Effect of Ultraviolet–Ozone Treatment on the Properties and Antibacterial Activity of Zinc Oxide Sol-Gel Film
Abstract
1. Introduction
2. Materials and Methods
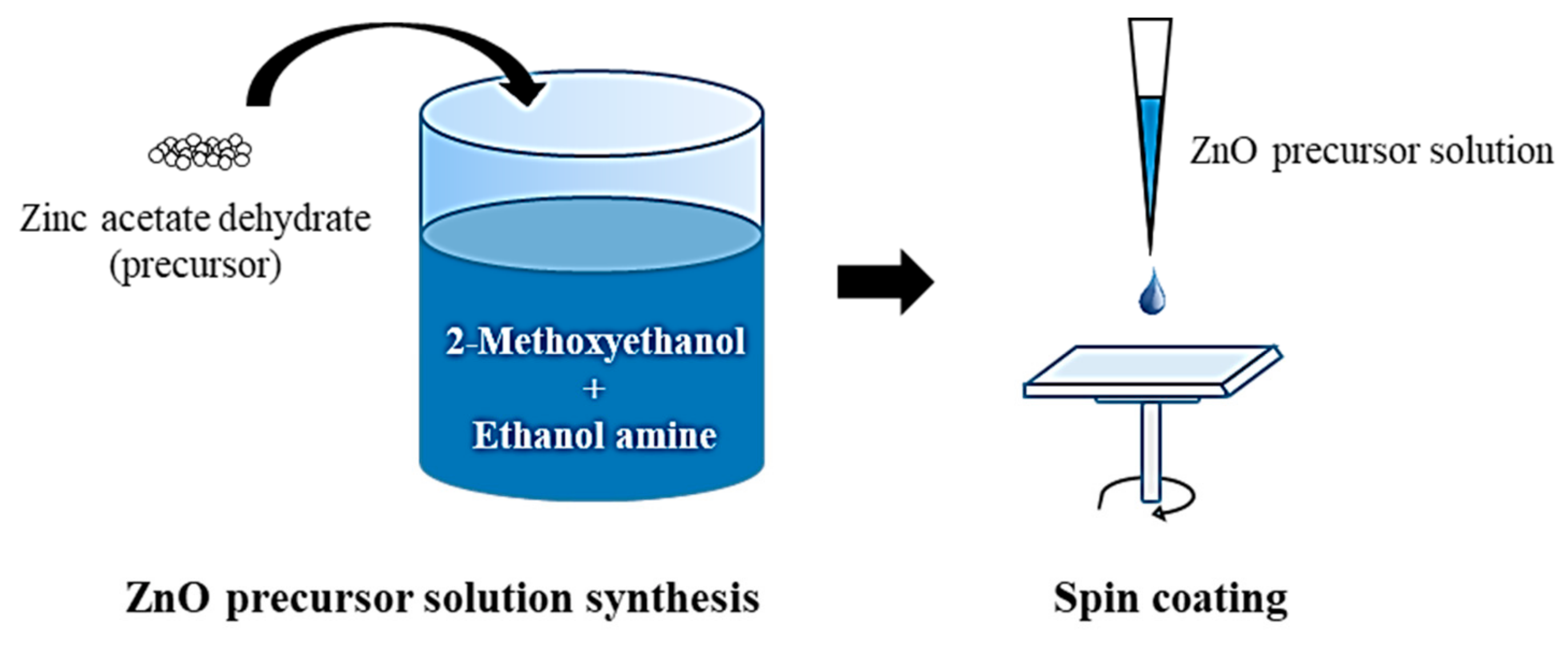
2.1. Preparation of the ZnO Film
2.2. UV–Ozone Treatment
2.3. Characterization
2.4. Antibacterial Test
3. Results and Discussion
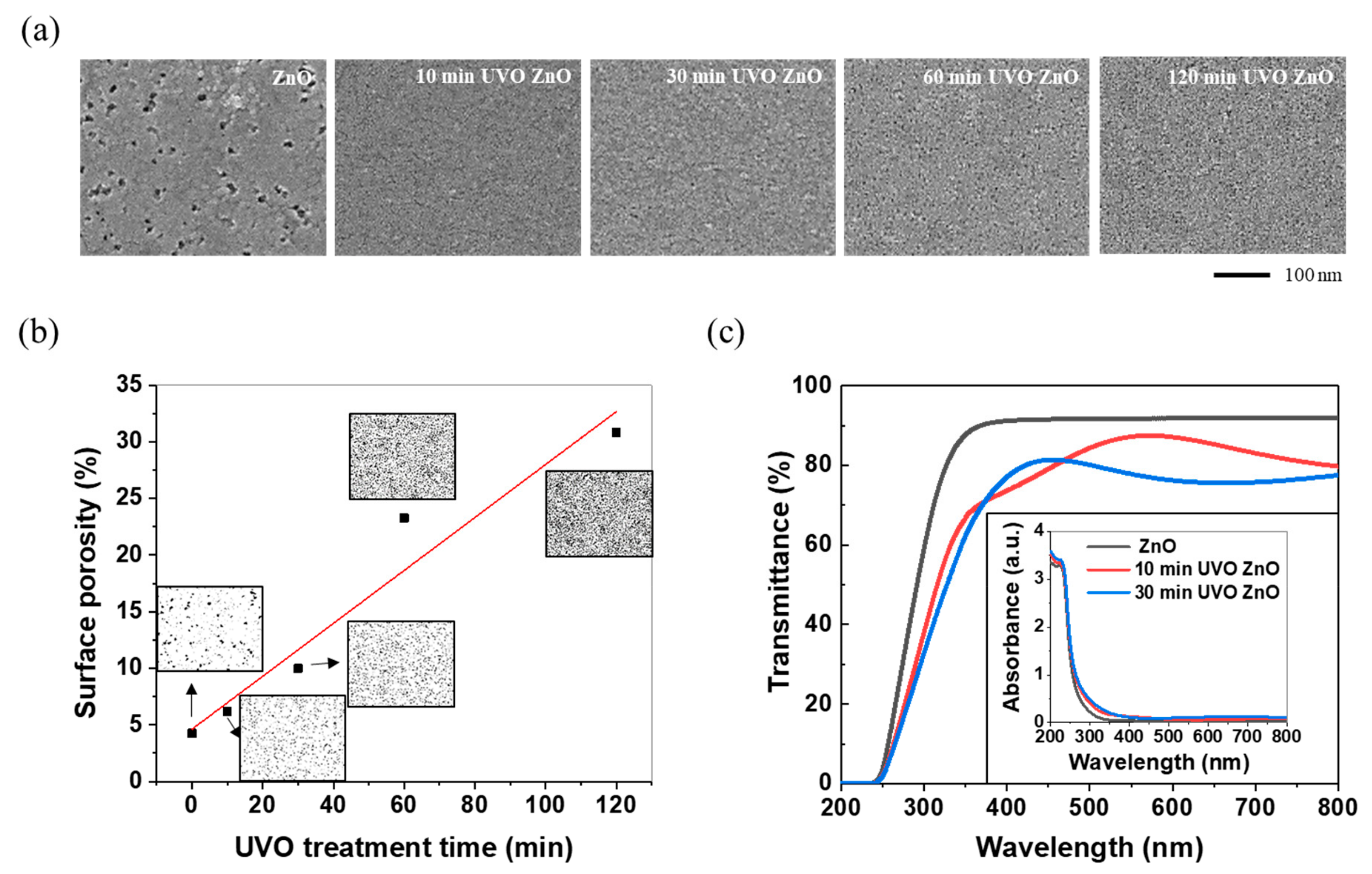
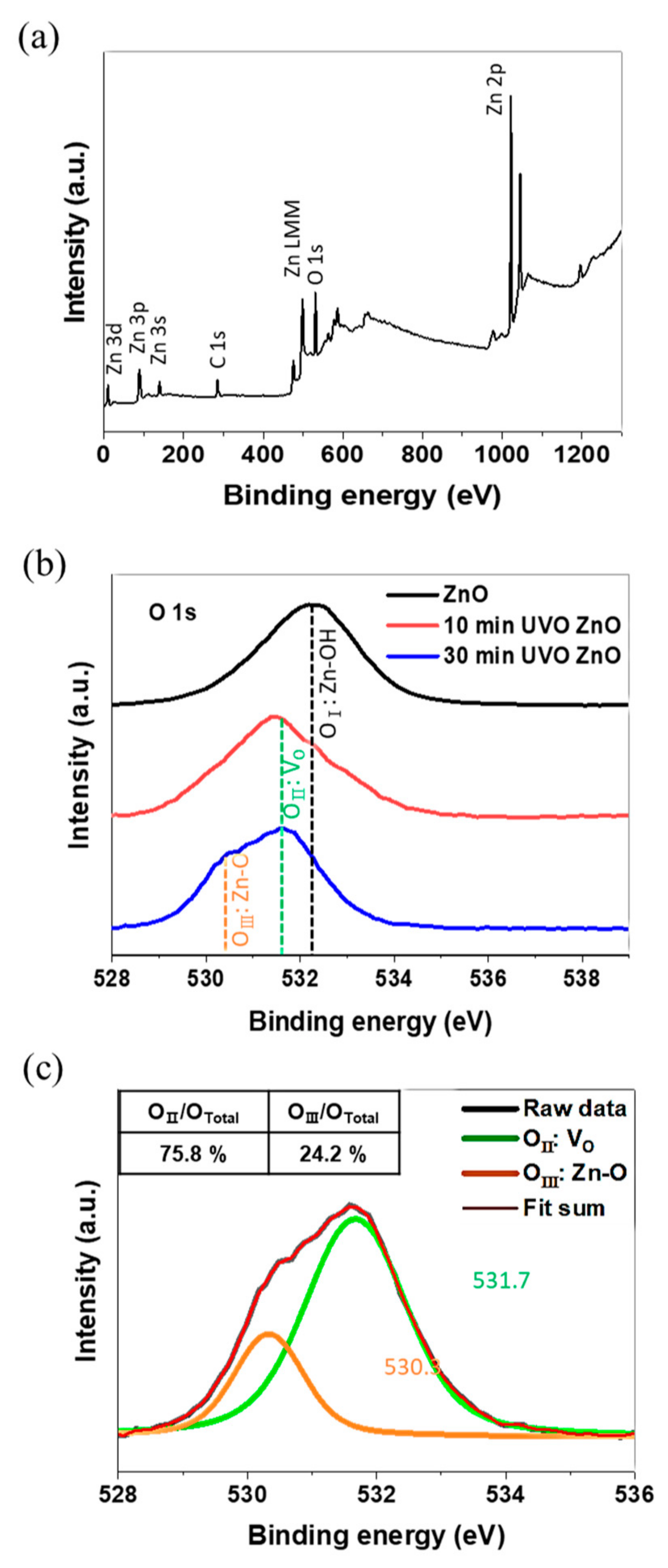
3.1. Morphological, Optical, and Chemical Properties
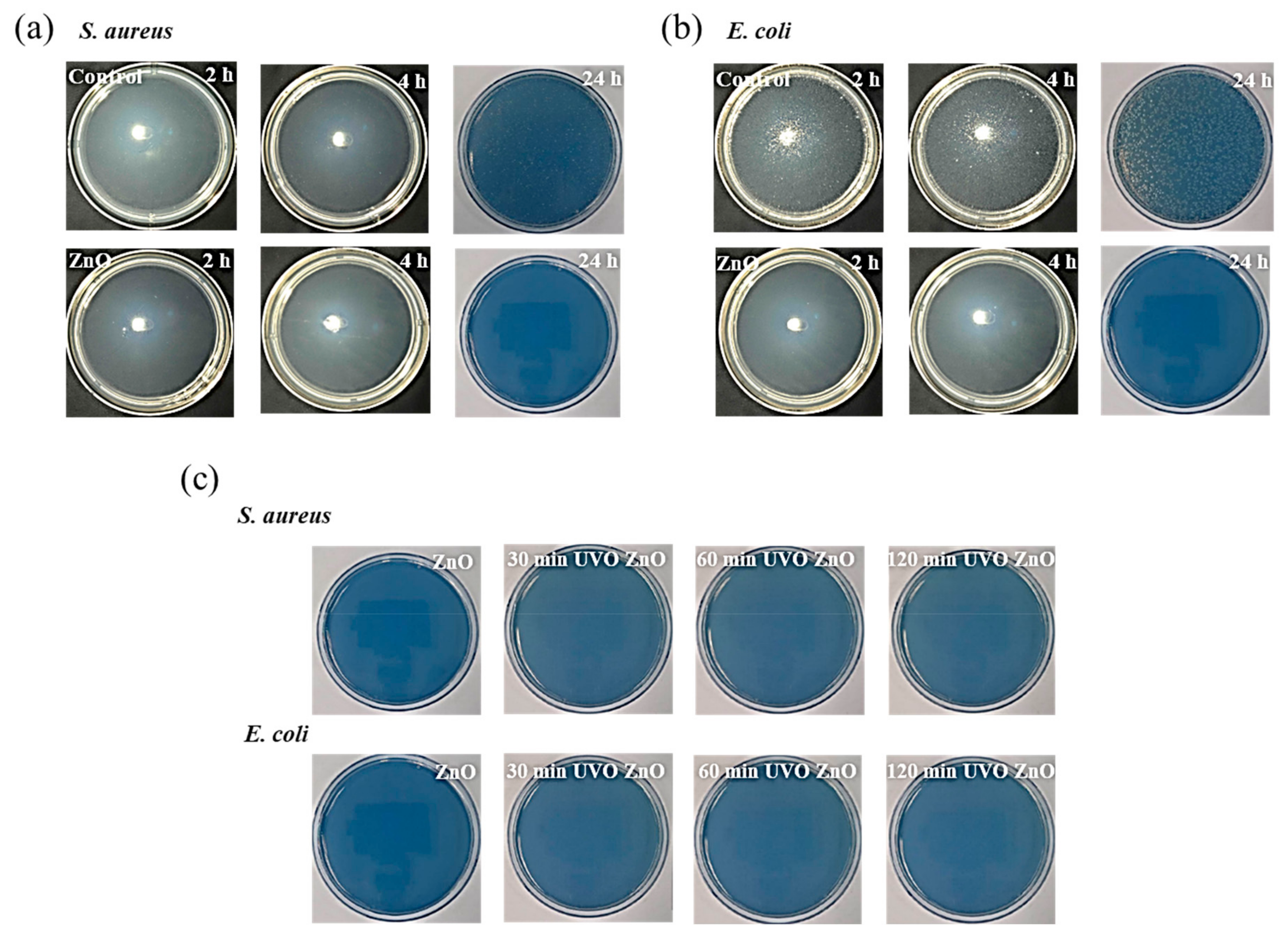
3.2. Bactericidal Effectiveness
4. Conclusions
- As the UVO treatment progressed, the film surface showed a decrease in the porosity size and an enormous increase in the porosity density, eventually resulting in an increase in the surface porosity and, thus, the specific surface area. The resulting surface, which was more porous, induced lower transmittance and higher absorbance in the visible light region.
- O 1s XPS spectrum analysis revealed that the chemical state of the film surface also changed via UVO treatment. Firstly, the Zn(OH)2 present in the prepared ZnO sol-gel film was transformed into ZnO containing oxygen vacancies. Afterwards, the oxygen vacancies were partially occupied with oxygen atoms as a consequence of ROS generation during UVO treatment. That is, a solid–solid transformation (or a dehydration reaction) and an oxidation reaction sequentially occurred on the ZnO film surface.
- However, the UVO treatment-induced changes over the ZnO film surface did not degrade its antibacterial effectiveness against S. aureus and E. coli. Rather, the porosification and Zn(OH)2 to ZnO transformation induced by the UVO treatment improved the bactericidal ability due to the specific surface area increase and the great antibacterial activity of ZnO being superior to that of Zn(OH)2..
Author Contributions
Funding
Conflicts of Interest
References
- McBryde, E.S.; Bradley, L.C.; Whitby, M.; McElwain, D.L.S. An investigation of contact transmission of methicillin-resistant Staphylococcus aureus. J. Hosp. Infect. 2004, 58, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Klevens, R.M.; Edwards, J.R.; Richards, C.L.; Horan, T.C.; Gaynes, R.P.; Pollock, D.A.; Cardo, D.M. Estimating health care-associated infections and deaths in U.S. Hospitals, 2002. Public Health Rep. 2007, 122, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Rutledge-Taylor, K.; Matlow, A.; Gravel, D.; Embree, J.; Le Saux, N.; Johnston, L.; Suh, K.; Embil, J.; Henderson, E.; John, M.; et al. A point prevalence survey of health care-associated infections in Canadian pediatric inpatients. Am. J. Infect. Control 2012, 40, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.Y.; Hooper, D.C. Hospital-acquired infections due to gram-negative bacteria. N. Engl. J. Med. 2010, 362, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Zarb, P.; Coignard, B.; Griskeviciene, J.; Muller, A.; Vankerckhoven, V.; Weist, K.; Goossens, M.M.; Vaerenberg, S.; Hopkins, S.; Catry, B.; et al. The european centre for disease prevention and control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Eurosurveillance 2012, 17, 20316. [Google Scholar] [CrossRef] [PubMed]
- Allegranzi, B.; Nejad, S.B.; Combescure, C.; Graafmans, W.; Attar, H.; Donaldson, L.; Pittet, D. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet 2011, 377, 228–241. [Google Scholar] [CrossRef]
- Siempos, I.I.; Fragoulis, K.N.; Falagas, M.E. World wide web resources on control of nosocomial infections. Crit. Care 2007, 11, 101–105. [Google Scholar] [CrossRef] [PubMed][Green Version]
- WHO’s first global report on antibiotic resistance reveals serious, worldwide threat to public health. Available online: https://www.who.int/mediacentre/news/releases/2014/amr-report/en/ (accessed on 29 July 2019).
- Yamamoto, O. Influence of particle size on the antibacterial activity of zinc oxide. Int. J. Inorg. Mater. 2001, 3, 643–646. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Sawai, J.; Kawada, E.; Kanou, F.; Igarashi, H.; Hashimoto, A.; Kokugan, T.; Shimizu, M. Detection of active oxygen generated from ceramic powders having antibacterial activity. J. Chem. Eng. Japan 1996, 29, 627–633. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomedicine 2012, 122, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Stanković, A.; Dimitrijević, S.; Uskoković, D. Influence of size scale and morphology on antibacterial properties of ZnO powders hydrothemally synthesized using different surface stabilizing agents. Colloids Surfaces B Biointerfaces 2013, 102, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Appierot, G.; Lipovsky, A.; Dror, R.; Perkas, N.; Nitzan, Y.; Lubart, R.; Gedanken, A. Enhanced antibacterial actiwity of nanocrystalline ZnO due to increased ROS-mediated cell injury. Adv. Funct. Mater. 2009, 19, 842–852. [Google Scholar] [CrossRef]
- Chang, Y.N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials (Basel) 2012, 5, 2850–2871. [Google Scholar] [CrossRef]
- Nair, S.; Sasidharan, A.; Divya Rani, V.V.; Menon, D.; Nair, S.; Manzoor, K.; Raina, S. Role of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cells. J. Mater. Sci. Mater. Med. 2008, 20, 235–241. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Memic, A. Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and -negative bacterial strains. Int. J. Nanomedicine 2012, 7, 3527. [Google Scholar] [CrossRef]
- Gupta, K.; Singh, R.P.; Pandey, A.; Pandey, A. Photocatalytic antibacterial performance of TiO2 and Ag-doped TiO2 against S. Aureus. P. Aeruginosa and E. Coli. Beilstein J. Nanotechnol. 2013, 4, 345–351. [Google Scholar] [CrossRef]
- Allahverdiyev, A.M.; Abamor, E.S.; Bagirova, M.; Rafailovich, M. Antimicrobial effects of TiO(2) and Ag(2)O nanoparticles against drug-resistant bacteria and leishmania parasites. Future Microbiol. 2011, 6, 933–940. [Google Scholar] [CrossRef]
- He, Y.; Ingudam, S.; Reed, S.; Gehring, A.; Strobaugh, T.P.; Irwin, P. Study on the mechanism of antibacterial action of magnesium oxide nanoparticles against foodborne pathogens. J. Nanobiotechnology 2016, 14, 54. [Google Scholar] [CrossRef]
- Jin, T.; He, Y. Antibacterial activities of magnesium oxide (MgO) nanoparticles against foodborne pathogens. J. Nanoparticle Res. 2011, 13, 6877–6885. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. J. Biol. Chem. 2015, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Jalal, R.; Goharshadi, E.K.; Abareshi, M.; Moosavi, M.; Yousefi, A.; Nancarrow, P. ZnO nanofluids: Green synthesis, characterization, and antibacterial activity. Mater. Chem. Phys. 2010, 121, 198–201. [Google Scholar] [CrossRef]
- Seil, J.T.; Webster, T.J. Antimicrobial applications of nanotechnology: Methods and literature. Int. J. Nanomedicine 2012, 7, 2767. [Google Scholar] [PubMed]
- Zarrindokht Emami-Karvani Antibacterial activity of ZnO nanoparticle on Gram-positive and Gram-negative bacteria. African J. Microbiol. Res. 2012, 5, 1368–1373.
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles-an antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef]
- Colon, G.; Ward, B.C.; Webster, T.J. Increased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2. J. Biomed. Mater. Res. Part A 2006, 78, 595–604. [Google Scholar] [CrossRef]
- Kasemets, K.; Ivask, A.; Dubourguier, H.C.; Kahru, A. Toxicity of nanoparticles of ZnO, CuO and TiO2 to yeast Saccharomyces cerevisiae. Toxicol. Vitr. 2009, 23, 1116–1122. [Google Scholar] [CrossRef]
- Brunner, T.J.; Wick, P.; Manser, P.; Spohn, P.; Grass, R.N.; Limbach, L.K.; Bruinink, A.; Stark, W.J. In vitro cytotoxicity of oxide nanoparticles: Comparison to asbestos, silica, and the effect of particle solubility. Environ. Sci. Technol. 2006, 40, 4374–4381. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhu, L.; Lin, D. Toxicity of ZnO nanoparticles to escherichia Coli: Mechanism and the influence of medium components. Environ. Sci. Technol. 2011, 45, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.K.; Lyon, D.Y.; Alvarez, P.J.J. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006, 40, 3527–3532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanoparticle Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Antifungal activity of ZnO nanoparticles-the role of ROS mediated cell injury. Nanotechnology 2011, 22, 105101. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, Y.; Povey, M.; York, D. ZnO nanofluids-A potential antibacterial agent. Prog. Nat. Sci. 2008, 18, 939–944. [Google Scholar] [CrossRef]
- Sagar, P.; Shishodia, P.K.; Mehra, R.M. Influence of pH value on the quality of sol–gel derived ZnO films. Appl. Surf. Sci. 2007, 253, 5419–5424. [Google Scholar] [CrossRef]
- Ghamsari, M.S.; Alamdari, S.; Han, W.; Park, H.H. Impact of nanostructured thin ZnO film in ultraviolet protection. Int. J. Nanomedicine 2017, 12, 207–216. [Google Scholar] [CrossRef]
- Znaidi, L. Sol-gel-deposited ZnO thin films: A review. Mater. Sci. Eng. B 2010, 174, 18–30. [Google Scholar] [CrossRef]
- Kamalasanan, M.N.; Chandra, S. Sol-gel synthesis of ZnO thin films. Thin Solid Films 1996, 288, 112–115. [Google Scholar] [CrossRef]
- Tynell, T.; Karppinen, M. Atomic layer deposition of ZnO: A review. Semicond. Sci. Technol. 2014, 29, 043001. [Google Scholar] [CrossRef]
- Yamada, A.; Sang, B.; Konagai, M. Atomic layer deposition of ZnO transparent conducting oxides. Appl. Surf. Sci. 1997, 112, 216–222. [Google Scholar] [CrossRef]
- Abrarov, S.M.; Yuldashev, S.U.; Kim, T.W.; Lee, S.B.; Kwon, Y.H.; Kang, T.W. Effect of photonic band-gap on photoluminescence of ZnO deposited inside the green synthetic opal. Opt. Commun. 2005, 250, 111–119. [Google Scholar] [CrossRef]
- Ayouchi, R.; Leinen, D.; Martı́n, F.; Gabas, M.; Dalchiele, E.; Ramos-Barrado, J.R. Preparation and characterization of transparent ZnO thin films obtained by spray pyrolysis. Thin Solid Films 2003, 426, 68–77. [Google Scholar] [CrossRef]
- Martin, P.M.; Good, M.S.; Johnston, J.W.; Posakony, G.J.; Bond, L.J.; Crawford, S.L. Piezoelectric films for 100-MHz ultrasonic transducers. Thin Solid Films 2000, 379, 253–258. [Google Scholar] [CrossRef]
- Hofman, G.L.; Domagala, R.F.; Copeland, G.L. Irradiation behavior of low-enriched U 6 Fe-Al dispersion fuel elements. J. Nucl. Mater. 1987, 150, 238–243. [Google Scholar] [CrossRef]
- Ondo-Ndong, R.; Pascal-Delannoy, F.; Boyer, A.; Giani, A.; Foucaran, A. Structural properties of zinc oxide thin films prepared by r.f. magnetron sputtering. Mater. Sci. Eng. B 2003, 97, 68–73. [Google Scholar] [CrossRef]
- Agarwal, D.C.; Chauhan, R.S.; Kumar, A.; Kabiraj, D.; Singh, F.; Khan, S.A.; Avasthi, D.K.; Pivin, J.C.; Kumar, M.; Ghatak, J.; et al. Synthesis and characterization of ZnO thin film grown by electron beam evaporation. J. Appl. Phys. 2006, 99, 123105. [Google Scholar] [CrossRef]
- Chen, Z.; Shum, K.; Salagaj, T.; Zhang, W.; Strobl, K. ZnO thin films synthesized by chemical vapor deposition. In Proceedings of the 2010 IEEE Long Island Systems, Applications and Technology Conference, Farmingdale, NY, USA, 7 May 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 1–6. [Google Scholar]
- Tan, S.T.; Chen, B.J.; Sun, X.W.; Fan, W.J.; Kwok, H.S.; Zhang, X.H.; Chua, S.J. Blueshift of optical band gap in ZnO thin films grown by metal-organic chemical-vapor deposition. J. Appl. Phys. 2005, 98, 013505. [Google Scholar] [CrossRef]
- Bagnall, D.M.; Chen, Y.F.; Shen, M.Y.; Zhu, Z.; Goto, T.; Yao, T. Room temperature excitonic stimulated emission from zinc oxide epilayers grown by plasma-assisted MBE. J. Cryst. Growth 1998, 184, 605–609. [Google Scholar] [CrossRef]
- Yu, P.; Tang, Z.K.; Wong, G.K.L.; Kawasaki, M.; Ohtomo, A.; Koinuma, H.; Segawa, Y. Room-temperature gain spectra and lasing in microcrystalline ZnO thin films. J. Cryst. Growth 1998, 184, 601–604. [Google Scholar] [CrossRef]
- Ohtomo, A.; Kawasaki, M.; Sakurai, Y.; Yoshida, Y.; Koinuma, H.; Yu, P.; Tang, Z.K.; Wong, G.K.L.; Segawa, Y. Room temperature ultraviolet laser emission from ZnO nanocrystal thin films grown by laser MBE. Mater. Sci. Eng. B 1998, 54, 24–28. [Google Scholar] [CrossRef]
- Kawasaki, M.; Ohtomo, A.; Ohkubo, I.; Koinuma, H.; Tang, Z.K.; Yu, P.; Wong, G.K.L.; Zhang, B.P.; Segawa, Y. Excitonic ultraviolet laser emission at room temperature from naturally made cavity in ZnO nanocrytal thin films. Mater. Sci. Eng. B 1998, 56, 239–245. [Google Scholar] [CrossRef]
- Pauporté, T.; Lincot, D. Electrodeposition of semiconductors for optoelectronic devices: Results on zinc oxide. Electrochim. Acta 2000, 45, 3345–3353. [Google Scholar] [CrossRef]
- Dodd, M.C.; Kohler, H.P.E.; Gunten, U. Von Oxidation of antibacterial compounds by ozone and hydroxyl radical: Elimination of biological activity during aqueous ozonation processes. Environ. Sci. Technol. 2009, 43, 2498–2504. [Google Scholar] [CrossRef]
- Guirguis, O.W.; El-Bassyouni, G.T.; Esawy, M.A.; Abd Elkader, N.R.; Mahmoud, H.M.; Mostafa, H.M.; Abdel-Zaher, N.A. Exposure of chitosan to UV/ozone, Structural information and antibacterial activity. J. Appl. Pharm. Sci. 2016, 6, 124–130. [Google Scholar] [CrossRef]
- Li, J.; Yang, D.; Zhu, X. Effects of aging time and annealing temperature on structural and optical properties of sol-gel ZnO thin films. Aip Adv. 2017, 7, 65213. [Google Scholar] [CrossRef]
- Khan, Z.R.; Khan, M.S.; Zulfequar, M.; Khan, M.S. Optical and structural properties of ZnO thin films fabricated by sol-gel method. Mater. Sci. Appl. 2011, 2, 340. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, A.; Suri, N.; Kumar, S.; Kumar, M.; Khanna, P.K.; Kumar, D. Structural and optical characterization of ZnO thin films deposited by sol-gel method. J. Optoelectron. Adv. M. 2009, 11, 790–793. [Google Scholar]
- Gómez Núñez, A.; Alonso-Gil, S.; López, C.; Roura, P.; Vilà, A. Role of Ethanolamine on the Stability of a Sol-Gel ZnO Ink. J. Phys. Chem. 2017, 121, 23839–23846. [Google Scholar] [CrossRef]
- JIS, Z. 2801: 2010 (SIAA/JSA) Antibacterial products—Test for antibacterial activity and efficacy. Available online: http://www.questin.org/sites/default/files/intl-codes/jis.z.2801.e.2010.pdf (accessed on 29 July 2019).
- Go, H.; Han, E.-M.; Hee Kang, M.; Hyun Kim, Y.; Yun, C. The coated porous polyimide layers for optical scattering films. AIMS Mater. Sci. 2018, 5, 1102–1111. [Google Scholar] [CrossRef]
- Liu, Z.; Jin, Z.; Li, W.; Liu, X. Ordered porous ZnO thin films formed by dip-coating method using PS templates. J. Sol-Gel Sci. Technol. 2006, 40, 25–30. [Google Scholar] [CrossRef]
- Shin, H.; Kang, C.; Baek, K.-H.; Kim, J.Y.; Do, L.-M.; Lee, C. Low-temperature solution-processed zinc oxide field effect transistor by blending zinc hydroxide and zinc oxide nanoparticle in aqueous solutions. Jpn. J. Appl. Phys. 2018, 57, 05GD04. [Google Scholar] [CrossRef]
- Karakawa, M.; Sugahara, T.; Hirose, Y.; Suganuma, K.; Aso, Y. Thin Film of Amorphous Zinc Hydroxide Semiconductor for Optical Devices with an Energy-Efficient Beneficial Coating by Metal Organic Decomposition Process. Sci. Rep. 2018, 8, 10839. [Google Scholar] [CrossRef]
- Hsu, J.-C.; Lin, Y.-H.; Wang, P.W.; Chen, Y.Y. Spectroscopic ellipsometry studies on various zinc oxide films deposited by ion beam sputtering at room temperature. Appl. Opt. 2012, 51, 1209–1215. [Google Scholar] [CrossRef]
- Sun, Y.; Seo, J.H.; Takacs, C.J.; Seifter, J.; Heeger, A.J. Inverted polymer solar cells integrated with a low-temperature-annealed sol-gel-derived ZnO film as an electron transport layer. Adv. Mater. 2011, 23, 1679–1683. [Google Scholar] [CrossRef]
- Ghobadi, A.; Ulusoy, T.G.; Garifullin, R.; Guler, M.O.; Okyay, A.K. A heterojunction design of single layer hole tunneling ZnO passivation wrapping around TiO2 nanowires for superior photocatalytic performance. Sci. Rep. 2016, 6, 30587. [Google Scholar] [CrossRef]
- Jung, Y.; Yang, W.; Koo, C.Y.; Song, K.; Moon, J. High performance and high stability low temperature aqueous solution-derived Li–Zr co-doped ZnO thin film transistors. J. Mater. Chem. 2012, 22, 5390–5397. [Google Scholar] [CrossRef]
- Kushwaha, A.; Aslam, M. Hydrogen-incorporated ZnO nanowire films: Stable and high electrical conductivity. J. Phys. D. Appl. Phys. 2013, 46, 485104. [Google Scholar] [CrossRef]
- Znaidi, L.; Illia, G.S.; Benyahia, S.; Sanchez, C.; Kanaev, A. V Oriented ZnO thin films synthesis by sol–gel process for laser application. Thin Solid Films 2003, 428, 257–262. [Google Scholar] [CrossRef]
- Jagadamma, L.K.; Abdelsamie, M.; El Labban, A.; Aresu, E.; Ndjawa, G.O.N.; Anjum, D.H.; Cha, D.; Beaujuge, P.M.; Amassian, A. Efficient inverted bulk-heterojunction solar cells from low-temperature processing of amorphous ZnO buffer layers. J. Mater. Chem. A 2014, 2, 13321–13331. [Google Scholar] [CrossRef]
- Nehmann, J.B.; Ehrmann, N.; Reineke-Koch, R.; Bahnemann, D.W. Aluminum-doped zinc oxide sol–gel thin films: Influence of the sol’s water content on the resistivity. Thin Solid Films 2014, 556, 168–173. [Google Scholar] [CrossRef]
- Yang, F.; Kang, D.-W.; Kim, Y.-S. Improved interface of ZnO/CH3NH3PbI3 by a dynamic spin-coating process for efficient perovskite solar cells. RSC Adv. 2017, 7, 19030–19038. [Google Scholar] [CrossRef]
- Briois, V.; Giorgetti, C.; Baudelet, F.; Blanchandin, S.; Tokumoto, M.S.; Pulcinelli, S.H.; Santilli, C.V. Dynamical study of ZnO nanocrystal and Zn-HDS layered basic zinc acetate formation from sol− gel route. J. Phys. Chem. C 2007, 111, 3253–3258. [Google Scholar] [CrossRef]
- Nistor, S.V.; Ghica, D.; Stefan, M.; Vlaicu, I.; Barascu, J.N.; Bartha, C. Magnetic defects in crystalline Zn(OH)2 and nanocrystalline ZnO resulting from its thermal decomposition. J. Alloys Compd. 2013, 548, 222–227. [Google Scholar] [CrossRef]
- Mihaiu, S.; Szilágyi, I.M.; Atkinson, I.; Mocioiu, O.C.; Hunyadi, D.; Pandele-Cusu, J.; Toader, A.; Munteanu, C.; Boyadjiev, S.; Madarász, J. Thermal study on the synthesis of the doped ZnO to be used in TCO films. J. Therm. Anal. Calorim. 2016, 124, 71–80. [Google Scholar] [CrossRef][Green Version]
- Stefan, M.; Ghica, D.; Nistor, S.V.; Maraloiu, A.V.; Plugaru, R. Mn2+ ions distribution in doped sol–gel deposited ZnO films. Appl. Surf. Sci. 2017, 396, 1880–1889. [Google Scholar] [CrossRef]
- Rochman, N.T.; Akwalia, P.R. Fabrication and characterization of Zinc Oxide (ZnO) nanoparticle by sol-gel method. In Proceedings of the Journal of Physics: Conference Series, Surabaya, Indonesia, 27 October 2016; IOP Publishing: Bristol, UK, 2017; 853, p. 12041. [Google Scholar]
- Fiedot, M.; Maliszewska, I.; Rac-Rumijowska, O.; Suchorska-Wózniak, P.; Lewínska, A.; Teterycz, H. The relationship between the mechanism of zinc oxide crystallization and its antimicrobial properties for the surface modification of surgical meshes. Materials (Basel). 2017, 10, 353. [Google Scholar] [CrossRef]
- Urwyler, P.; Pascual, A.; Müller, B.; Schift, H. Ultraviolet–ozone surface cleaning of injection-molded, thermoplastic microcantilevers. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Vig, J.R. UV/ozone cleaning of surfaces. J. Vac. Sci. Technol. Vacuum Surfaces Film 1985, 3, 1027–1034. [Google Scholar] [CrossRef]
- Wei, W.; Yang, C.; Mai, J.; Gong, Y.; Yan, L.; Zhao, K.; Ning, H.; Wu, S.; Gao, J.; Gao, X. High mobility solution-processed C 8-BTBT organic thin-film transistors via UV-ozone interface modification. J. Mater. Chem. C 2017, 5, 10652–10659. [Google Scholar] [CrossRef]
- Summerfelt, S.T. Ozonation and UV irradiation—An introduction and examples of current applications. Aquac. Eng. 2003, 28, 21–36. [Google Scholar] [CrossRef]
- Zoschke, K.; Börnick, H.; Worch, E. Vacuum-UV radiation at 185 nm in water treatment—A review. Water Res. 2014, 52, 131–145. [Google Scholar] [CrossRef]
- Yasuda, K.; Okazaki, Y.; Abe, Y.; Tsuga, K. Effective UV/Ozone irradiation method for decontamination of hydroxyapatite surfaces. Heliyon 2017, 3, e00372. [Google Scholar] [CrossRef]
- Catauro, M.; Barrino, F.; Bononi, M.; Colombini, E.; Giovanardi, R.; Veronesi, P.; Tranquillo, E. Coating of Titanium Substrates with ZrO2 and ZrO2-SiO2 Composites by Sol-Gel Synthesis for Biomedical Applications: Structural Characterization, Mechanical and Corrosive Behavior. Coatings 2019, 9, 200. [Google Scholar] [CrossRef]
- Tranquillo, E.; Barrino, F.; Dal Poggetto, G.; Blanco, I. Sol–Gel Synthesis of Silica-Based Materials with Different Percentages of PEG or PCL and High Chlorogenic Acid Content. Materials (Basel) 2019, 12, 155. [Google Scholar] [CrossRef]
- Verdier, T.; Coutand, M.; Bertron, A.; Roques, C. Antibacterial activity of TiO2 photocatalyst alone or in coatings on E. coli: The influence of methodological aspects. Coatings 2014, 4, 670–686. [Google Scholar] [CrossRef]
| Percent reduction (%) | ||||||
|---|---|---|---|---|---|---|
| Test Organism | S. aureus | E. coli | ||||
| Contact Time | 2 h | 4 h | 24 h | 2 h | 4 h | 24 h |
| ZnO | 99.9 | 99.9 | 99.9 | 99.8 | 99.9 | 99.9 |
| 30-min UVO ZnO | – | – | 99.9 | – | – | 99.9 |
| 60-min UVO ZnO | – | – | 99.9 | – | – | 99.9 |
| 120-min UVO ZnO | – | – | 99.8 | – | – | 99.9 |
| Control | 2.0 | 0.6 | −41.2 | 6.0 | 52.0 | −7547.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Ma, J.; Lee, S.; Jo, S.; Kim, C.S. Effect of Ultraviolet–Ozone Treatment on the Properties and Antibacterial Activity of Zinc Oxide Sol-Gel Film. Materials 2019, 12, 2422. https://doi.org/10.3390/ma12152422
Kim J-H, Ma J, Lee S, Jo S, Kim CS. Effect of Ultraviolet–Ozone Treatment on the Properties and Antibacterial Activity of Zinc Oxide Sol-Gel Film. Materials. 2019; 12(15):2422. https://doi.org/10.3390/ma12152422
Chicago/Turabian StyleKim, Ji-Hyeon, Junfei Ma, Seunghun Lee, Sungjin Jo, and Chang Su Kim. 2019. "Effect of Ultraviolet–Ozone Treatment on the Properties and Antibacterial Activity of Zinc Oxide Sol-Gel Film" Materials 12, no. 15: 2422. https://doi.org/10.3390/ma12152422
APA StyleKim, J.-H., Ma, J., Lee, S., Jo, S., & Kim, C. S. (2019). Effect of Ultraviolet–Ozone Treatment on the Properties and Antibacterial Activity of Zinc Oxide Sol-Gel Film. Materials, 12(15), 2422. https://doi.org/10.3390/ma12152422




