Research on Erosion-Corrosion Rate of 304 Stainless Steel in Acidic Slurry via Experimental Design Method
Abstract
1. Introduction
2. Experimental Method
2.1. Specimen Preparation
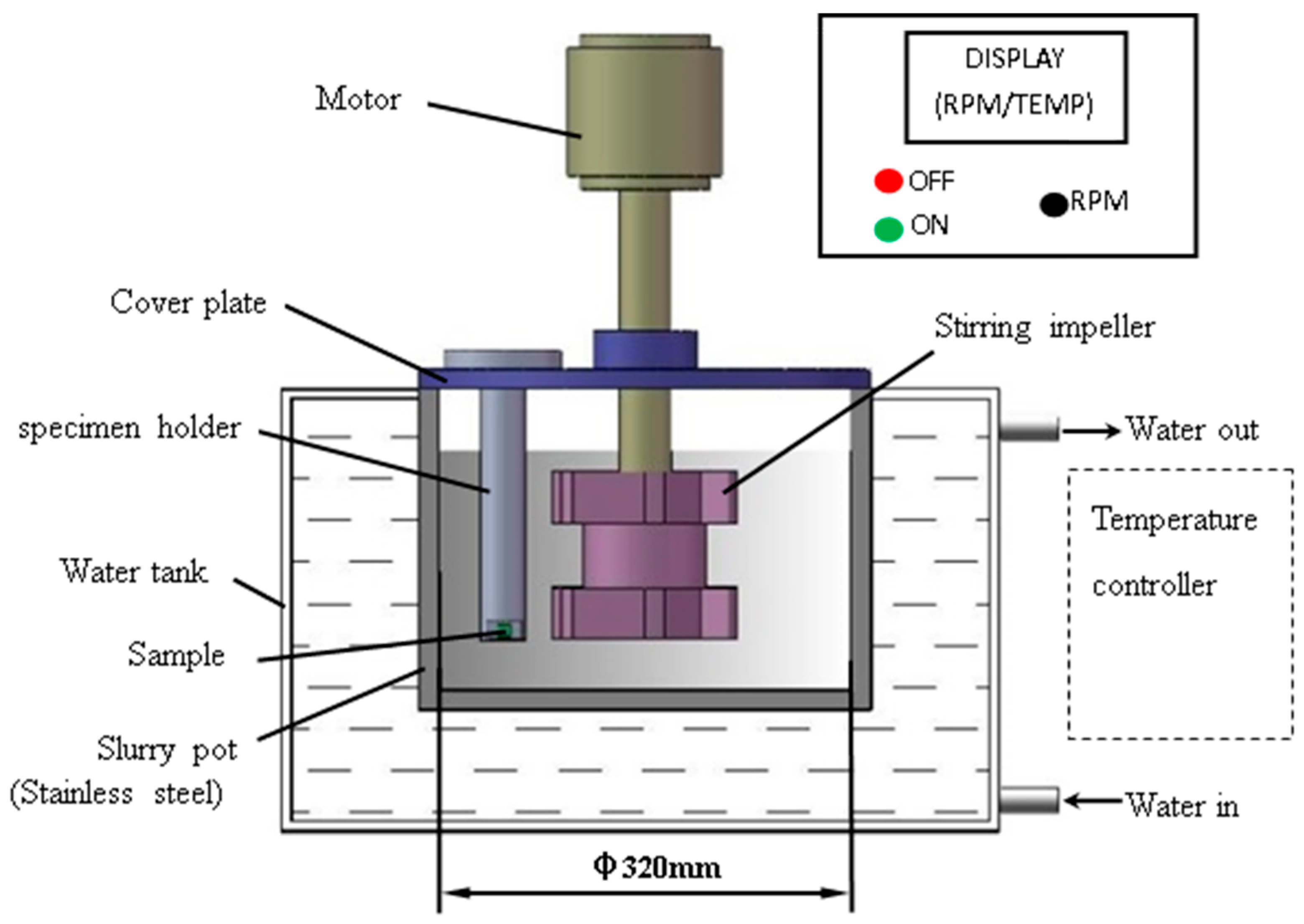
2.2. Test Apparatus
2.3. Experimental Methodology
3. Results and Discussion
3.1. Weight Loss Rate
3.2. Quantitative Analysis of Data
3.3. Qualitative Analysis of Data
4. Conclusions
- Temperature has the largest contribution percentage of more than 20% in the range of three considered factors, i.e., temperature, rotational speed, and sulfuric acid concentration. At the same time, temperature plays a remarkable role on erosion wear rate variation of 304 stainless steel;
- According to the results of quantitative analysis, besides temperature, percentage contributions of three parameters (STA, TA, and ST) are higher than 20%, which are the interactions of temperature and other factors. Moreover, their values are higher than that of single temperature;
- All the three factors increase erosion wear rate of 304 SS (A > 0, T > 0, S > 0), namely from Table 3 where all three examined factors that show the accelerating effect on erosion wear under keeping two other factors at the fixed level;
- Under the range of our research, the contribution percentages of the interactions for two factors (ST, SA, TA) are higher than single-acting ones, the contribution percentage of the interaction for three factors (STA) are higher than that of two factors or single factor;
- The combining contributions of factors are larger than that of a single factor by quantitative analysis. Nevertheless, every factor exhibits different intensity on erosion wear rate by qualitative analysis.
Author Contributions
Funding
Conflicts of Interest
References
- Ariely, S.; Khentov, A. Erosion corrosion of pump impeller of cyclic cooling water system. Eng. Fail. Anal. 2006, 13, 925–932. [Google Scholar] [CrossRef]
- Li, P.; Cai, Q.Z.; Wei, B.K. Failure analysis of the impeller of slurry pump used in zinc hydrometallurgy process. Eng. Fail. Anal. 2006, 13, 876–885. [Google Scholar] [CrossRef]
- Adnan, A.N.; Kim, M.H. Erosion wear on centrifugal pump casing due to slurry flow. Wear 2016, 364–365, 103–111. [Google Scholar]
- Lindgren, M.; Perolainen, J. Slurry pot investigation of the influence of erodant characteristics on the erosion resistance of titanium. Wear 2014, 321, 64–69. [Google Scholar] [CrossRef]
- Islam, M.A.; Farhat, Z.N.; Ahmed, E.M.; Alfantazi, A.M. Erosion enhanced corrosion and corrosion enhanced erosion of API X-70 pipeline steel. Wear 2013, 302, 1592–1601. [Google Scholar] [CrossRef]
- Zheng, Y.G.; Yao, Z.M.; Wei, X.Y.; Ke, Y. The synergistic effect between erosion and corrosion in acidic slurry medium. Wear 1995, 186–187, 555–561. [Google Scholar] [CrossRef]
- Celis, J.P.; Ponthiaux, P.; Wenger, F. Tribo-corrosion of materials: Interplay between chemical, electrochemical, and mechanical reactivity of surfaces. Wear 2006, 261, 939–946. [Google Scholar] [CrossRef]
- Ji, X.L.; Yan, C.Y.; Duan, H.; Luo, C.Y. Effect of phosphorous content on the microstructure and erosion-corrosion resistance of electrodeposited Ni-Co-Fe-P coatings. Surf. Coat. Technol. 2016, 302, 208–214. [Google Scholar] [CrossRef]
- Liu, Y.C.; Zhang, B.; Zhang, Y.L.; Ma, L.L.; Yang, P. Electrochemical polarization study on crude oil pipeline corrosion by the produced water with high salinity. Eng. Fail. Anal. 2016, 60, 307–315. [Google Scholar] [CrossRef]
- Calderón, J.A.; Henao, J.E.; Gómez, M.A. Erosion–corrosion resistance of Ni composite coatings with embedded SiC nanoparticles. Electrochim. Acta 2014, 124, 190–198. [Google Scholar] [CrossRef]
- Li, S.R.; Zuo, Y. Erosion-corrosion behavior of Pd–Co and Pd–Cu films on 316L stainless steel in simulated PTA slurry environment. Trans. Nonferrous Met. Soc. Chin. 2016, 26, 167–174. [Google Scholar] [CrossRef]
- López, D.; Congote, J.P.; Cano, J.R.; Toro, A.; Tschiptschin, A.P. Effect of particle velocity and impact angle on the corrosion–erosion of AISI 304 and AISI 420 stainless steels. Wear 2005, 259, 118–124. [Google Scholar] [CrossRef]
- Jana, B.D.; Stack, M.M. Modelling impact angle effects on erosion-corrosion of pure metals: Construction of materials performance maps. Wear 2005, 259, 243–255. [Google Scholar] [CrossRef]
- Hussain, E.A.M.; Robinson, M.J. Erosion-corrosion of 2205 duplex stainless steel in flowing seawater containing sand particles. Corros. Sci. 2007, 49, 1737–1754. [Google Scholar] [CrossRef]
- Burstein, G.T.; Sasaki, K. Effect of impact angle on the slurry erosion–corrosion of 304L stainless steel. Wear 2000, 240, 80–94. [Google Scholar] [CrossRef]
- Telfer, C.G.; Stack, M.M.; Jana, B.D. Particle concentration and size effects on the erosion-corrosion of pure metals in aqueous slurries. Tribol. Int. 2012, 53, 35–44. [Google Scholar] [CrossRef]
- Dong, C.F.; Luo, H.; Xiao, K.; Sun, T.; Liu, Q.; Li, X.G. Effect of temperature and Cl− concentration on pitting of 2205 duplex stainless steel. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2011, 26, 641–647. [Google Scholar] [CrossRef]
- Mesa, D.H.; Toro, A.; Sinatora, A.; Tschiptschin, A.P. The effect of testing temperature on corrosion-erosion resistance of martensitic stainless steels. Wear 2003, 255, 139–145. [Google Scholar] [CrossRef]
- Meng, H.; Hu, X.; Neville, A. A systematic erosion-corrosion study of two stainless steels in marine conditions via experimental design. Wear 2007, 263, 355–362. [Google Scholar] [CrossRef]
- Rajahram, S.S.; Harvey, T.J.; Wood, R.J.K. Full factorial investigation on the erosion-corrosion resistance of UNS S31603. Tribol. Int. 2010, 43, 2072–2083. [Google Scholar] [CrossRef]
- Dalbehera, S.; Acharya, S.K. Effect of cenosphere addition on erosive wear behaviour of Jute-glass reinforced composite using Taguchi experimental design. Mater. Today Proc. 2015, 2, 2389–2398. [Google Scholar] [CrossRef]
- López, D.; Falleiros, N.A.; Tschiptschin, A.P. Effect of nitrogen on the corrosion-erosion synergism in an austenitic stainless steel. Tribol. Int. 2011, 44, 610–616. [Google Scholar] [CrossRef]
- Abdel Aziz, M.; Mahmoud, T.S.; Abdel Aal, A. Modeling and optimizing of factors affecting erosion-corrosion of AA6063-(TiC/Al2O3) hybrid composites by experimental design method. Mater. Sci. Eng. A 2008, 486, 313–320. [Google Scholar] [CrossRef]
- Desale, G.R.; Paul, C.P.; Gandhi, B.K.; Jainc, S.C. Erosion wear behavior of laser clad surfaces of low carbon austenitic steel. Wear 2009, 266, 975–987. [Google Scholar] [CrossRef]
- Mondal, D.P.; Das, S.; Prasad, B.K. Study of erosive-corrosive wear characteristics of an aluminium alloy composite through factorial design of experiments. Wear 1998, 217, 1–6. [Google Scholar] [CrossRef]
- Aguirre, J.; Walczak, M. Multifactorial study of erosion–corrosion wear of a X65 steel by slurry of simulated copper tailing. Tribol. Int. 2018, 126, 177–185. [Google Scholar] [CrossRef]
- Atashin, S.; Pakshir, M.; Yazdani, A. Synergistic investigation into the marine parameters’ effect on the corrosion rate of AISI 316 stainless steel. Mater. Des. 2011, 32, 1315–1324. [Google Scholar] [CrossRef]
- Jones, M.; Llewellyn, R.J. Erosion–corrosion assessment of materials for use in the resources industry. Wear 2009, 267, 2003–2009. [Google Scholar] [CrossRef]
- Singhn, J.; Kumar, S.; Mohapatra, S.K. Tribological analysis of WC-10Co-4Cr and Ni-20Cr2O3 coating on stainless steel 304. Wear 2017, 376–377, 1105–1111. [Google Scholar] [CrossRef]
- Escrivà-Cerdán, C.; Blasco-Tamarit, E.; García-García, D.M.; García-Antón, J.; Guenbour, A. Effect of potential formation on the electrochemical behaviour of a highly alloyed austenitic stainless steel in contaminated phosphoric acid at different temperatures. Electrochim. Acta 2012, 80, 248–256. [Google Scholar] [CrossRef]
- Blasco-Tamarit, E.; Igual-Muñoz, A.; García Antón, J.; García-García, D. Effect of temperature on the corrosion resistance and pitting behavior of Alloy 31 in LiBr solutions. Corros. Sci. 2008, 50, 1848–1857. [Google Scholar] [CrossRef]
- Sánchez-Tovara, R.; Montañésa, M.T.; García-Antón, J.; Guenbour, A. Influence of temperature and hydrodynamic conditions on the corrosion behavior of AISI 316L stainless steel in pure and polluted H3PO4: Application of the response surface methodology. Mater. Chem. Phys. 2012, 133, 289–298. [Google Scholar] [CrossRef]
- Hu, X.M.; Neville, A. CO2 erosion corrosion of pipeline steel (API X65) in oil and gas conditions—A systematic approach. Wear 2009, 267, 2027–2032. [Google Scholar] [CrossRef]
- Jana, B.D.; Stack, M.M. A note on threshold velocity criteria for modelling the solid particle erosion of WC/Co MMCs. Wear 2011, 270, 439–445. [Google Scholar] [CrossRef][Green Version]
- González, M.A.; Rodríguez, E.; Mojardín, E.; Jiménez, O.; Guillen, H.; Ibarra, J. Study of the erosive wear behaviour of cryogenically and tempered WC-CoCr coating deposited by HVOF. Wear 2017, 376–377, 595–607. [Google Scholar] [CrossRef]
- Biswas, S.; Williams, K.; Jones, M. Development of a constitutive model for erosion based on dissipated particle energy to predict the wear rate of ductile metals. Wear 2018, 404–405, 166–175. [Google Scholar] [CrossRef]
- Arabnejad, H.; Mansouri, A.; Shirazi, S.A.; McLaury, B.S. Development of mechanistic erosion equation for solid particles. Wear 2015, 332–333, 1044–1050. [Google Scholar] [CrossRef]
- Parsi, M.; Najmi, K.; Najafifard, H.F.S.; McLaury, B.S.; Shirazi, S.A. A comprehensive review of solid particle erosion modeling for oil and gas wells and pipelines applications. J. Nat. Gas Sci. Eng. 2015, 21, 850–873. [Google Scholar] [CrossRef]
- Badr, H.M.; Habib, M.A.; Ben-Mansour, R.; Said, S.A.M. Numerical investigation of erosion threshold velocity in a pipe with sudden contraction. Comput. Fluids 2005, 34, 721–742. [Google Scholar] [CrossRef]
- Lindsley, B.A.; Marder, A.R. The effect of velocity on the solid particle erosion rate of alloys. Wear 1999, 225–229, 510–516. [Google Scholar] [CrossRef]
- Liu, T.G.; Wu, J.; Tang, X.H.; Yang, Z.Y. Qualitative ferrographic analysis method by quantitative parameters of wear debris characteristics. Ind. Lubr. Tribol. 2012, 64, 367–375. [Google Scholar]
- Toro, J.; Requena, I.; Duarte, O.R.; Zamorano, M. A qualitative method proposal to improve environmental impact assessment. Environ. Impact Assess. Rev. 2013, 43, 9–20. [Google Scholar] [CrossRef]
- Voss, B.M.; Pereira, M.P.; Rolfe, B.F.; Doolan, M.C. A new methodology for measuring galling wear severity in high strength steels. Wear 2017, 390–391, 334–345. [Google Scholar] [CrossRef]
- Baena, J.C.; Peng, Z. 3D quantitative characterization of degraded surfaces of human knee cartilages affected by osteoarthritis. Wear 2014, 319, 1–11. [Google Scholar] [CrossRef]
- Dong, W.P.; Stout, K.J. An integrated approach to the characterization of surface wear I: Qualitative characterization. Wear 1995, 181–183, 700–716. [Google Scholar] [CrossRef]
- Tiana, Y.; Wang, J.; Peng, Z.; Jiang, X. A new approach to numerical characterisation of wear particle surfaces in three-dimensions for wear study. Wear 2012, 282–283, 59–68. [Google Scholar] [CrossRef]
| C | V | Cr | Mn | Ni | Cu | Fe |
|---|---|---|---|---|---|---|
| 0.05 | 0.12 | 17.84 | 1.32 | 7.93 | 0.2 | Bal. |
| Factors | Speed /S (rpm) | Temperature /T (°C) | Acid Concentration/A (mol/L) | |
|---|---|---|---|---|
| Levels | ||||
| 1 | 800 | 25 ± 3 | 0.25 | |
| 2 | 1200 | 45 ± 3 | 0.50 | |
| Test No | Variables | Outputs | ||
|---|---|---|---|---|
| S | T | A | Weight Loss Rate | |
| 1 | 1 ∗ | 1 | 1 | W1 |
| 2 | 1 | 1 | 2 | W2 |
| 3 | 1 | 2 | 1 | W3 |
| 4 | 1 | 2 | 2 | W4 |
| 5 | 2 ∗ | 1 | 1 | W5 |
| 6 | 2 | 1 | 2 | W6 |
| 7 | 2 | 2 | 1 | W7 |
| 8 | 2 | 2 | 2 | W8 |
| Parameter | W1 | W2 | W3 | W4 | W5 | W6 | W7 | W8 | |
|---|---|---|---|---|---|---|---|---|---|
| Weight Loss (mg) | |||||||||
| 1 | 5.6 | 7.4 | 14.0 | 22.6 | 7.5 | 9.1 | 15.4 | 24.6 | |
| 2 | 5.2 | 8.4 | 14.7 | 24.6 | 6.7 | 9.4 | 15.5 | 25.5 | |
| 3 | 6.1 | 7.6 | 13.0 | 22.2 | 6.6 | 9.9 | 16.6 | 23.5 | |
| Average | 5.6 | 7.8 | 13.9 | 23.1 | 6.9 | 9.5 | 15.8 | 24.5 | |
| Standard deviations | 0.5 | 0.5 | 0.9 | 1.3 | 0.5 | 0.4 | 0.7 | 1.0 | |
| Weight loss rate (g⋅m−2⋅h−1) | 28 | 39 | 70 | 116 | 35 | 47 | 79 | 123 | |
| Factors | Calculating Formula | Calculating Value |
|---|---|---|
| WlS | 63 | |
| WhS | 71 | |
| WlT | 37 | |
| WhT | 97 | |
| WlA | 53 | |
| WhA | 81 |
| Parameters | Calculating Formula | Calculating Value |
|---|---|---|
| SSS | 2(WlS − WG)2 + 2(WhS − WG)2 | 62 |
| SST | 2(WlT − WG)2 + 2(WhT − WG)2 | 3538 |
| SSA | 2(WlA − WG)2 + 2(WhA − WG)2 | 802 |
| SSST | 2(WlS − WG)2 + 2(WhS − WG)2 + 2(WlT − WG)2 + 2(WhT − WG)2 | 3600 |
| SSSA | 2(WlS − WG)2 + 2(WhS − WG)2 + 2(WlA − WG)2 + 2(WhA − WG)2 | 865 |
| SSTA | 2(WlT − WG)2 + 2(WhT − WG)2 + 2(WlA − WG)2 + 2(WhA − WG)2 | 4340 |
| SSSTA | 2(WlS − WG)2 + 2(WhS − WG)2 + 2(WlT − WG)2 + 2(WhT − WG)2 + 2(WlA − WG)2 + 2(WhA − WG)2 | 4402 |
| Parameters | S | T | A | ST | SA | TA | STA |
|---|---|---|---|---|---|---|---|
| Contribution (K%) | 0.4 | 20 | 4.6 | 20.4 | 4.9 | 24.7 | 25 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1 | ||||||||
| 2 | A > 0 | |||||||
| 3 | T > 0 | T > A | ||||||
| 4 | TA > 0 | ↑TA » A | ↑TA > T | |||||
| 5 | S > 0 | S ≈ A | S < T | S < TA | ||||
| 6 | SA > 0 | °SA ≈ A | SA < T | SA « TA | °SA ≈ S | |||
| 7 | ST > 0 | ST > A | °ST ≈ T | ST < TA | ↑ST > S | ST > SA | ||
| 8 | STA > 0 | ↑STA » A | ↑STA » T | °STA ≈ TA | ↑STA » S | ↑STA » SA | ↑STA > ST |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Zhao, Y.; Wang, L. Research on Erosion-Corrosion Rate of 304 Stainless Steel in Acidic Slurry via Experimental Design Method. Materials 2019, 12, 2330. https://doi.org/10.3390/ma12142330
Li P, Zhao Y, Wang L. Research on Erosion-Corrosion Rate of 304 Stainless Steel in Acidic Slurry via Experimental Design Method. Materials. 2019; 12(14):2330. https://doi.org/10.3390/ma12142330
Chicago/Turabian StyleLi, Ping, Yanjie Zhao, and Libo Wang. 2019. "Research on Erosion-Corrosion Rate of 304 Stainless Steel in Acidic Slurry via Experimental Design Method" Materials 12, no. 14: 2330. https://doi.org/10.3390/ma12142330
APA StyleLi, P., Zhao, Y., & Wang, L. (2019). Research on Erosion-Corrosion Rate of 304 Stainless Steel in Acidic Slurry via Experimental Design Method. Materials, 12(14), 2330. https://doi.org/10.3390/ma12142330





