Biosorption of Methylene Blue Dye Using Natural Biosorbents Made from Weeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Biosorbent
2.2. Solutions and Reagents
2.3. Used Equipments at the Characterization
2.4. Biosorption Experimental Procedure
- = Percentage of removal
- = Initial concentration (mg/L)
- = Concentration at time t
- = Volume (L)
- = Biosorption capacity at time t
- m = Biosorbent mass.
3. Results
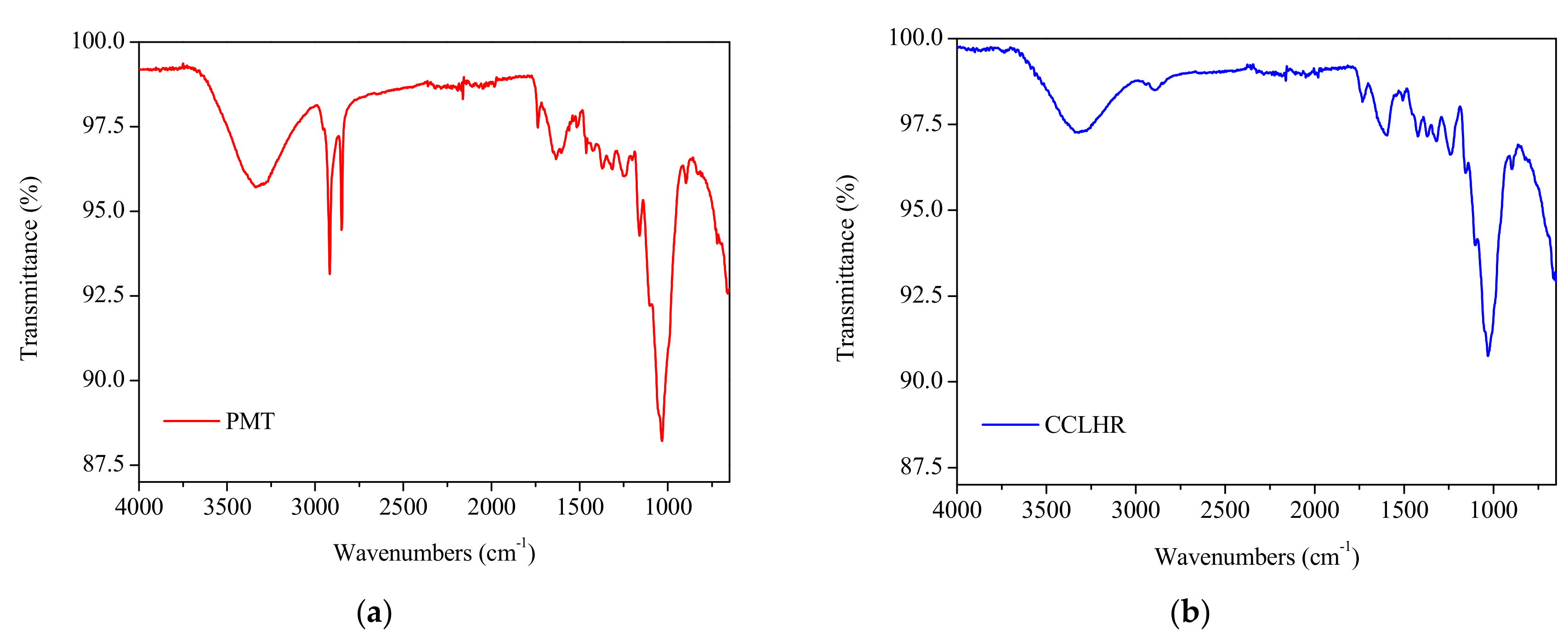
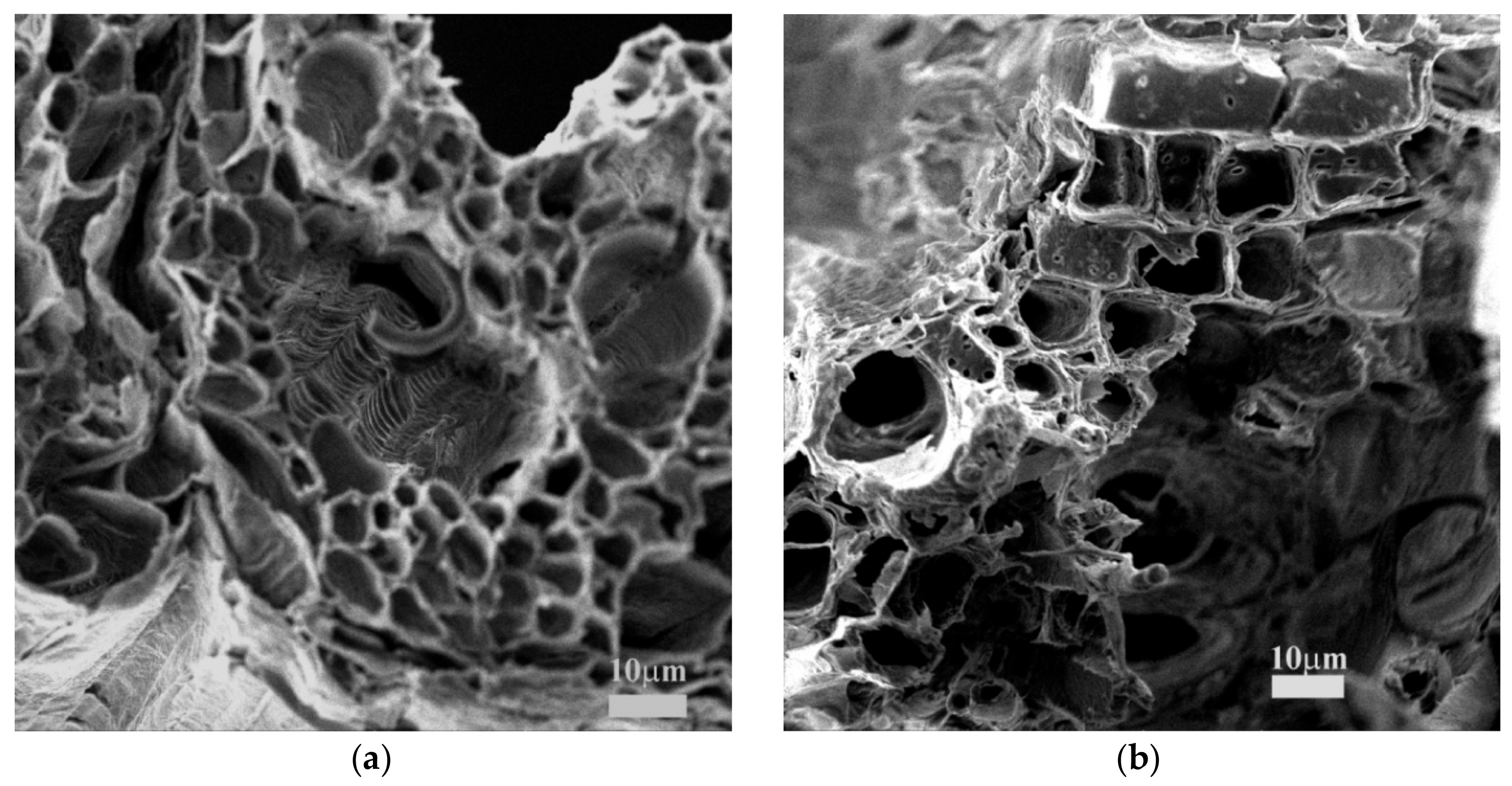
3.1. Characterization of PMT and CCLHR Biosorbents
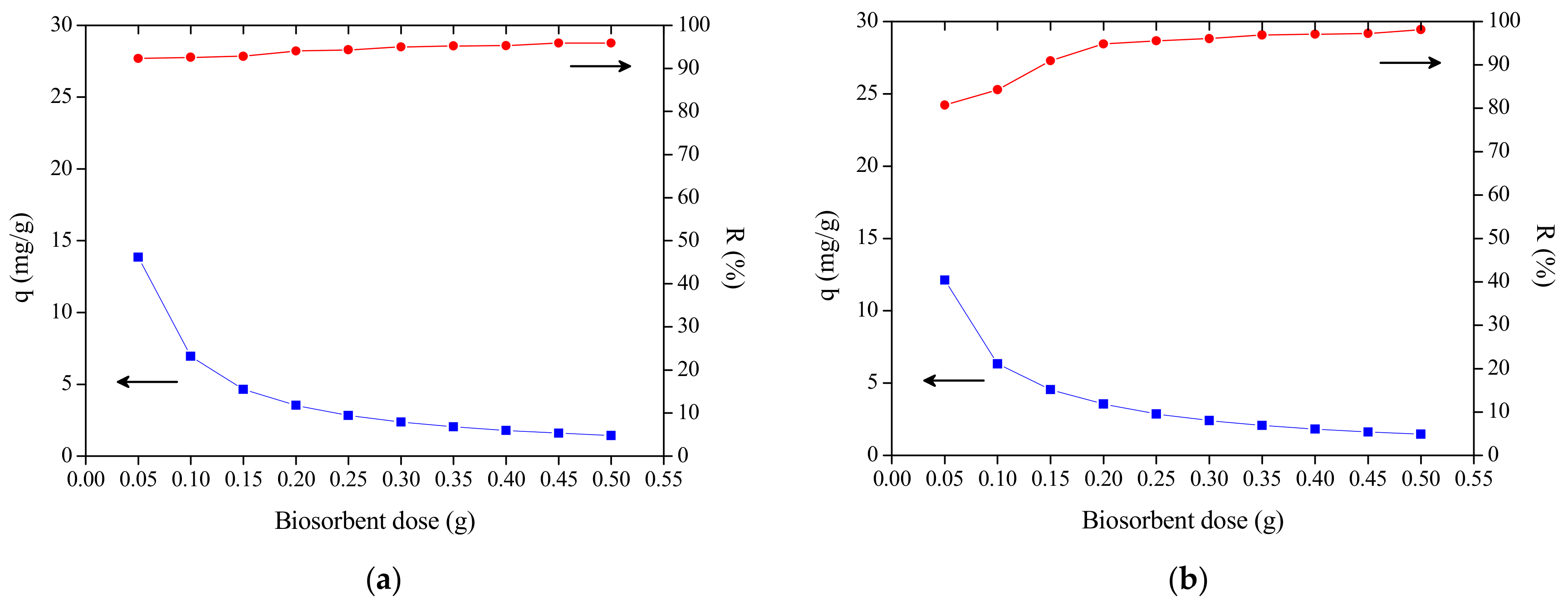
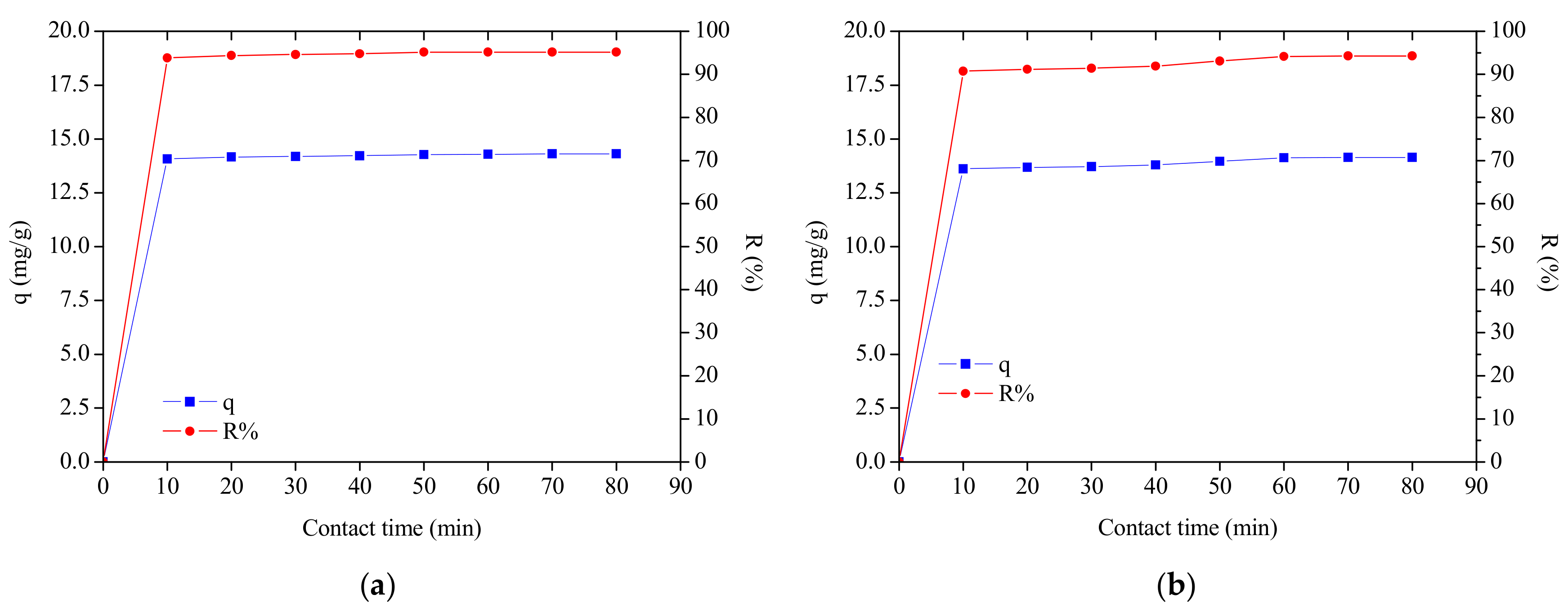
3.2. The Effect of Dosage
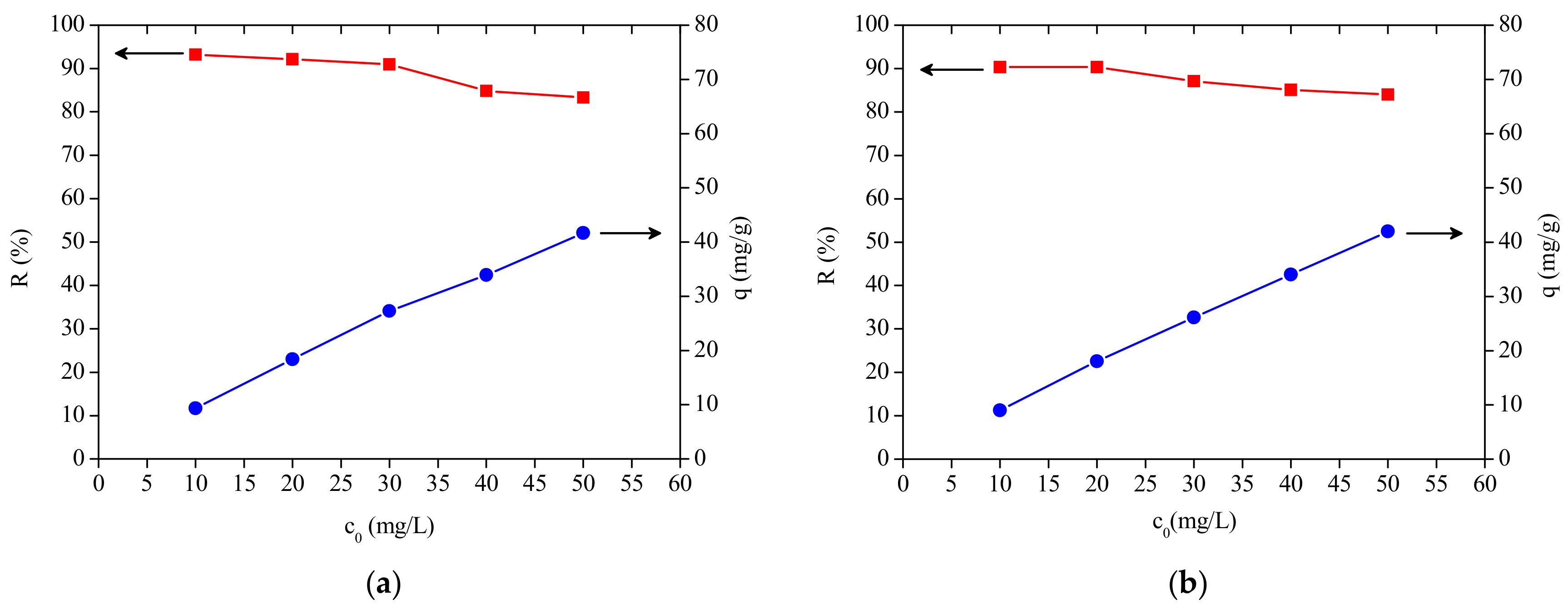
3.3. Effect of Initial Dye Concentration
3.4. Adsorption Isotherms
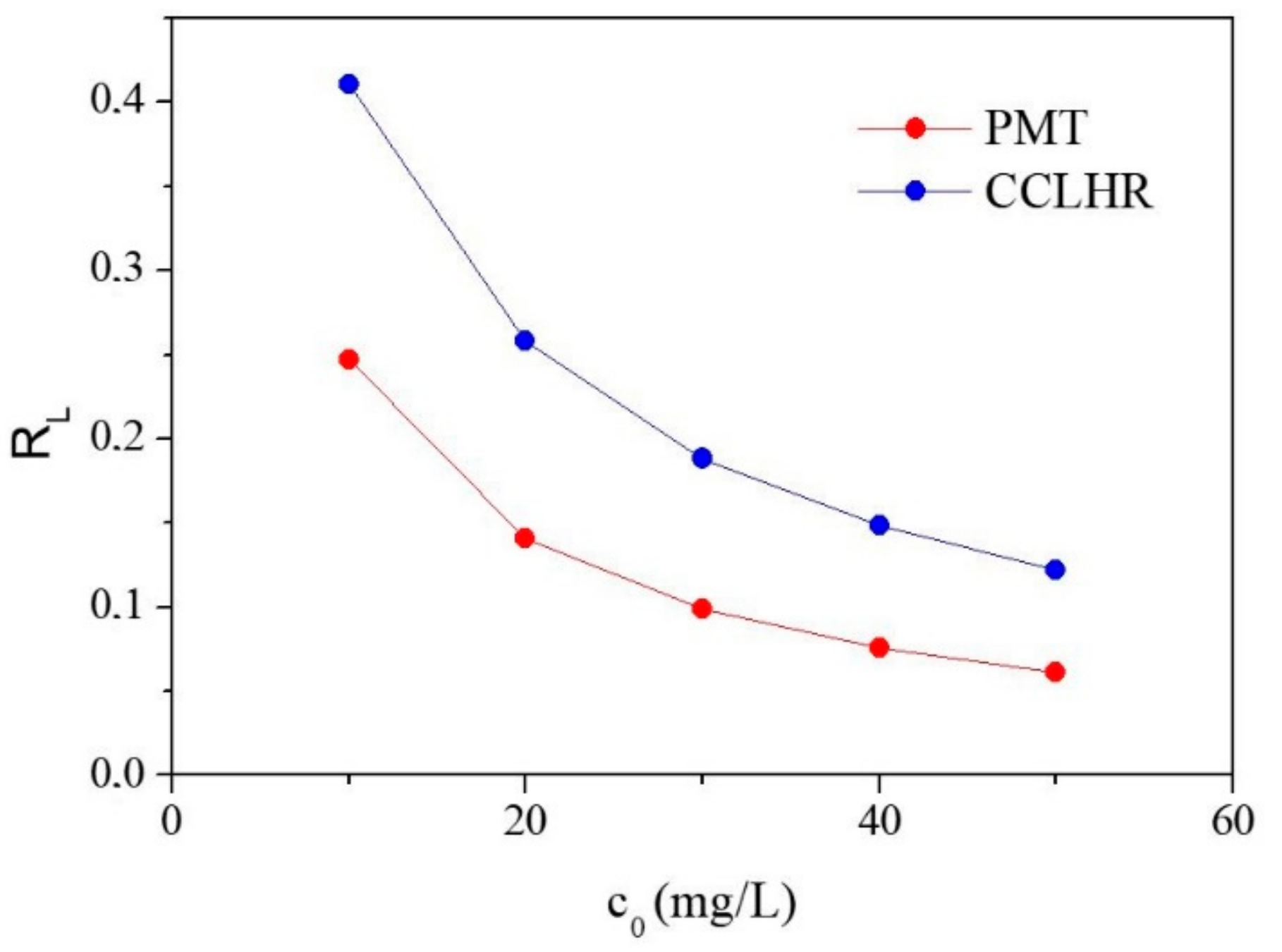
3.4.1. Langmuir Isotherm
- = amount of solute adsorbed per gram of adsorbent at equilibrium (mg/g);
- : maximum biosorption capacity (mg/g);
- interaction constant of adsorbate/adsorbent (L/mg);
- : equilibrium concentration of adsorbate (mg/L).
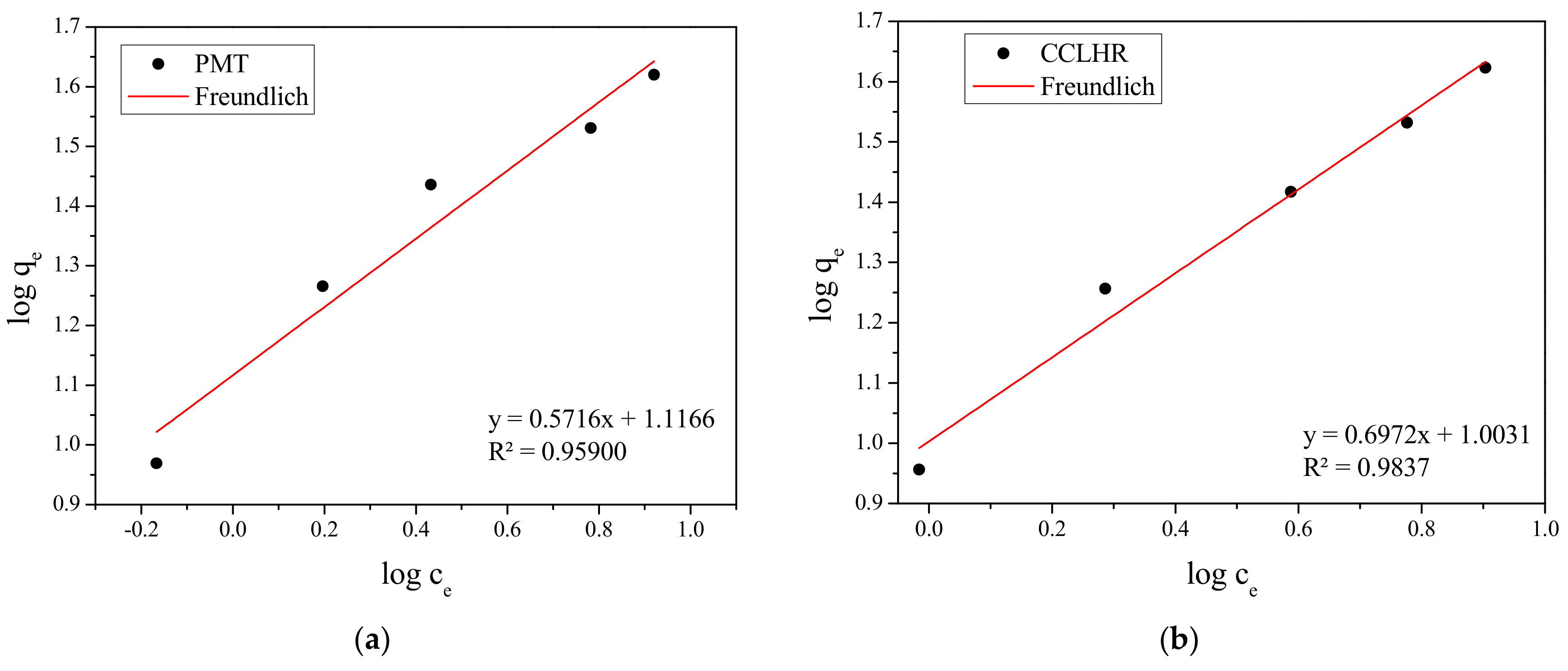
3.4.2. Freundlich Isotherm
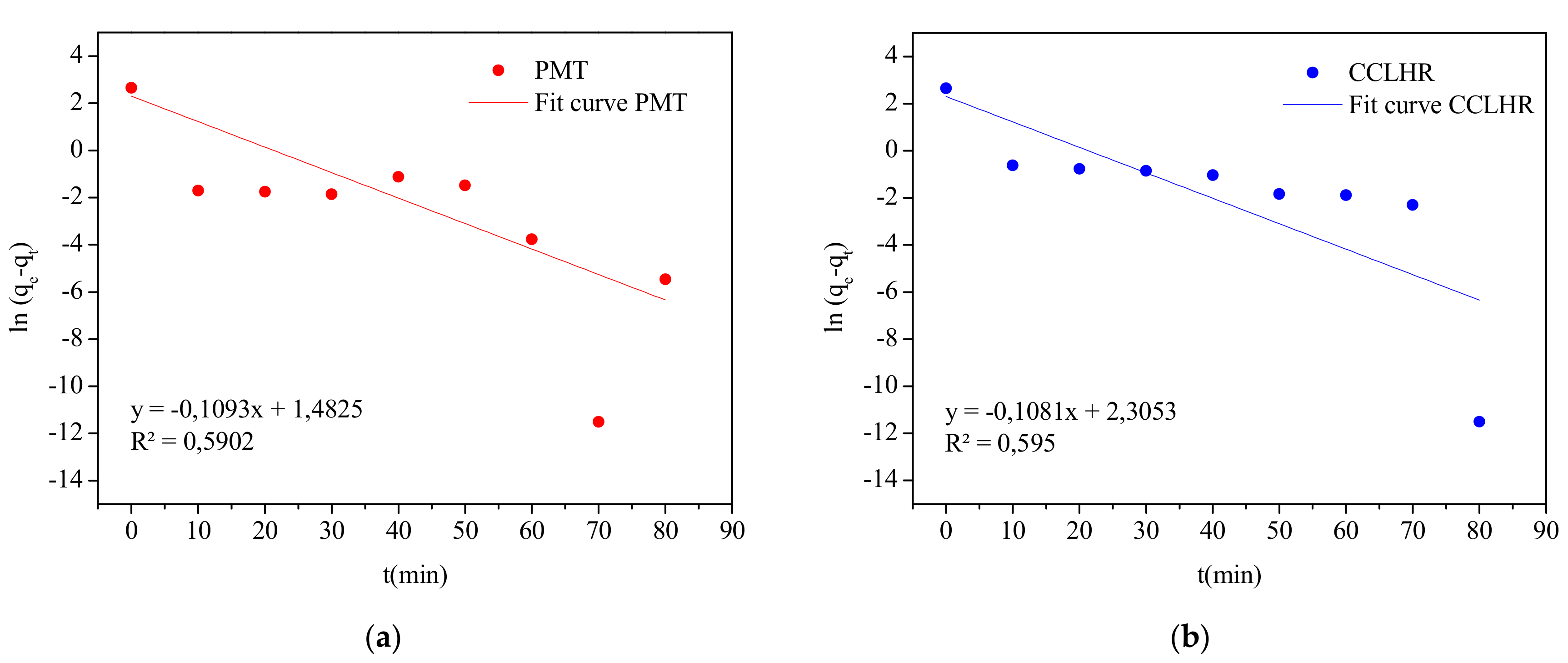
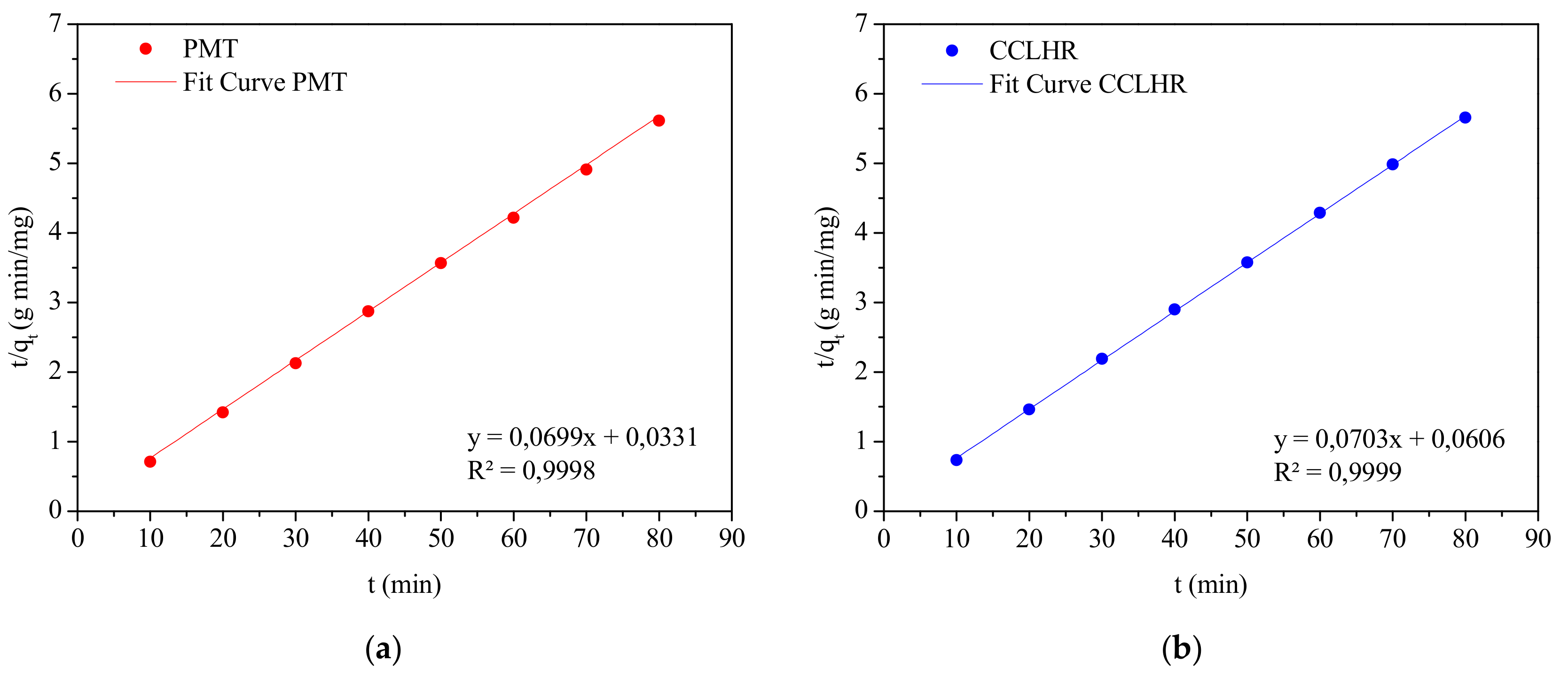
3.5. Adsorption Kinetics
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Borges, F.C.; Santos, L.S.; Correa, M.J.C. Aleopathic potential of two neolignans isolated from Virola suminanensis (Myristicaceae) leaves. Planta Daninha 2007, 25, 51–59. [Google Scholar] [CrossRef]
- Vidotti, E.C.; Rollemberg, M.C. Algae: From economics in aquatic environments to bioremediation and analytical chemistry. Quím. Nova 2004, 27, 1–7. [Google Scholar]
- Pereira, L.; Alves, M. Dyes—Environmental Impact and Remediation. In Environmental Protection Strategies for Sustainable Development; Strategies for Sustainability; Malik, A., Grohmann, E., Eds.; Springer: Dordrecht, The Netherlands, 2012; p. 111. [Google Scholar]
- Tundisi, J.G.; Tundisi, T.M. Limnology, 1st ed.; Oficina de Textos: São Paulo, Brazil, 2008; p. 632. [Google Scholar]
- Souza, N.K. Adsorption of Cationic and Anionic Dyes in Aqueous Solution Using New Bi-Functional Materials of Sugarcane Bagasse. Master’s Thesis, Federal University of Ouro Preto, Ouro Preto, Brazil, 2013. [Google Scholar]
- Ali, N.; Hameed, A.; Ahmed, S. Physicochemical characterization and bioremediation perspective of textile effluent, dyes and metals by indigenous bacteria. J. Hazard Mater. 2009, 164, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Suhas Ali, I.; Saini, V.K. Removal of rhodamine B, fast green, and methylene blue from wastewater using red mud, an aluminum industry waste. Ind. Eng. Chem. Res. 2004, 43, 1740–1747. [Google Scholar] [CrossRef]
- Yamashita, M.S.J.; Kikutani, T.; Hashimoto, T. Activated carbon fibers and films derived from poly (vinylidene fluoride). Carbon 2001, 39, 207–214. [Google Scholar] [CrossRef]
- Hu, D.D.; Lin, J.; Zhang, Q.; Lu, J.N.; Wang, X.Y.; Wang, Y.W.; Bu, F.; Ding, L.F.; Wang, L.; Wu, T. Multi-step host–guest energy transfer between inorganic chalcogenide-based semiconductor zeolite material and organic dye molecules. Chem. Mater. 2015, 27, 4099–4104. [Google Scholar] [CrossRef]
- Shang, L.; Bian, T.; Zhang, B.; Zhang, D.; Wu, L.Z.; Tung, C.H.; Yin, Y.; Zhang, T. Graphene-supported ultrafine metal nanoparticles encapsulated by mesoporous silica: robust catalysts for oxidation and reduction reactions, Angew. Chem. Int. Ed. 2014, 53, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Hossaini, H.; Moussavi, G.; Farrokhi, M. The investigation of the LED-activated FeFNS-TiO2 nanocatalyst for photocatalytic degradation and mineralization of organophosphate pesticides in water. Water Res. 2014, 59, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Echavia, G.R.; Matzusawa, F.; Negishi, N. Photocatalytic degradation of organophosphate and phosphonoglycine pesticides using TiO2 immobilized on silica gel. Chemosphere 2009, 76, 595–600. [Google Scholar] [CrossRef]
- Ahmed, S.M.; El-Dib, F.I.; El-Gendy, N.; Sayed, W.M.; El-Khodary, M. A kinetic study for the removal of anionic sulphonated dye from aqueous solution using nano-polyaniline and Baker’s yeast. Arab. J. Chem. 2016, 9, S1721–S1728. [Google Scholar] [CrossRef]
- Dabrowski, A. Adsorption e from theory to practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Fan, L.; Luo, C.; Sun, M.; Qiu, H.; Li, X. Synthesis of magnetic beta-cyclodextrin-chitosan/graphene oxide as nanoadsorbent and its application in dye adsorption and removal. Colloids Surf. B 2013, 103, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lin, F.; Chen, J.; Li, F.; Weng, W. Metal-organic framework MIL-125(Ti) for efficient adsorptive removal of Rhodamine B from aqueous solution. Appl. Organomet. Chem. 2015, 29, 12–19. [Google Scholar] [CrossRef]
- Cui, Y.-Y.; Zhang, J.; Ren, L.-L.; Cheng, A.-L.; Gao, E.-Q. A functional anionic metal–organic framework for selective adsorption and separation of organic dyes. Polyhedron 2018, 161, 71–77. [Google Scholar]
- Gupta, V.K.; Suhas. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Gusmão, G.K.A.; Gurgel, A.L.V.; Melo, S.T.M.; Gil, L.F. Application of succinylated sugarcane bagasse as adsorbent to remove methylene blue and gentian violet from aqueous solutions—Kinetic and equilibrium studies. Dyes Pigment. 2012, 92, 967–974. [Google Scholar] [CrossRef]
- Calvete, T. Pinatura in Natura and Active Coal Shells—Adsorbents for Dye Removal in Aqueous Effluents. Ph.D. Thesis, Chemistry, Federal University of Rio Grande do Sul, Porto Alegre, Brazil, 2011. [Google Scholar]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Boudechiche, N.; Mokaddem, H.; Sadaoui, Z.; Trari, M. Biosorption of cationic dye from aqueous solutions onto lignocellulosic biomass (Luffa cylindrica): Characterization, equilibrium, kinetic and thermodynamic studies. Int. J. Ind. Chem. 2016, 7, 167–180. [Google Scholar] [CrossRef]
- Kissmann, K.G. Weed and Noxious Plants, 1st ed.; BASF: São Paulo, Brazil, 1991; pp. 1–126. [Google Scholar]
- Swetha Bindu, C.H.; Prathibha, B. Evaluation of antioxidant activity of ethanolic extrat of leaves of Cyanthilium cinereum (L). H. Rob. by using isolated frog heart. IJPPR Hum. 2018, 12, 458–465. [Google Scholar]
- Essawy, A.A.; Ali, A.E.-H.; Abdel-Mottaleb, M.S.A. Application of novel copolymer-TiO2 membranes for some textile dyes adsorptive removal from aqueous solution and photocatalytic decolorization. J. Hazard. Mater. 2008, 157, 547–552. [Google Scholar] [CrossRef]
- Sun, Q.Y.; Yang, L.Z. The adsorption of basic dyes from aqueous solution on modified peat-resin particle. Water Res. 2003, 37, 1535–1544. [Google Scholar] [CrossRef]
- Gupta, V.K.; Mittal, A.; Krishnan, L.; Gajbe, V. Adsorption kinetics and column operations for the removal and recovery of malachite green from wastewater using bottom ash. Sep. Purif. Technol. 2004, 40, 87–96. [Google Scholar] [CrossRef]
- Kunz, A.; Zamora, P.P.; Moraes, S.G.; Durán, N. New trends in effluent treatment of textile effluents. Quím. Nova 2002, 25, 78–82. [Google Scholar] [CrossRef]
- Silva, W.L.L.D.; Oliveira, S.P.D. Modification of the adsorption characteristics of the sugarcane bagasse for the removal of methylene blue from aqueous solutions. Sci. Plena 2012, 8, 1–9. [Google Scholar]
- Abdolali, A.; Guo, W.S.; Ngo, H.H.; Chen, S.S.; Nguyen, N.C.; Tung, K.L. Typical lignocellulosic wastes and by-products for biosorption process in water and wastewatertreatment: A critical review. Bioresour. Technol. 2014, 160, 57–66. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Rangabhashiyam, S.; Ashokkumar, T.; Arockiaraj, J. Assessment of samarium biosorption from aqueous solution by brown macroalga Turbinaria conoides. J. Taiwan Inst. Chem. E 2017, 74, 113–120. [Google Scholar] [CrossRef]
- Wong, Y.C.; Senan, M.S.R.; Atiqah, N.A. Removal of methylene blue and malachite green dye using different form of coconut fibre as absorbent. J. Basic Appl. Sci. 2013, 9, 172–177. [Google Scholar] [CrossRef]
- Mourarak, F.; Atmani, R.; Maghri, I.; Elkouali, M.; Talbi, M.; Bouamrani, M.L.; Salouhi, M.; Kenz, A. Elimination of methylene blue dye with natural adsorbent-banana peels powder. GJSFR 2014, 14, 38–44. [Google Scholar]
- Ainane, T.; Khammour, F.; Belghazi, O.; Kabbaj, M.; Yousfi, S.; Talbi, M.M. Elkouali. Study and modelling of kinetics biosorption of methylene blue on biomass material from waste mint. Biotechnology 2015, 11, 281–285. [Google Scholar]
- Weng, C.-H.; Lin, Y.-T.; Tzeng, T.-W. Removal of methylene blue from aqueous solution by adsorption onto pineapple leaf powder. J. Hazard. Mater. 2009, 170, 417–424. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Abhinaya, R.V.; Gayathri Lashmi, K.; Arthi, V.; Pavithra, R.; Sathyaselvabala, V.; Sivanesan, S. Adsorption of methylene blue dye from aqueous solution by agricultural waste: Equilibrium, thermodynamics, kinetics, mechanism and process design. Colloid. J. 2011, 73, 651–661. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Ang, H.M. Equilibrium, kinetics, and thermodynamics of methylene blue adsorption by Pine tree leaves. Water Air Soil Pollut. 2012, 223, 5267–5282. [Google Scholar] [CrossRef]
- Uddin, M.T.; Islam, M.A.; Mahmud, S.; Rukanuzzaman, M. Adsorptive removal of methylene blue by tea waste. J. Hazard. Mater. 2009, 164, 53–60. [Google Scholar] [CrossRef]
- Honorato, A.C.; Machado, J.M.; Celante, G.; Borges, W.G.P.; Dragunski, D.C.; Caetano, J. Biosorption of methylene blue using agroindustrial residues. Rev. Bras. Eng. Agric. Amb. 2015, 19, 705–710. [Google Scholar] [CrossRef][Green Version]
- Rangabhashiyam, S.; Lata, S.; Balasubramanian, P. Biosorption characteristics of methylene blue and malachite green from simulated wastewater onto Carica papaya wood biosorbent. Surf. Interfaces 2018, 10, 197–215. [Google Scholar]
- Carvalho, L.B. Weeds, 1st ed.; Leonardo Bianco de Carvalho: Lages, Brazil, 2013; p. 82. [Google Scholar]
- Pimentel, A.M.R. Removal of Co (II) and Mn (II) from Aqueous Solutions Using the Biomass R. opacus. Master’s Thesis, PUC—Rio, Rio de Janeiro, Brazil, 2011. [Google Scholar]
- Cai, L.; Zhang, Y.; Zhou, Y.; Zhang, X.; Ji, L.; Song, W.; Liu, J. Effective adsorption of diesel oil by Crab-Shell-Derived biochar nanomaterials. Materials 2019, 12, 236. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, Y.; Wang, X.; Chen, Z. Rapid removal of zinc(II) from aqueous solutions using a mesoporous activated carbon prepared from agricultural waste. Materials 2017, 10, 1002. [Google Scholar] [CrossRef]
- Saeed, A.; Sharif, M.; Iqbal, M. Application potential of grapefruit peel as dye sorbent: Kinetics, equilibrium and mechanism of crystal violet adsorption. J. Hazard. Mater. 2010, 179, 564–572. [Google Scholar] [CrossRef]
- Alencar, W.S.; Acayanka, E.; Lima, E.C.; Royer, B.; de Souza, F.E.; Lameira, J.; Alves, C.N. Application of Mangifera indica (mango) seeds as a biosorbent for removal of Victazol Orange 3R dye from aqueous solution and study of the biosorption mechanism. Chem. Eng. J. 2012, 209, 577–588. [Google Scholar] [CrossRef]
- Tural, B.; Ertaş, E.; Enez, B.; Fincan, S.A.; Tural, S. Preparation and characterization of a novel magnetic biosorbent functionalized with biomass of Bacillus Subtilis: Kinetic and isotherm studies of biosorption processes in the removal of Methylene Blue. J. Environ. Chem. Eng. 2017, 5, 4795–4802. [Google Scholar] [CrossRef]
- Royer, B.; Cardoso, N.F.; Lima, E.C.; Ruiz, V.S.O.; Macedo, T.R.; Airoldi, C. Organofunctionalized kenyaite for dye removal from aqueous solution. J. Colloid Interface Sci. 2009, 336, 398–405. [Google Scholar] [CrossRef]
- Royer, B.; Cardoso, N.F.; Lima, E.C.; Macedo, T.R.; Airoldi, C. A useful organofunctionalized layered silicate for textile dye removal. J. Hazard. Mater. 2010, 181, 366–374. [Google Scholar] [CrossRef]
- Da Silva, L.G.; Ruggiero, R.; Gontijo, P.M.; Pinto, R.B.; Royer, B.; Lima, E.C.; Calvete, T. Adsorption of Brilliant Red 2BE dye from water solutions by a chemically modified sugarcane bagasse lignin. Chem. Eng. J. 2011, 168, 620–628. [Google Scholar] [CrossRef]
- Cardoso, N.F.; Pinto, R.B.; Lima, E.C.; Calvete, T.; Amavisca, C.V.; Royer, B.; Pinto, I.S. Removal of remazol black B textile dye from aqueous solution by adsorption. Desalination 2011, 269, 92–103. [Google Scholar] [CrossRef]
- Calvete, T.; Lima, E.C.; Cardoso, N.F.; Dias, S.L.P.; Pavan, F.A. Application of carbon adsorbents prepared from the Brazilian pine-fruit-shell for the removal of Procion Red MX 3B from aqueous solution—Kinetic, equilibrium, and thermodynamic studies. Chem. Eng. J. 2009, 155, 627–636. [Google Scholar] [CrossRef]
- Calvete, T.; Lima, E.C.; Cardoso, N.F.; Vaghetti, J.C.P.; Dias, S.L.P.; Pavan, F.A. Application of carbon adsorbents prepared from Brazilian-pine fruit shell for the removal of reactive orange 16 from aqueous solution: Kinetic, equilibrium, and thermodynamic studies. J. Environ. Manag. 2010, 91, 1695–1706. [Google Scholar] [CrossRef]
- Hassan, W.; Farooq, U.; Ahmad, M.; Athar, M.; Khan, M.A. Potential biosorbent, Haloxylon recurvum plant stems, for the removal of methylene blue dye. Arab. J. Chem. 2017, 10, 1512–1522. [Google Scholar] [CrossRef]
- Miraboutalebi, S.M.; Nikouzad, S.K.; Peydayesh, M.; Allahgholi, N.; Vafajoo, L.; McKay, G. Methylene blue adsorption via maize silk powder: Kinetic, equilibrium, thermodynamic studies and residual error analysis. Process Saf. Environ. Prot. 2017, 106, 191–202. [Google Scholar] [CrossRef]
- Leal, P.V.B.; Gregório, A.M.; Otoni, E.; da Silva, P.R.; de Krauser, M.O.; Holzbach, J.C. Study of adsorption of methylene blue dye on babassu. J. Biotec. Biodiv. 2012, 3, 166–171. [Google Scholar]
- Nascimento, R.F.; Lima, A.C.A.; Vidal, C.B.; Melo, D.Q.; Raulino, G.S.C. Adsorption: Theoretical Aspects and Environmental Applications, 1st ed.; UFC: Fortaleza, Brazil, 2014; p. 256. [Google Scholar]
- Tang, Y.; Zeng, Y.; Hu, T.; Zhou, Q.; Peng, Y. Preparation of lignin sulfonate-based mesoporous materials for adsorbing malachite green from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 2900–2910. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Limousin, G.; Gaudet, J.-P.; Charlet, L.; Szenknect, S.; Barthès, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Pal, S.; Ghorai, S.; Das, C.; Samrat, S.; Ghosh, A.; Panda, A.B. Carboxymethyl tamarind-g-poly (acrylamide)/silica: A high performance hybrid nanocomposite for adsorption of methylene blue dye. Ind. Eng. Chem. Res. 2012, 51, 15546–15556. [Google Scholar] [CrossRef]
- Freundlich, H.M. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Alfredo, A.P.C.; Gonçalves, G.C.; Lobo, V.S.; Montanher, S.F. Adsorção de azul de metileno em casca de batata utilizando sistemas em batelada e coluna de leito fixo. Rev. Virtual Quim. 2015, 7, 1909–1920. [Google Scholar] [CrossRef]
- Afshariani, F.; Roosta, A. Experimental study and mathematical modeling of biosorption of methylene blue from aqueous solution in a packed bed of microalgae Scenedesmus. J. Clean. Prod. 2019, 225, 133–142. [Google Scholar] [CrossRef]
- Kumar, A.; Jena, H.M. Removal of methylene blue and phenol onto prepared activated carbon from Fox nutshell by chemical activation in batch and fixed-bed column. J. Clean. Prod. 2016, 137, 1246–1259. [Google Scholar] [CrossRef]
- Pang, J.; Fu, F.; Ding, Z.; Lu, J.; Li, N.; Tang, B. Adsorption behaviors of methylene blue from aqueous solution on mesoporous birnessite. J. Taiwan Inst. Chem. E 2017, 77, 168–176. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Rahmanian, O.; Fazlzadeh, M.; Heidari, M. Enhancement of methylene blue adsorption onto activated carbon prepared from Date Press Cake by low frequency ultrasound. J. Mol. Liq. 2018, 264, 591–599. [Google Scholar] [CrossRef]
- Da Rocha, O.R.S.; do Nascimento, G.E.; Campos, N.F.; da Silva, V.L.; Duarte, M.M.M.B. Evaluation of the adsorptive process using green coconut mesocarp for the removal of the reactive gray dye BF-2R. Quím. Nova 2012, 35, 1369–1374. [Google Scholar]



| Cationic Dye | Molecular Structure | Chemical Formula |
|---|---|---|
| Methylene blue | 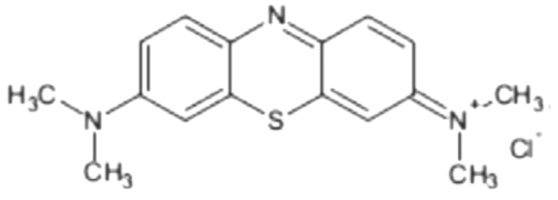 | C16H18N3SCl |
| Sample | Freundlich | Langmuir | |||||
|---|---|---|---|---|---|---|---|
| n | kF | R² | qmax (mg/g) | kL | R² | RL | |
| PMT | 1.7495 | 13.0798 | 0.9590 | 56.1798 | 0.3048 | 0.9806 | 0.2470–0.0616 |
| CCLHR | 1.4343 | 10.0716 | 0.9837 | 76.3359 | 0.1436 | 0.9657 | 0.4104–0.1222 |
| Model | Equation | Linearized Formula |
|---|---|---|
| Pseudo-first-order | ||
| Pseudo-second-order |
| Kinetic Model | Parameter | PMT | CCLHR |
|---|---|---|---|
| Pseudo-first-order | k1 (mg·min/g) | 0.1093 | 0.1081 |
| qe (mg/g) | 14.2554 | 14.1435 | |
| R2 | 0.5902 | 0.5950 | |
| Pseudo-second-order | k2 (mg·min/g) | 0.1476 | 0.0815 |
| qe (mg/g) | 14.3062 | 14.2248 | |
| R2 | 0.9998 | 0.9999 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, F.; Nascimento, L.; Brito, M.; da Silva, K.; Paschoal, W.; Fujiyama, R. Biosorption of Methylene Blue Dye Using Natural Biosorbents Made from Weeds. Materials 2019, 12, 2486. https://doi.org/10.3390/ma12152486
Silva F, Nascimento L, Brito M, da Silva K, Paschoal W, Fujiyama R. Biosorption of Methylene Blue Dye Using Natural Biosorbents Made from Weeds. Materials. 2019; 12(15):2486. https://doi.org/10.3390/ma12152486
Chicago/Turabian StyleSilva, Francisco, Lorena Nascimento, Matheus Brito, Kleber da Silva, Waldomiro Paschoal, and Roberto Fujiyama. 2019. "Biosorption of Methylene Blue Dye Using Natural Biosorbents Made from Weeds" Materials 12, no. 15: 2486. https://doi.org/10.3390/ma12152486
APA StyleSilva, F., Nascimento, L., Brito, M., da Silva, K., Paschoal, W., & Fujiyama, R. (2019). Biosorption of Methylene Blue Dye Using Natural Biosorbents Made from Weeds. Materials, 12(15), 2486. https://doi.org/10.3390/ma12152486






