The Durability of an Organic–Inorganic Sol–Gel Interlayer in Al-GFRP-CFRP Laminates in a Saline Environment
Abstract
:1. Introduction
2. Materials and Methods
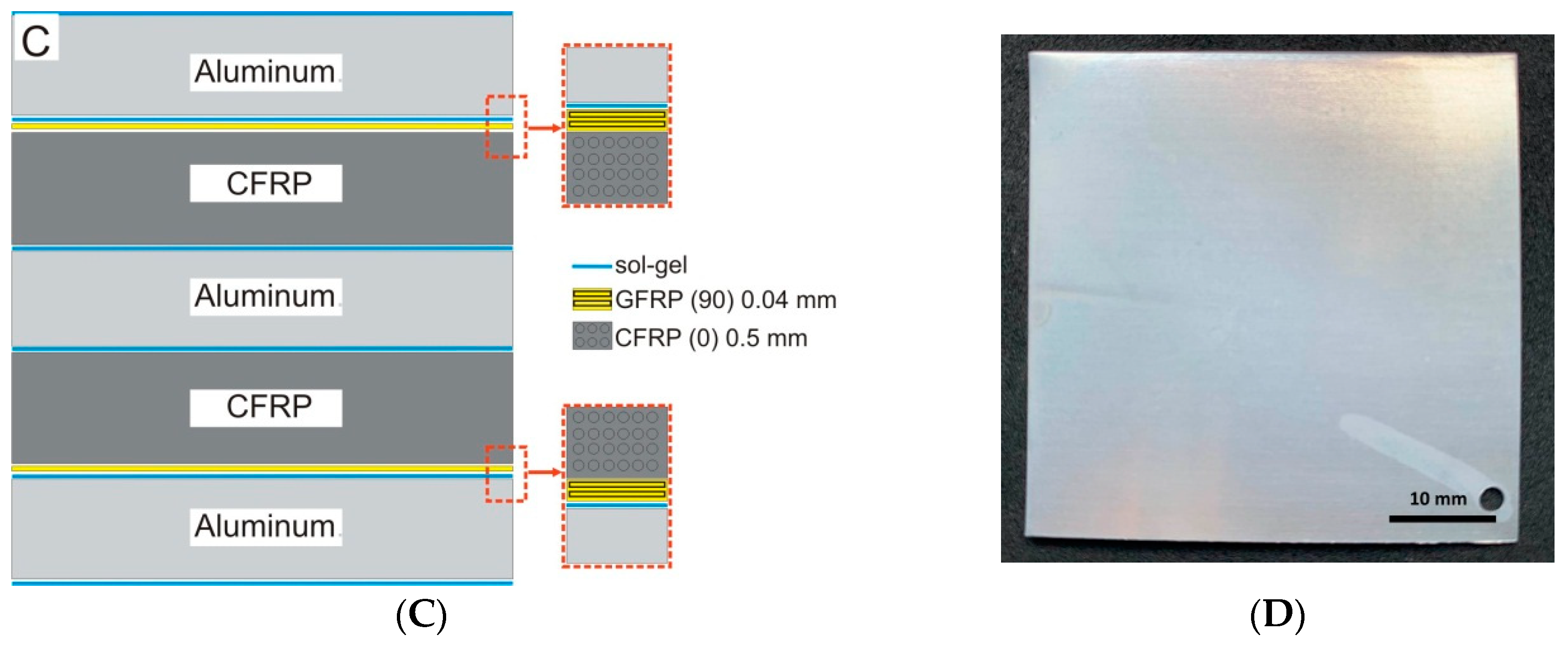
2.1. Materials
2.2. Methods
3. Results and Discussion
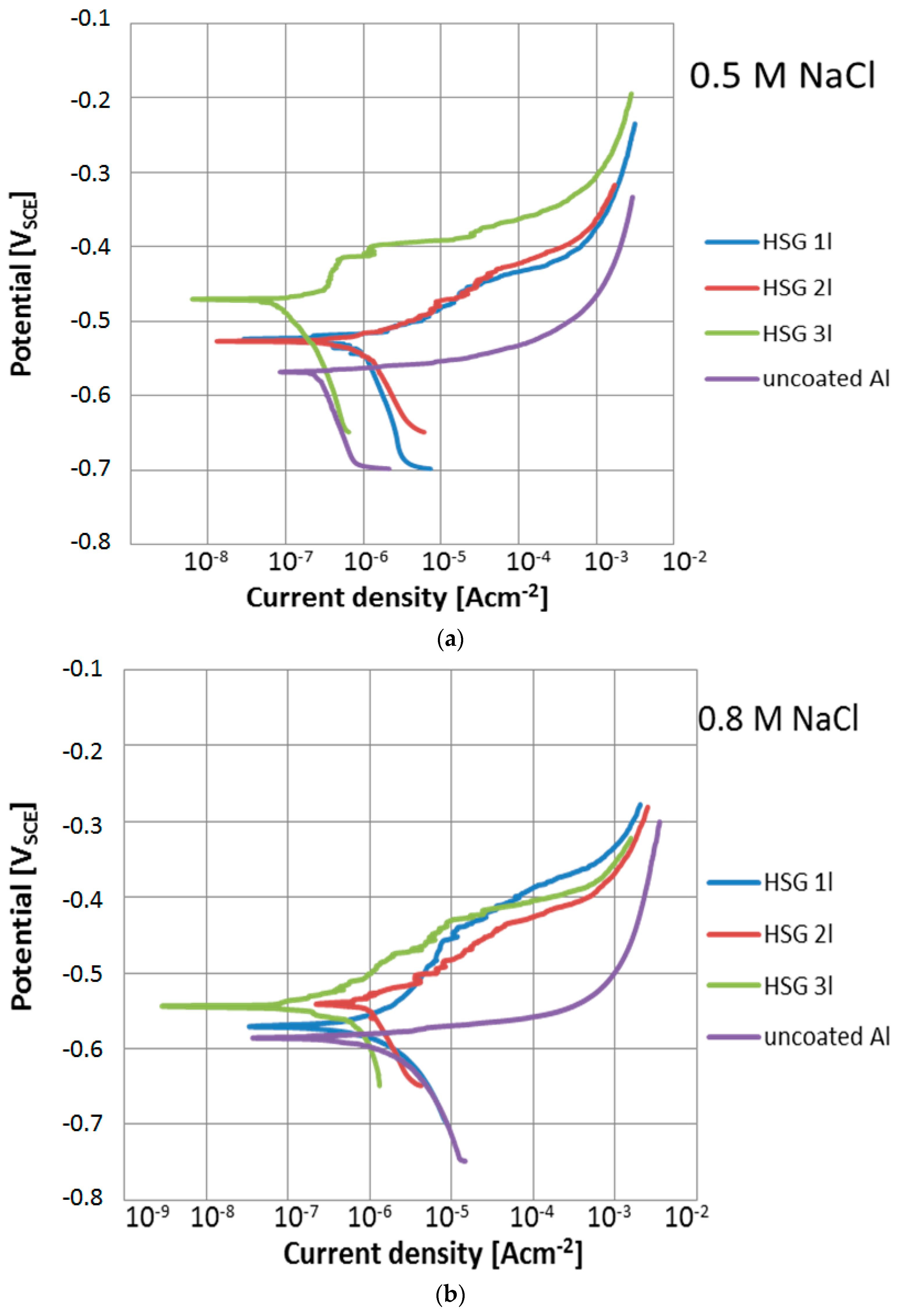
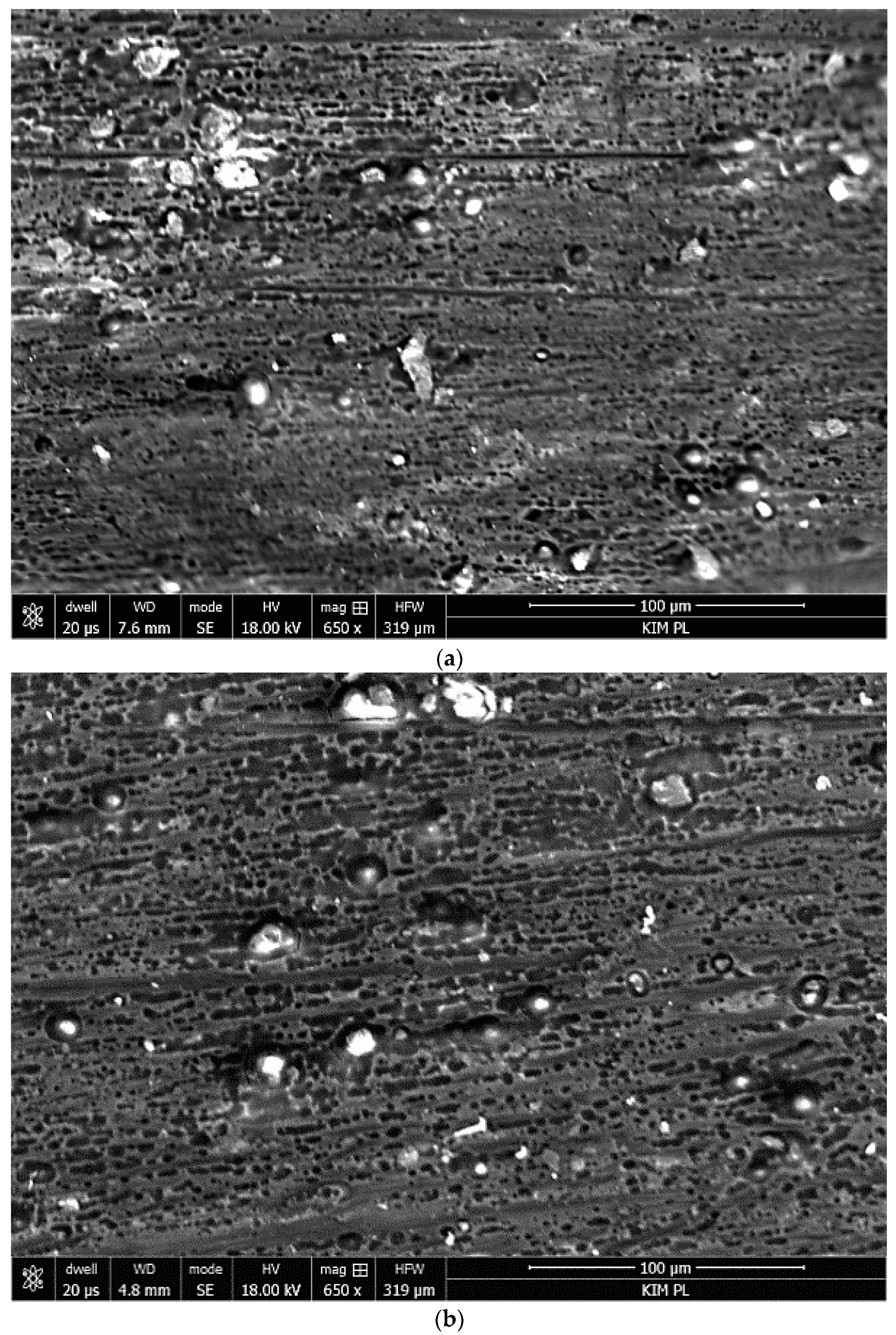
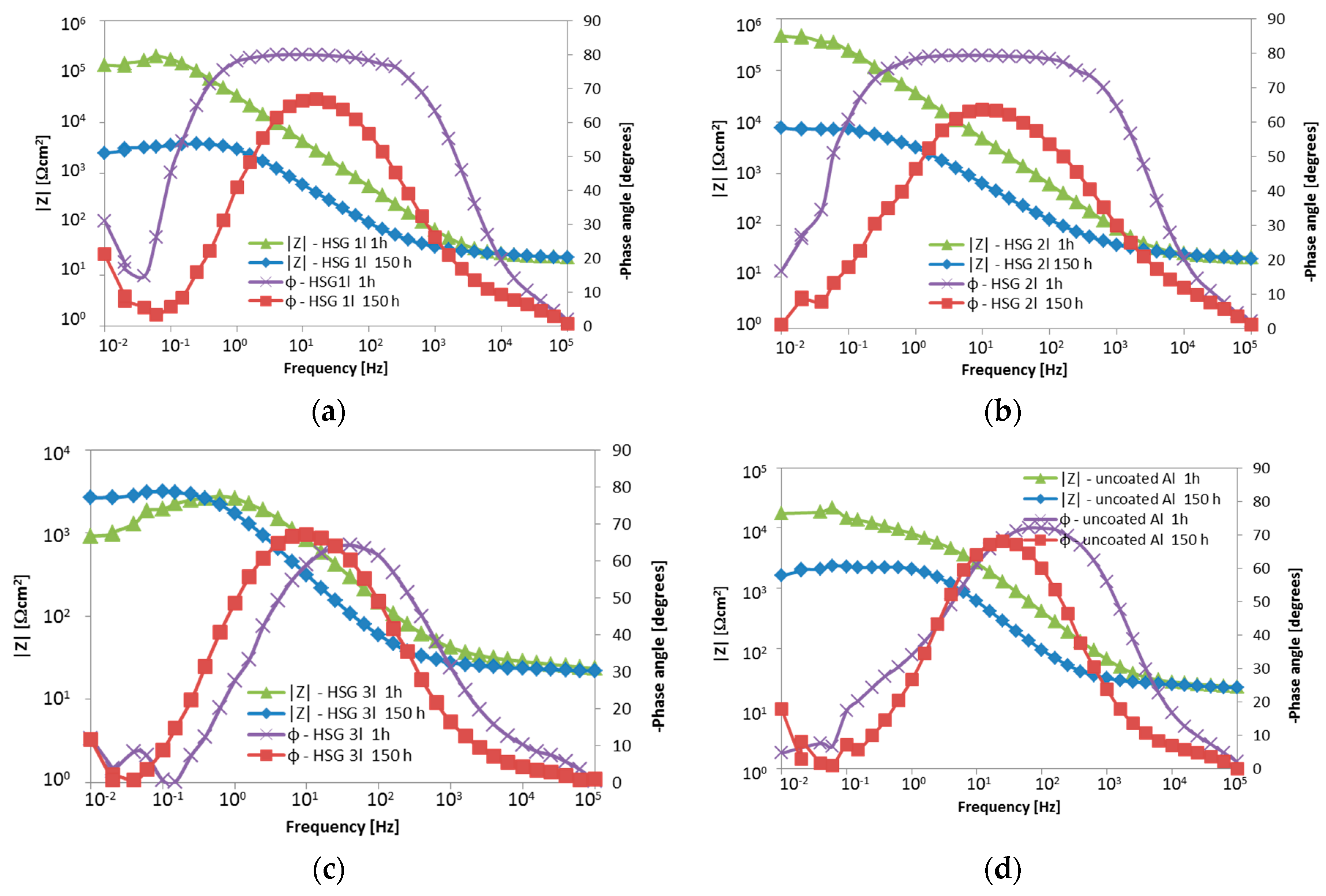
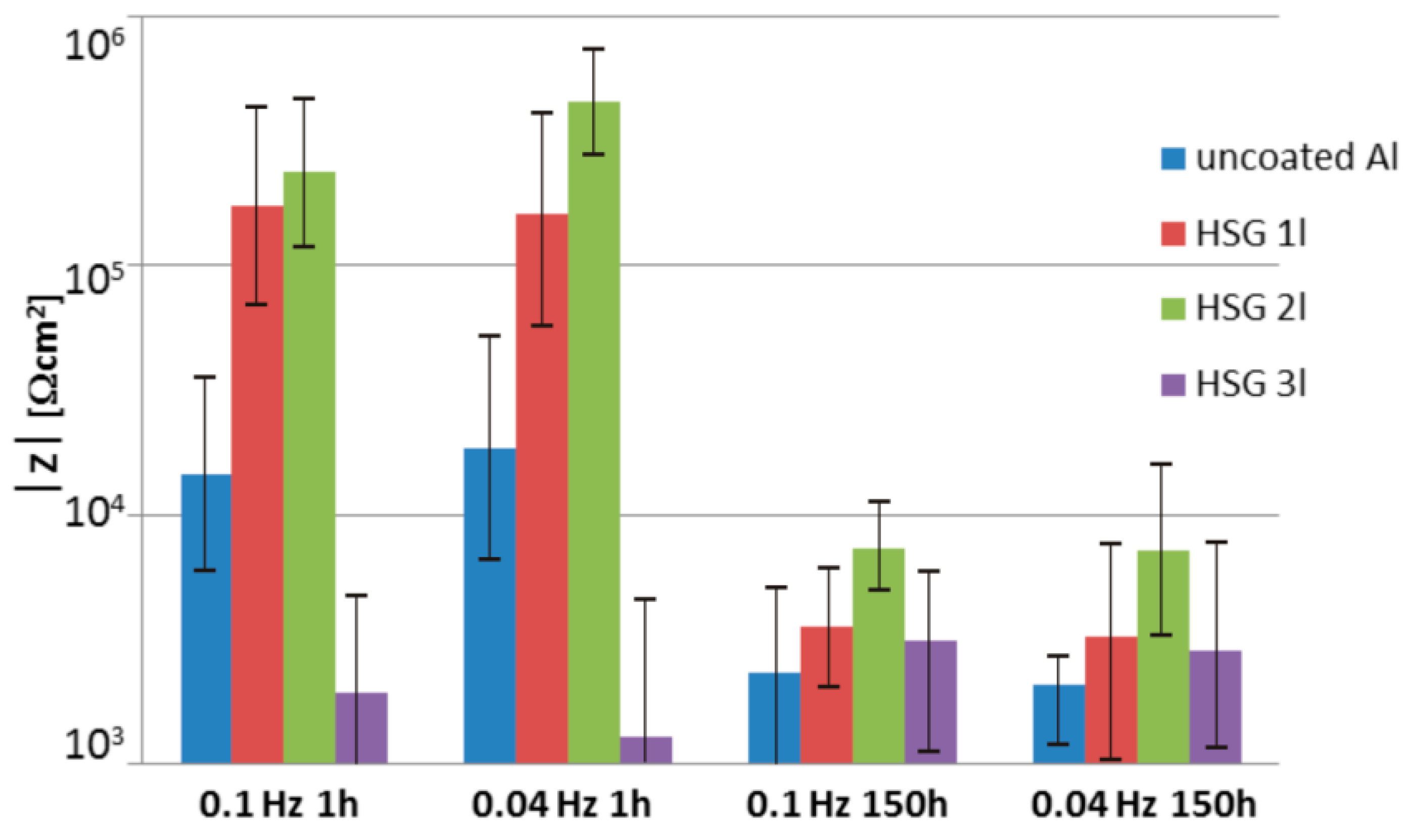
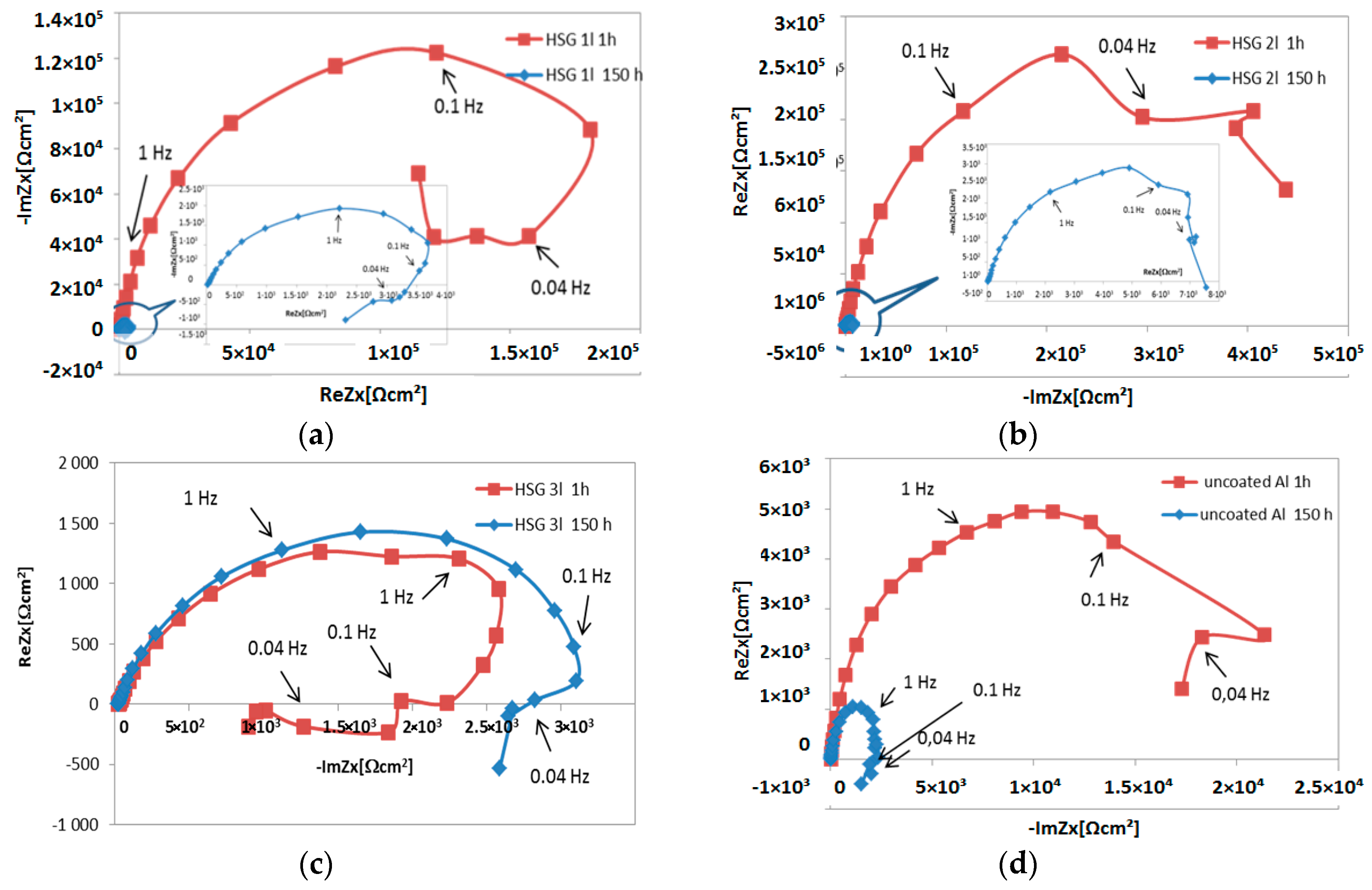
3.1. Microstructure and Electrochemical Properties of Sol–Gel Coatings
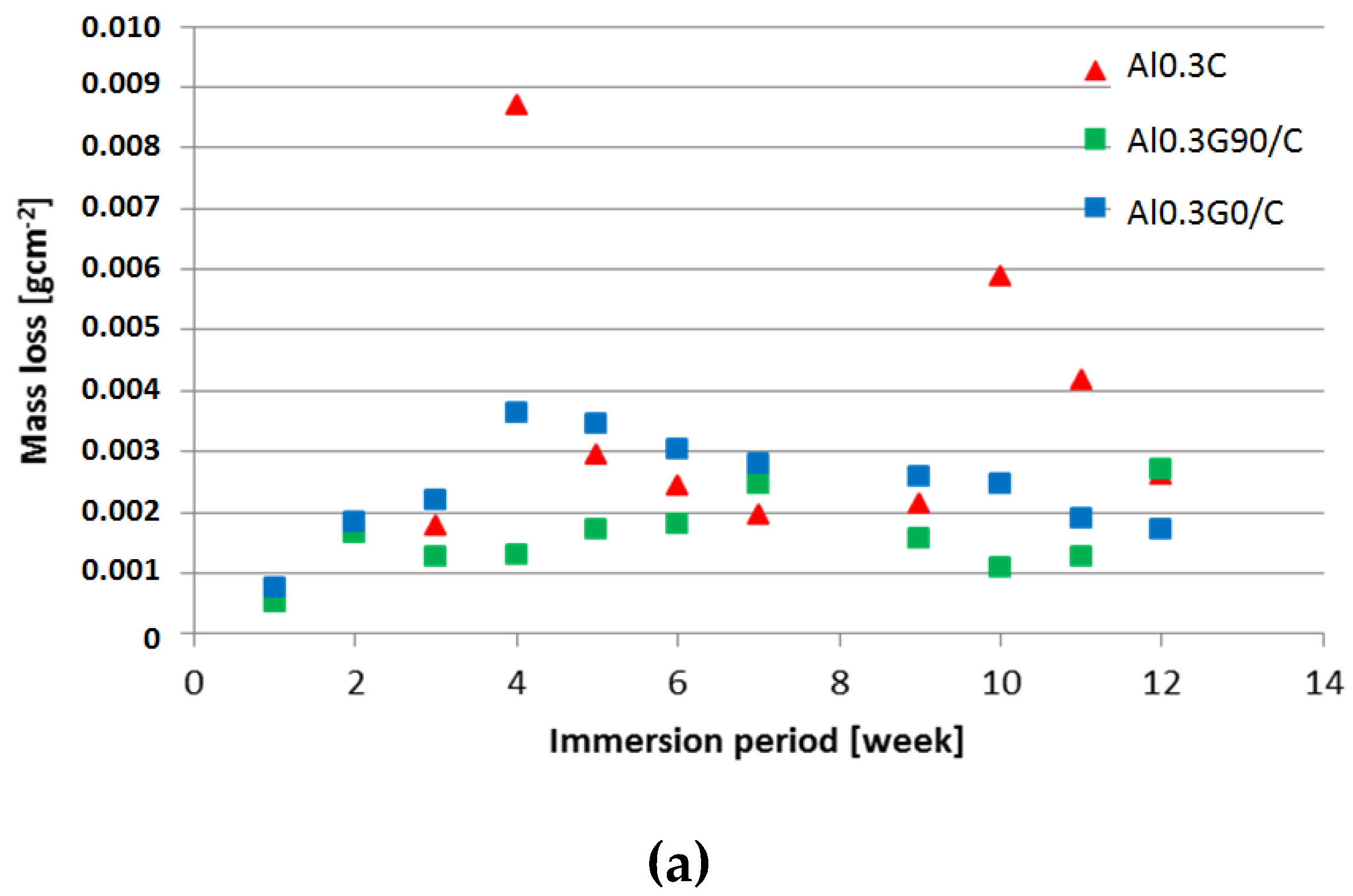
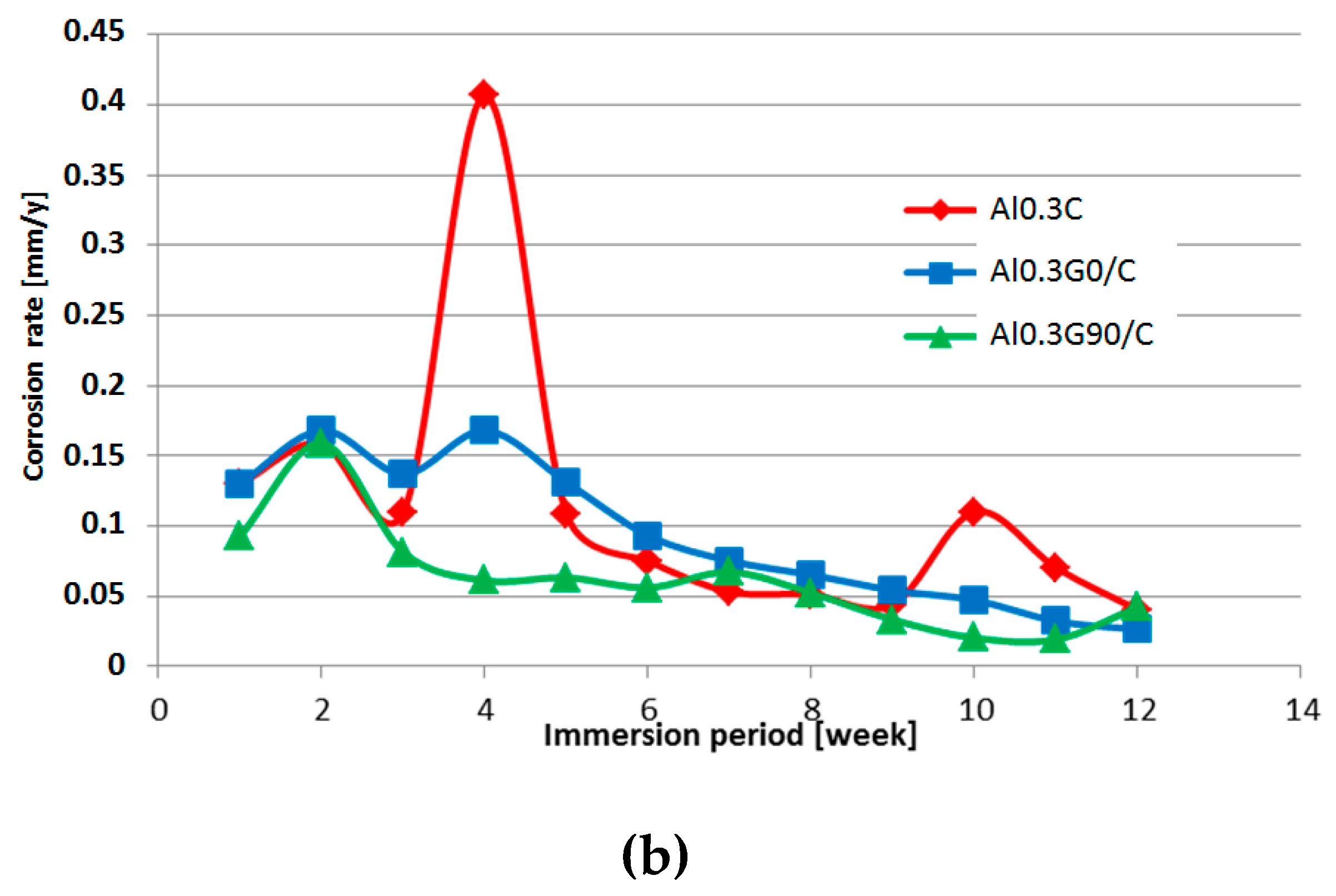
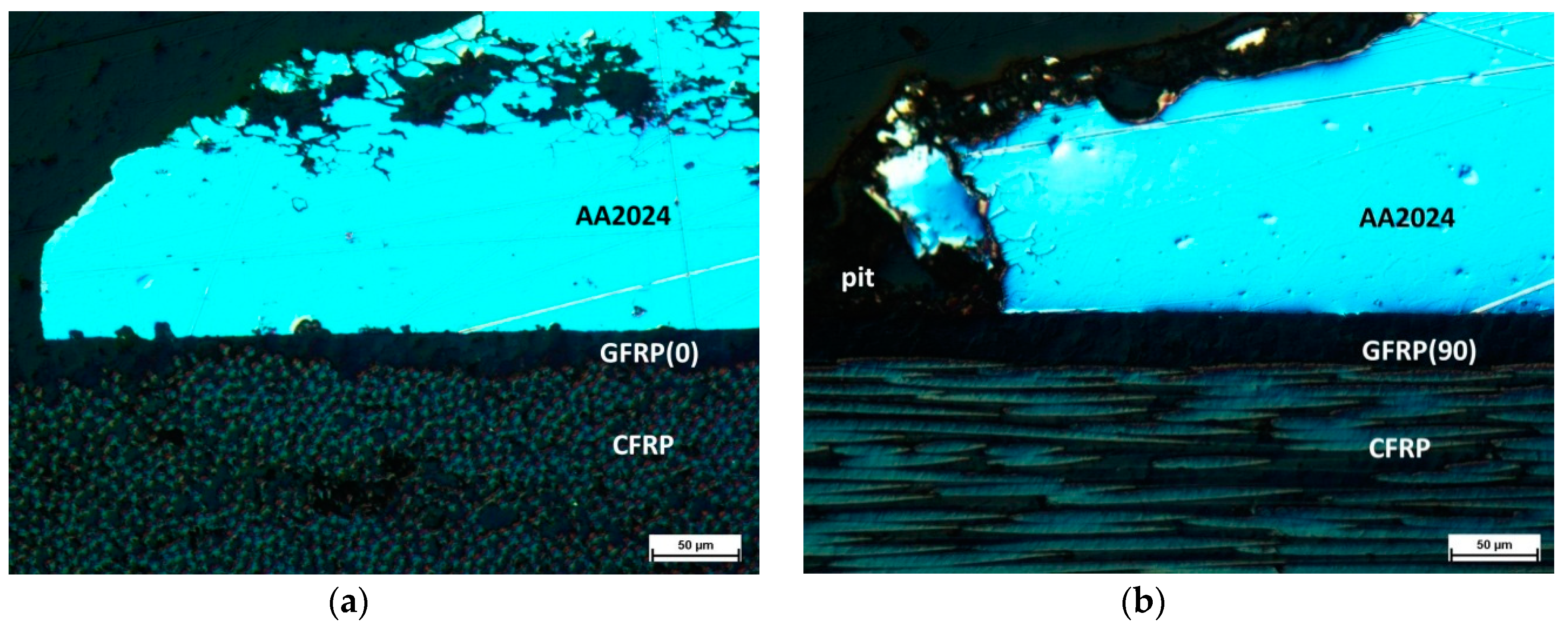
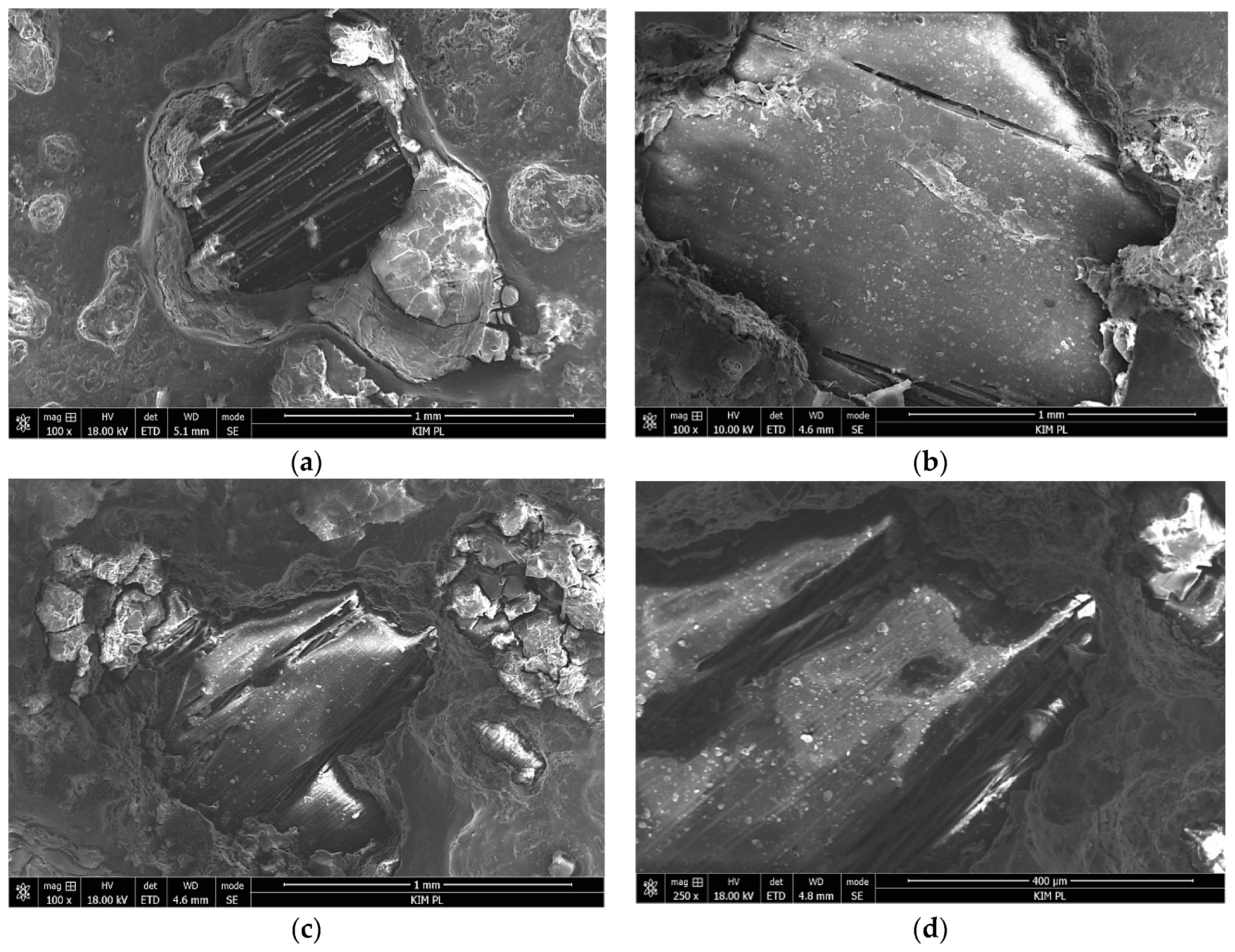
3.2. Salt Spray Test of Laminates
4. Summary and Conclusions
- An HSG coating does not provide sufficient protection against corrosion for an aluminum alloy in direct contact with an aggressive environment, but it is effective as an interlayer.
- Impedance spectra indicate the complex structure of the coating that contains a reactive epoxy-silane layer and a conversion Al-O-Zr interlayer. After long-term contact with an environment containing chloride ions, water penetrates the porous structure of the cured sol–gel, and hydrolysis may occur. Migrating chloride ions react with the conversion interlayer and lead to the initiation of pitting corrosion in the metal.
- Local aluminum layer perforation does not lead to delamination at the metal–composite interface despite the type or configuration of the composite. This confirms the durability of the HSG used in FMLs.
Author Contributions
Funding
Conflicts of Interest
References
- Sinke, J. Manufacturing of glare parts and structures. Appl. Compos. Mater. 2003, 10, 293–305. [Google Scholar] [CrossRef]
- Botelho, E.C.; Silva, R.A.; Pardini, L.C.; Rezende, M.C. A review on the development and properties of continuous fiber/epoxy/aluminum hybrid composites for aircraft structures. Mat. Res. 2006, 9, 247–256. [Google Scholar] [CrossRef]
- Borgonje, B.; Ypma, M.S. Long Term Behaviour of Glare. Appl. Compos. Mater. 2003, 10, 243–255. [Google Scholar] [CrossRef]
- Peng, Z.; Nie, X. Galvanic corrosion property of contacts between carbon fiber cloth materials and typical metal alloys in an aggressive environment. Surf. Coat. Technol. 2013, 215, 85–89. [Google Scholar] [CrossRef]
- Liu, Z.; Curioni, M.; Jamshidi, P.; Walker, A.; Prengnell, P.; Thompson, G.E.; Skeldon, P. Electrochemical characteristics of a carbon fibre composite and the associated galvanic effects with aluminium alloys. Appl. Surf. Sci. 2014, 314, 233–240. [Google Scholar] [CrossRef]
- Santos, T.F.A.; Vasconcelos, G.C.; de Souza, W.A.; Costa, M.L.; Botelho, E.C. Suitability of carbon fiber-reinforced polymers as power cable cores: Galvanic corrosion and thermal stability evaluation. Mater. Des. 2015, 65, 780–788. [Google Scholar] [CrossRef]
- Håkansson, E.; Hoffman, J.; Predecki, P.; Kumosa, M. The role of corrosion product deposition in galvanic corrosion of aluminum/carbon systems. Corros. Sci. 2017, 114, 10–16. [Google Scholar] [CrossRef]
- Cai, B.P.; Liu, Y.H.; Ren, C.K.; Liu, Z.K.; Tian, X.J.; Abulimiti, A.B. Experimental study of galvanic corrosion behaviour of carbon fibre composite coupled to aluminium in artificial seawater. Corros. Eng. Sci. Technol. 2012, 47, 289–296. [Google Scholar] [CrossRef]
- Surowska, B.; Jakubczak, P.; Bieniaś, J. Structure and chemistry of fibre metal laminates. In Hybrid Polymer Composite Materials: Structure and Chemistry; Thakur, V.K., Thakur, M.K., Gupta, R.K., Eds.; Woodhead Publishing Elsevier: Cambridge, UK, 2017; Chapter 8; pp. 193–234. [Google Scholar]
- Sinmazçelik, T.; Avcu, E.; Bora, M.O.; Çoban, O. A review: Fibre metal laminates, background, bonding types and applied test methods. Mater. Des. 2011, 32, 3671–3685. [Google Scholar] [CrossRef]
- Bieniaś, J.; Jakubczak, P.; Surowska, B. Properties and characterization of fiber metal laminates. In Hybrid Polymer Composite Materials: Properties and Characterisation; Thakur, V.K., Thakur, M.K., Pappu, A., Eds.; Woodhead Publishing Elsevier: Cambridge, UK, 2017; Chapter 11; pp. 253–278. [Google Scholar]
- Twite, R.L.; Bierwagen, G.P. Review of alternatives to chromate for corrosion protection of aluminum aerospace alloys. Prog. Org. Coat. 1998, 33, 91–100. [Google Scholar] [CrossRef]
- Qi, J.-T.; Hashimoto, T.; Walton, J.R.; Zhou, X.; Skeldon, P.; Thompson, G.E. Trivalent chromium conversion coating formation on aluminium. Surf. Coat. Technol. 2015, 280, 317–329. [Google Scholar] [CrossRef]
- Santa Coloma, P.; Izagirre, U.; Belaustegi, Y.; Jorcin, J.B.; Cano, F.J.; Lapena, N. Chromium-free conversion coatings based on inorganic salts (Zr/Ti/Mn/Mo) for aluminum alloys used in aircraft applications. Appl. Surf. Sci. 2015, 345, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Organic–inorganic hybrid sol–gel coatings for metal corrosion protection: A review of recent progress. J. Coat. Technol. Res. 2015, 12, 1–35. [Google Scholar] [CrossRef]
- Fontinha, I.R.; Salta, M.M.; Zheludkevich, M.L.; Ferreira, M.G.S. EIS Study of Amine Cured Epoxy-silica-zirconia Sol-gel Coatings for Corrosion Protection of the Aluminium Alloy EN AW 6063. Port. Electrochim. Acta 2013, 31, 307–319. [Google Scholar] [CrossRef]
- Wang, D.; Bierwagen, G.P. Sol–gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Ebnesajjad, S. Surface Treatment of Materials for Adhesive Bonding, 2nd ed.; William Andrew Elsevier: Amsterdam, The Netherlands, 2014; Chapter 7; pp. 139–183. [Google Scholar] [CrossRef]
- Milosev, I.; Kapun, B.; Rodic, P.; Iskra, J. Hybrid sol–gel coating agents based on zirconium(IV) propoxide and epoxysilane. J. Sol-Gel Sci. Technol. 2015, 74, 447–459. [Google Scholar] [CrossRef]
- Purcar, V.; Somoghi, R.; Niţu, S.G.; Nicolae, C.-A.; Alexandrescu, E.; Gîfu, I.C.; Gabor, A.R.; Stroescu, H.; Ianchiş, R.; Căprărescu, S.; et al. Effect of coupling agent on nano-ZnO materials obtained by sol-gel process. Nanomaterials 2017, 7, 2–13. [Google Scholar] [CrossRef]
- Oliver, M.S.; Blohowiak, K.Y.; Dauskardt, R.H. Molecular structure and fracture properties of ZrOX/Epoxysilane hybrid films. J. Sol-Gel Sci. Technol. 2010, 55, 360–368. [Google Scholar] [CrossRef]
- Rodic, P.; Iskra, J.; Milosev, I. A hybrid organic–inorganic sol–gel coating for protecting aluminium alloy 7075-T6 against corrosion in Harrison’s solution. J. Sol-Gel Sci. Technol. 2014, 70, 90–103. [Google Scholar] [CrossRef]
- Purcar, V.; Donescu, D.; Ghiurea, M.; Serban, S.; Petcu, C.; Podina, C. The effect of different crosslinking agents on the properties of hybrid films. Optoelectron. Adv. Mater. Rapid Commun. 2009, 3, 204–208. [Google Scholar]
- Zheng, S.X.; Li, J.H. Inorganic–organic sol gel hybrid coatings for corrosion protection of metals. J. Sol. Gel. Sci. Technol. 2010, 54, 174–187. [Google Scholar] [CrossRef]
- Park, S.Y.; Choi, W.J.; Choi, H.S. A review of the recent developments in surface treatment techniques for bonded repair of aluminum airframe structures. Int. J. Adhes. Adhes. 2018, 80, 16–29. [Google Scholar] [CrossRef]
- Aakkula, J.; Saarela, O. Silane based field level surface treatment methods for aluminium, titanium and steel bonding. Int. J. Adhes. Adhes. 2014, 48, 268–279. [Google Scholar] [CrossRef]
- 3M™ Surface Pre-Treatment AC-130-2. Available online: https://www.3m.com/3M/en_US/company-us/all-3m-products/~/3M-Surface-Pre-Treatment-AC-130-2/?N=5002385+3292667587&preselect=8710645+8710683&rt=rud (accessed on 24 July 2019).
- Jakubczak, P.; Bieniaś, J.; Surowska, B. Interlaminar shear strength of fibre metal laminates after thermal cycles. Compos Struct 2018, 206, 876–887. [Google Scholar] [CrossRef]
- International Organization for Standardization. EN ISO 9227:2017 Corrosion Tests in Artificial Atmospheres—Salt Spray Tests; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- International Organization for Standardization. EN ISO 8407:2009 Corrosion of Metals and Alloys—Removal of Corrosion Products from Corrosion Test Specimens; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Nwosu, F.O.; Owate, I.O.; Osarolube, E. Acidic corrosion inhibition mechanism of aluminum alloy using green inhibitors. Am. J. Mater. Sci. 2018, 8, 45–50. [Google Scholar]
- Murthy, H.C.A. Electroanalytical Study on the Corrosion Behaviour of TiO2 Particulate Reinforced Al 6061 Composites. Mat. Sci. Res. India 2015, 12, 112–126. [Google Scholar] [CrossRef]
- Charitha, B.P.; Rao, P. Protection of 6061 Al-15%(v) SiC(P) Composite from Corrosion by a Biopolymer and Surface Morphology Studies. Prot. Met. Phys. Chem. Surf. 2016, 52, 704–713. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Li, S.; Zhao, P. Influence of adipic acid on anodic film formation and corrosion resistance of 2024 aluminum alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 492–500. [Google Scholar] [CrossRef]
- Veys-Renaux, D.; Chahboun, N.; Rocca, E. Anodizing of multiphase aluminium alloys in sulfuric acid: In-situ electrochemical behaviour and oxide properties. Electrochim. Acta 2016, 211, 1056–1065. [Google Scholar] [CrossRef]
- Liu, S.D.; Chen, B.; Li, C.B.; Dai, Y.; Deng, Y.L.; Zhang, X.M. Mechanism of low exfoliation corrosion resistance due to slow quenching in high strength aluminium alloy. Corros. Sci. 2015, 91, 203–212. [Google Scholar] [CrossRef]
- Klotz, D. Negative capacitance or inductive loop?—A general assessment of a common low frequency impedance feature. Electrochem. Commun. 2019, 98, 58–62. [Google Scholar] [CrossRef]
- Kordas, G.; Efthimiadou, E.K. Self-Healing Coatings for Corrosion Protection of Metals. In The Sol-Gel Handbook: Synthesis, Characterization and Applications; Levy, D., Zayat, M., Eds.; Wiley VCH Verlag GmbH: Weinheim, Germany, 2015; Chapter 44; pp. 1371–1384. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Zheludkevich, M.L.; Karavai, O.V.; Ferreira, M.G.S. Influence of inhibitor addition on the corrosion protection performance of sol–gel coatings on AA2024. Prog. Org. Coat. 2008, 63, 352–361. [Google Scholar] [CrossRef]
- Juan-Díaz, M.J.; Martínez-Ibánez, M.; Hernández-Escolano, M.; Cabedo, L.; Izquierdo, R.; Suay, J.; Gurruchaga, M.; Goni, I. Study of the degradation of hybrid sol–gel coatings in aqueous medium. Prog. Org. Coat. 2014, 77, 1799–1806. [Google Scholar] [CrossRef]
- Fernandez-Sanchez, C.; McNeil, C.J.; Rawson, K. Electrochemical impedance spectroscopy studies of polymer degradation: application to biosensor development. Trends Anal. Chem. 2005, 24, 37–48. [Google Scholar] [CrossRef]
- Gobara, M.; Kamel, H.; Akid, R.; Baraka, A. Corrosion behaviour of AA2024 coated with an acid-soluble collagen/hybrid silica sol–gel matrix. Prog. Org. Coat. 2015, 89, 57–66. [Google Scholar] [CrossRef]
- Poznyak, S.K.; Zheludkevich, M.L.; Raps, D.; Gammelc, F.; Yasakau, K.A.; Ferreira, M.G.S. Preparation and corrosion protective properties of nanostructured titania-containing hybrid sol–gel coatings on AA2024. Prog. Org. Coat. 2008, 62, 226–235. [Google Scholar] [CrossRef]
- Capelossi, V.R.; Poelman, M.; Recloux, I.; Hernandez, R.P.B.; de Melo, H.G.; Olivier, M.G. Corrosion protection of clad 2024 aluminum alloy anodized in tartaric-sulfuric acid bath and protected with hybrid sol–gel coating. Electrochim. Acta 2014, 124, 69–79. [Google Scholar] [CrossRef]







| Abbreviation | Molecular Formula | Structural Formula |
|---|---|---|
| GPTMS | C9H20O5Si | 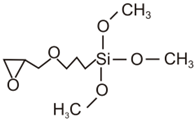 |
| TPOZ | C12H28O4Zr | 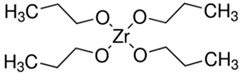 |
| Sol–Gel Coating | Thickness Layer [μm] | EOCP [VSCE] | Ecorr [VSCE] | icorr [mAcm−2] | Rp [kΩcm2] | ||||
|---|---|---|---|---|---|---|---|---|---|
| 0.5 M NaCl | 0.8 M NaCl | 0.5 M NaCl | 0.8 M NaCl | 0.5 M NaCl | 0.8 M NaCl | 0.5 M NaCl | 0.8 M NaCl | ||
| 1 layer | 0.5 ± 0.2 | −0.52 ± 0.01 | −0.57 ± 0.04 | −0.52 ± 0.02 | −0.57 ± 0.02 | 0.39 ± 0.09 | 0.69 ± 0.1 | 66.7 ± 8.5 | 37.7 ± 5.3 |
| 2 layers | 0.8 ± 0.16 | −0.53 ± 0.02 | −0.56 ± 0.02 | −0.52 ± 0.01 | −0.54 ± 0.03 | 0.35 ± 0.08 | 0.39 ± 0.12 | 74.3 ± 7.2 | 66.7 ± 6.8 |
| 3 layers | 1.2 ± 0.2 | −0.55 ± 0.02 | −0.56 ± 0.03 | −0.49 ± 0.02 | −0.54 ± 0.02 | 0.018 ± 0.01 | 0.044 ± 0.02 | 1444 ± 152 | 591 ± 75 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surowska, B.; Ostapiuk, M.; Jakubczak, P.; Droździel, M. The Durability of an Organic–Inorganic Sol–Gel Interlayer in Al-GFRP-CFRP Laminates in a Saline Environment. Materials 2019, 12, 2362. https://doi.org/10.3390/ma12152362
Surowska B, Ostapiuk M, Jakubczak P, Droździel M. The Durability of an Organic–Inorganic Sol–Gel Interlayer in Al-GFRP-CFRP Laminates in a Saline Environment. Materials. 2019; 12(15):2362. https://doi.org/10.3390/ma12152362
Chicago/Turabian StyleSurowska, Barbara, Monika Ostapiuk, Patryk Jakubczak, and Magda Droździel. 2019. "The Durability of an Organic–Inorganic Sol–Gel Interlayer in Al-GFRP-CFRP Laminates in a Saline Environment" Materials 12, no. 15: 2362. https://doi.org/10.3390/ma12152362
APA StyleSurowska, B., Ostapiuk, M., Jakubczak, P., & Droździel, M. (2019). The Durability of an Organic–Inorganic Sol–Gel Interlayer in Al-GFRP-CFRP Laminates in a Saline Environment. Materials, 12(15), 2362. https://doi.org/10.3390/ma12152362






