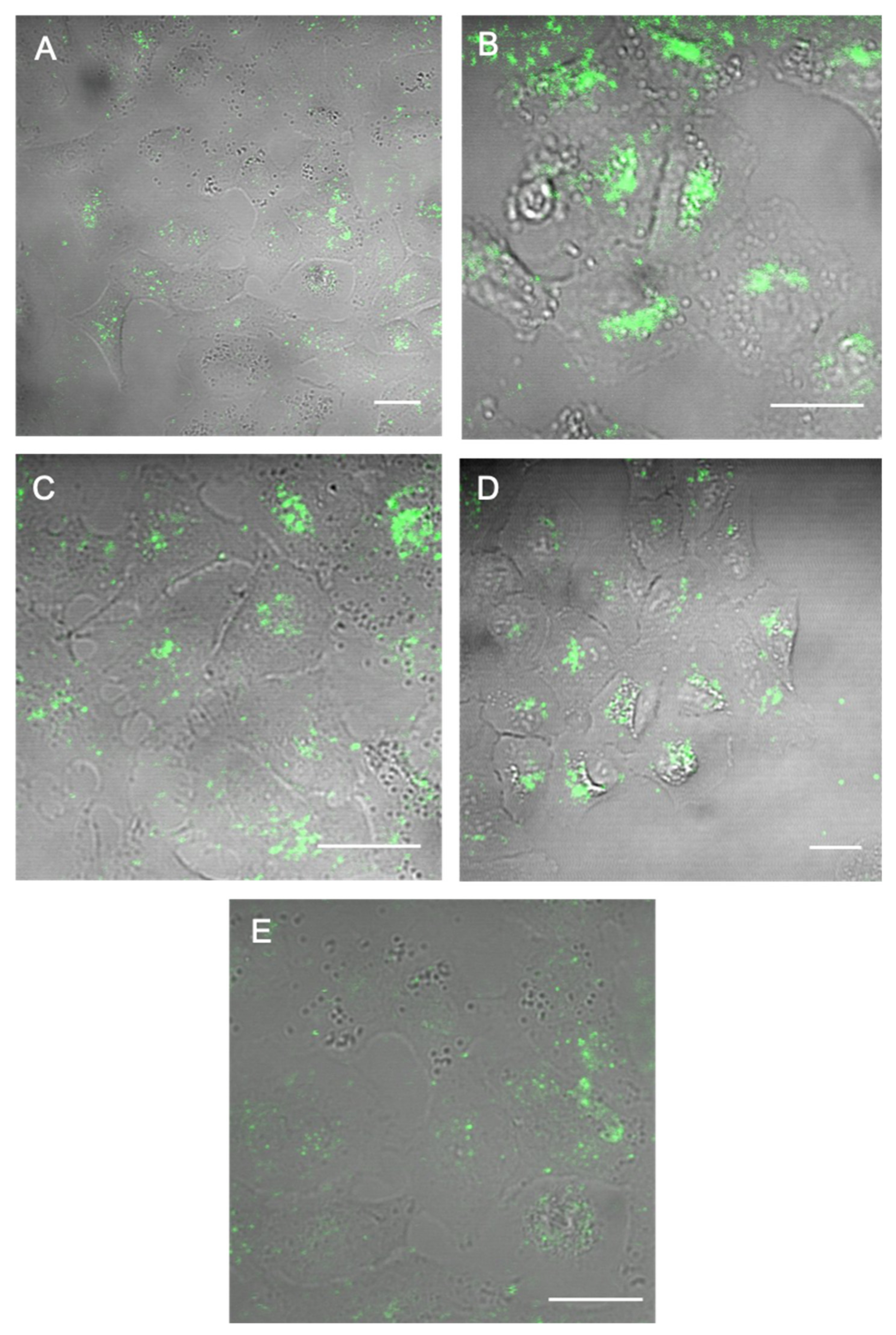
3.4. In Vitro Imaging
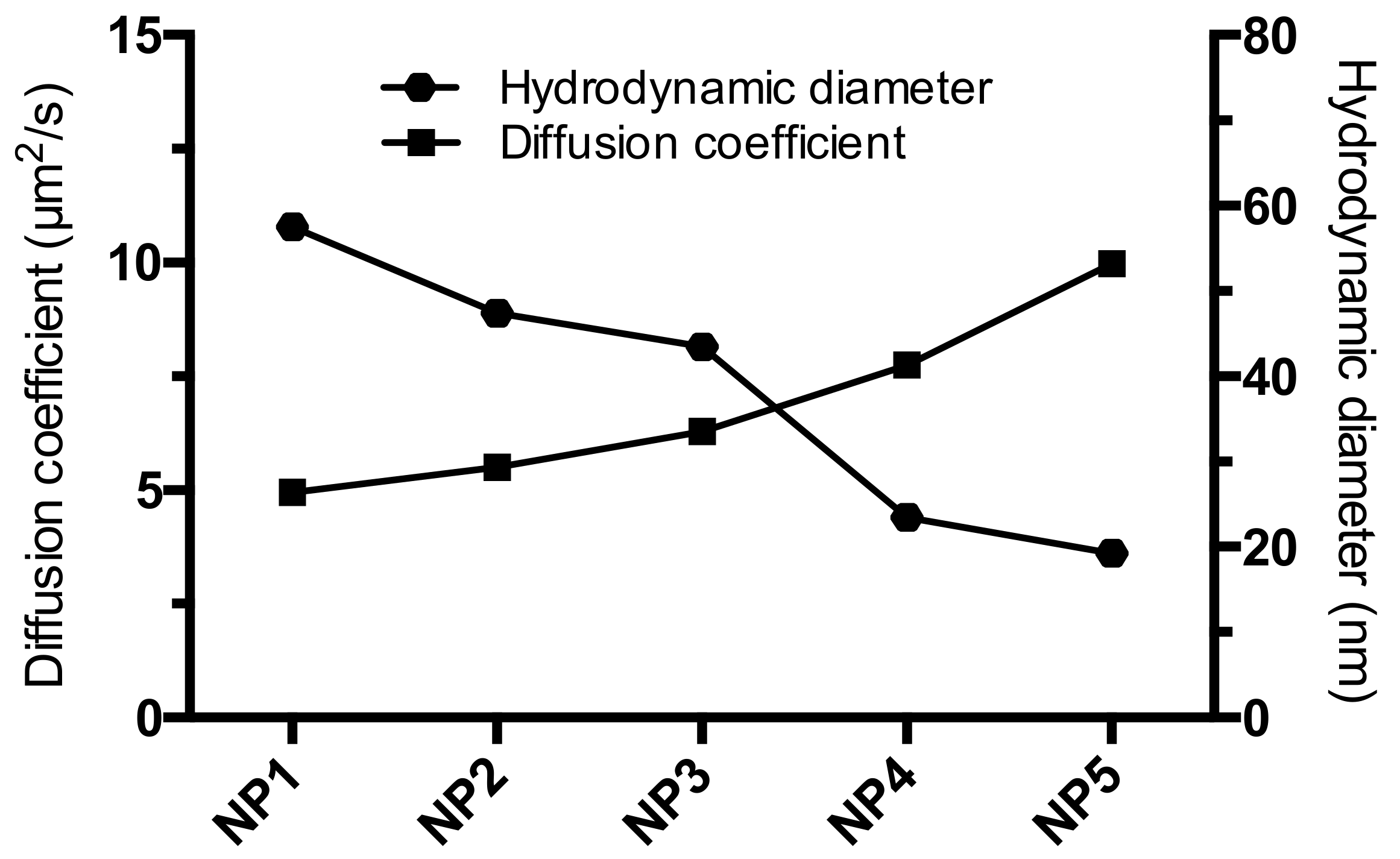
3.5. Raster Image Correlation Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tuncel, D.; Demir, H.V. Conjugated polymer nanoparticles. Nanoscale 2010, 2, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhu, C.; Yuan, H.; Liu, L.; Lv, F.; Wang, S. Conjugated polymer nanoparticles: Preparation, properties, functionalization and biological applications. Chem. Soc. Rev. 2013, 42, 6620–6633. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.H.; Cho, M.J.; Cheong, O.J.; Jang, W.D.; Ohulchanskyy, T.Y.; Lee, S.; Choi, D.H.; Prasad, P.N.; Kim, S. Low-bandgap biophotonic nanoblend: A platform for systemic disease targeting and functional imaging. Biomaterials 2015, 39, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lim, C.K.; Na, J.; Lee, Y.D.; Kim, K.; Choi, K.; Leary, J.F.; Kwon, I.C. Conjugated polymer nanoparticles for biomedical in vivo imaging. Chem. Commun. 2010, 46, 1617–1619. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.H.; Wu, P.J. Semiconducting Polymer Nanoparticles as Fluorescent Probes for Biological Imaging and Sensing. Part. Part. Syst. Charact. 2015, 32, 11–28. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, H.; Yin, S.Y.; Chang, J.J.; Li, Y.H.; Guo, X.Y.; Yuan, Z. Bright red-emitting polymer dots for specific cellular imaging. J. Mater. Sci. 2015, 50, 5571–5577. [Google Scholar] [CrossRef]
- Doshi, M.; Krienke, M.; Khederzadeh, S.; Sanchez, H.; Copik, A.; Oyer, J.; Gesquiere, A.J. Conducting polymer nanoparticles for targeted cancer therapy. RSC Adv. 2015, 5, 37943–37956. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, Y.; Cheng, G.; Liu, S.; Dong, M.; Huang, C. ‘Imperfect’ conjugated polymer nanoparticles from MEH-PPV for bioimaging and Fe(III) sensing. Luminescence 2015, 30, 451–456. [Google Scholar] [CrossRef]
- Sun, K.; Chen, H.; Wang, L.; Yin, S.; Wang, H.; Xu, G.; Chen, D.; Zhang, X.; Wu, C.; Qin, W. Size-dependent property and cell labeling of semiconducting polymer dots. ACS Appl. Mater. Interfaces 2014, 6, 10802–10812. [Google Scholar] [CrossRef]
- Peters, M.; Seneca, S.; Hellings, N.; Junkers, T.; Ethirajan, A. Size-dependent properties of functional PPV-based conjugated polymer nanoparticles for bioimaging. Colloids Surf. B 2018, 169, 494–501. [Google Scholar] [CrossRef]
- D’Olieslaeger, L.; Braeken, Y.; Cheruku, S.; Smits, J.; Ameloot, M.; Vanderzande, D.; Maes, W.; Ethirajan, A. Tuning the optical properties of poly(p-phenylene ethynylene) nanoparticles as bio-imaging probes by side chain functionalization. J. Colloid Interface Sci. 2017, 504, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Zaquen, N.; D’Olieslaeger, L.; Bove, H.; Vanderzande, D.; Hellings, N.; Junkers, T.; Ethirajan, A. PPV-Based Conjugated Polymer Nanoparticles as a Versatile Bioimaging Probe: A Closer Look at the Inherent Optical Properties and Nanoparticle-Cell Interactions. Biomacromolecules 2016, 17, 2562–2571. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Sun, C.; Liu, J.; Liao, L.D.; Yuan, Y.; Thakor, N.; Wang, J.; Liu, B. Biocompatible conjugated polymer nanoparticles for efficient photothermal tumor therapy. Small 2015, 11, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.C.; Yu, J.; Ye, F.; Rong, Y.; Gallina, M.E.; Fujimoto, B.S.; Zhang, Y.; Chan, Y.H.; Sun, W.; Zhou, X.H.; et al. Squaraine-based polymer dots with narrow, bright near-infrared fluorescence for biological applications. J. Am. Chem. Soc. 2015, 137, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Zou, Y.; Antaris, A.L.; Diao, S.; Wu, D.; Cheng, K.; Zhang, X.; Chen, C.; Liu, B.; He, Y.; et al. Ultrafast fluorescence imaging in vivo with conjugated polymer fluorophores in the second near-infrared window. Nat. Commun. 2014, 5, 4206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, J.; Rong, Y.; Ye, F.; Chiu, D.T.; Uvdal, K. High-intensity near-IR fluorescence in semiconducting polymer dots achieved by cascade FRET strategy. Chem. Sci. 2013, 4, 2143–2151. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Liu, J.; Feng, G.; Li, K.; Hu, Y.; Liu, B. Bright far-red/near-infrared conjugated polymer nanoparticles for in vivo bioimaging. Small 2013, 9, 3093–3102. [Google Scholar] [CrossRef] [PubMed]
- Frangioni, J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Braeken, Y.; Cheruku, S.; Ethirajan, A.; Maes, W. Conjugated Polymer Nanoparticles for Bioimaging. Materials 2017, 10, 1420. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, P.; Ding, H.; Wu, Y.; Yan, Y.; Liu, H.; Wang, X.; Huang, F.; Zhao, Y.; Tian, Z. Conjugated Polymer-Based Hybrid Nanoparticles with Two-Photon Excitation and Near-Infrared Emission Features for Fluorescence Bioimaging within the Biological Window. ACS Appl. Mater. Interfaces 2015, 7, 20640–20648. [Google Scholar] [CrossRef]
- Bundgaard, E.; Krebs, F.C. Low band gap polymers for organic photovoltaics. Sol. Energy Mater. Sol. Cells 2007, 91, 954–985. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Gong, X.; Heeger, A.J. Low bandgap semiconducting polymers for polymeric photovoltaics. Chem. Soc. Rev. 2016, 45, 4825–4846. [Google Scholar] [CrossRef] [PubMed]
- Etzold, F.; Howard, I.A.; Mauer, R.; Meister, M.; Kim, T.D.; Lee, K.S.; Baek, N.S.; Laquai, F. Ultrafast exciton dissociation followed by nongeminate charge recombination in PCDTBT:PCBM photovoltaic blends. J. Am. Chem. Soc. 2011, 133, 9469–9479. [Google Scholar] [CrossRef] [PubMed]
- Banerji, N.; Cowan, S.; Leclerc, M.; Vauthey, E.; Heeger, A.J. Exciton formation, relaxation, and decay in PCDTBT. J. Am. Chem. Soc. 2010, 132, 17459–17470. [Google Scholar] [CrossRef] [PubMed]
- Landfester, K. Miniemulsion polymerization and the structure of polymer and hybrid nanoparticles. Angew. Chem. Int. Ed. Engl. 2009, 48, 4488–4507. [Google Scholar] [CrossRef] [PubMed]
- Kietzke, T.; Neher, D.; Kumke, M.; Ghazy, O.; Ziener, U.; Landfester, K. Phase separation of binary blends in polymer nanoparticles. Small 2007, 3, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Kietzke, T.; Neher, D.; Landfester, K.; Montenegro, R.; Guntner, R.; Scherf, U. Novel approaches to polymer blends based on polymer nanoparticles. Nat. Mater. 2003, 2, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.J.; Gesquiere, A.J. PCBM concentration dependent morphology of P3HT in composite P3HT/PCBM nanoparticles. Chem. Phys. Lett. 2009, 476, 51–55. [Google Scholar] [CrossRef]
- Hu, Z.J.; Tenery, D.; Bonner, M.S.; Gesquiere, A.J. Correlation between spectroscopic and morphological properties of composite P3HT/PCBM nanoparticles studied by single particle spectroscopy. J. Lumin. 2010, 130, 771–780. [Google Scholar] [CrossRef]
- Tenery, D.; Worden, J.G.; Hu, Z.J.; Gesquiere, A.J. Single particle spectroscopy on composite MEH-PPV/PCBM nanoparticles. J. Lumin. 2009, 129, 423–429. [Google Scholar] [CrossRef]
- D’Olieslaeger, L.; Pfannmöller, M.; Fron, E.; Cardinaletti, I.; Van Der Auweraer, M.; Van Tendeloo, G.; Bals, S.; Maes, W.; Vanderzande, D.; Manca, J.; et al. Tuning of PCDTBT:PC 71 BM blend nanoparticles for eco-friendly processing of polymer solar cells. Sol. Energy Mater. Sol. Cells 2017, 159, 179–188. [Google Scholar] [CrossRef]
- Patil, A.; Heeger, A.; Wudl, F. Optical properties of conducting polymers. Chem. Rev. 1988, 88, 183–200. [Google Scholar] [CrossRef]
- Cornil, J.; Beljonne, D.; Calbert, J.P.; Brédas, J.L. Interchain interactions in organic π-conjugated materials: Impact on electronic structure, optical response, and charge transport. Adv. Mater. 2001, 13, 1053–1067. [Google Scholar] [CrossRef]
- Braeken, Y.; Cheruku, S.; Seneca, S.; Smisdom, N.; Berden, L.; Kruyfhooft, L.; Penxten, H.; Lutsen, L.; Fron, E.; Vanderzande, D.; et al. Effect of Branching on the Optical Properties of Poly(p-phenylene ethynylene) Conjugated Polymer Nanoparticles for Bioimaging. ACS Biomater. Sci. Eng. 2019, 5, 1967–1977. [Google Scholar] [CrossRef]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, N.; Gray, S.; Han, H.B.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Rurack, K.; Spieles, M. Fluorescence Quantum Yields of a Series of Red and Near-Infrared Dyes Emitting at 600–1000 nm. Anal. Chem. 2011, 83, 1232–1242. [Google Scholar] [CrossRef]
- Penjweini, R.; Deville, S.; D’Olieslaeger, L.; Berden, M.; Ameloot, M.; Ethirajan, A. Intracellular localization and dynamics of Hypericin loaded PLLA nanocarriers by image correlation spectroscopy. J. Control. Release 2015, 218, 82–93. [Google Scholar] [CrossRef]
- Slenders, E.; Bové, H.; Urbain, M.; Mugnier, Y.; Sonay, A.Y.; Pantazis, P.; Bonacina, L.; Vanden Berghe, P.; vandeVen, M.; Ameloot, M. Image Correlation Spectroscopy with Second Harmonic Generating Nanoparticles in Suspension and in Cells. J. Phys. Chem. Lett. 2018, 9, 6112–6118. [Google Scholar] [CrossRef]
- Hendrix, J.; Dekens, T.; Schrimpf, W.; Lamb, D.C. Arbitrary-Region Raster Image Correlation Spectroscopy. Biophys. J. 2016, 111, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Schrimpf, W.; Barth, A.; Hendrix, J.; Lamb, D.C. PAM: A Framework for Integrated Analysis of Imaging, Single-Molecule, and Ensemble Fluorescence Data. Biophys. J. 2018, 114, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Spano, F.C.; Silva, C. H-and J-Aggregate Behavior in Polymeric Semiconductors. Annu. Rev. Phys. Chem. 2014, 65, 477–500. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, C.; Wu, C.; Hooper, J.; Salazar, M.A.; Perdomo, A.; Dukes, A.; McNeill, J. Single molecule nanoparticles of the conjugated polymer MEH-PPV, preparation and characterization by near-field scanning optical microscopy. J. Phys. Chem. B 2005, 109, 8543–8546. [Google Scholar] [CrossRef] [PubMed]
- Grey, J.K.; Kim, D.Y.; Norris, B.C.; Miller, W.L.; Barbara, P.F. Size-dependent spectroscopic properties of conjugated polymer nanoparticles. J. Phys. Chem. B 2006, 110, 25568–25572. [Google Scholar] [CrossRef] [PubMed]
- Notelaers, K.; Smisdom, N.; Rocha, S.; Janssen, D.; Meier, J.C.; Rigo, J.M.; Hofkens, J.; Ameloot, M. Ensemble and single particle fluorimetric techniques in concerted action to study the diffusion and aggregation of the glycine receptor alpha3 isoforms in the cell plasma membrane. Biochim. Biophys. Acta 2012, 1818, 3131–3140. [Google Scholar] [CrossRef] [PubMed]
- Rossow, M.J.; Sasaki, J.M.; Digman, M.A.; Gratton, E. Raster image correlation spectroscopy in live cells. Nat. Protoc. 2010, 5, 1761–1774. [Google Scholar] [CrossRef] [PubMed]
- Hemmerich, P.H.; von Mikecz, A.H. Defining the Subcellular Interface of Nanoparticles by Live-Cell Imaging. PLoS ONE 2013, 8, e62018. [Google Scholar] [CrossRef]
- Mahowald, J.; Arcizet, D.; Heinrich, D. Impact of External Stimuli and Cell Micro-Architecture on Intracellular Transport States. Chemphyschem 2009, 10, 1559–1566. [Google Scholar] [CrossRef]
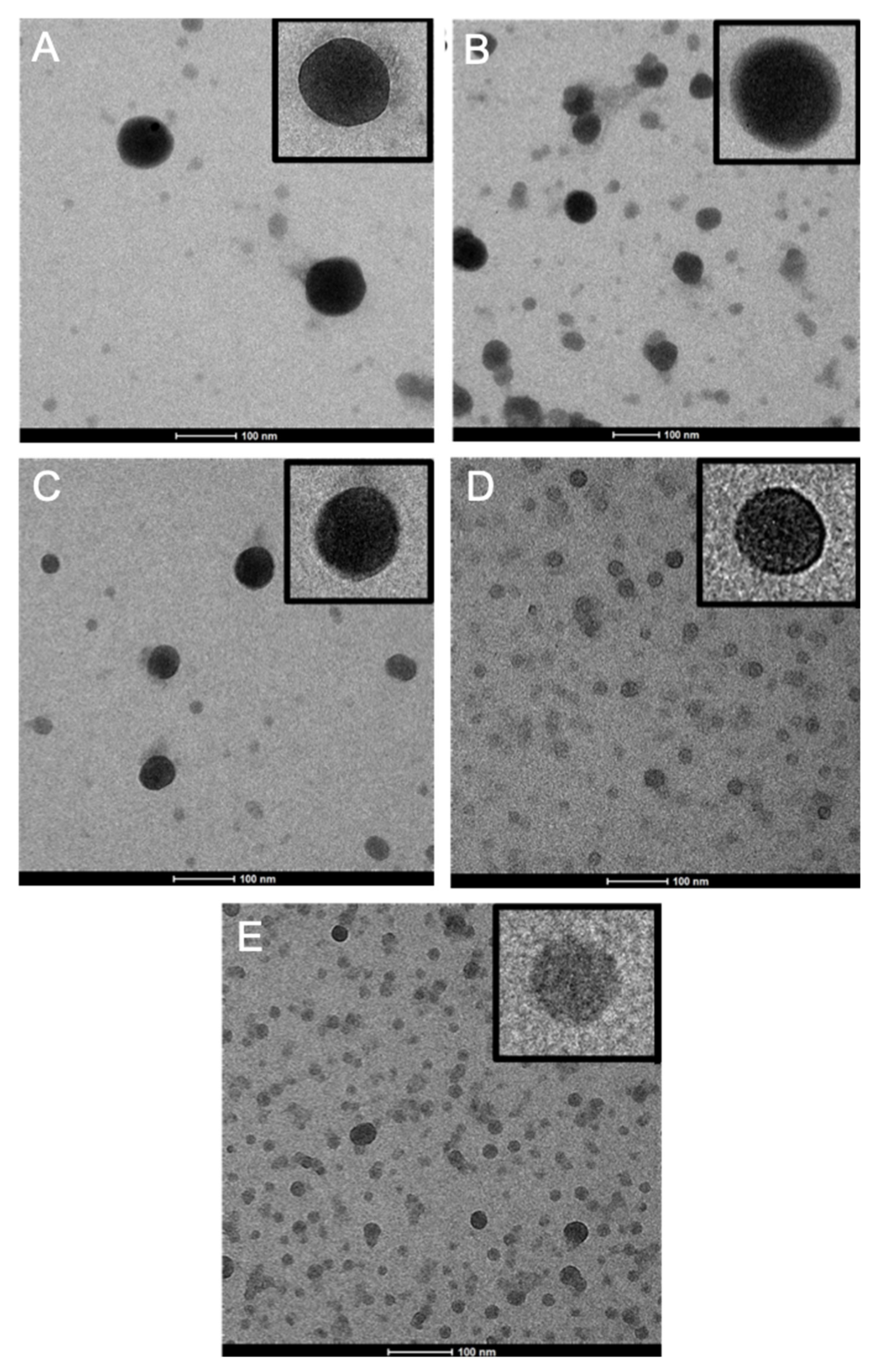
| Sample | PCDTBT (mg) | Size in DLS (nm) * | Geometric Standard Deviation | PDI |
|---|---|---|---|---|
| NP1 | 25 | 57 | 1.4 | 0.138 |
| NP2 | 15 | 47 | 1.5 | 0.179 |
| NP3 | 10 | 44 | 1.5 | 0.189 |
| NP4 | 5 | 24 | 1.6 | 0.211 |
| NP5 | 2 | 19 | 1.6 | 0.212 |
| Sample | λmax Excitation (nm) | λmax Emission (nm) | Stokes Shift (nm) | PLQY (%) |
|---|---|---|---|---|
| MD | 398–571 | 687 | 116 | 41 |
| NP1 | 391–543 | 711 | 168 | 6 |
| NP2 | 391–541 | 709 | 168 | 4 |
| NP3 | 389–541 | 709 | 168 | 3 |
| NP4 | 388–563 | 710 | 174 | 3 |
| NP5 | 388–538 | 711 | 173 | 3 |
| Sample | Diffusion Coefficient (µm2/s) |
|---|---|
| NP1 | 4.95 |
| NP2 | 5.51 |
| NP3 | 6.30 |
| NP4 | 7.76 |
| NP5 | 9.98 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheruku, S.; D’Olieslaeger, L.; Smisdom, N.; Smits, J.; Vanderzande, D.; Maes, W.; Ameloot, M.; Ethirajan, A. Fluorescent PCDTBT Nanoparticles with Tunable Size for Versatile Bioimaging. Materials 2019, 12, 2497. https://doi.org/10.3390/ma12152497
Cheruku S, D’Olieslaeger L, Smisdom N, Smits J, Vanderzande D, Maes W, Ameloot M, Ethirajan A. Fluorescent PCDTBT Nanoparticles with Tunable Size for Versatile Bioimaging. Materials. 2019; 12(15):2497. https://doi.org/10.3390/ma12152497
Chicago/Turabian StyleCheruku, Srujan, Lien D’Olieslaeger, Nick Smisdom, Joeri Smits, Dirk Vanderzande, Wouter Maes, Marcel Ameloot, and Anitha Ethirajan. 2019. "Fluorescent PCDTBT Nanoparticles with Tunable Size for Versatile Bioimaging" Materials 12, no. 15: 2497. https://doi.org/10.3390/ma12152497
APA StyleCheruku, S., D’Olieslaeger, L., Smisdom, N., Smits, J., Vanderzande, D., Maes, W., Ameloot, M., & Ethirajan, A. (2019). Fluorescent PCDTBT Nanoparticles with Tunable Size for Versatile Bioimaging. Materials, 12(15), 2497. https://doi.org/10.3390/ma12152497