Impact of Electrocautery on Fatigue Life of Spinal Fusion Constructs—An In Vitro Biomechanical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Spinal Fusion Constructs and Vertebrectomy Models
2.2. Electrocautery Contact
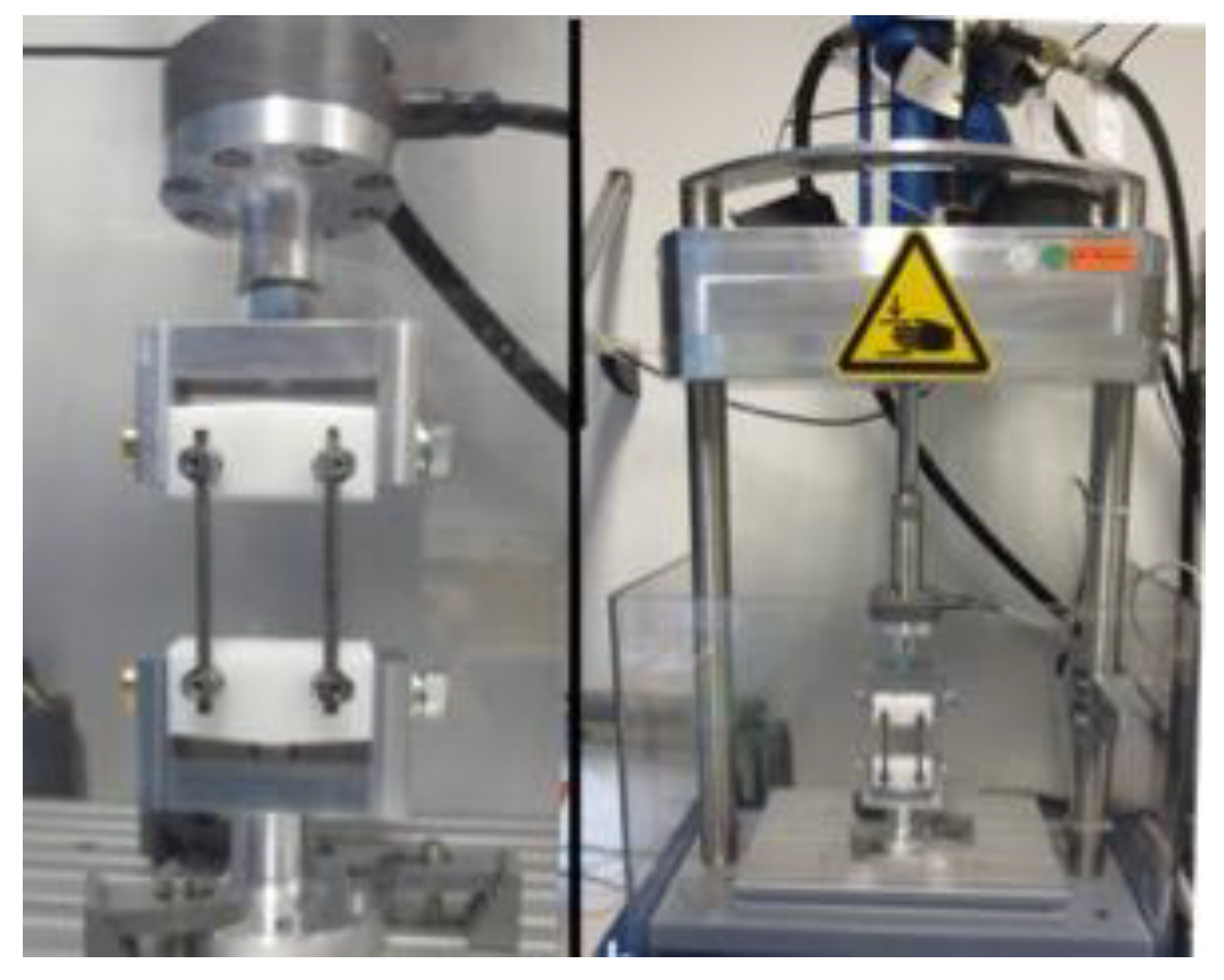
2.3. Dynamic Mechanical Testing
2.4. Outcome Measures
2.5. Statistical Analysis
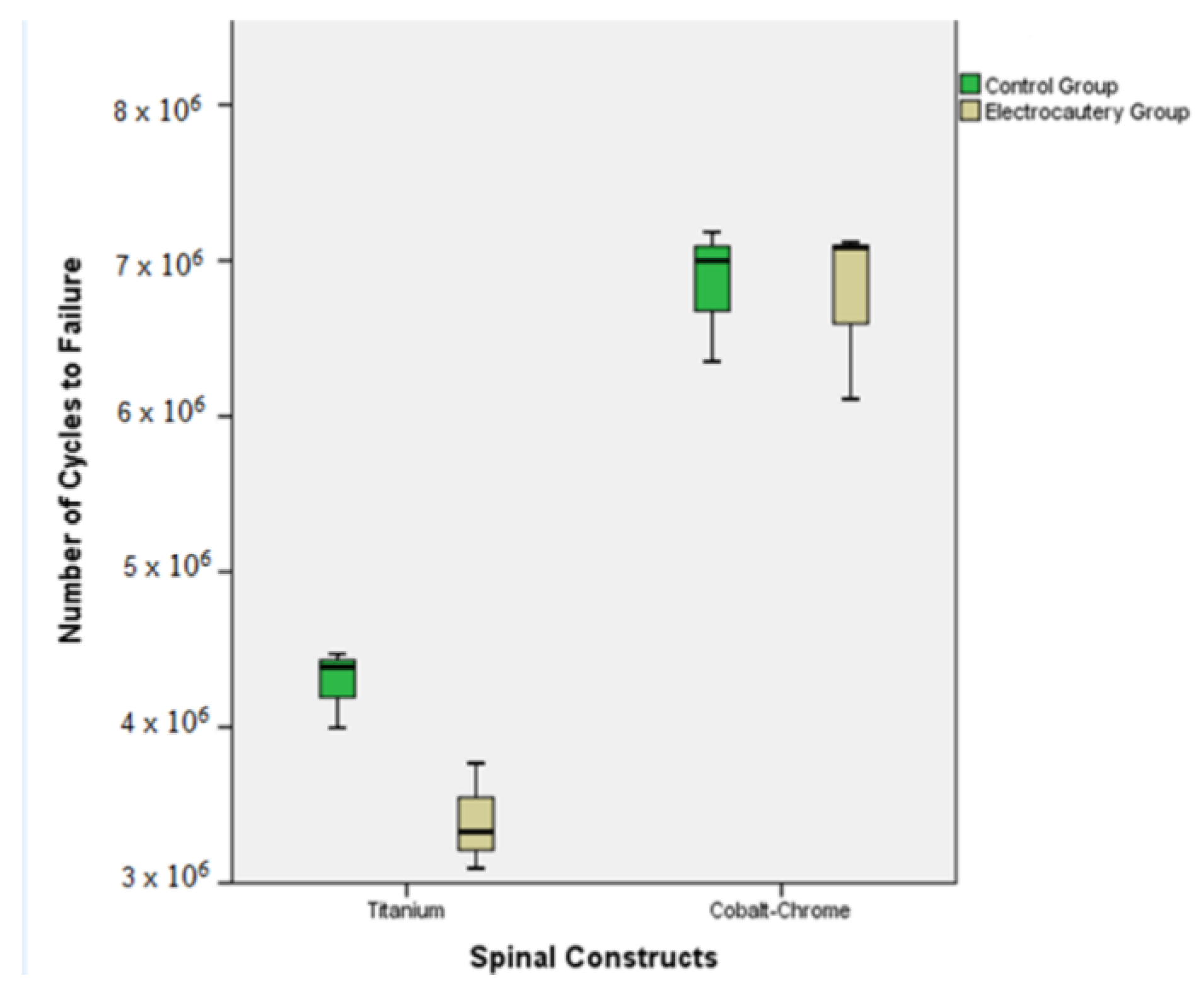
3. Results
3.1. Titanium Control Group (Ti-CG)
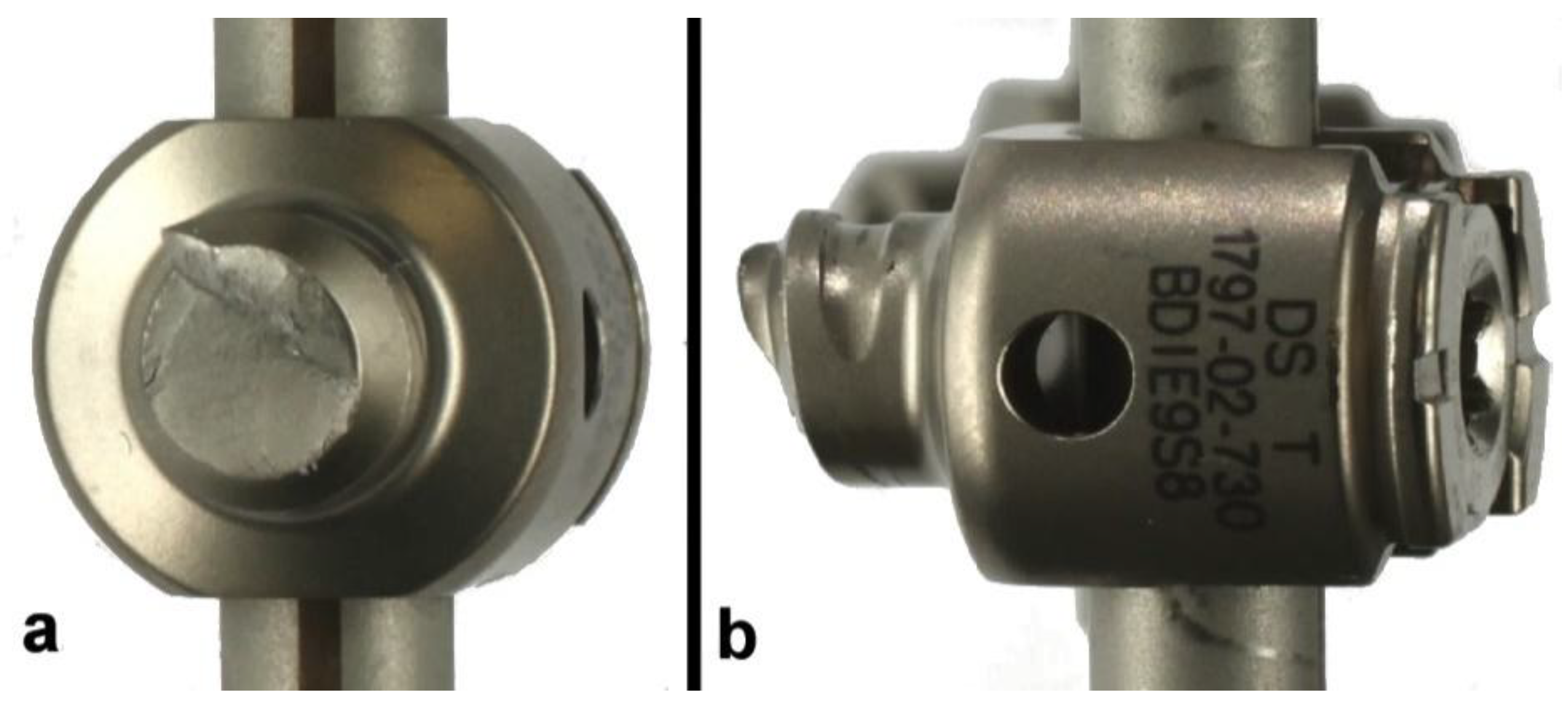
3.2. Titanium Electrocautery Group (Ti-EG)
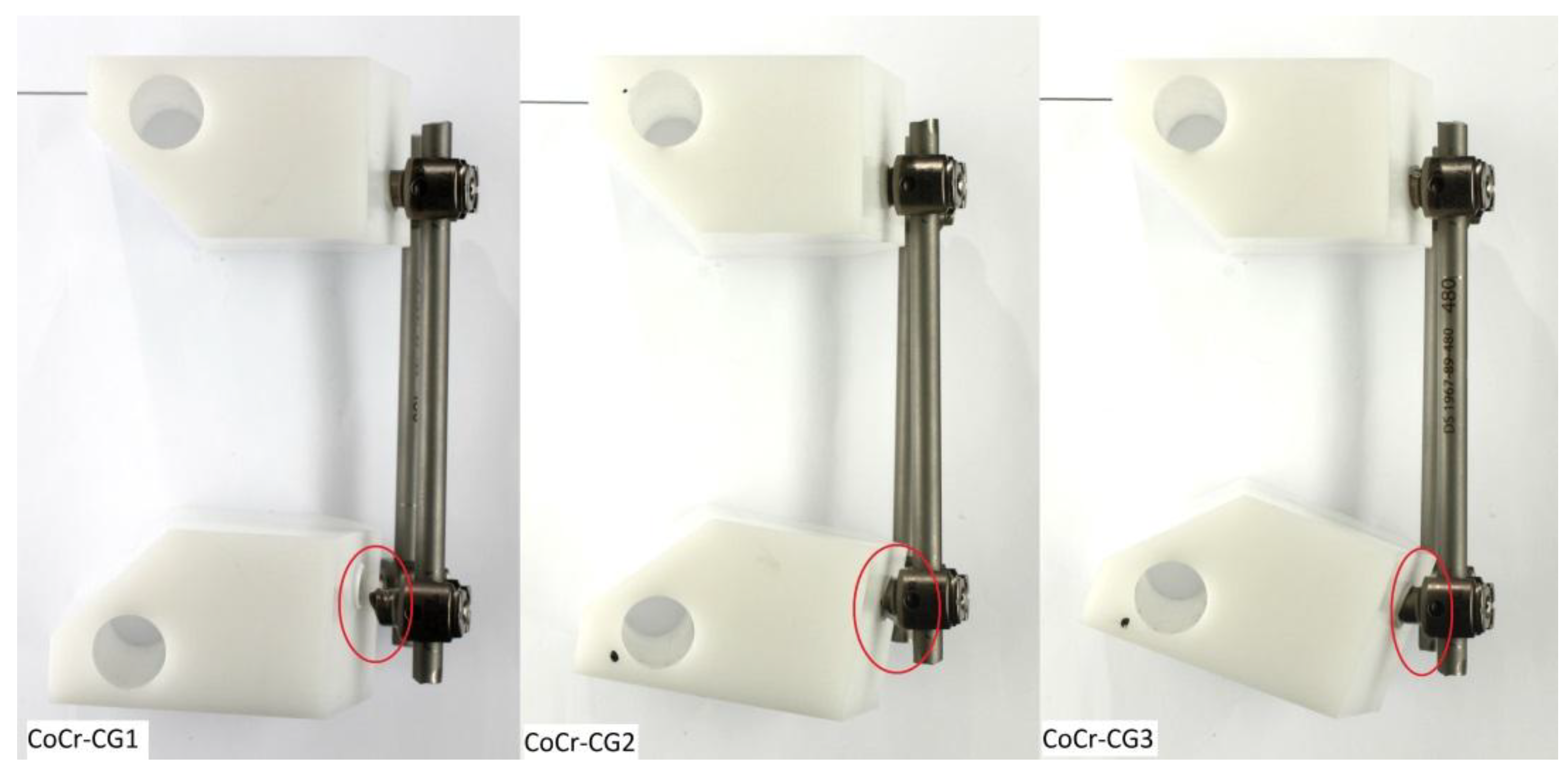
3.3. CoCr Control Group (CoCr-CG) and Electrocautery Group (CoCr-EG)
4. Discussion
4.1. Thermal Damage and Notch Sensitivity: A Threat to Ti Biomechanical Integrity
4.2. Pathogenesis of Ti Rod Fractures at the Rod-Screw Junction
4.3. CoCr Versus Ti Rods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Soroceanu, A.; Diebo, B.G.; Burton, D.; Smith, J.S.; Deviren, V.; Shaffrey, C.; Kim, H.J.; Mundis, G.; Ames, C.; Errico, T. Radiographical and implant-related complications in adult spinal deformity surgery: Incidence, patient risk factors, and impact on health-related quality of life. Spine 2015, 40, 1414–1421. [Google Scholar] [CrossRef]
- Sciubba, D.M.; Yurter, A.; Smith, J.S.; Kelly, M.P.; Scheer, J.K.; Goodwin, C.R.; Lafage, V.; Hart, R.A.; Bess, S.; Kebaish, K.; et al. A Comprehensive Review of Complication Rates after Surgery for Adult Deformity: A Reference for Informed Consent. Spine Deform. 2015, 3, 575–594. [Google Scholar] [CrossRef]
- Hibbs, R.A.; Peltier, L.F. A report of fifty-nine cases of scoliosis treated by the fusion operation. Clin. Orthop. Relat. Res. 1988, 229, 4–19. [Google Scholar]
- Heary, R.F.; Madhavan, K. The History of Spinal Deformity. Neurosurgery 2008, 63, A5–A15. [Google Scholar] [CrossRef]
- Albee, F.H. The classic: Transplantation of a portion of the tibia into the spine for Pott's disease: A preliminary report. Clin. Orthop. Relat. Res. 2007, 460, 14–16. [Google Scholar] [CrossRef]
- King, D. Internal fixation for lumbosacral fusion. JBJS 1948, 30, 560–578. [Google Scholar] [CrossRef]
- Lange, F. Support for the spondylitic spine by means of buried steel bars, attached to the vertebrae. JBJS 1910, 2, 344–361. [Google Scholar]
- Harrington, P.R. Treatment of scoliosis: Correction and internal fixation by spine instrumentation. JBJS 1962, 44, 591–634. [Google Scholar] [CrossRef]
- Cotrel, Y.; Dubousset, J. A new technic for segmental spinal osteosynthesis using the posterior approach. Revue de Chirurgie Orthopédique et Réparatrice de L'appareil Moteur 1984, 70, 489–494. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Liu, W.; Liu, S.; Wang, L. Surface Modification of Biomedical Titanium Alloy: Micromorphology, Microstructure Evolution and Biomedical Applications. Coatings 2019, 9, 249. [Google Scholar] [CrossRef]
- Kyzioł, K.; Kaczmarek, Ł.; Brzezinka, G.; Kyzioł, A. Structure, characterization and cytotoxicity study on plasma surface modified Ti–6Al–4V and γ-TiAl alloys. Chem. Eng. J. 2014, 240, 516–526. [Google Scholar] [CrossRef]
- Smith, J.S.; Sansur, C.A.; Donaldson, W.F., 3rd; Perra, J.H.; Mudiyam, R.; Choma, T.J.; Zeller, R.D.; Knapp, D.R., Jr.; Noordeen, H.H.; Berven, S.H.; et al. Short-term morbidity and mortality associated with correction of thoracolumbar fixed sagittal plane deformity: A report from the Scoliosis Research Society Morbidity and Mortality Committee. Spine 2011, 36, 958–964. [Google Scholar] [CrossRef]
- Scheer, J.K.; Tang, J.A.; Deviren, V.; Acosta, F.; Buckley, J.M.; Pekmezci, M.; McClellan, R.T.; Ames, C.P. Biomechanical analysis of cervicothoracic junction osteotomy in cadaveric model of ankylosing spondylitis: Effect of rod material and diameter. J. Neurosurg. Spine 2011, 14, 330–335. [Google Scholar] [CrossRef]
- Smith, J.S.; Klineberg, E.; Lafage, V.; Shaffrey, C.I.; Schwab, F.; Lafage, R.; Hostin, R.; Mundis, G.M.; Errico, T.J.; Kim, H.J.; et al. Prospective multicenter assessment of perioperative and minimum 2-year postoperative complication rates associated with adult spinal deformity surgery. J. Neurosurg. Spine 2016, 25, 1–14. [Google Scholar] [CrossRef]
- Bridwell, K.H.; Baldus, C.; Berven, S.; Edwards, C., 2nd; Glassman, S.; Hamill, C.; Horton, W.; Lenke, L.G.; Ondra, S.; Schwab, F.; et al. Changes in radiographic and clinical outcomes with primary treatment adult spinal deformity surgeries from two years to three- to five-years follow-up. Spine 2010, 35, 1849–1854. [Google Scholar] [CrossRef]
- Cho, S.K.; Bridwell, K.H.; Lenke, L.G.; Yi, J.S.; Pahys, J.M.; Zebala, L.P.; Kang, M.M.; Cho, W.; Baldus, C.R. Major complications in revision adult deformity surgery: Risk factors and clinical outcomes with 2- to 7-year follow-up. Spine 2012, 37, 489–500. [Google Scholar] [CrossRef]
- Bagchi, K.; Mohaideen, A.; Thomson, J.D.; Foley, L.C. Hardware complications in scoliosis surgery. Pediatr. Radiol. 2002, 32, 465–475. [Google Scholar] [CrossRef]
- Bago, J.; Ramirez, M.; Pellise, F.; Villanueva, C. Survivorship analysis of Cotrel-Dubousset instrumentation in idiopathic scoliosis. Eur. Spine J. 2003, 12, 435–439. [Google Scholar] [CrossRef][Green Version]
- Lindsey, C.; Deviren, V.; Xu, Z.; Yeh, R.F.; Puttlitz, C.M. The effects of rod contouring on spinal construct fatigue strength. Spine 2006, 31, 1680–1687. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Buckley, J.M.; Ames, C.; Deviren, V. The fatigue life of contoured cobalt chrome posterior spinal fusion rods. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2011, 225, 194–198. [Google Scholar] [CrossRef]
- Smith, J.S.; Shaffrey, C.I.; Ames, C.P.; Demakakos, J.; Fu, K.M.; Keshavarzi, S.; Li, C.M.; Deviren, V.; Schwab, F.J.; Lafage, V.; et al. Assessment of symptomatic rod fracture after posterior instrumented fusion for adult spinal deformity. Neurosurgery 2012, 71, 862–867. [Google Scholar] [CrossRef]
- Tang, J.A.; Leasure, J.M.; Smith, J.S.; Buckley, J.M.; Kondrashov, D.; Ames, C.P. Effect of severity of rod contour on posterior rod failure in the setting of lumbar pedicle subtraction osteotomy (PSO): A biomechanical study. Neurosurgery 2013, 72, 276–282. [Google Scholar] [CrossRef]
- Huber, G.; Weik, T.; Morlock, M.M. Schädigung eines Hüftendoprothesenschafts durch Einsatz eines Hochfrequenzmessers. Der Orthopäde 2009, 38, 622–625. [Google Scholar] [CrossRef]
- Konrads, C.; Wente, M.N.; Plitz, W.; Rudert, M.; Hoberg, M. Implantatschädigung durch Einsatz eines Hochfrequenzmessers. Der Orthopäde 2014, 43, 1106–1111. [Google Scholar] [CrossRef]
- Yuan, N.; Park, S.H.; Luck, J.V.; Campbell, P.A. Revisiting the concept of inflammatory cell-induced corrosion. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106B, 1148–1155. [Google Scholar] [CrossRef]
- Sonntag, R.G.J.; Pulvermacher, S.; Mueller, U.; Eckert, J.; Braun, S.; Reichkendler, M.; Kretzer, J.P. Electrocautery damage can reduce implant fatigue strength: Cases and in vitro investigation. J. Bone Jt. Surg. 2019, 101, 868–878. [Google Scholar] [CrossRef]
- ASTM-International. Standard Test Methods for Spinal Implant Constructs in a Vertebrectomy Model. Available online: https://www.astm.org/Standards/F1717.htm (accessed on 20 May 2019).
- Ashman, R. Mechanical testing of spinal implants. Semin. Spine Surg. 1993, 5, 73–80. [Google Scholar]
- Dick, J.; Bourgeault, C. Notch sensitivity of titanium alloy, commercially pure titanium, and stainless steel spinal implants. Spine 2001, 26, 1668–1672. [Google Scholar] [CrossRef]
- Wang, S.; Wei, M. Tensile properties of gas tungsten arc weldments in commercially pure titanium, Ti–6Al–4V and Ti–15V–3Al–3Sn–3Cr alloys at different strain rates. Sci. Technol. Weld. Join. 2004, 9, 415–422. [Google Scholar] [CrossRef]
- Lütjering, G. Influence of processing on microstructure and mechanical properties of (α + β) titanium alloys. Mater. Sci. Eng. A 1998, 243, 32–45. [Google Scholar] [CrossRef]
- Rohlmann, A.; Graichen, F.; Bender, A.; Kayser, R.; Bergmann, G. Loads on a telemeterized vertebral body replacement measured in three patients within the first postoperative month. Clin. Biomech. 2008, 23, 147–158. [Google Scholar] [CrossRef]
- Rohlmann, A.; Bergmann, G.; Graichen, F. Loads on an internal spinal fixation device during walking. J. Biomech. 1997, 30, 41–47. [Google Scholar] [CrossRef]
- Rohlmann, A.; Claes, L.; Bergmann, G.; Graichen, F.; Neef, P.; Wilke, H.-J. Comparison of intradiscal pressures and spinal fixator loads for different body positions and exercises. Ergonomics 2001, 44, 781–794. [Google Scholar] [CrossRef]
- D’Lima, D.D.; Fregly, B.J.; Colwell, C.W. Implantable sensor technology: Measuring bone and joint biomechanics of daily life in vivo. Arthritis Res. Ther. 2013, 15, 203. [Google Scholar] [CrossRef]
- Tang, J.A. Comparison of a novel pedicle subtraction osteotomy model using the traditional American Society of Testing and Materials standard for spinal biomechanics fatigue testing [RETRACTED]. J. Neurosurg. Spine 2012. [Google Scholar] [CrossRef]
- Chen, P.Q.; Lin, S.J.; Wu, S.S.; So, H. Mechanical performance of the new posterior spinal implant: Effect of materials, connecting plate, and pedicle screw design. Spine 2003, 28, 881–886. [Google Scholar] [CrossRef]
- Yamanaka, K.; Mori, M.; Yamazaki, K.; Kumagai, R.; Doita, M.; Chiba, A. Analysis of the Fracture Mechanism of Ti-6Al-4V Alloy Rods That Failed Clinically After Spinal Instrumentation Surgery. Spine 2015, 40, E767–E773. [Google Scholar] [CrossRef]
- Ravichandran, K. Three-dimensional crack-shape effects during the growth of small surface fatigue cracks in a titanium-base alloy. Fatigue Fract. Eng. Mater. Struct. 1997, 20, 1423–1442. [Google Scholar] [CrossRef]
- Cook, R.D.; Young, W.C. Advanced Mechanics of Materials; Prentice Hall: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Jang, B.; Kanawati, A.; Brazil, D.; Bruce, W. Laser etching causing fatigue fracture at the neck–shoulder junction of an uncemented femoral stem: A case report. J. Orthop. 2013, 10, 95–98. [Google Scholar] [CrossRef]
- Malinov, S.; Sha, W. Application of artificial neural networks for modelling correlations in titanium alloys. Mater. Sci. Eng. A 2004, 365, 202–211. [Google Scholar] [CrossRef]
- Balasubramanian, M.; Jayabalan, V.; Balasubramanian, V. Effect of microstructure on impact toughness of pulsed current GTA welded α–β titanium alloy. Mater. Lett. 2008, 62, 1102–1106. [Google Scholar] [CrossRef]
- Pederson, R.; Babushkin, O.; Skystedt, F.; Warren, R. Use of high temperature X-ray diffractometry to study phase transitions and thermal expansion properties in Ti-6Al-4V. Mater. Sci. Technol. 2003, 19, 1533–1538. [Google Scholar] [CrossRef]
- Oguma, H.; Nakamura, T. The effect of microstructure on very high cycle fatigue properties in Ti–6Al–4V. Scr. Mater. 2010, 63, 32–34. [Google Scholar] [CrossRef]
- Everaerts, J.; Verlinden, B.; Wevers, M. The influence of the alpha grain size on internal fatigue crack initiation in drawn Ti-6Al-4V wires. Procedia Struct. Integr. 2016, 2, 1055–1062. [Google Scholar] [CrossRef]
- Gil, F.; Rodriguez, D.; Planell, J. Grain growth kinetics of pure titanium. Scr. Metall. Mater. 1995, 33. [Google Scholar] [CrossRef]
- Shinohara, K.; Takigawa, T.; Tanaka, M.; Sugimoto, Y.; Arataki, S.; Yamane, K.; Watanabe, N.; Ozaki, T.; Sarai, T. Implant Failure of Titanium Versus Cobalt-Chromium Growing Rods in Early-onset Scoliosis. Spine 2016, 41, 502–507. [Google Scholar] [CrossRef]
- Doulgeris, J.J.; Aghayev, K.; Gonzalez-Blohm, S.A.; Del Valle, M.; Waddell, J.; Lee, W.E.; Vrionis, F.D. Comparative analysis of posterior fusion constructs as treatments for middle and posterior column injuries: An in vitro biomechanical investigation. Clin. Biomech. 2013, 28, 483–489. [Google Scholar] [CrossRef]
- Wojnar, L.; Dąbrowski, J.R.; Oksiuta, Z. Porosity structure and mechanical properties of vitalium-type alloy for implants. Mater. Charact. 2001, 46, 221–225. [Google Scholar] [CrossRef]
- Serhan, H.; Mhatre, D.; Newton, P.; Giorgio, P.; Sturm, P. Would CoCr rods provide better correctional forces than stainless steel or titanium for rigid scoliosis curves? J. Spinal Disord. Tech. 2013, 26, E70–E74. [Google Scholar] [CrossRef]
- Lamerain, M.; Bachy, M.; Delpont, M.; Kabbaj, R.; Mary, P.; Vialle, R. CoCr rods provide better frontal correction of adolescent idiopathic scoliosis treated by all-pedicle screw fixation. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2014, 23, 1190–1196. [Google Scholar] [CrossRef]
- Han, S.; Hyun, S.J.; Kim, K.J.; Jahng, T.A.; Kim, H.J. Comparative Study Between Cobalt Chrome and Titanium Alloy Rods for Multilevel Spinal Fusion: Proximal Junctional Kyphosis More Frequently Occurred in Patients Having Cobalt Chrome Rods. World Neurosurg. 2017, 103, 404–409. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants–A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Niinomi, M.; Liu, Y.; Nakai, M.; Liu, H.; Li, H. Biomedical titanium alloys with Young’s moduli close to that of cortical bone. Regen. Biomater. 2016, 3, 173–185. [Google Scholar] [CrossRef]
- Saban, K.L.; Penckofer, S.M. Patient expectations of quality of life following lumbar spinal surgery. J. Neurosci. Nurs. 2007, 39, 180–189. [Google Scholar] [CrossRef]
- Cho, K.-J.; Suk, S.-I.; Park, S.-R.; Kim, J.-H.; Kim, S.-S.; Choi, W.-K.; Lee, K.-Y.; Lee, S.-R. Complications in Posterior Fusion and Instrumentation for Degenerative Lumbar Scoliosis. Spine 2007, 32, 2232–2237. [Google Scholar] [CrossRef]
- McCarthy, I.; Hostin, R.; O’Brien, M.; Saigal, R.; Ames, C.P. Health Economic Analysis of Adult Deformity Surgery. Neurosurg. Clin. N. Am. 2013, 24, 293–304. [Google Scholar] [CrossRef]
- Yeramaneni, S.; Robinson, C.; Hostin, R. Impact of spine surgery complications on costs associated with management of adult spinal deformity. Curr. Rev. Musculoskelet. Med. 2016, 9, 327–332. [Google Scholar] [CrossRef]





| Group | Description | Samples |
|---|---|---|
| Ti-CG | Titanium rods control group (without electrocautery application) | n = 3 |
| Ti-EG | Titanium electrocautery group | n = 3 |
| CoCr-CG | Cobalt-Chrome control group (without electrocautery application) | n = 3 |
| CoCr-EG | Cobalt-chrome electrocautery group | n = 3 |
| Spinal Construct | Electrocautery Application | Completed Load Level | Min/Max Load at Failure (N) | No. Cycles to Failure | Failure Site | Fatigue Strength (N) |
|---|---|---|---|---|---|---|
| Ti-CG1 | No | #4 | 30/300 | 4,473,034 | Unilateral rod-screw junction | 273.6 |
| Ti-CG2 | No | #4 | 30/300 | 4,388,472 | Bilateral rod-screw junction | 269.4 |
| Ti-CG3 | No | #3 | 25/250 | 3,995,938 | Unilateral rod-screw junction | 249.8 |
| Ti-EG1 | Yes | #3 | 25/250 | 3,093,921 | Bilateral peripheral rod fractures | 204.7 |
| Ti-EG2 | Yes | #3 | 25/250 | 3,328,583 | Unilateral peripheral rod fracture * | 216.4 |
| Ti-EG3 | Yes | #3 | 25/250 | 3,770,073 | Bilateral central rod fractures | 238.5 |
| CoCr-CG1 | No | #6 | 40/400 | 6,351,621 | Bilateral pedicle screws ** | 367.6 |
| CoCr-CG2 | No | #7 | 40/400 | 7,000,000 | Bilateral pedicle screws ** | 400 |
| CoCr-CG3 | No | #7 | 45/450 | 7,183,433 | Bilateral pedicle screws ** | 409.2 |
| CoCr-EG1 | Yes | #7 | 45/450 | 7,079,071 | Bilateral pedicle screws ** | 403.9 |
| CoCr-EG2 | Yes | #7 | 45/450 | 7,118,378 | Bilateral pedicle screws ** | 405.9189 |
| CoCr-EG3 | Yes | #6 | 40/400 | 6,112,167 | Bilateral pedicle screws ** | 355.60835 |
| Load to Failure (N) * | 95% CI | p | No. Cycles to Failure * | 95% CI | p | |
|---|---|---|---|---|---|---|
| Ti-CG | 264.3 ± 12.7 | [232.7–295.9] | p= 0.02 | 4.3 × 106 ± 25 × 103 | [3.6 × 106–4.9 × 106] | p= 0.03 |
| Ti-EG | 219.8 ± 17.2 | [177.2–262.5] | 3.4 × 106 ± 34 × 103 | [2.5 × 106–4.2 × 106] | ||
| CoCr-CG | 392.2 ± 21.8 | [338.1–446.5] | p > 0.05 | 6.8 × 106 ± 43 × 103 | [5.7 × 106–7.9 × 106] | p > 0.05 |
| CoCr-EG | 388.5 ± 28.5 | [317.7–459.3] | 6.8 × 106 ± 57 × 103 | [5.3 × 106–8.2 × 106] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almansour, H.; Sonntag, R.; Pepke, W.; Bruckner, T.; Kretzer, J.P.; Akbar, M. Impact of Electrocautery on Fatigue Life of Spinal Fusion Constructs—An In Vitro Biomechanical Study. Materials 2019, 12, 2471. https://doi.org/10.3390/ma12152471
Almansour H, Sonntag R, Pepke W, Bruckner T, Kretzer JP, Akbar M. Impact of Electrocautery on Fatigue Life of Spinal Fusion Constructs—An In Vitro Biomechanical Study. Materials. 2019; 12(15):2471. https://doi.org/10.3390/ma12152471
Chicago/Turabian StyleAlmansour, Haidara, Robert Sonntag, Wojciech Pepke, Thomas Bruckner, Jan Philippe Kretzer, and Michael Akbar. 2019. "Impact of Electrocautery on Fatigue Life of Spinal Fusion Constructs—An In Vitro Biomechanical Study" Materials 12, no. 15: 2471. https://doi.org/10.3390/ma12152471
APA StyleAlmansour, H., Sonntag, R., Pepke, W., Bruckner, T., Kretzer, J. P., & Akbar, M. (2019). Impact of Electrocautery on Fatigue Life of Spinal Fusion Constructs—An In Vitro Biomechanical Study. Materials, 12(15), 2471. https://doi.org/10.3390/ma12152471






