Effect of Eggshell Powder on the Hydration of Cement Paste
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
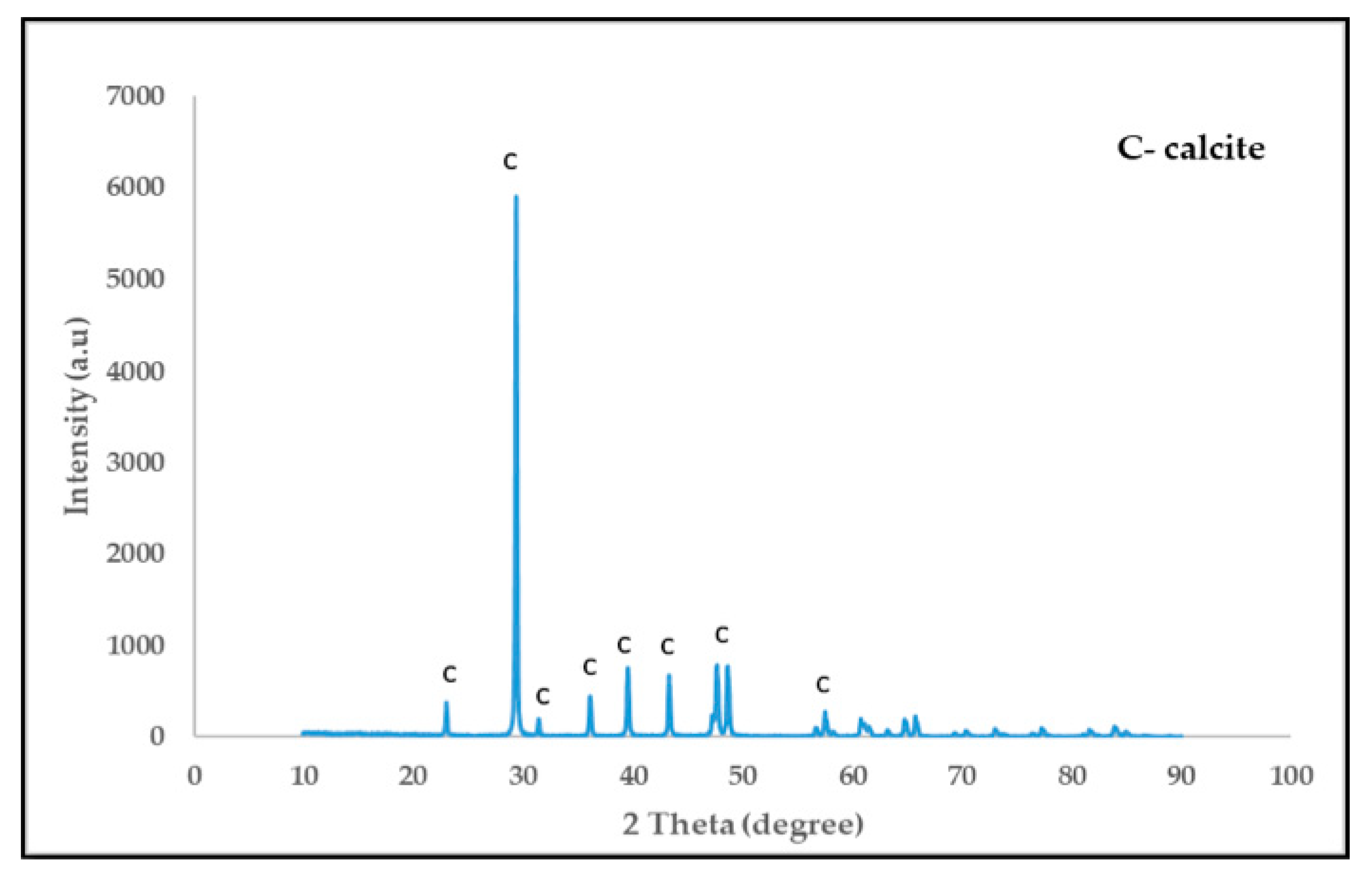
2.3. Characterization
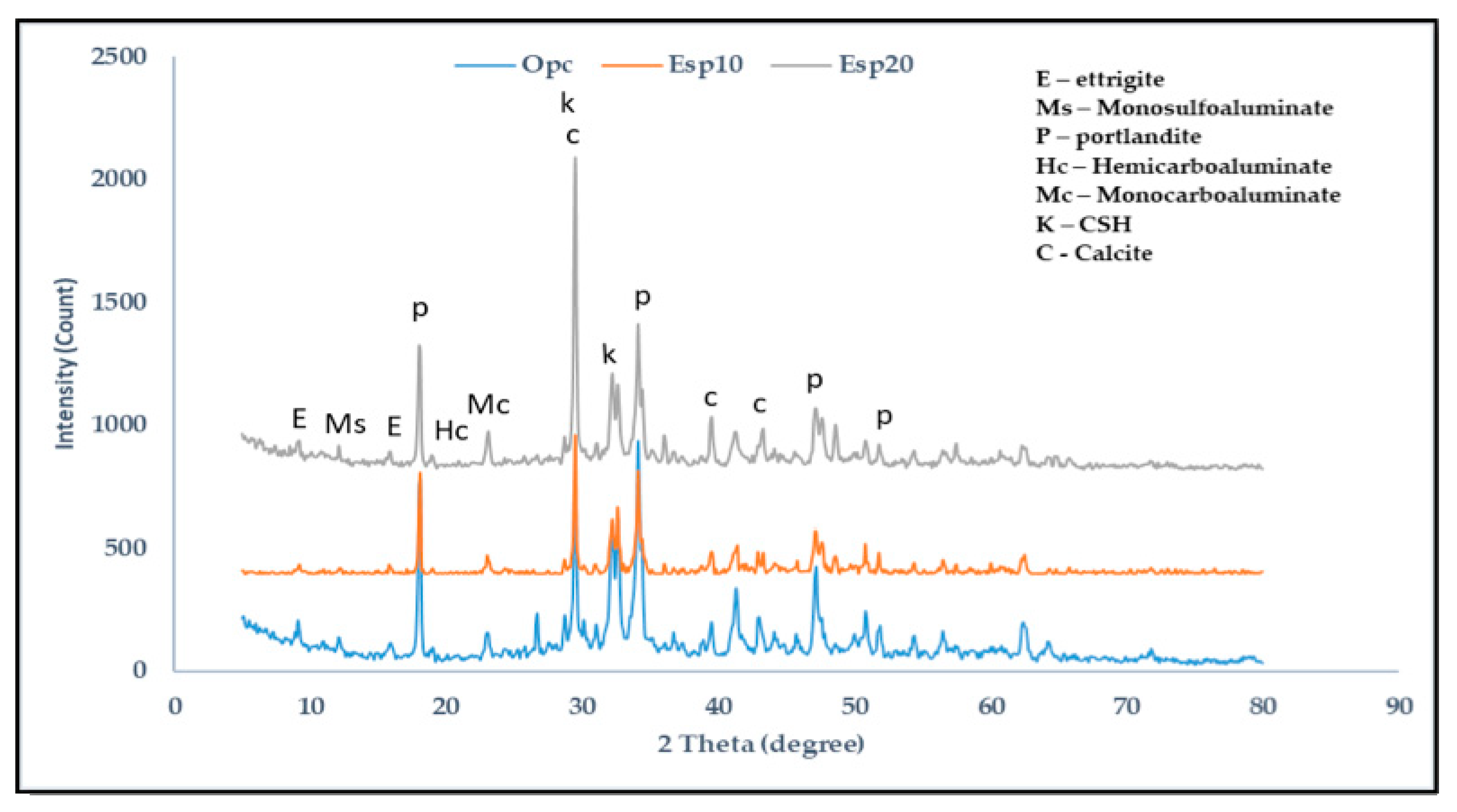
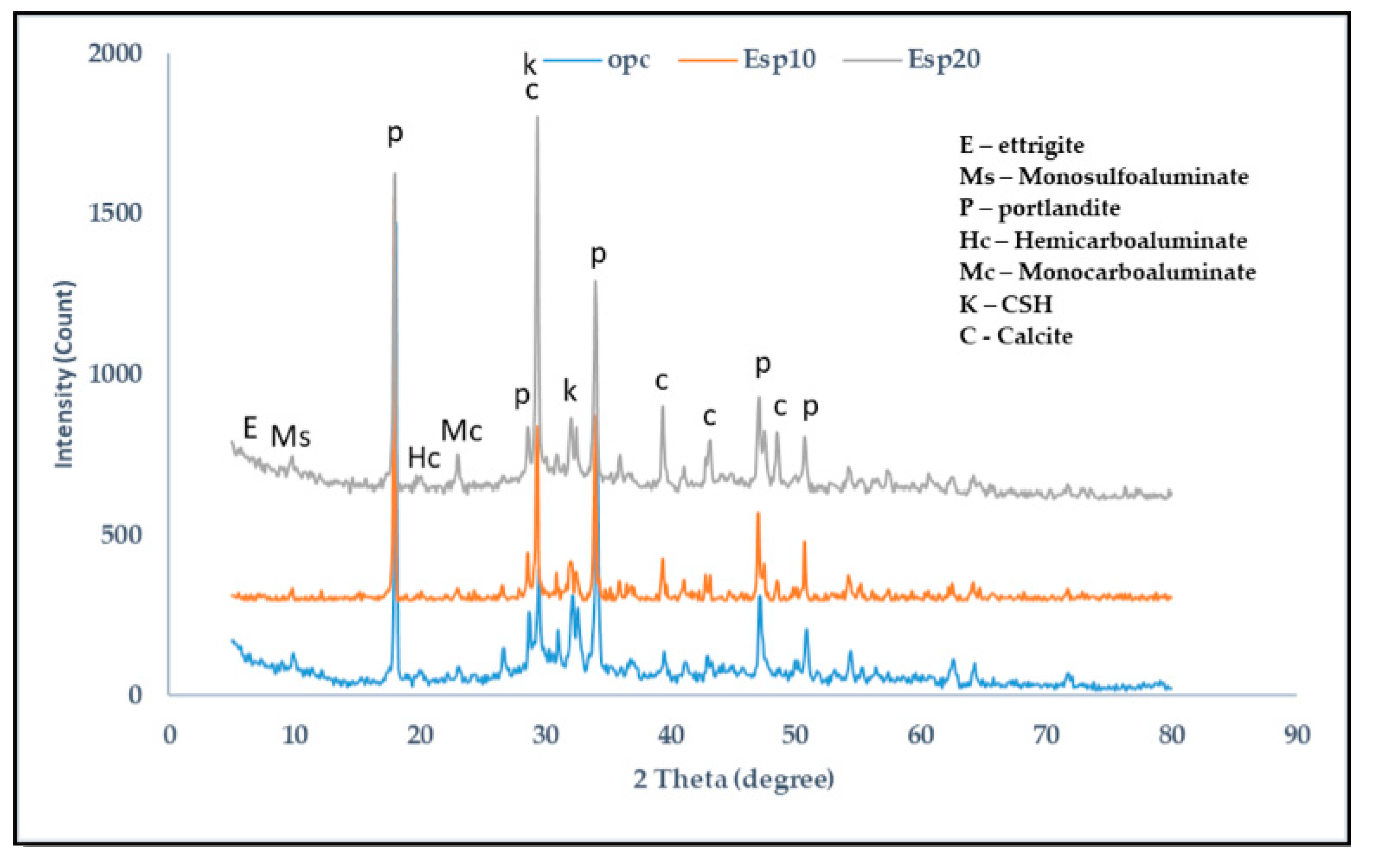
3. Results and Discussion
3.1. Activity Test
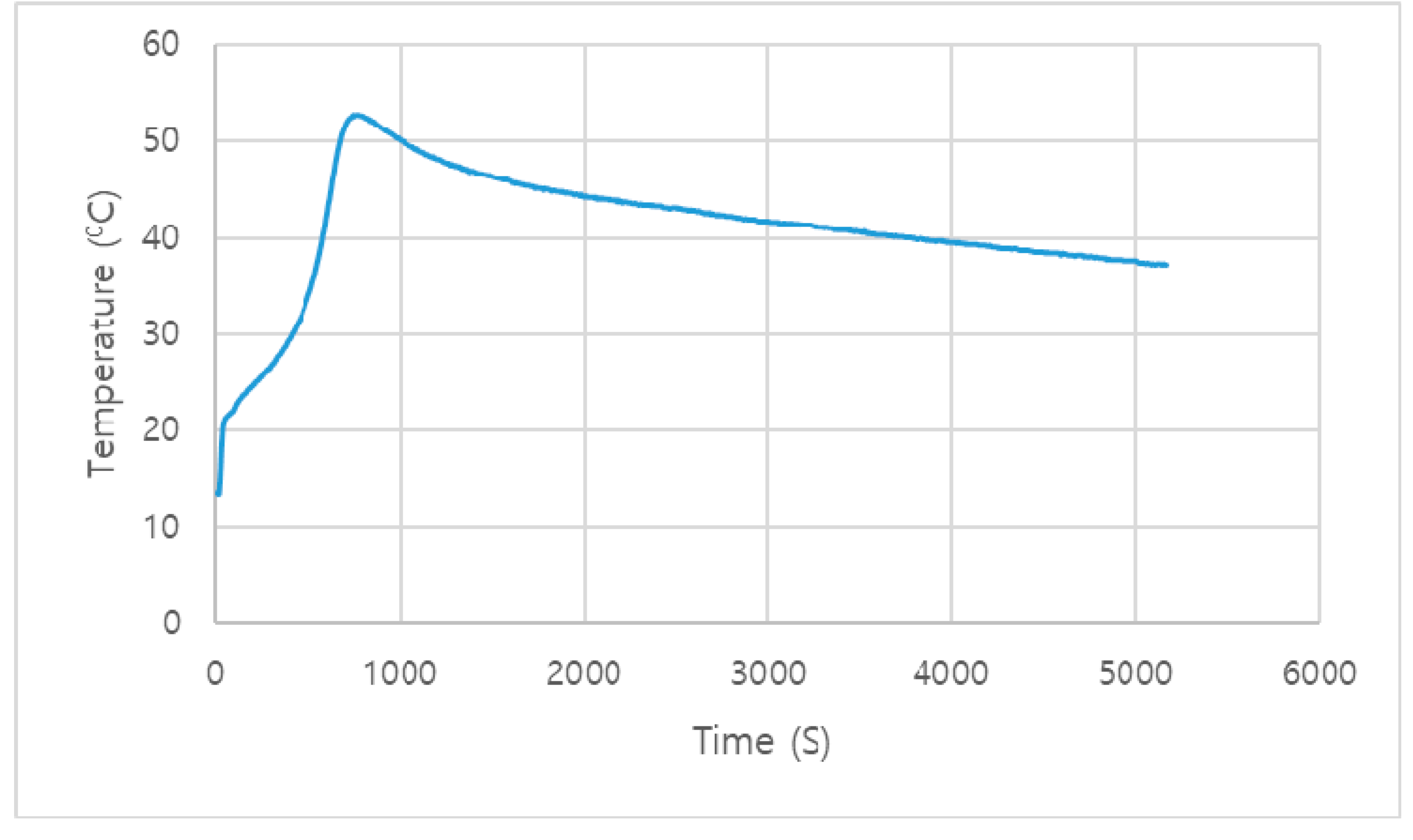
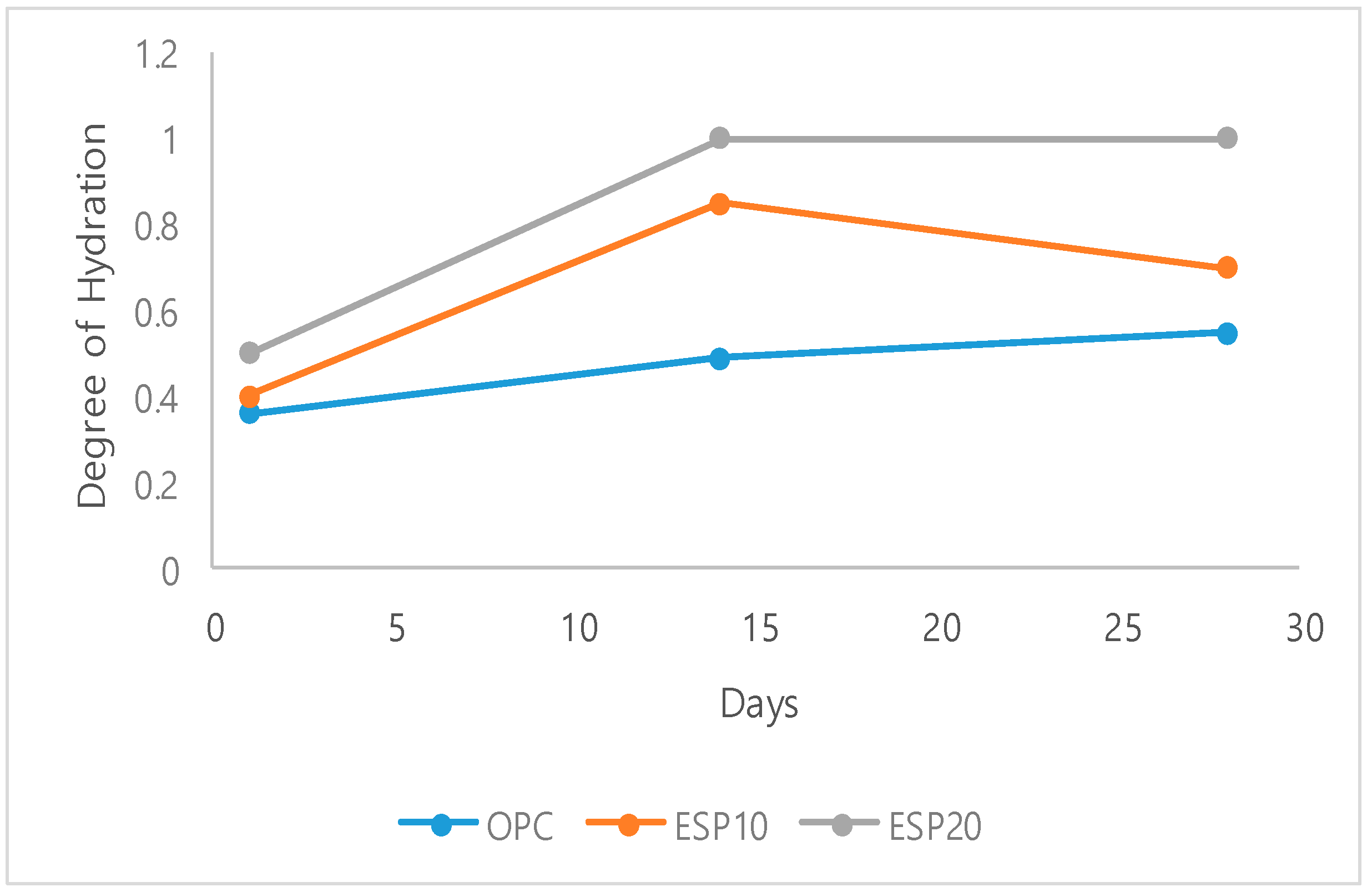
3.2. Aging Test
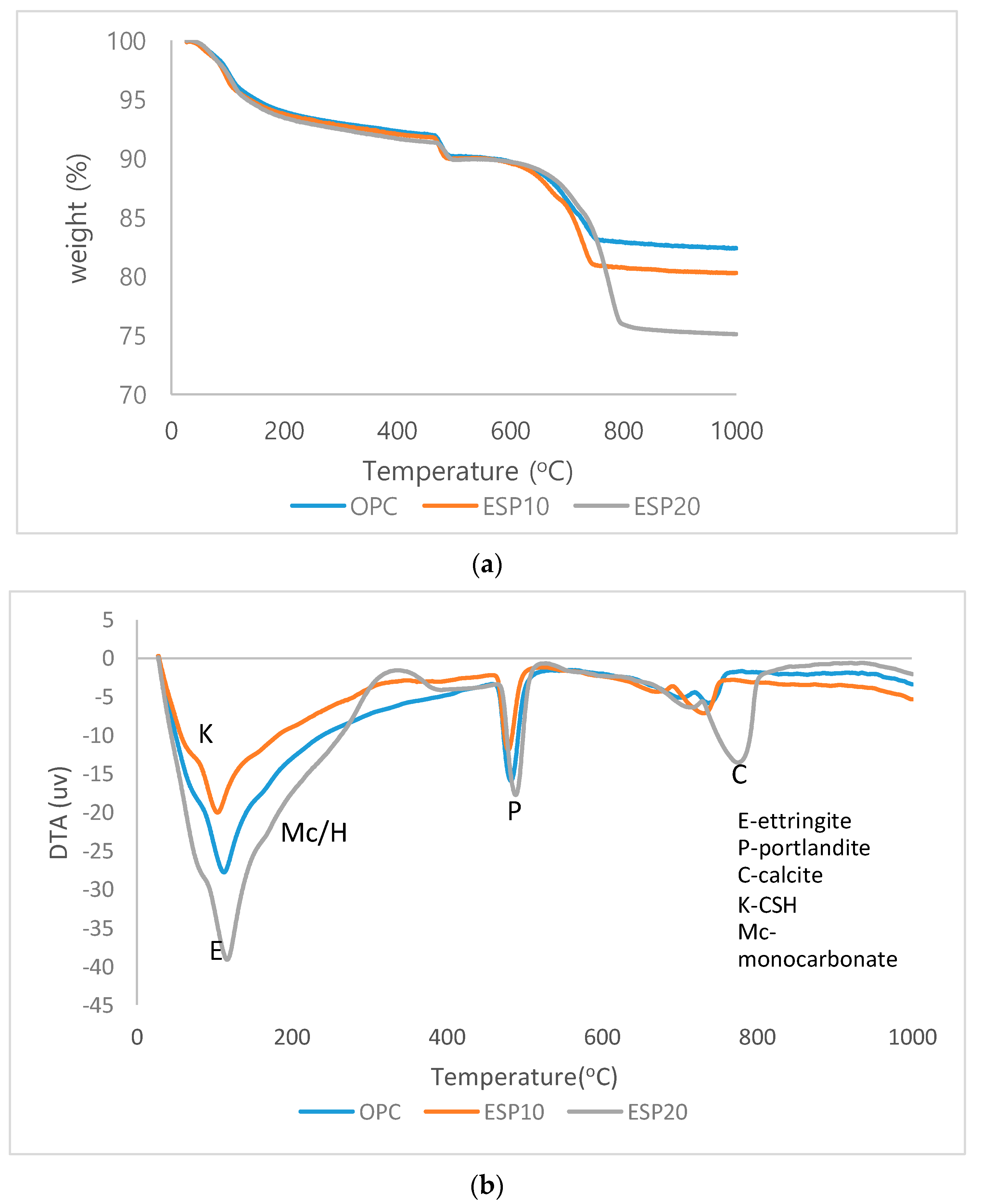
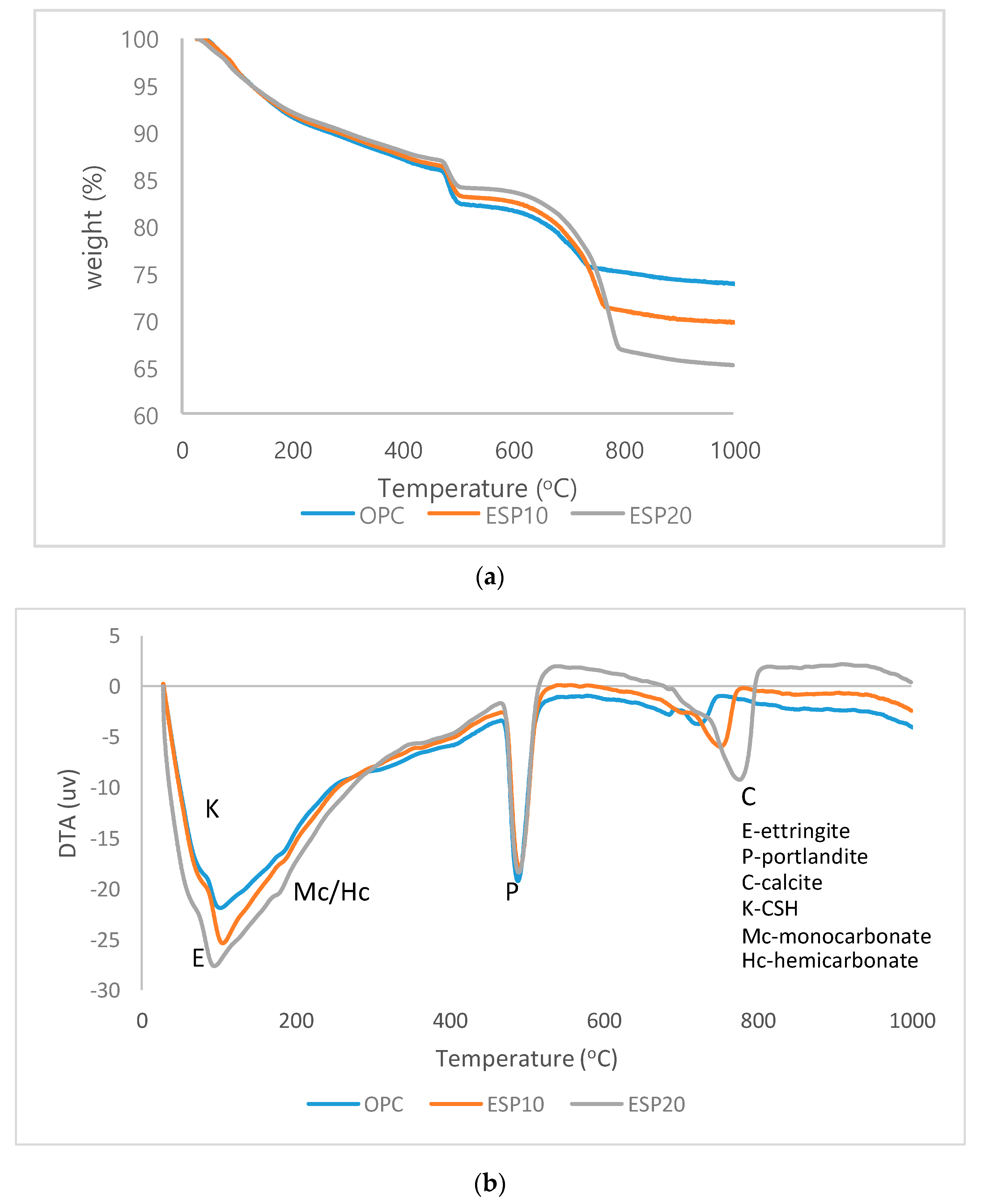
3.3. TGA/DTA Analysis
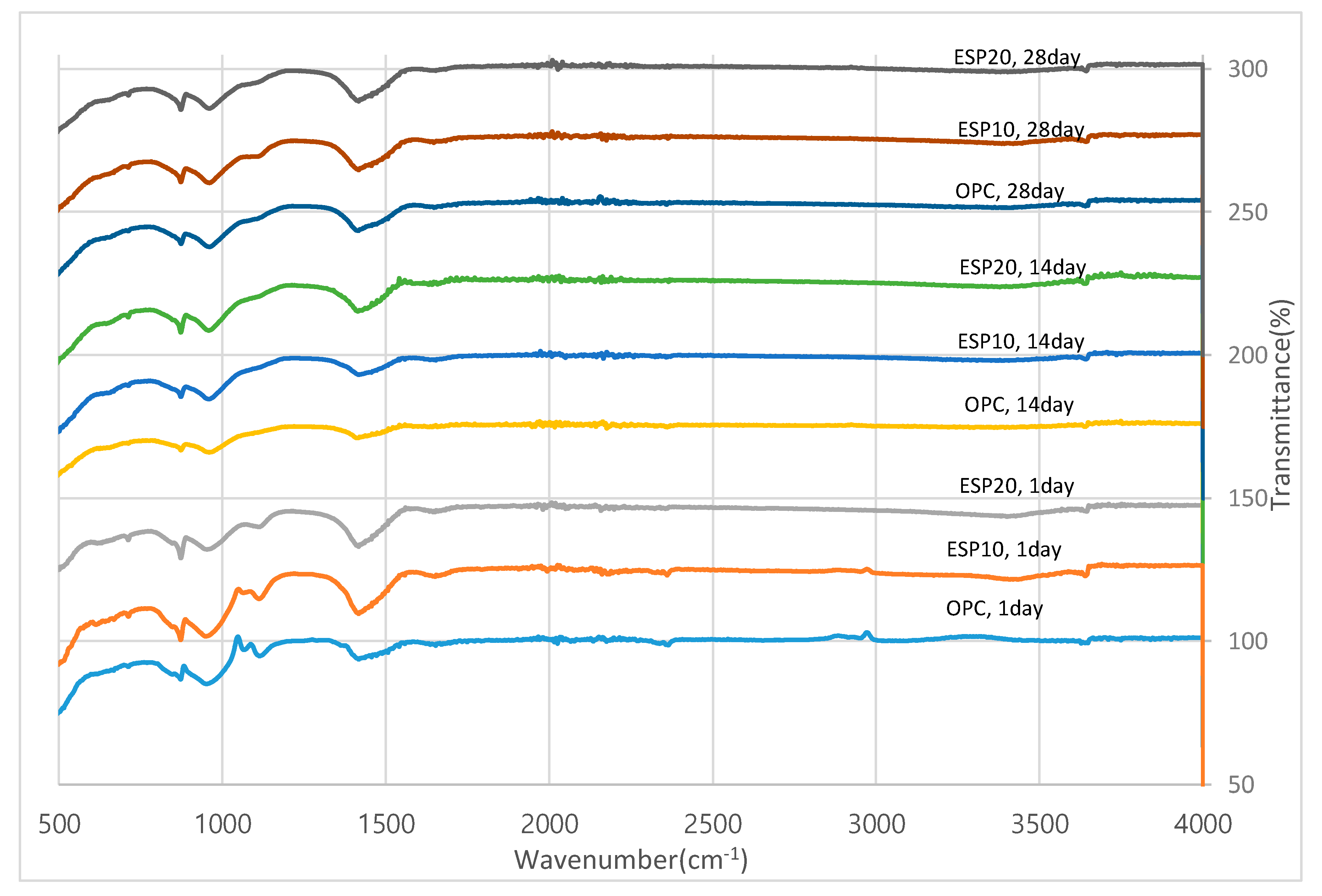
3.4. FT-IR Analysis
3.5. SEM Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barcelo, L.; Kline, J. Cement and carbon emissions. Mater. Struct. 2014, 47, 1055–1065. [Google Scholar] [CrossRef]
- Ejaz, N.; Akhtar, N.; Nisar, H.; Naeem, U.A. Environmental impacts of improper solid waste management in developing countries: A case study of Rawalpindi City. WIT Trans. Ecol. Environ. 2010, 142, 379–387. [Google Scholar] [CrossRef]
- Golewski, G.L. Effect of fly ash addition on the fracture toughness of plain concrete at third model of fracture. J. Civ. Eng. Manag. 2017, 23, 613–620. [Google Scholar] [CrossRef]
- Golewski, G.L. The influence of microcrack width on the mechanical parameters in concrete with the addition of fly ash: Consideration of technological and ecological benefits. Constr. Build. Mater. 2019, 197, 849–861. [Google Scholar] [CrossRef]
- Cree, D.; Rutter, A. Sustainable Bio-Inspired Limestone Eggshell Powder for Potential Industrialized Applications. ACS Sustain. Chem. Eng. 2015, 3, 941–949. [Google Scholar] [CrossRef]
- Kitchen Waste Piling up in Edmonton Landfill Since Compost Facility Closed Last Fall. Available online: https://www.cbc.ca/news/canada/edmonton/landfill-edmonton-waste-management-system-1.4565158 (accessed on 26 July 2019).
- Intharapat, P.; Kongnoo, A. The Potential of Chicken Eggshell Waste as a Bio-filler Filled Epoxidized Natural Rubber (ENR) Composite and its Properties. J. Polym. Environ. 2013, 21, 245–258. [Google Scholar] [CrossRef]
- Bonavetti, V.; Donza, H.; Menendez, G.; Cabrera, O.; Irassar, E.F. Limestone filler cement in low w/c concrete: A Rational Use of Energy. Cem. Concr. Res. 2003, 33, 865–871. [Google Scholar] [CrossRef]
- Pliya, P.; Cree, D. Limestone derived eggshell powder as a replacement in Portland cement mortar. Constr. Build. Mater. 2015, 95, 1–9. [Google Scholar] [CrossRef]
- Algin, H.M.; Turgut, P. Cotton and limestone powder wastes as brick material. Constr. Build. Mater. 2008, 22, 1074–1080. [Google Scholar] [CrossRef]
- Aziz, H.A.; Adlan, M.N.; Ariffin, K.S. Heavy metals (Cd, Pb. Zn, Ni, Cu and Cr(Ⅲ)) removal from water in Malaysia: Post treatment by high quality limestone. Bioresour. Technol. 2008, 99, 1578–1583. [Google Scholar] [CrossRef]
- European Committee for Standardization EN 197-1. Cement—Part 1, Composition, Specifications and Uniformity Criteria for Common Cements; European Committee for Standardization: Brussels, Belgium, 2000. [Google Scholar]
- Mtallib, M.O.; Rabiu, A. Effects of eggshell ash (ESA) on the setting time of cement. Niger. J. Technol. 2009, 28, 29–38. [Google Scholar]
- Moon, G.D.; Oh, S.; Jung, S.H.; Choi, Y.C. Effects of the fineness of limestone powder and cement on the hydration and strength development of PLC concrete. Constr. Build. Mater. 2017, 135, 129–136. [Google Scholar] [CrossRef]
- Ellerbrock, H.G.; Spung, S.; Kuhlmann, K. Particle size distribution and properties of cement: Part Ⅲ. Influence of grinding process. Zem. Kalk Gips 1990, 43, 13–19. [Google Scholar]
- Ramachandran, V.S. Hydration Kinetics and microstructural development in the 3CaO. Al2O3-CaSO4. 2H2O-CaCO3-H2O system. Mater. Struct. 1986, 19, 437–444. [Google Scholar] [CrossRef]
- Gonzalez, M.A.; Irassar, E.F. Effect of Limestone Filler on the Sulfate Resistance of Low C3A Portland cement. Cem. Concr. Res. 1998, 28, 1655–1667. [Google Scholar] [CrossRef]
- Stark, J.; Freyburg, E.; Lohmer, K. Investigation into the influence of limestone additions to Portland cement clinker phases on the early phase of hydration. In Proceedings of the Modern Concrete Material: Binders, Additions and Admixtures, Scotland, UK, 8–10 September 1999; pp. 69–77. [Google Scholar]
- Bonavetti, V.L.; Rahhal, V.F.; Irassar, E.F. Studies on the carboaluminate formation in limestone filler-blended cements. Cem. Concr. Res. 2001, 31, 853–859. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The AFm phase in Portland cement. Cem. Concr. Res. 2007, 37, 118–130. [Google Scholar] [CrossRef]
- Lothenbach, B.; Saout, G.L.; Gallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cem. Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]
- Feldman, R.F.; Ramachandran, V.S.; Sereda, P.J. Influence of CaCO3 on the Hydration of 3CaO·Al2O3. J. Am. Ceram. Soc. 1965, 48, 25–30. [Google Scholar] [CrossRef]
- Ramachandran, V.S. Thermal Analysis of Cement Components Hydrated in the Presence of Calcium Carbonate. Thermochim. Acta 1988, 127, 385–394. [Google Scholar] [CrossRef]
- Kakali, G.; Tsivilis, S.; Aggeli, E.; Bati, M. Hydration products of C3A, C3S and Portland cement in the presence of CaCO3. Cem. Concr. Res. 2000, 30, 1073–1077. [Google Scholar] [CrossRef]
- Gabrovsek, R.; Vuk, T.; Kaucic, V. Evaluation of the Hydration of Portland Cement Containing Various Carbonates by Means of Thermal Analysis. Acta Chim. Slov. 2006, 53, 159–165. [Google Scholar]
- Musa, N.M. Thermal Analysis of Cement Paste Partially Replaced with Neem Seed Husk Ash. Int. J. Sci. Eng. Res. 2014, 5, 1101–1105. [Google Scholar]
- Darweesh, H.H.; Abou-El-Anwar, E.A.; Mekky, H.S. Addition of Limestone at the Expense of Gypsum in Portland Cement. Interceram 2018, 67, 18–27. [Google Scholar] [CrossRef]
- Trezza, M.A.; Lavat, A.E. Analysis of the system 3CaO.Al2O3-CaSO4.2H2O-CaCO3-H2O by FT-IR spectroscopy. Cem. Concr. Res. 2001, 31, 869–872. [Google Scholar] [CrossRef]
- Yu, P.; Kirkpatrick, R.J.; Poe, B.; McMillan, P.F.; Cong, X. Structure of calcium silicate hydrate (C-S-H): Near, mid, and far-infrared spectroscopy. J. Am. Ceram. Soc. 1999, 82, 742–748. [Google Scholar] [CrossRef]
- Baquerizo, L.G.; Matschei, T.; Scrivener, K.L.; Saeidpour, M.; Wadso, L. Hdration states of AFm cement phases. Cem. Concr. Res. 2015, 73, 143–157. [Google Scholar] [CrossRef]




| Chemical Composition | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | LOI |
|---|---|---|---|---|---|---|---|---|
| OPC | 20.99 | 6.19 | 3.86 | 65.96 | 0.22 | 0.17 | 0.60 | 1.73 |
| Eggshell | 0.01 | 0.01 | 0.01 | 52.75 | 0.51 | 0.05 | 0.04 | 46.62 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiferaw, N.; Habte, L.; Thenepalli, T.; Ahn, J.W. Effect of Eggshell Powder on the Hydration of Cement Paste. Materials 2019, 12, 2483. https://doi.org/10.3390/ma12152483
Shiferaw N, Habte L, Thenepalli T, Ahn JW. Effect of Eggshell Powder on the Hydration of Cement Paste. Materials. 2019; 12(15):2483. https://doi.org/10.3390/ma12152483
Chicago/Turabian StyleShiferaw, Natnael, Lulit Habte, Thriveni Thenepalli, and Ji Whan Ahn. 2019. "Effect of Eggshell Powder on the Hydration of Cement Paste" Materials 12, no. 15: 2483. https://doi.org/10.3390/ma12152483
APA StyleShiferaw, N., Habte, L., Thenepalli, T., & Ahn, J. W. (2019). Effect of Eggshell Powder on the Hydration of Cement Paste. Materials, 12(15), 2483. https://doi.org/10.3390/ma12152483






