Development and Characterization of Bacterial Cellulose Reinforced with Natural Rubber
Abstract
1. Introduction
2. Materials and Methods
2.1. Film Preparation
2.2. Characterization
2.2.1. Field Emission Scanning Electron Microscopy (FESEM)
2.2.2. Laser Particle Size Distribution (PSD)
2.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.4. Water Absorption Capacity (WAC)
2.2.5. Toluene Uptake (TU)
2.2.6. X-Ray Diffraction (XRD)
2.2.7. Differential Scanning Calorimetry (DSC)
2.2.8. Thermal Gravimetric Analysis (TGA)
2.2.9. Mechanical Properties Testing
2.2.10. Biodegradation in Soil
3. Results
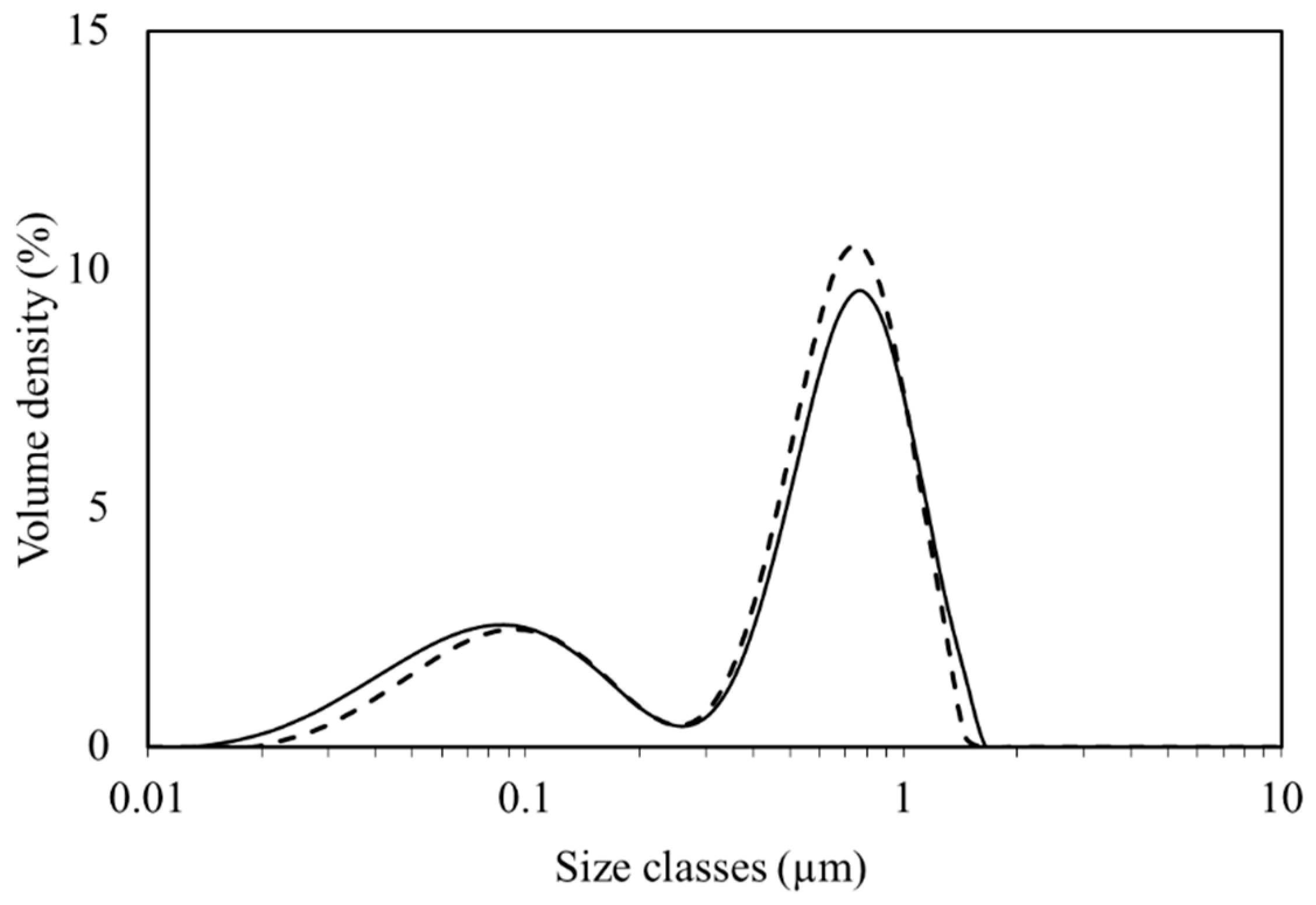
3.1. NR Particle Size Distribution
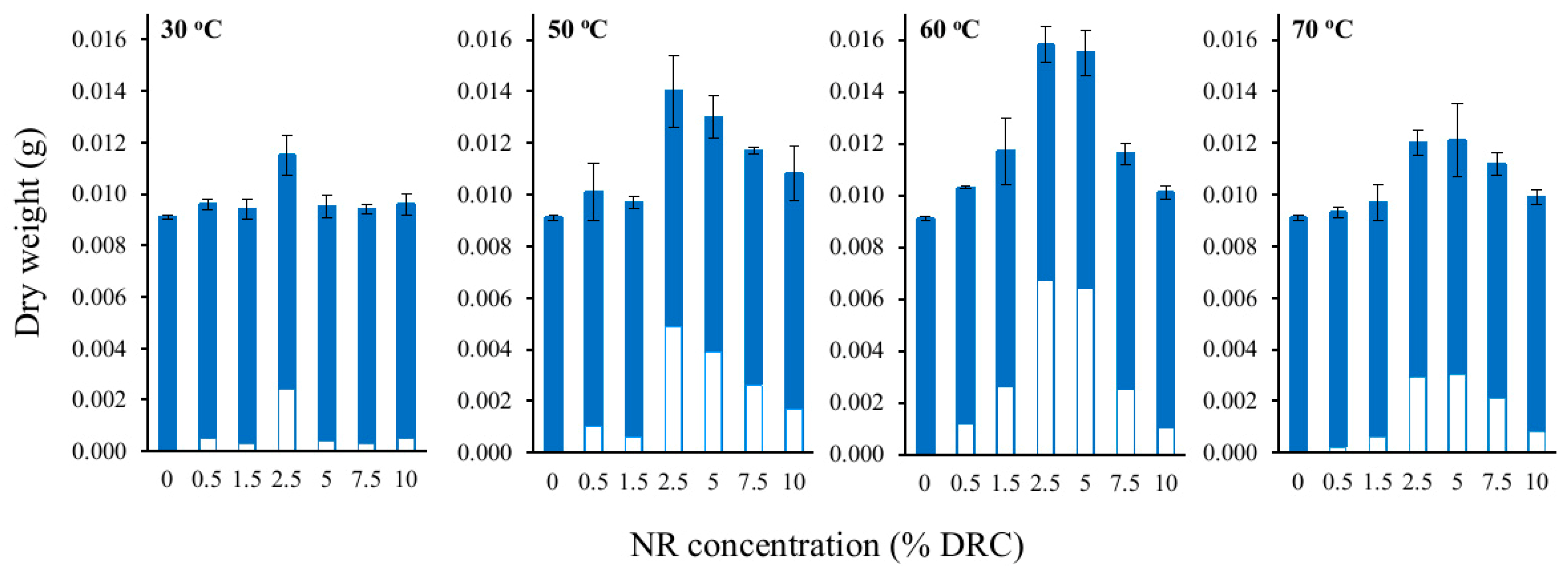
3.2. Effects of NRL Concentration and Temperature on Integration of NR into BC
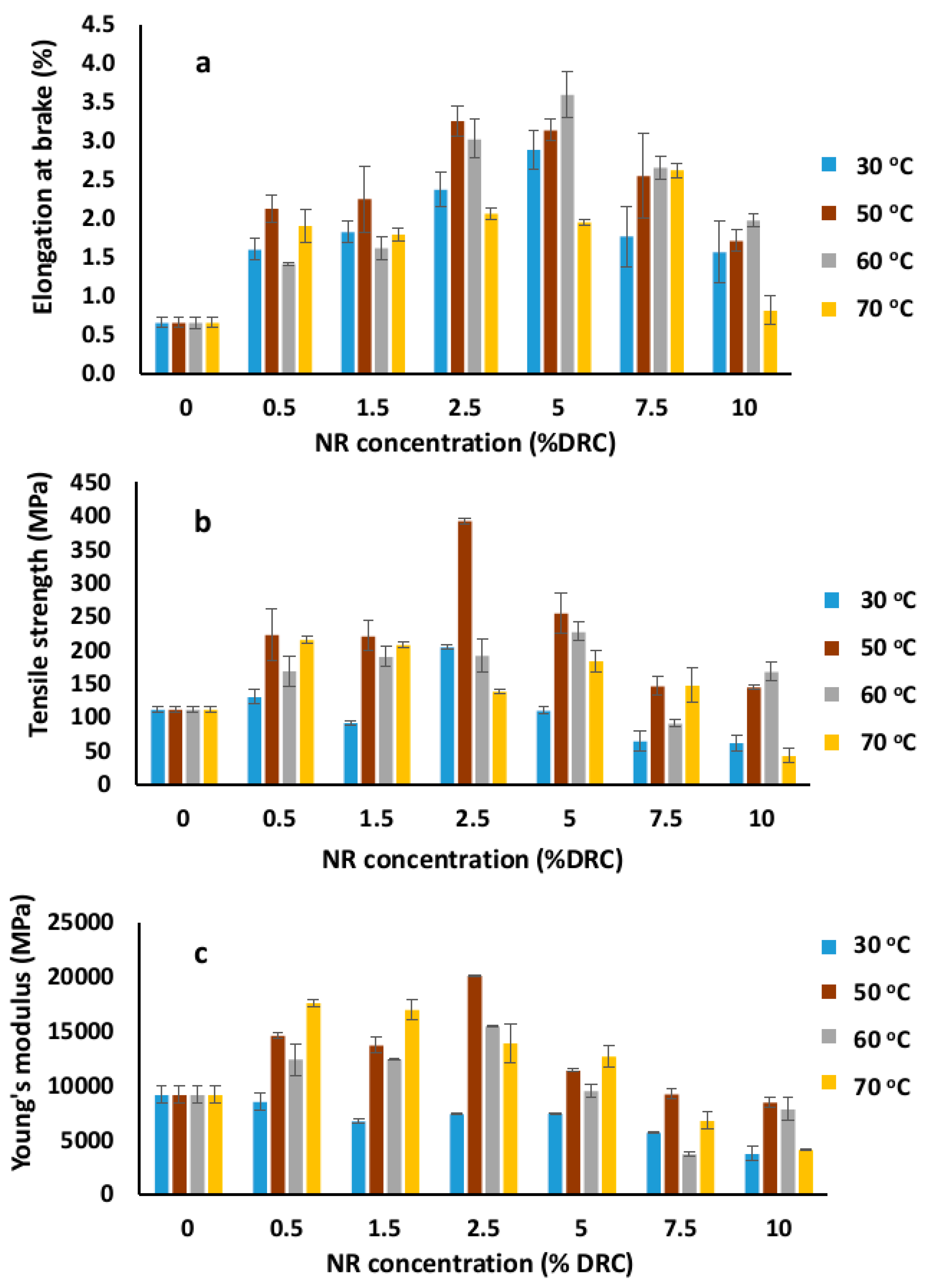
3.3. Mechanical Properties
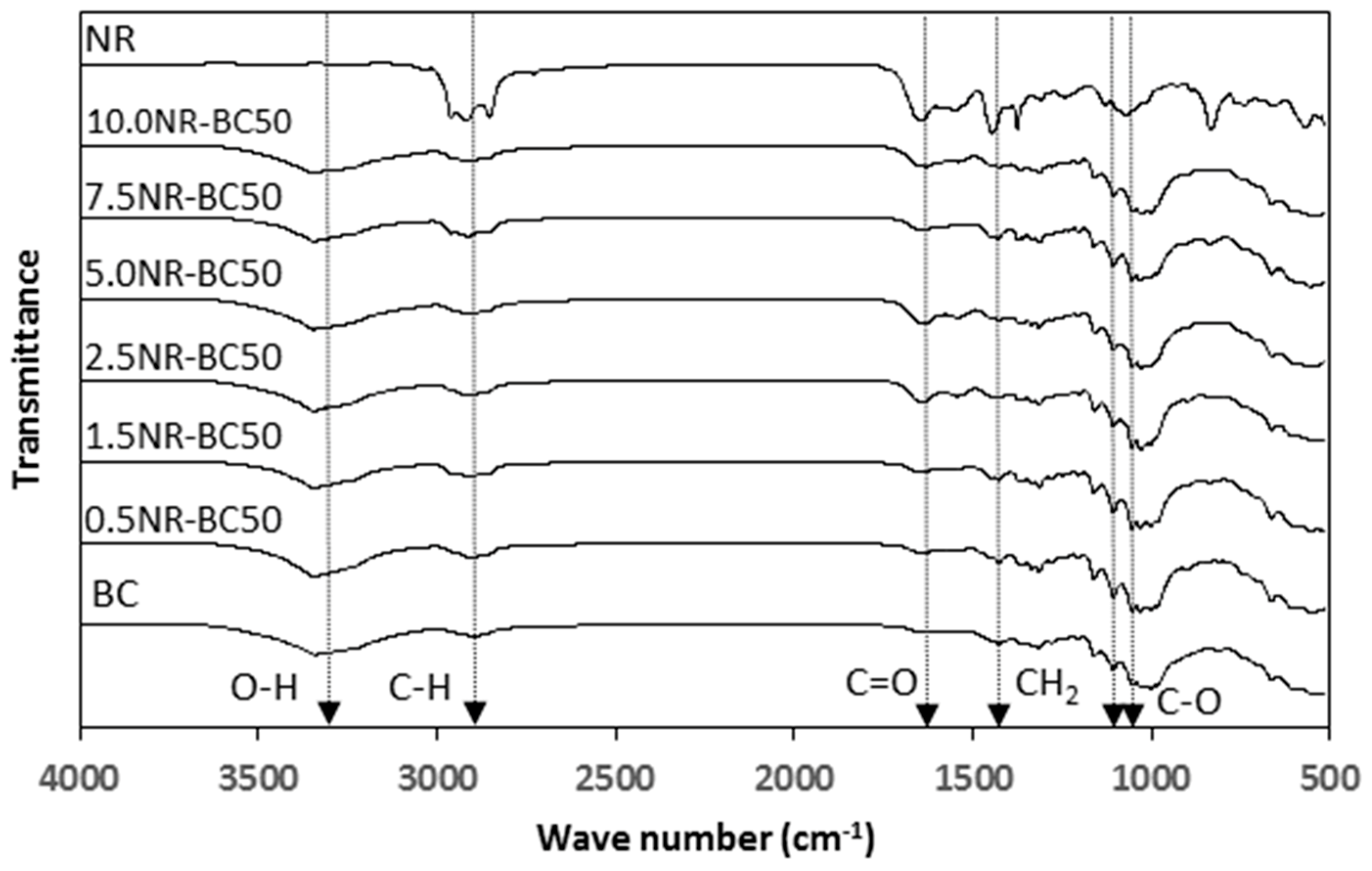
3.4. FTIR Analysis
3.5. X-Ray Diffraction (XRD) Analysis
3.6. Differential Scanning Calorimetry (DSC)
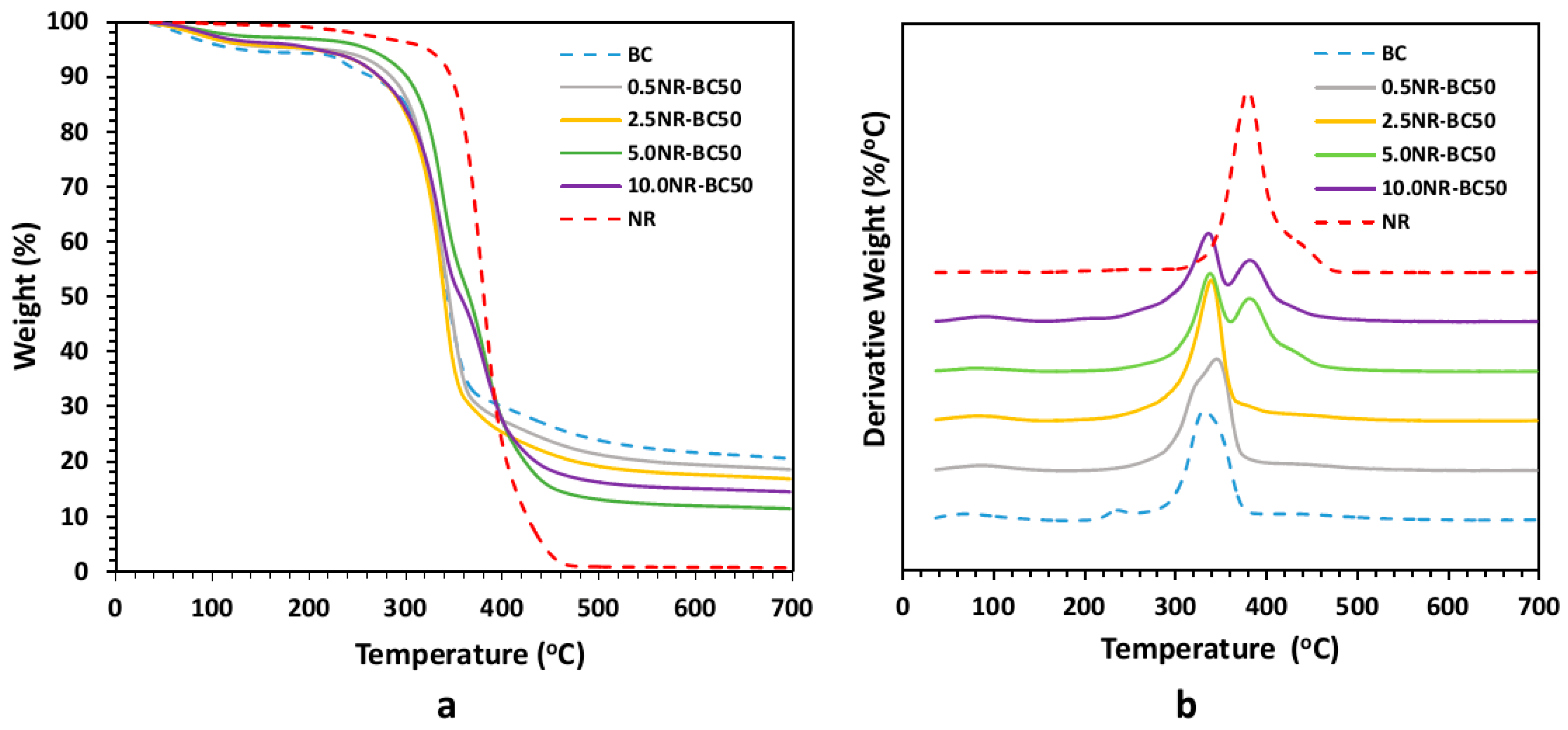
3.7. Thermal Gravimetric Analysis (TGA) and Differential Thermal Analysis (DTA)
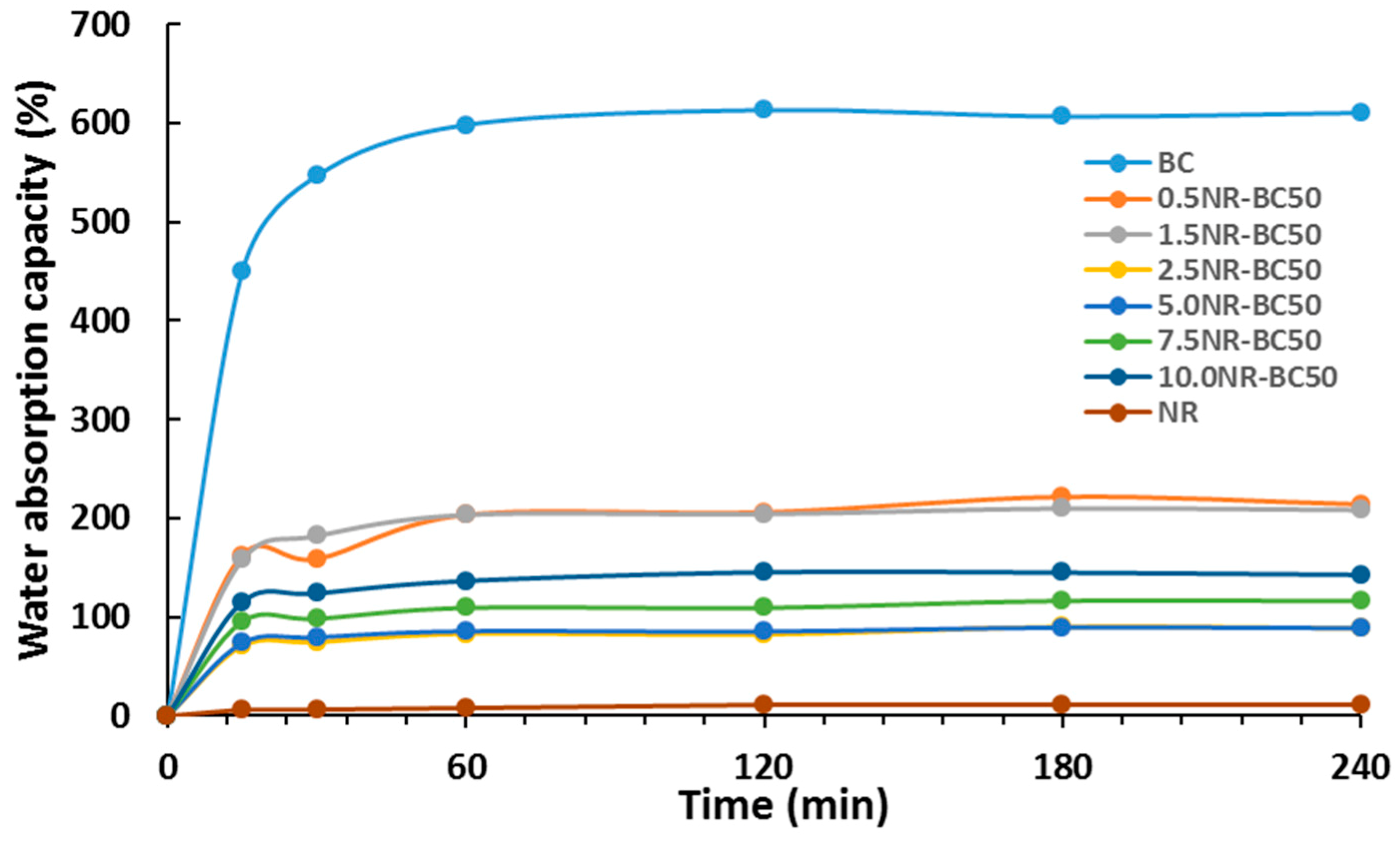
3.8. Water Absorption Capacity (WAC)
3.9. Toluene Uptake (TU)
3.10. Biodegradation in Soil
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Industry Focus. Thailand: The World’s Leader in Natural Rubber Production. Thailand Investment Review. Available online: https://www.boi.go.th/upload/content/TIR_AUGUST_PROOF_10_44271.pdf (accessed on 28 May 2019).
- Natural Rubber Production Up, but Consumption Dips in 2017, European Rubber Journal Report (2018). Available online: http://www.rubbernews.com/article/20180118/NEWS/180119950/natural-rubber-production-up-but-consumption-dips-in-2017 (accessed on 28 May 2019).
- Zhang, Y.; Liu, Q.; Zhang, Q.; Lu, Y. Gas barrier properties of natural rubber/kaolin composites prepared by melt blending. Appl. Clay Sci. 2010, 50, 255–259. [Google Scholar] [CrossRef]
- Jacob, M.; Thomas, S.; Varughese, K.T. Mechanical properties of sisal/oil palm hybrid fiber reinforced natural rubber composites. Compos. Sci. Technol. 2004, 64, 955–965. [Google Scholar] [CrossRef]
- George, J.; Bhagawan, S.S.; Prabhakaran, N.; Thomas, S. Short pineapple-leaf-fiber-reinforced low-density polyethylene composites. J. Appl. Polym. Sci. 1995, 57, 843–854. [Google Scholar] [CrossRef]
- Arumugam, N.; Selvy, K.T.; Rao, K.V.; Rajalingam, P. Coconut-fiber-reinforced rubber composites. J. Appl. Polym. Sci. 1989, 37, 2645–2659. [Google Scholar] [CrossRef]
- Ismail, H.; Edyham, M.; Wirjosentono, B. Bamboo fibre filled natural rubber composites: The effects of filler loading and bonding agent. Polym. Test. 2002, 21, 139–144. [Google Scholar] [CrossRef]
- De, D.; De, D.; Adhikari, B. Curing characteristics and mechanical properties of alkali-treated grass-fiber-filled natural rubber composites and effects of bonding agent. J. Appl. Polym. Sci. 2006, 101, 3151–3160. [Google Scholar] [CrossRef]
- Shoda, M.; Sugano, Y. Recent advances in bacterial cellulose production. Biotechnol. Bioproc. E. 2005, 10, 1–8. [Google Scholar] [CrossRef]
- Jiji, S.; Thenmozhi, S.; Kadirvelu, K. Comparison on properties and efficiency of bacterial and electrospun cellulose nanofibers. Fiber. Polym. 2018, 19, 2498–2506. [Google Scholar] [CrossRef]
- Ougiya, H.; Watanabe, K.; Morinaga, Y.; Yoshinaga, F. Emulsion-stabilizing effect of bacterial cellulose. Biosci. Biotech. Bioch. 1997, 61, 1541–1545. [Google Scholar] [CrossRef]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial synthesized cellulose-artificial blood vessels for microsurgery. Prog. Polym. Sci. 2001, 26, 1561–1603. [Google Scholar] [CrossRef]
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.L.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005, 26, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lin, T.F.; Tang, Z.H.; Guo, B.C.; Huang, G.S. Natural rubber/graphene oxide composites: effect of sheet size on mechanical properties and strain induced crystallization behavior. Express Polym. Lett. 2015, 9, 672–685. [Google Scholar] [CrossRef]
- Fu, D.H.; Zhan, Y.H.; Yan, N.; Xia, H.S. A comparative investigation on strain induced crystallization for graphene and carbon nanotubes filled natural rubber composites. Express Polym. Lett. 2015, 9, 597–607. [Google Scholar] [CrossRef]
- Trovatti, E.; Carvalho, A.J.F.; Ribeiro, S.J.L.; Gandini, A. Simple green approach to reinforce natural rubber with bacterial cellulose nanofibers. Biomacromolecules 2013, 14, 2667–2674. [Google Scholar] [CrossRef] [PubMed]
- Phomrak, S.; Phisalaphong, M. Reinforcement of natural rubber with bacterial cellulose via a latex aqueous microdispersion process. J. Nanomater. 2017, 4739793. [Google Scholar] [CrossRef]
- Pircher, N.; Veigel, S.; Aigner, N.; Nedelec, J.M.; Rosenau, T.; Liebner, F. Reinforcement of bacterial cellulose aerogels with biocompatible polymers. Carbohyd. Polym. 2014, 111, 505–513. [Google Scholar] [CrossRef]
- Suratago, T.; Taokaew, S.; Kanjanamosit, N.; Kanjanaprapakul, K.; Burapatana, V.; Phisalaphong, M. Development of bacterial cellulose/alginate nanocomposite membrane for separation of ethanol-water mixtures. J. Ind. Eng. Chem. 2015, 32, 305–312. [Google Scholar] [CrossRef]
- Taokaew, S.; Seetabhawang, S.; Phisalaphong, M. Biosynthesis and characterization of nanocellulose-gelatin films. Materials 2013, 6, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Subtaweesin, C.; Woraharn, W.; Taokaew, S.; Chiaoprakobkij, N.; Sereemaspun, A.; Phisalaphong, M. Characteristics of curcumin-loaded bacterial cellulose films and anticancer properties against malignant melanoma skin cancer cells. Appl. Sci. 2018, 8, 1188. [Google Scholar] [CrossRef]
- Panitchakarn, P.; Wikranvanich, J.; Phisalaphong, M. Synthesis and characterization of natural rubber/coal fly ash composites via latex aqueous microdispersion. J. Ind. Eng. Chem. 2019, 21, 134. [Google Scholar] [CrossRef]
- Taokaew, S.; Nunkaew, N.; Siripong, P.; Phisalaphong, M. Characteristics and anticancer properties of bacterial cellulose films containing ethanolic extract of mangosteen peel. J. Biomat. Sci. Polym. E. 2014, 25, 907–922. [Google Scholar] [CrossRef] [PubMed]
- Halib, N.; Amin, M.C.I.M.; Ahmad, I. Physicochemical properties and characterization of nata de coco from local food industries as a source of cellulose. Sains Malays. 2012, 41, 205–211. [Google Scholar]
- Guidelli, E.J.; Ramos, A.P.; Zaniquelli, M.E.D.; Baffa, O. Green synthesis of colloidal silver nanoparticles using natural rubber latex extracted from Hevea brasiliensis. Spectrochim. Acta A 2011, 82, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Czaja, W.; Romanovicz, D.; Malcolm Brown, R. Structural investigations of microbial cellulose produced in stationary and agitated culture. Cellulose 2004, 11, 403–411. [Google Scholar] [CrossRef]
- Barud, H.S.; Ribeiro, C.A.; Crespi, M.S.; Martines, M.A.U.; Dexpert-Ghys, J.; Marques, R.F.C.; Messaddeq, Y.; Ribeiro, S.J.L. Thermal characterization of bacterial cellulose-phosphate composite membranes. J. Therm. Anal. Calorim. 2007, 87, 815–818. [Google Scholar] [CrossRef]
- Oliveira, R.L.; Vieira, J.G.; Barud, H.S.; Assunção, R.M.N.; Filho, G.R.; Ribeiro, S.J.L.; Messadeqq, Y. Synthesis and characterization of methylcellulose produced from bacterial cellulose under heterogeneous condition. J. Brazil. Chem. Soc. 2015, 26, 1861–1870. [Google Scholar] [CrossRef]
- George, J.; Ramana, K.V.; Sabapathy, S.N.; Jagannath, J.H.; Bawa, A.S. Characterization of chemically treated bacterial (Acetobacter xylinum) biopolymer: Some thermo-mechanical properties. Int. J. Biol. Macromol. 2005, 37, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Barud, H.S.; de Araújo Júnior, A.M.; Santos, D.B.; de Assunção, R.M.N.; Meireles, C.S.; Cerqueira, D.A.; Filho, G.R.; Ribeiro, C.A.; Messaddeq, Y.; Ribeiro, S.J.L. Thermal behavior of cellulose acetate produced from homogeneous acetylation of bacterial cellulose. Thermochim. Acta 2008, 471, 61–69. [Google Scholar] [CrossRef]
- Kanjanamosit, N.; Muangnapoh, C.; Phisalaphong, M. Biosynthesis and characterization of bacteria cellulose-alginate film. J. Appl. Polym. Sci. 2010, 115, 1581–1588. [Google Scholar] [CrossRef]
- Lin, W.-C.; Lien, C.-C.; Yeh, H.-J.; Yu, C.-M.; Hsu, S.-h. Bacterial cellulose and bacterial cellulose-chitosan membranes for wound dressing applications. Carbohyd. Polym. 2013, 94, 603–611. [Google Scholar] [CrossRef]
- Obasi, H.C.; Ogbobe, O.; Igwe, I.O. Diffusion characteristics of toluene into natural rubber/linear low density polyethylene blends. Int. J. Polym. Sci. 2009, 140682. [Google Scholar] [CrossRef]
- Pérez, J.; Munoz-Dorado, J.; de la Rubia, T.; Martinez, J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin: An overview. Int. Microbiol 2002, 5, 53–63. [Google Scholar] [CrossRef]
- Béguin, P.; Aubert, J.-P. The biological degradation of cellulose. FEMS Microbiol. Rev. 1994, 13, 25–58. [Google Scholar] [CrossRef] [PubMed]
- Cherian, E.; Jayachandran, K. Microbial degradation of natural rubber latex by a novel species of Bacillus sp. SBS25 isolated from soil. Int. J. Environ. Res. 2009, 3, 599–604. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Shah, Z.; Kanwal, N.; Zeb, S. Biodegradation of natural and synthetic rubbers: A review. Int. Biodeter. Biodegr. 2013, 83, 145–157. [Google Scholar] [CrossRef]
- Nawong, C.; Umsakul, K.; Sermwittayawong, N. Rubber gloves biodegradation by a consortium, mixed culture and pure culture isolated from soil samples. Braz. J. Microbiol. 2018, 49, 481–488. [Google Scholar] [CrossRef]
- Chaikumpollert, O.; Yamamoto, Y.; Suchiva, K.; Kawahara, S. Protein-free natural rubber. Colloid Polym. Sci. 2012, 290, 331–338. [Google Scholar] [CrossRef]
- Kawahara, S.; Kakubo, T.; Nishiyama, N.; Tanaka, Y.; Isono, Y.; Sakdapipanich, J.T. Crystallization behavior and strength of natural rubber: Skim rubber, deproteinized natural rubber, and pale crepe. J. Appl. Polym. Sci. 2000, 78, 1510–1516. [Google Scholar] [CrossRef]
- Arayapranee, W. Rubber abrasion resistance. In Abrasion Resistance of Materials, 1st ed.; Adamiak, M., Ed.; In Tech: Rijeka, Croatia, 2012; Volume 8, pp. 147–166. [Google Scholar]
- Ahmad, M.R.; Ahmad, N.A.; Suhaimi, S.A.; Bakar, N.A.A.; Ahmad, W.Y.W.; Salleh, J. Tensile and tearing strength of uncoated and natural rubber latex coated high strength woven fabrics. In Proceedings of the 2012 IEEE Symposium on Humanities, Science and Engineering Research, Kuala Lumpur, Malaysia, 24–27 June 2012; pp. 541–545. [Google Scholar]
- Rose, K.; Steinbüchel, A. Biodegradation of natural rubber and related compounds: recent insights into a hardly understood catabolic capability of microorganisms. Appl. Environ. Microb. 2005, 71, 2803–2812. [Google Scholar] [CrossRef]






| Samples | Thickness (µm) | Samples | Thickness (µm) |
|---|---|---|---|
| 0.5NR–BC30 | 12.74 ± 0.58 | 0.5NR–BC60 | 13.86 ± 0.13 |
| 2.5NR–BC30 | 19.08 ± 0.38 | 2.5NR–BC60 | 24.97 ± 0.90 |
| 5NR–BC30 | 15.85 ± 0.53 | 5NR–BC60 | 27.21 ± 0.39 |
| 10NR–BC30 | 14.66 ± 0.49 | 10NR–BC60 | 24.15 ± 1.62 |
| 0.5NR–BC50 | 14.84 ± 0.64 | 0.5NR–BC70 | 13.32 ± 0.58 |
| 2.5NR–BC50 | 20.61 ± 0.24 | 2.5NR–BC70 | 20.98 ± 0.69 |
| 5NR–BC50 | 21.99 ± 0.96 | 5NR–BC70 | 25.27 ± 0.29 |
| 10NR–BC50 | 18.54 ± 0.63 | 10NR–BC70 | 21.73 ± 0.35 |
| Solvent | Absorption (WAC%, TU%) | |||||||
|---|---|---|---|---|---|---|---|---|
| BC | 0.5 NR–BC | 1.5 NR–BC | 2.5 NR–BC | 5 NR–BC | 7.5 NR–BC | 10 NR–BC | NR | |
| Water | 610.5 ± 2.6 | 213.9 ± 6.4 | 208.2 ± 2.4 | 88.4 ± 3.3 | 88.6 ± 1.8 | 115.9 ± 3.3 | 142.5 ± 1.2 | 10.9 ± 0.3 |
| Toluene | 8.7 ± 1.0 | 33.2 ± 6.8 | 34.2 ± 12.6 | 70.9 ± 24.0 | 68.6 ± 13.5 | 30.5 ± 11.7 | 47.3 ± 3.4 | 2,652.8 ± 300.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potivara, K.; Phisalaphong, M. Development and Characterization of Bacterial Cellulose Reinforced with Natural Rubber. Materials 2019, 12, 2323. https://doi.org/10.3390/ma12142323
Potivara K, Phisalaphong M. Development and Characterization of Bacterial Cellulose Reinforced with Natural Rubber. Materials. 2019; 12(14):2323. https://doi.org/10.3390/ma12142323
Chicago/Turabian StylePotivara, Kornkamol, and Muenduen Phisalaphong. 2019. "Development and Characterization of Bacterial Cellulose Reinforced with Natural Rubber" Materials 12, no. 14: 2323. https://doi.org/10.3390/ma12142323
APA StylePotivara, K., & Phisalaphong, M. (2019). Development and Characterization of Bacterial Cellulose Reinforced with Natural Rubber. Materials, 12(14), 2323. https://doi.org/10.3390/ma12142323




