Biocompatibility and Mineralization Activity of Three Calcium Silicate-Based Root Canal Sealers Compared to Conventional Resin-Based Sealer in Human Dental Pulp Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Dental Pulp Stem Cells (hDPSCs)
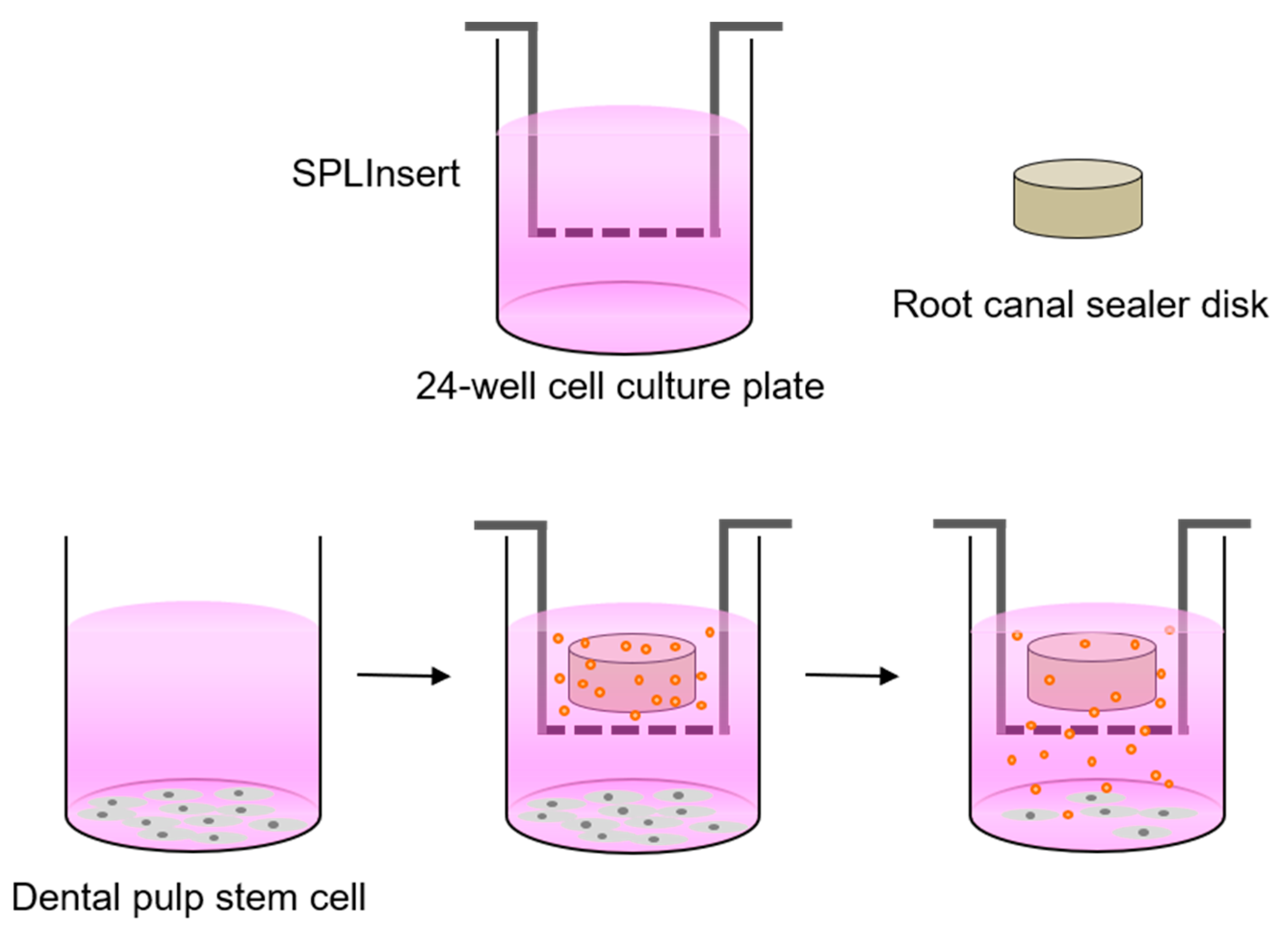
2.2. Experimental Disks of Various Root Canal Sealers
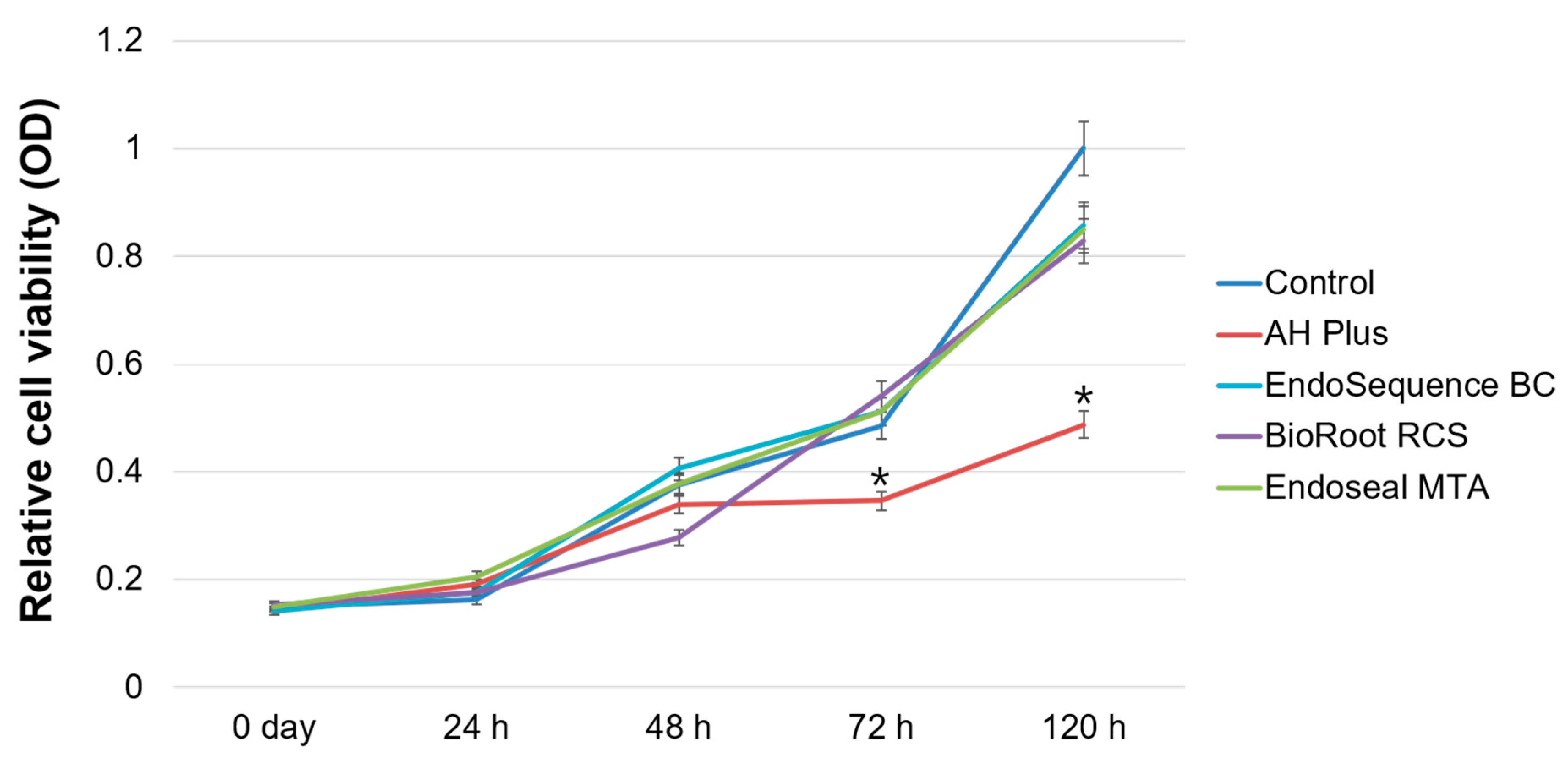
2.3. Cell Viability Assay
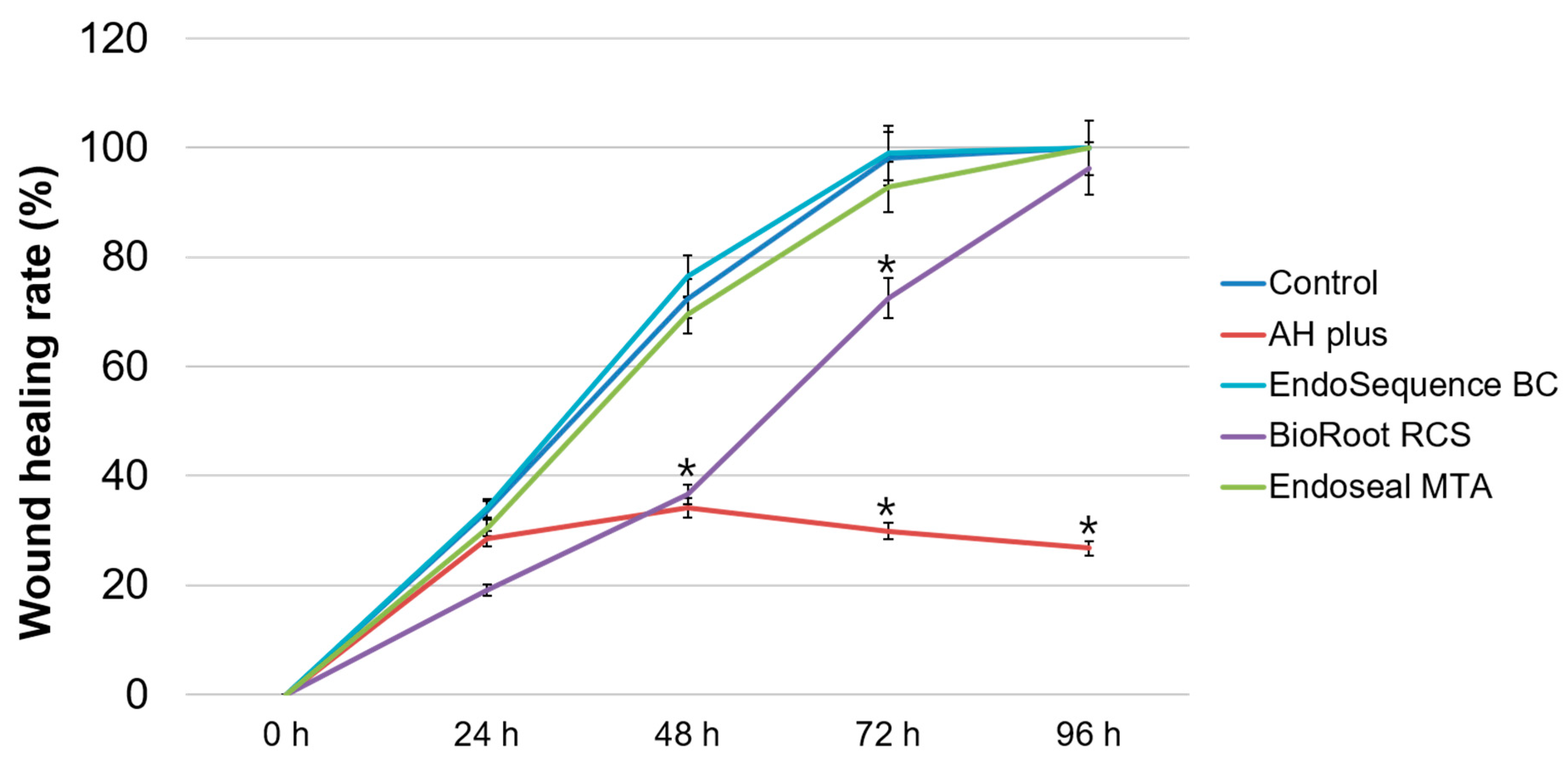
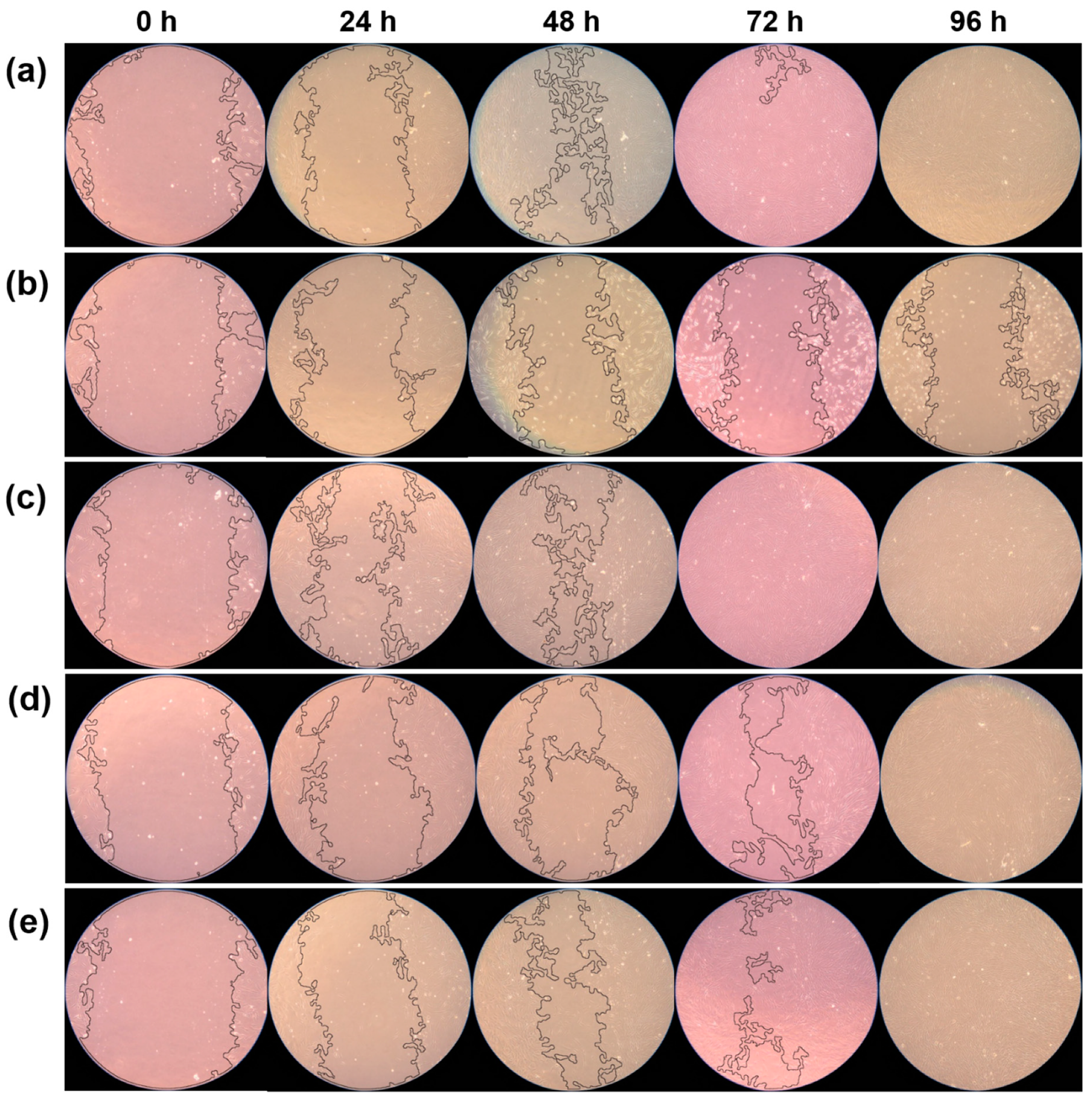
2.4. Cell Migration Assay
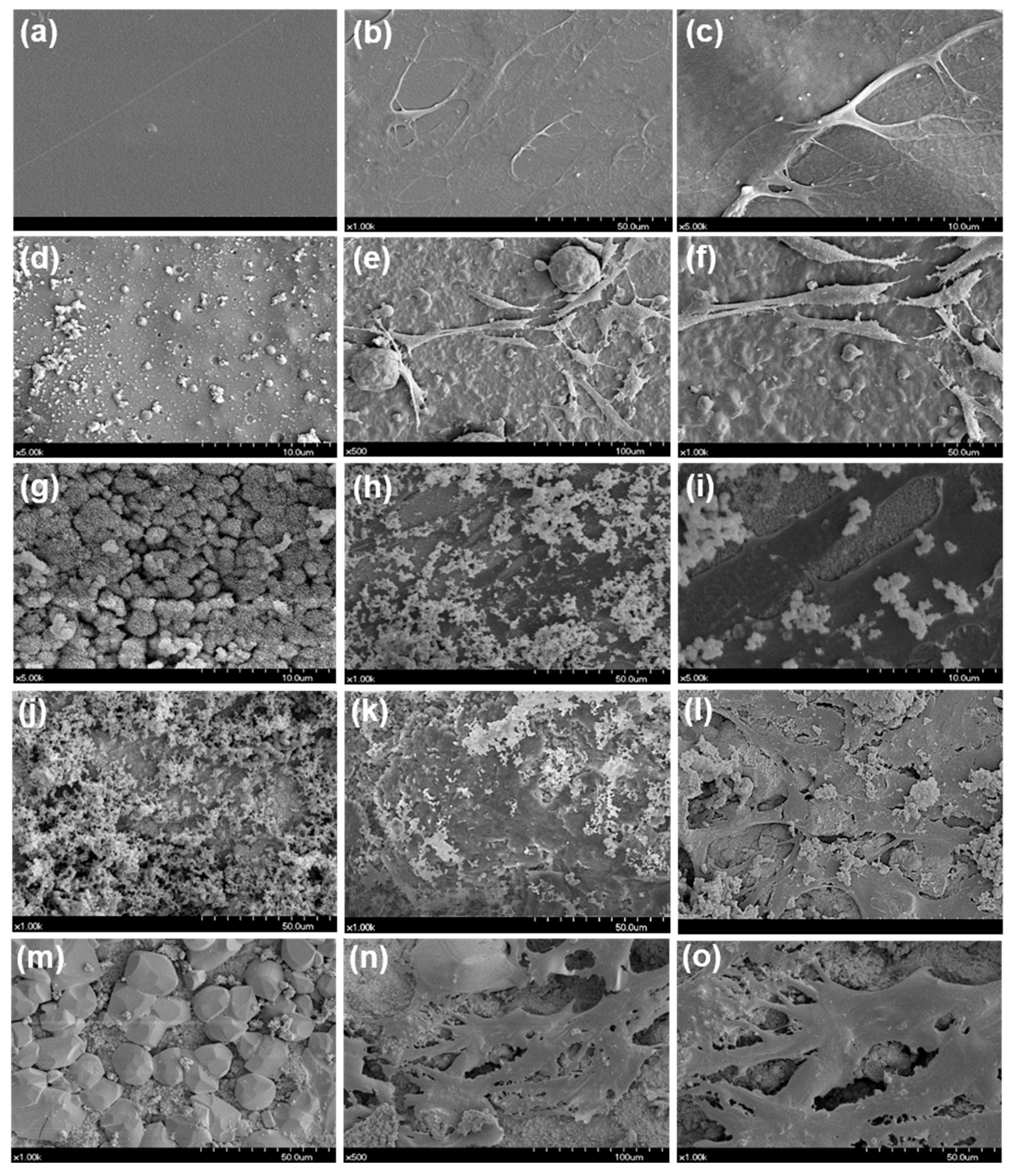
2.5. Cellular Morphology Evaluation
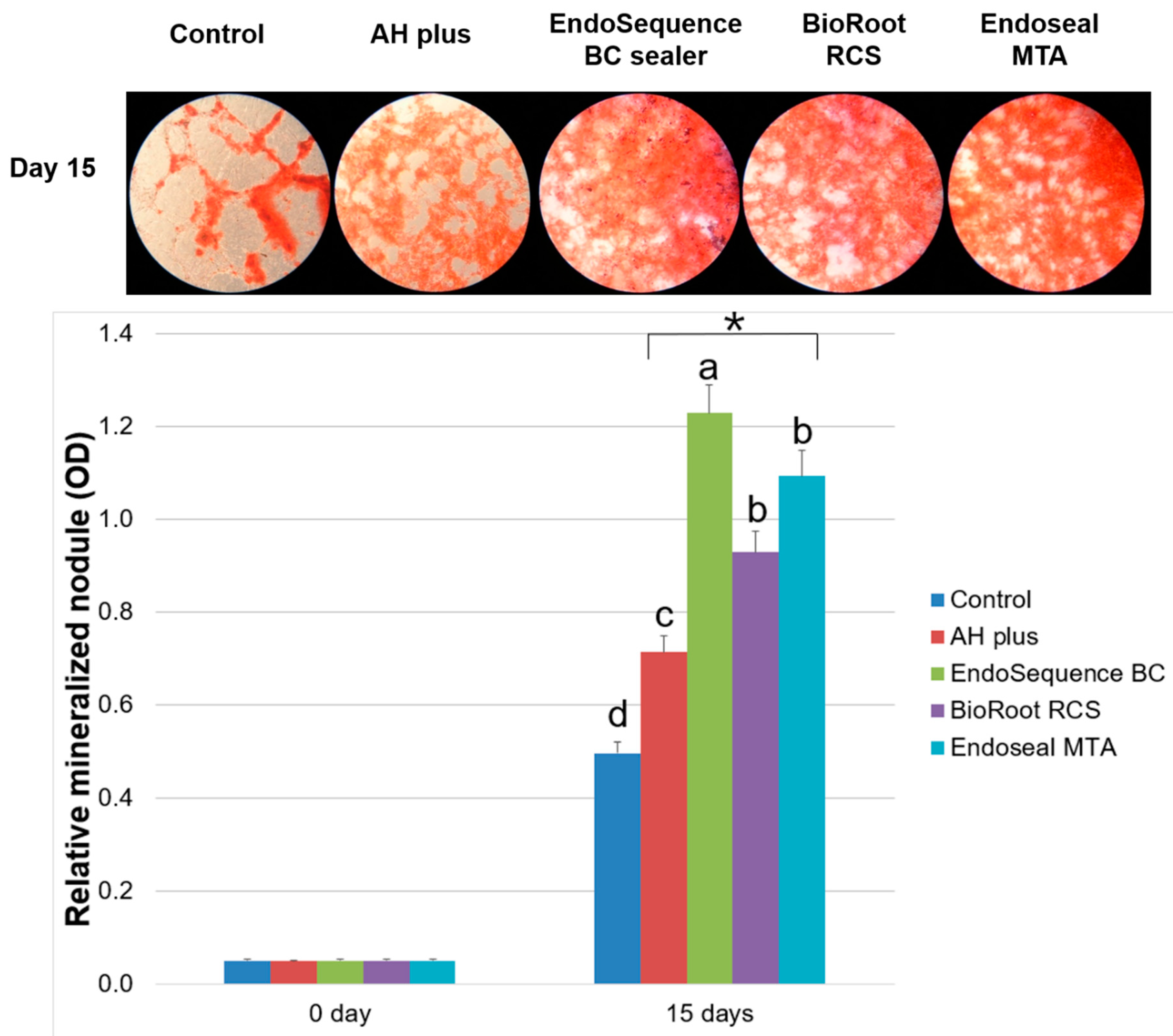
2.6. Alizarin Red Staining Assay
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kirkevang, L.L.; Orstavik, D.; Horsted-Bindslev, P.; Wenzel, A. Periapical status and quality of root fillings and coronal restorations in a Danish population. Int. Endod. J. 2000, 33, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F.; Rocas, I.N.; Alves, F.R.; Campos, L.C. Periradicular status related to the quality of coronal restorations and root canal fillings in a Brazilian population. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 100, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Tavares, P.B.; Bonte, E.; Boukpessi, T.; Siqueira, J.F.; Lasfargues, J.J. Prevalence of apical periodontitis in root canal-treated teeth from an urban French population: Influence of the quality of root canal fillings and coronal restorations. J. Endod. 2009, 35, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Mamootil, K.; Messer, H.H. Penetration of dentinal tubules by endodontic sealer cements in extracted teeth and in vivo. Int. Endod. J. 2007, 40, 873–881. [Google Scholar] [CrossRef]
- Bouillaguet, S.; Shaw, L.; Barthelemy, J.; Krejci, I.; Wataha, J.C. Long-term sealing ability of Pulp Canal Sealer, AH-Plus, GuttaFlow and Epiphany. Int. Endod. J. 2008, 41, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.G.; Araujo, S.P.R.; Silveira, M.T.; Sobral, V.A.P.; Carvalho, M.V. Comparison of the biocompatibility of calcium silicate-based materials to mineral trioxide aggregate: Systematic review. Eur. J. Dent. 2018, 12, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Sielker, S.; Hanisch, M.R.; Libricht, V.; Schafer, E.; Dammaschke, T. Cytotoxic effects of four different root canal sealers on human osteoblasts. PLoS ONE 2018, 13, e0194467. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, B.S.; Kim, Y.M.; Lee, D.; Kim, S.Y. The penetration ability of calcium silicate root canal sealers into dentinal tubules compared to conventional resin-based sealer: A confocal laser scanning microscopy study. Materials (Basel) 2019, 12, 531. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, K.J.; Yi, Y.A.; Seo, D.G. Quantitative microleakage analysis of root canal filling materials in single-rooted canals. Scanning 2015, 37, 237–245. [Google Scholar] [CrossRef]
- Zoufan, K.; Jiang, J.; Komabayashi, T.; Wang, Y.H.; Safavi, K.E.; Zhu, Q. Cytotoxicity evaluation of Gutta Flow and Endo Sequence BC sealers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, 657–661. [Google Scholar] [CrossRef]
- Bernath, M.; Szabo, J. Tissue reaction initiated by different sealers. Int. Endod. J. 2003, 36, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.M.; Shen, Y.; Zheng, W.; Li, L.; Zheng, Y.F.; Haapasalo, M. Physical properties of 5 root canal sealers. J. Endod. 2013, 39, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Giacomino, C.M.; Wealleans, J.A.; Kuhn, N.; Diogenes, A. Comparative biocompatibility and osteogenic potential of two bioceramic sealers. J. Endod. 2019, 45, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.N.; Hong, J.U.; Kim, S.M.; Jang, J.H.; Chang, H.S.; Hwang, Y.C.; Hwang, I.N.; Oh, W.M. Anti-inflammatory and osteogenic effects of calcium silicate-based root canal sealers. J. Endod. 2019, 45, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Zaia, A.A.; Peters, O.A. Cytocompatibility of calcium silicate-based sealers in a three-dimensional cell culture model. Clin. Oral Investig. 2017, 21, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Candeiro, G.T.M.; Moura-Netto, C.; D’Almeida-Couto, R.S.; Azambuja-Junior, N.; Marques, M.M.; Cai, S.; Gavini, G. Cytotoxicity, genotoxicity and antibacterial effectiveness of a bioceramic endodontic sealer. Int. Endod. J. 2016, 49, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Alsubait, S.A.; Al Ajlan, R.; Mitwalli, H.; Aburaisi, N.; Mahmood, A.; Muthurangan, M.; Almadhri, R.; Alfayez, M.; Anil, S. Cytotoxicity of different concentrations of three root canal sealers on human mesenchymal stem cells. Biomolecules 2018, 8, 68. [Google Scholar] [CrossRef]
- Camps, J.; Jeanneau, C.; Ayachi, I.; Laurent, P.; About, I. Bioactivity of a calcium silicate-based endodontic cement (Bioroot RCS): Interactions with human periodontal ligament cells in vitro. J. Endod. 2015, 41, 1469–1473. [Google Scholar] [CrossRef]
- Dimitrova-Nakov, S.; Uzunoglu, E.; Ardila-Osorio, H.; Baudry, A.; Richard, G.; Kellermann, O.; Goldberg, M. In vitro bioactivity of Bioroot RCS, via A4 mouse pulpal stem cells. Dent. Mater. 2015, 31, 1290–1297. [Google Scholar] [CrossRef]
- Lim, E.S.; Park, Y.B.; Kwon, Y.S.; Shon, W.J.; Lee, K.W.; Min, K.S. Physical properties and biocompatibility of an injectable calcium-silicate-based root canal sealer: In vitro and in vivo study. BMC Oral Health 2015, 15, 129. [Google Scholar] [CrossRef]
- Kim, J.A.; Hwang, Y.C.; Rosa, V.; Yu, M.K.; Lee, K.W.; Min, K.S. Root canal filling quality of a premixed calcium silicate endodontic sealer applied using gutta-percha cone-mediated ultrasonic activation. J. Endod. 2018, 44, 133–138. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.; Park, J.W.; Jung, I.Y.; Shin, S.J. Comparison of the percentage of voids in the canal filling of a calcium silicate-based sealer and gutta percha cones using two obturation techniques. Materials (Basel) 2017, 10, 1170. [Google Scholar] [CrossRef]
- Lee, J.K.; Kwak, S.W.; Ha, J.H.; Lee, W.; Kim, H.C. Physicochemical properties of epoxy resin-based and bioceramic-based root canal sealers. Bioinorg. Chem. Appl. 2017, 2017, 2582849. [Google Scholar] [CrossRef]
- Heino, T.J.; Hentunen, T.A. Differentiation of osteoblasts and osteocytes from mesenchymal stem cells. Curr. Stem Cell Res. Ther. 2008, 3, 131–145. [Google Scholar] [CrossRef]
- Souza, G.L.; Rosatto, C.M.P.; Silva, M.J.B.; Silva, M.V.; Rodrigues, R.D.B.; Moura, C.C.G. Evaluation of apoptosis/necrosis and cytokine release provoked by three root canal sealers in human polymorphonuclears and monocytes. Int. Endod. J. 2018. [Google Scholar] [CrossRef]
- Silva, E.J.; Carvalho, N.K.; Ronconi, C.T.; De-Deus, G.; Zuolo, M.L.; Zaia, A.A. Cytotoxicity profile of endodontic sealers provided by 3D cell culture experimental model. Braz. Dent. J. 2016, 27, 652–656. [Google Scholar] [CrossRef]
- Schweikl, H.; Schmalz, G.; Federlin, M. Mutagenicity of the root canal sealer AH Plus in the Ames test. Clin. Oral Investig. 1998, 2, 125–129. [Google Scholar] [CrossRef]
- Eldeniz, A.U.; Shehata, M.; Hogg, C.; Reichl, F.X. DNA double-strand breaks caused by new and contemporary endodontic sealers. Int. Endod. J. 2016, 49, 1141–1151. [Google Scholar] [CrossRef]
- Collado-González, M.; Garcia-Bernal, D.; Onate-Sanchez, R.E.; Ortolani-Seltenerich, P.S.; Lozano, A.; Forner, L.; Llena, C.; Rodríguez-Lozano, F.J. Biocompatibility of three new calcium silicate-based endodontic sealers on human periodontal ligament stem cells. Int. Endod. J. 2017, 50, 875–884. [Google Scholar] [CrossRef]
- Kim, R.J.; Shin, J.H. Cytotoxicity of a novel mineral trioxide aggregate-based root canal sealer [corrected]. Dent. Mater. J. 2014, 33, 313–318. [Google Scholar] [CrossRef][Green Version]
- Benezra, K.M.; Wismayer, S.P.; Camilleri, J. Interfacial characteristics and cytocompatibility of hydraulic sealer cements. J. Endod. 2018, 44, 1007–1017. [Google Scholar] [CrossRef]
- Eldeniz, A.U.; Mustafa, K.; Orstavik, D.; Dahl, J.E. Cytotoxicity of new resin-, calcium hydroxide and silicone-based root canal sealers on fibroblasts derived from human gingiva and L929 cell lines. Int. Endod. J. 2007, 40, 329–337. [Google Scholar] [CrossRef]
- Candeiro, G.T.; Correia, F.C.; Duarte, M.A.; Ribeiro-Siqueira, D.C.; Gavini, G. Evaluation of radiopacity, pH, release of calcium ions, and flow of a bioceramic root canal sealer. J. Endod. 2012, 38, 842–845. [Google Scholar] [CrossRef]
- Khan, S.; Kaleem, M.; Fareed, M.A.; Habib, A.; Iqbal, K.; Aslam, A.; Din, S. Chemical and morphological characteristics of mineral trioxide aggregate and Portland cements. Dent. Mater. J. 2016, 35, 112–117. [Google Scholar] [CrossRef][Green Version]
- Formosa, L.M.; Mallia, B.; Bull, T.; Camilleri, J. The microstructure and surface morphology of radiopaque tricalcium silicate cement exposed to different curing conditions. Dent. Mater. 2012, 28, 584–595. [Google Scholar] [CrossRef]
- Almeida, S.L.H.; Moraes, R.R.; Morgental, R.D.; Pappen, F.G. Are premixed calcium silicate-based endodontic sealers comparable to conventional materials? A systematic review of in vitro studies. J. Endod. 2017, 43, 527–535. [Google Scholar] [CrossRef]
- Loushine, B.A.; Bryan, T.E.; Looney, S.W.; Gillen, B.M.; Loushine, R.J.; Weller, R.N.; Pashley, D.H.; Tay, F.R. Setting properties and cytotoxicity evaluation of a premixed bioceramic root canal sealer. J. Endod. 2011, 37, 673–677. [Google Scholar] [CrossRef]
| Table | Manufacturer | Composition | Batch Number |
|---|---|---|---|
| AH Plus | Dentsply DeTrey GmbH, Konstanz, Germany | Epoxide paste: diepoxide, calcium tungstate, zirconium oxide, aerosil, pigment; amine paste: 1-adamantane amine, N, N’-dibenzyl-5-oxa-nonandiamin-1,9, TCD-diamine, calcium tungstate, zirconium oxide, aerosil, and silicon oil | 1703000226 |
| EndoSequence BC Sealer | Brasseler, Savannah, GA, USA | Zirconium oxide, calcium silicates, calcium phosphate monobasic, calcium hydroxide, filler and thickening agents | 17004SP |
| BioRoot RCS | Septodont, Saint Maur-des-Fossés, France | Tricalcium silicate, zirconium oxide (opacifier) and excipients in its powder form, and calcium chloride and excipients as an aqueous liquid | B16422 |
| Endoseal MTA | Maruchi, Wonju, Korea | Calcium silicates, calcium aluminates, calcium aluminoferrite, calcium sulfates, radiopacifier, and thickening agents | CD180327D |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, D.-G.; Lee, D.; Kim, Y.-M.; Song, D.; Kim, S.-Y. Biocompatibility and Mineralization Activity of Three Calcium Silicate-Based Root Canal Sealers Compared to Conventional Resin-Based Sealer in Human Dental Pulp Stem Cells. Materials 2019, 12, 2482. https://doi.org/10.3390/ma12152482
Seo D-G, Lee D, Kim Y-M, Song D, Kim S-Y. Biocompatibility and Mineralization Activity of Three Calcium Silicate-Based Root Canal Sealers Compared to Conventional Resin-Based Sealer in Human Dental Pulp Stem Cells. Materials. 2019; 12(15):2482. https://doi.org/10.3390/ma12152482
Chicago/Turabian StyleSeo, Deog-Gyu, Donghee Lee, Yong-Min Kim, Dani Song, and Sin-Young Kim. 2019. "Biocompatibility and Mineralization Activity of Three Calcium Silicate-Based Root Canal Sealers Compared to Conventional Resin-Based Sealer in Human Dental Pulp Stem Cells" Materials 12, no. 15: 2482. https://doi.org/10.3390/ma12152482
APA StyleSeo, D.-G., Lee, D., Kim, Y.-M., Song, D., & Kim, S.-Y. (2019). Biocompatibility and Mineralization Activity of Three Calcium Silicate-Based Root Canal Sealers Compared to Conventional Resin-Based Sealer in Human Dental Pulp Stem Cells. Materials, 12(15), 2482. https://doi.org/10.3390/ma12152482






