Mg/Al LDH Enhances Sulfate removal and Clarification of AMD Wastewater in Precipitation Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. LDH Synthesis
2.3. Sulfate Removal
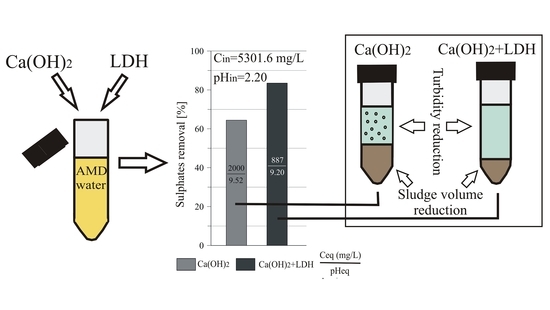
2.3.1. The Sulfate Removal by LDH, Ca(OH)2, and a Two-Step Experiment
2.3.2. The Sulfate Removal by a Mixture of Ca(OH)2 and LDH.
2.3.3. The Kinetic Experiment.
2.4. Analysis of Solid Sedimentation Rate
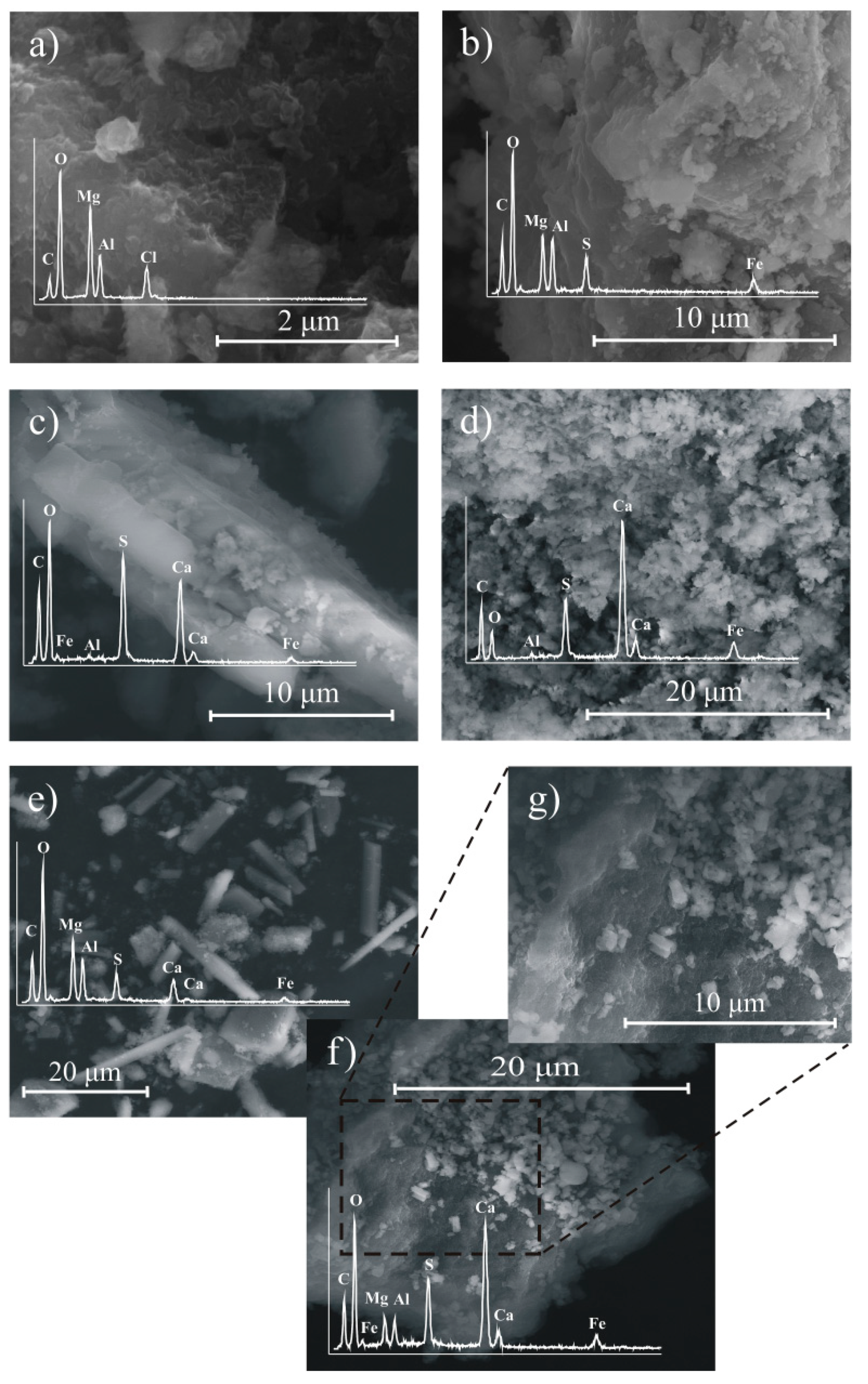
2.5. Characterization Methods
3. Results and Discussion.
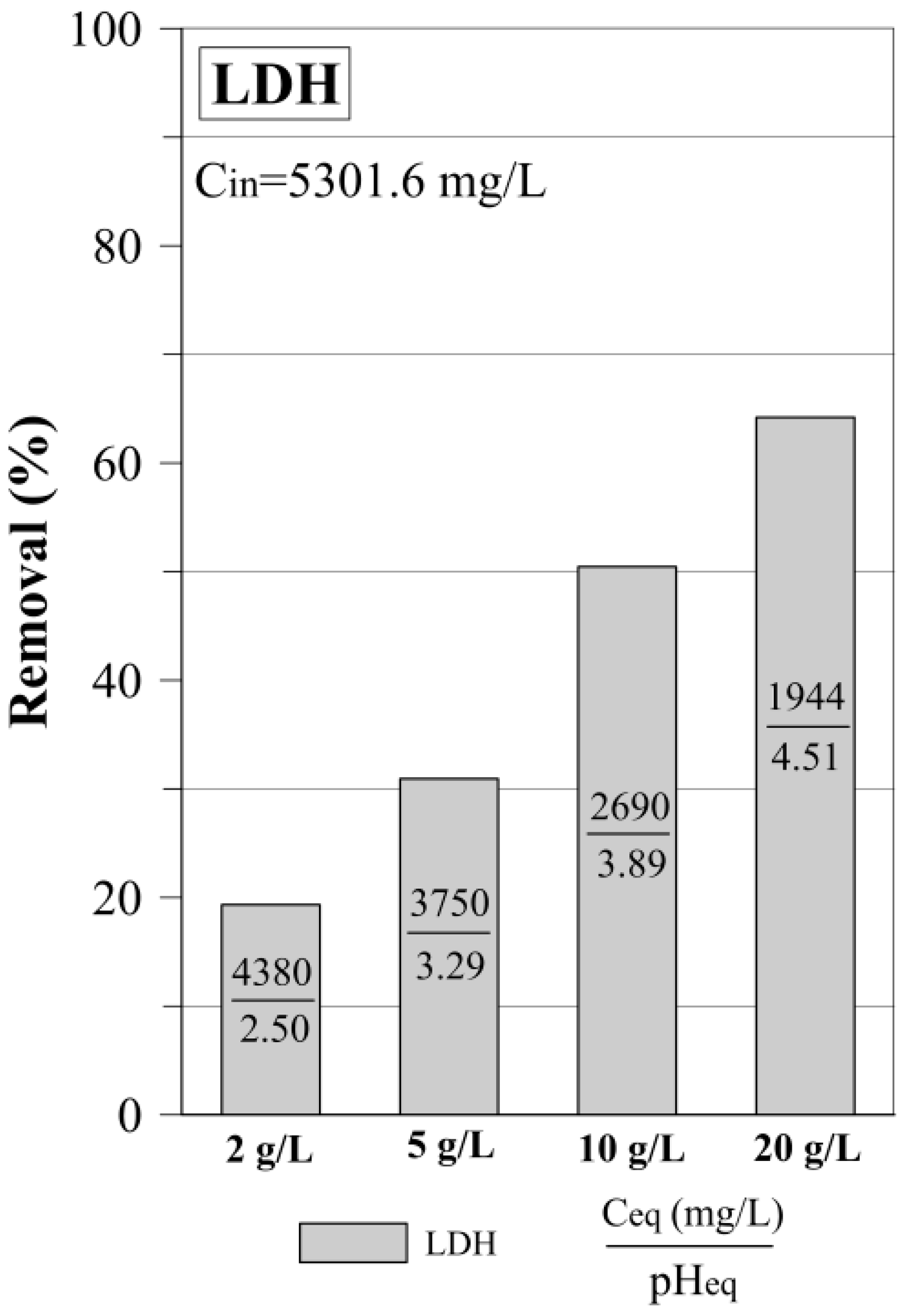
3.1. The Sulfate Removal by the LDH: Dosage Experiment
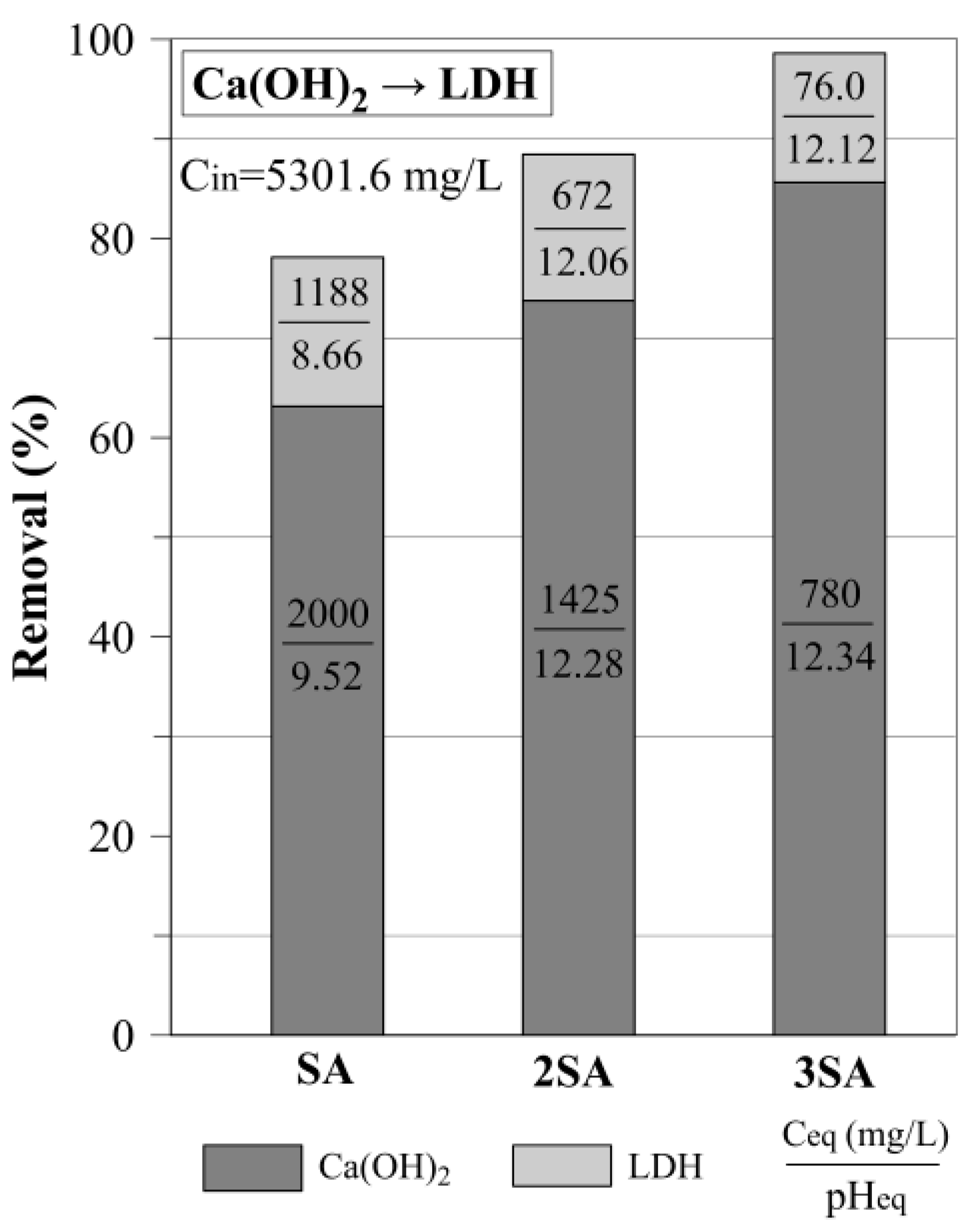
3.2. The Two-Step Sulfate Removal by Ca(OH)2 and LDH
3.2.1. The Sulfate Removal by Precipitation, Using Ca(OH)2: Dosage Effect
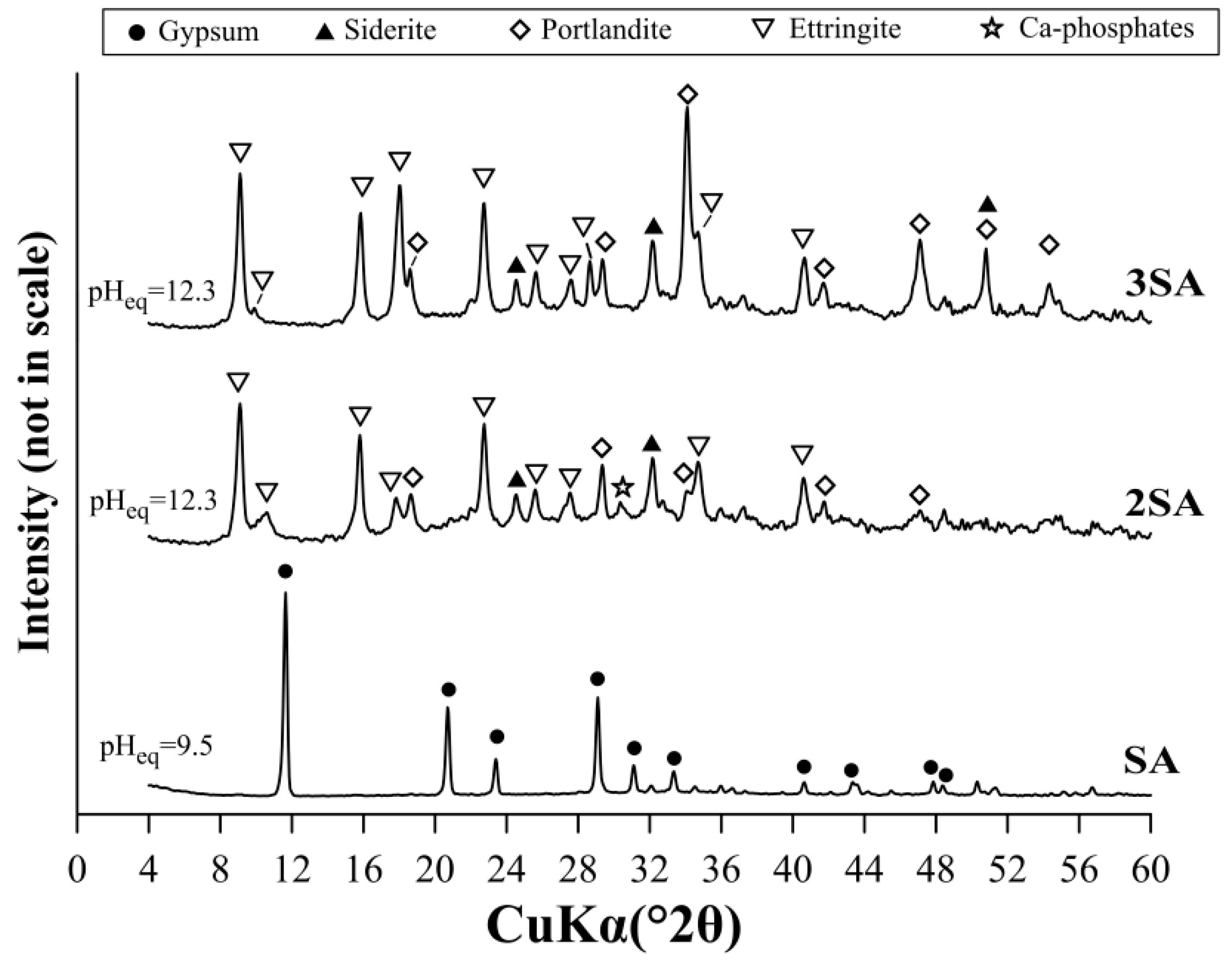
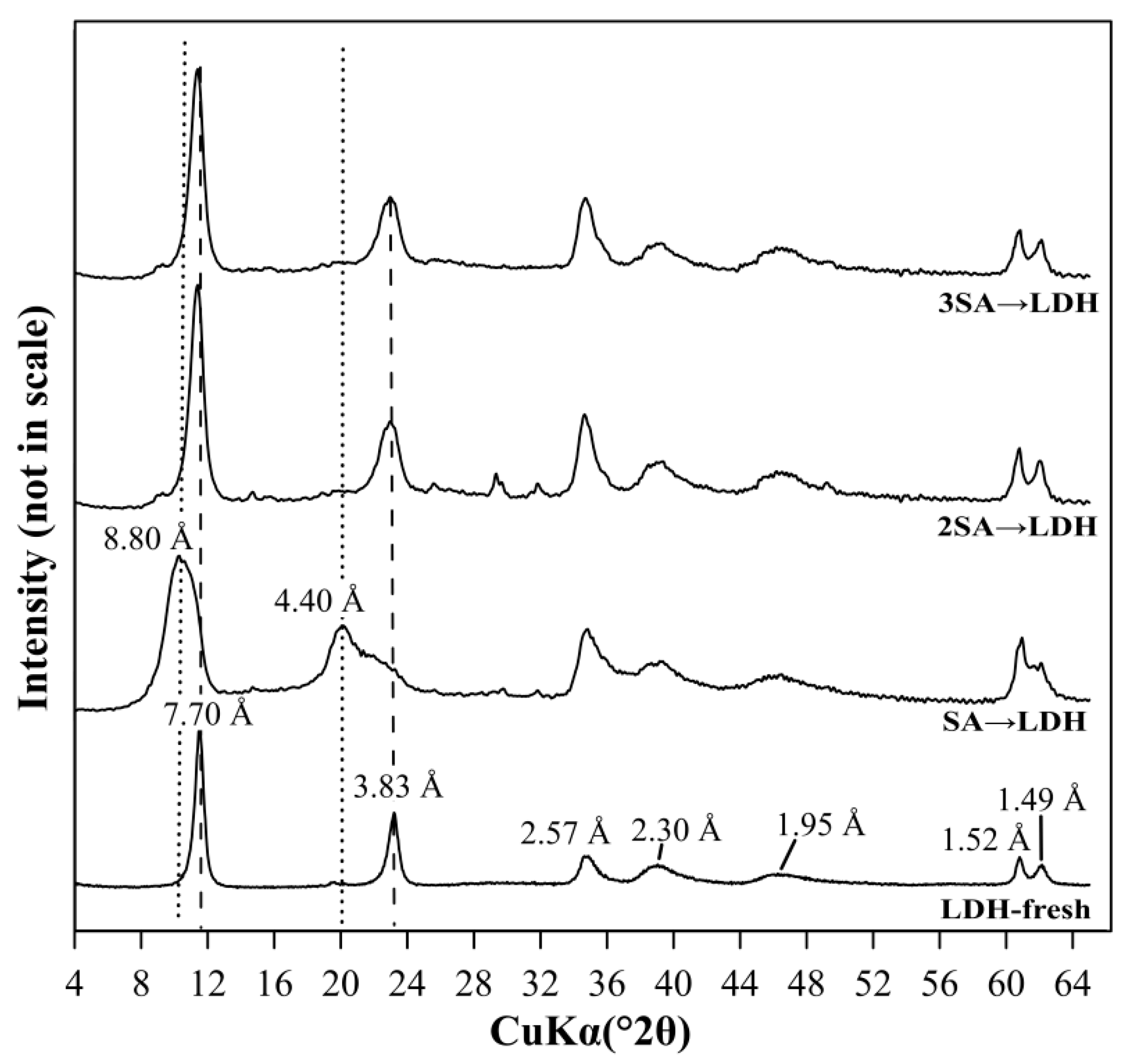
3.2.2. The Use of LDH for Sulfate Removal After Precipitation with Ca(OH)2
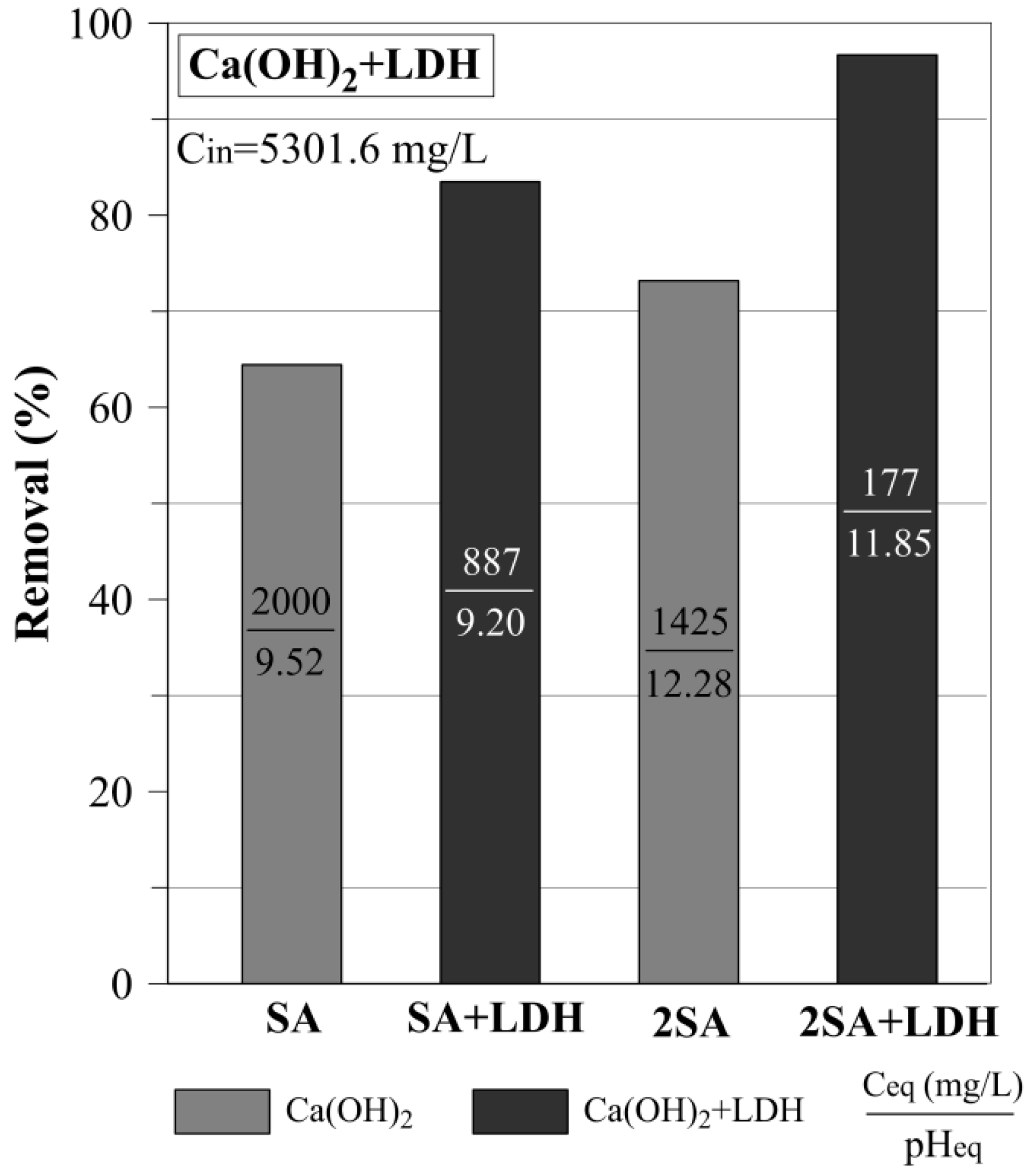
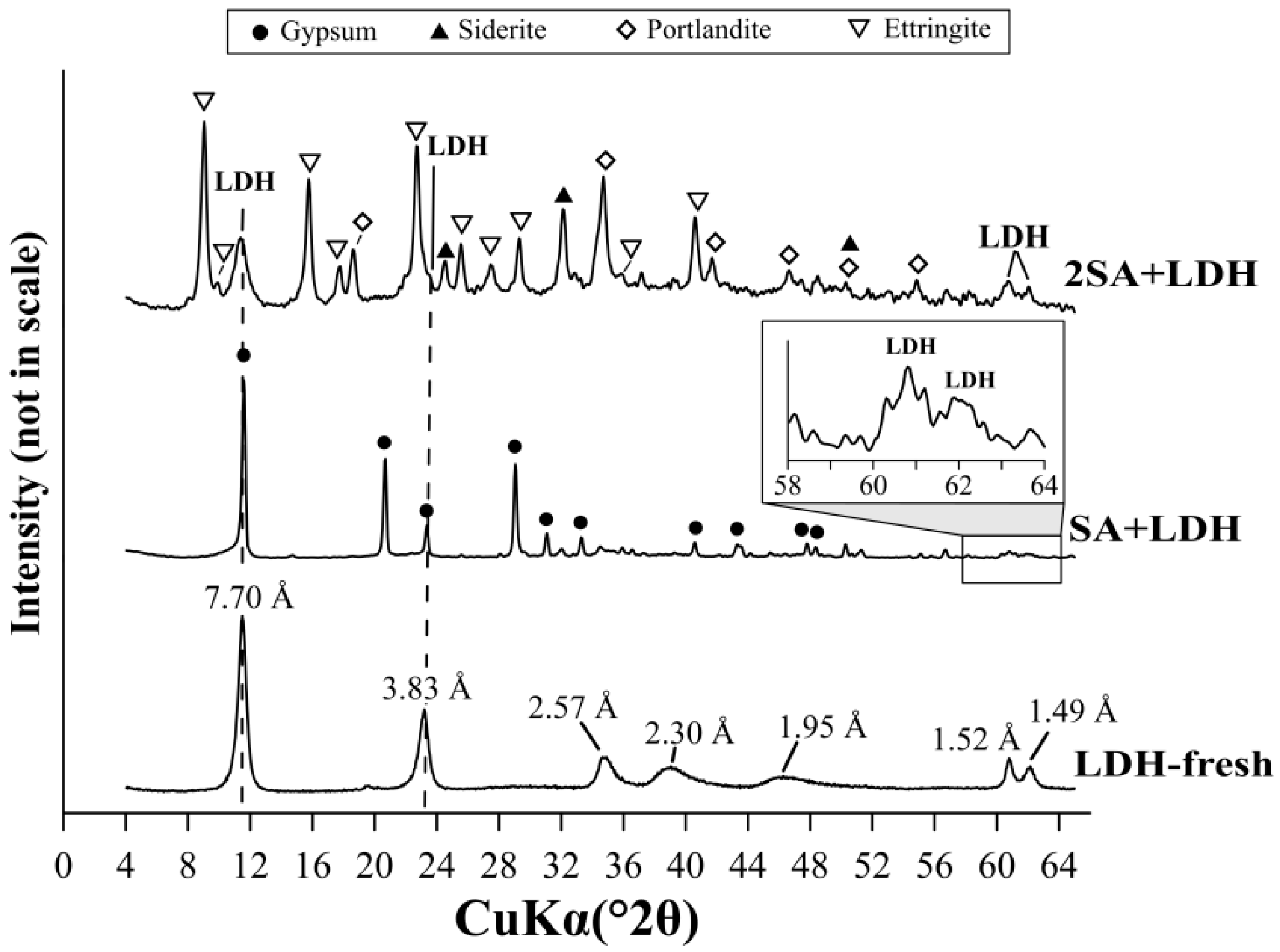
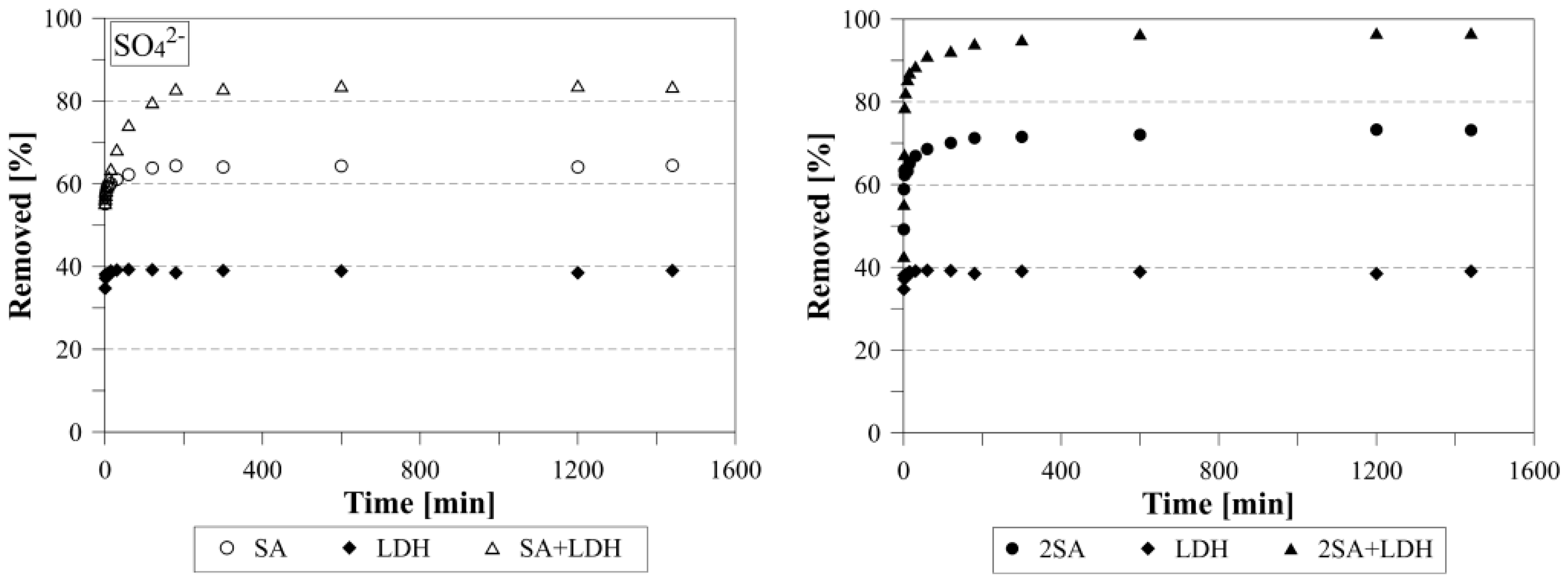
3.3. The Sulfate Removal Using a Mixture of Ca(OH)2 and LDH
3.4. Kinetics Studies of Sulfate Removal
3.5. Sedimentation and Sludge Volume Reduction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rozporządzenie Ministra Infrastruktury i Rozwoju z dnia 25 sierpnia 2015 r. zmieniające rozporządzenie w sprawie sposobu realizacji obowiązków dostawców ścieków przemysłowych oraz warunków wprowadzania ścieków do urządzeń kanalizacyjnych (Regulation of Minister of infrastructure and development concerning amending the regulation on the manner of fulfilling the duties of industrial wastewater suppliers and the conditions for the introduction of sewage into sewage systems. Dz. U. z 2015 r. poz. 1456. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20150001456 (accessed on 22 July 2019).
- Gu, B.; Ku, Y.K.; Jardine, P.M. Sorption and Binary Exchange of Nitrate, Sulfate, and Uranium on an Anion-Exchange Resin. Environ. Sci. Technol. 2004, 38, 3184–3188. [Google Scholar] [CrossRef] [PubMed]
- Bódalo, A.; Gómez, J.L.; Gómez, E.; León, G.; Tejera, M. Reduction of sulphate content in aqueous solutions by reverse osmosis using cellulose acetate membranes. Desalination 2004, 162, 55–60. [Google Scholar] [CrossRef]
- Murugananthan, M. Removal of sulfide, sulfate and sulfite ions by electro coagulation. J. Hazard. Mater. 2004, 109, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Benatti, C.T.; Tavares, C.R.G.; Lenzi, E. Sulfate removal from waste chemicals by precipitation. J. Environ. Manag. 2009, 90, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.S.; Shin, P.K.; Kim, B.H. Biological Treatment Of Acid Mine Drainage Under Sulphate-Reducing Conditions With Solid Waste Materials As Substrate. Water Res. 2000, 34, 1269–1277. [Google Scholar] [CrossRef]
- Maziarz, P.; Matusik, J.; Strączek, T.; Kapusta, C.; Woch, M.W.; Tokarz, W.; Radziszewska, A.; Leiviskä, T. Highly effective magnet-responsive LDH-Fe oxide composite adsorbents for As(V) removal. Chem. Eng. J. 2019, 362, 207–216. [Google Scholar] [CrossRef]
- Xu, Y.; Dai, Y.; Zhou, J.; Xu, Z.P.; Qian, G.; Lu, G.Q.M. Removal efficiency of arsenate and phosphate from aqueous solution using layered double hydroxide materials: Intercalation vs. precipitation. J. Mater. Chem. 2010, 20, 4684–4691. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, B.; Fang, L.; Ling, F.; Gao, J.; Wu, F.; Zhang, X. High performance NiFe layered double hydroxide for methyl orange dye and Cr(VI) adsorption. Chemosphere 2016, 152, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Forano, C.; Costantino, U.; Prévot, V.; Gueho, C.T. Layered Double Hydroxides (LDH). In Handbook of Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier Science Ltd.: Oxford, UK, 2013; Volume 5, pp. 745–782. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA, 1992. [Google Scholar]
- Constantino, V.R.L.; Pinnavaia, T.J. Basic Properties of Mg2+1-x, AI3+x, Layered Double Hydroxides Intercalated by Carbonate, Hydroxide, Chloride, and Sulfate Anions. Inorg. Chem. 1995, 34, 883–892. [Google Scholar] [CrossRef]
- Myneni, S.C.B.; Traina, S.J.; Waychunas, G.A.; Logan, T.J. Vibrational spectroscopy of functional group chemistry and arsenate coordination in ettringite. Geochim. Cosmochim. Acta 1998, 62, 3499–3514. [Google Scholar] [CrossRef]
- Mandal, P.K.; Mandal, T.K. Anion water in gypsum (CaSO4·2H2O) and hemihydrate (CaSO4 ·1/2H2O). Cem. Concr. Res. 2002, 32, 313–316. [Google Scholar] [CrossRef]
- Böke, H.; Akkurt, S.; Özdemir, S.; Göktürk, E.H.; Saltik, E.N.C. Quantification of CaCO3–CaSO3·0.5H2O–CaSO4·2H2O mixtures by FTIR analysis and its ANN model. Mater. Lett. 2004, 58, 723–726. [Google Scholar] [CrossRef]




| Sample | Turbidity after 30 min of Settling [NTU] |
|---|---|
| 2SA | 66.3 |
| SA | 10.8 |
| 2SA+LDH | 6.2 |
| SA+LDH | 1.1 |
| Sulfate Removal (%) | |||
|---|---|---|---|
| LDH Dosage Experiment | |||
| 2 g/L | 19.3 | ||
| 5 g/L | 30.9 | ||
| 10 g/L | 50.4 | ||
| 20 g/L | 64.2 | ||
| Two step experiment (Ca(OH)2→LDH) | |||
| Ca(OH)2 | LDH | Σ | |
| SA→LDH | 63.1 | 15.0 | 78.1 |
| 2SA→LDH | 73.7 | 14.7 | 88.4 |
| 3SA→LDH | 85.6 | 13.0 | 98.6 |
| Ca(OH)2+LDH mixture | |||
| SA+LDH | 83.5 | ||
| 2SA+LDH | 96.7 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maziarz, P.; Matusik, J.; Leiviskä, T. Mg/Al LDH Enhances Sulfate removal and Clarification of AMD Wastewater in Precipitation Processes. Materials 2019, 12, 2334. https://doi.org/10.3390/ma12142334
Maziarz P, Matusik J, Leiviskä T. Mg/Al LDH Enhances Sulfate removal and Clarification of AMD Wastewater in Precipitation Processes. Materials. 2019; 12(14):2334. https://doi.org/10.3390/ma12142334
Chicago/Turabian StyleMaziarz, Paulina, Jakub Matusik, and Tiina Leiviskä. 2019. "Mg/Al LDH Enhances Sulfate removal and Clarification of AMD Wastewater in Precipitation Processes" Materials 12, no. 14: 2334. https://doi.org/10.3390/ma12142334
APA StyleMaziarz, P., Matusik, J., & Leiviskä, T. (2019). Mg/Al LDH Enhances Sulfate removal and Clarification of AMD Wastewater in Precipitation Processes. Materials, 12(14), 2334. https://doi.org/10.3390/ma12142334