Induction of Osteogenic Differentiation of Mesenchymal Stem Cells by Bioceramic Root Repair Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Extract Preparation
2.2. Preparation of Rat Bone Marrow-Derived MSCs
2.3. Cell Viability and Proliferation Assay
2.4. Alkaline Phosphatase Staining
2.5. Reverse-Transcription Polymerase Chain Reaction (RT-PCR) and Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.6. Statistical Analysis
3. Results
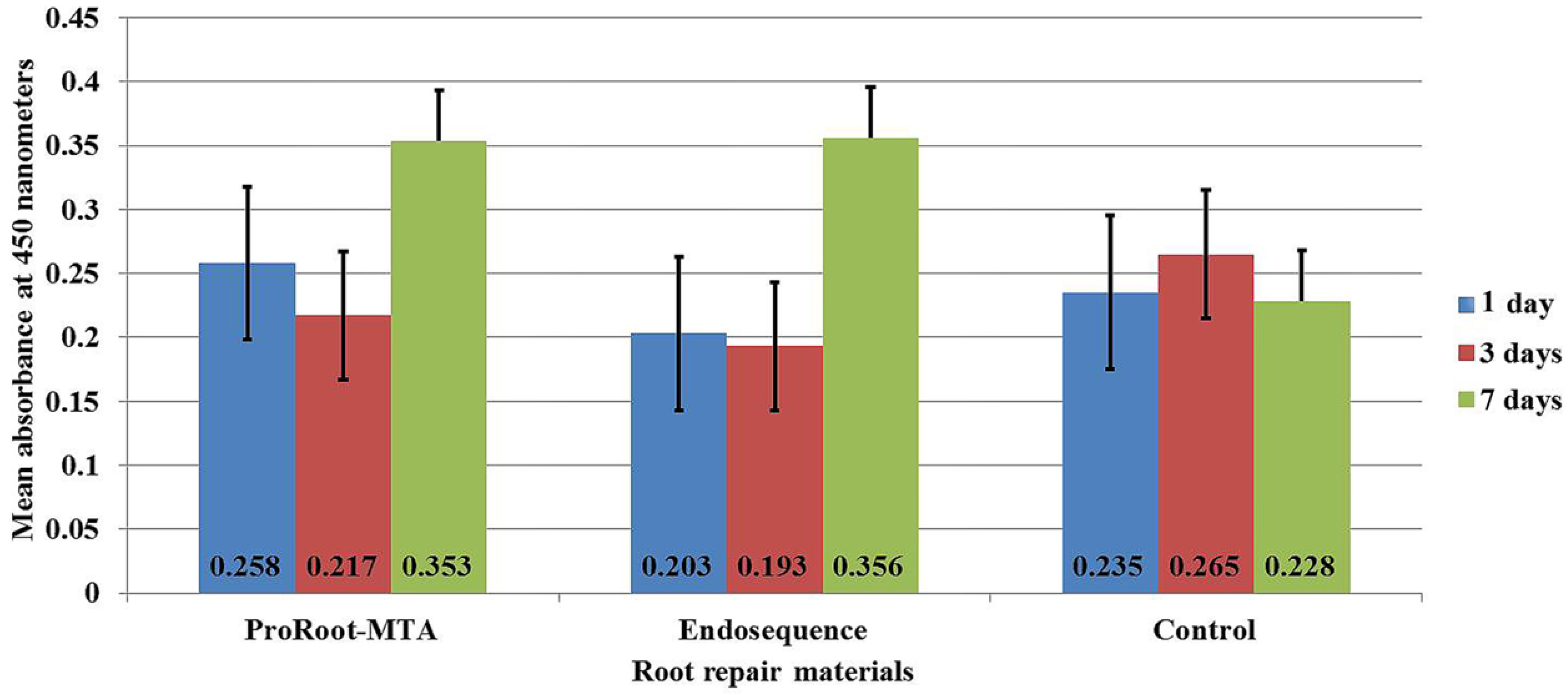
3.1. Cell Viability and Proliferation
3.2. Scanning Electron Microscopy
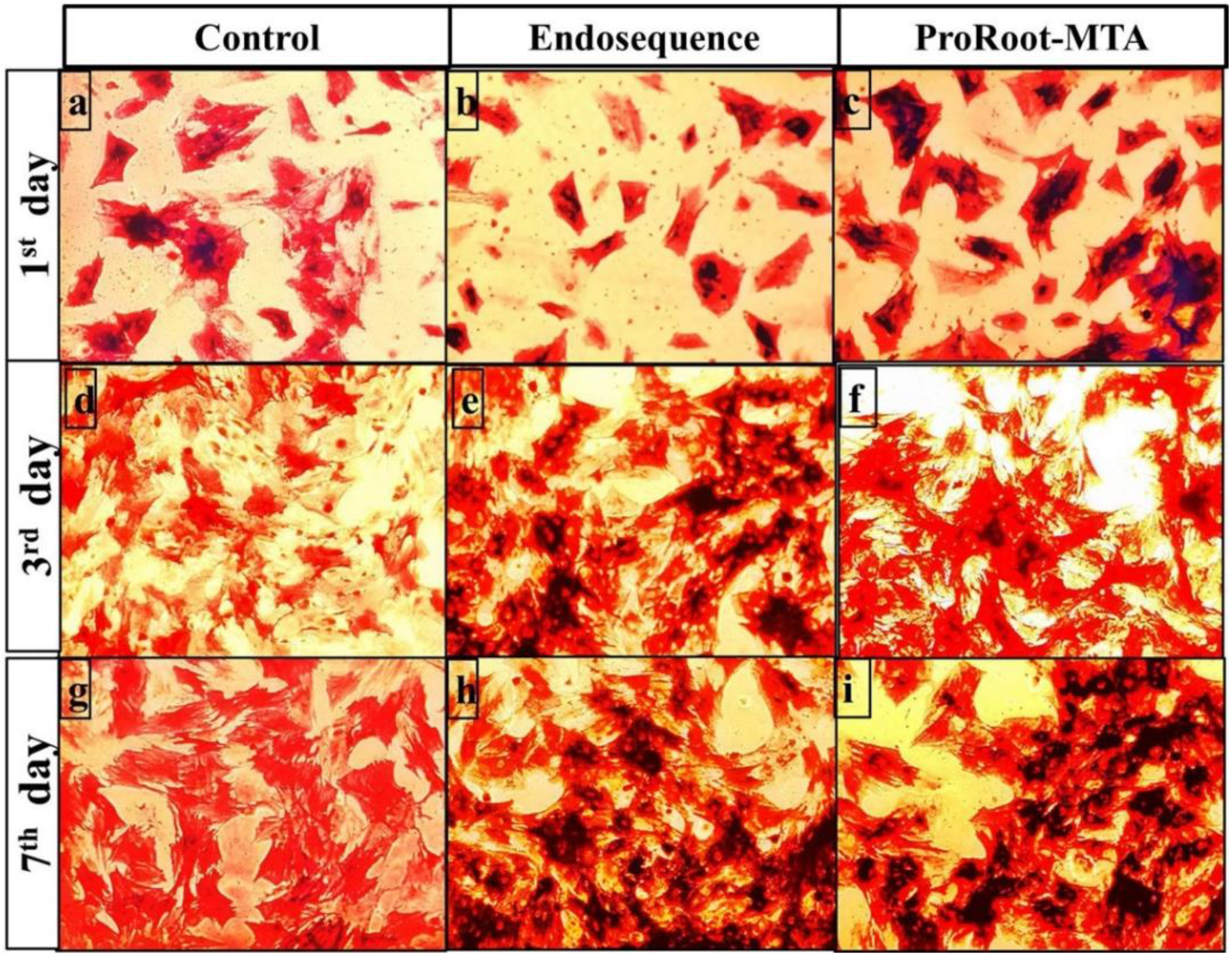
3.3. Alkaline Phosphatase Staining Analysis
3.4. Reverse-Transcription Polymerase Chain Reaction and Quantitative Real-Time Polymerase Chain Reaction
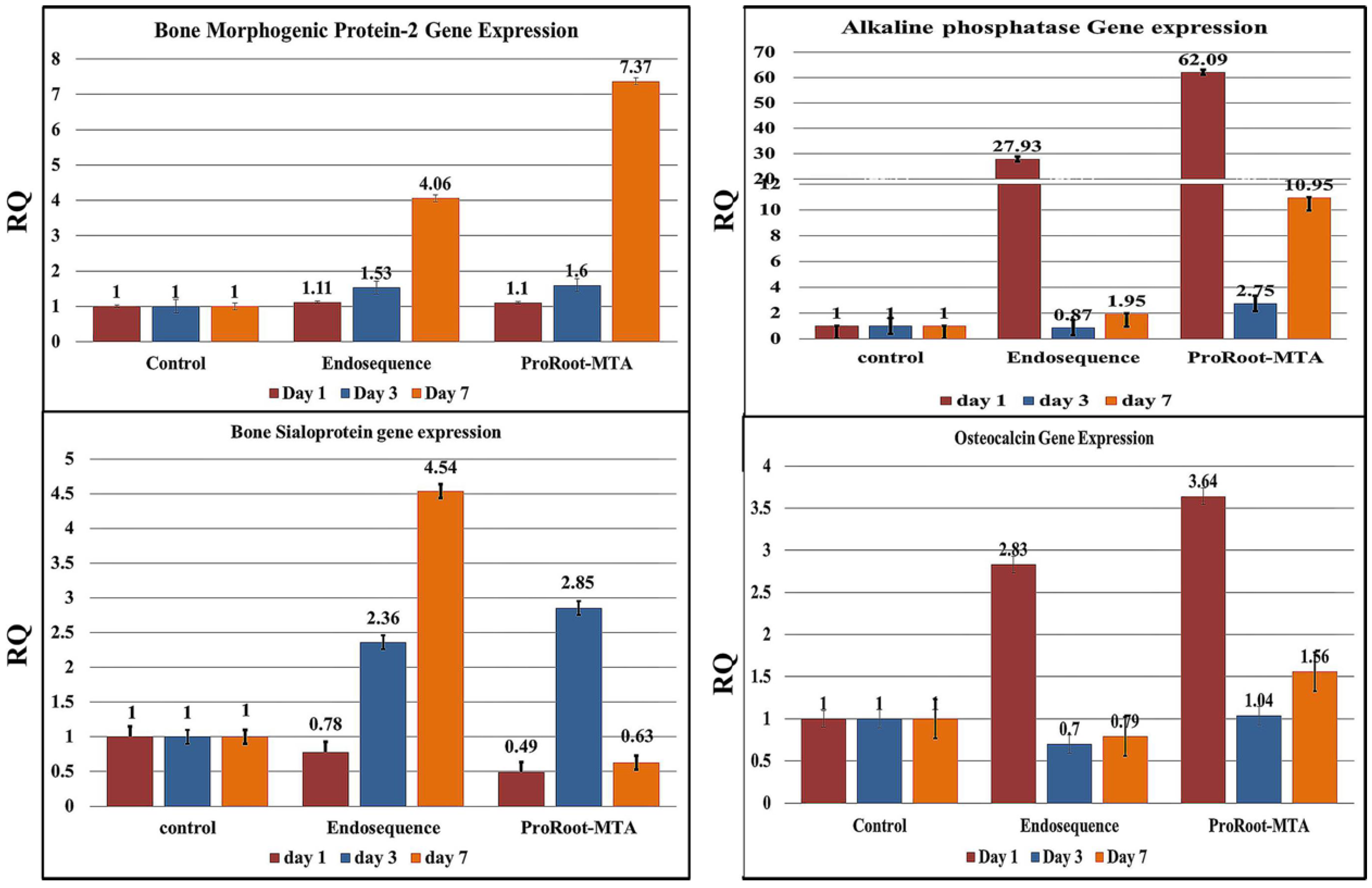
3.4.1. Bone Morphogenetic Protein-2 (BMP-2)
3.4.2. Alkaline Phosphatase
3.4.3. Bone Sialoprotein
3.4.4. Osteocalcin
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nair, P.N.R.; Duncan, H.F.; Pitt Ford, T.R.; Luder, H.U. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: A randomized controlled trial. Int. Endod. J. 2008, 41, 128–150. [Google Scholar] [PubMed]
- Parirokh, M.; Torabinejad, M. Mineral trioxide aggregate: A comprehensive literature review-part III: Clinical applications, drawbacks, and mechanism of action. J. Endod. 2010, 36, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Montesin, F.E.; Di Silvio, L.; Pitt Ford, T.R. The chemical constitution and biocompatibility of accelerated Portland cement for endodontic use. Int. Endod. J. 2005, 38, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Montesin, F.E.; Papaioannou, S.; McDonald, F.; Pitt Ford, T.R. Biocompatibility of two commercial forms of mineral trioxide aggregate. Int. Endod. J. 2004, 37, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.L.; Spears, R.; Gutmann, J.L.; Opperman, L.A. Osteoblasts and MG-63 osteosarcoma cells behave differently when in contact with ProRoot-MTA and White MTA. Int. Endod. J. 2003, 36, 564–570. [Google Scholar] [CrossRef]
- Lovato, K.F.; Sedgley, C.M. Antibacterial activity of endosequence root repair material and proroot MTA against clinical isolates of Enterococcus faecalis. J. Endod. 2011, 37, 1542–1546. [Google Scholar] [CrossRef] [PubMed]
- Alsalleeh, F.; Chung, N.; Stephenson, L. Antifungal Activity of Endosequence Root Repair Material and Mineral Trioxide Aggregate. J. Endod. 2014, 40, 1815–1819. [Google Scholar] [CrossRef]
- Willershausen, I.; Wolf, T.; Kasaj, A.; Weyer, V.; Willershausen, B.; Marroquin, B.B. Influence of a bioceramic root end material and mineral trioxide aggregates on fibroblasts and osteoblasts. Arch. Oral Biol. 2013, 58, 1232–1237. [Google Scholar] [CrossRef]
- Khalil, I.; Naaman, A.; Camilleri, J. Investigation of a novel mechanically mixed mineral trioxide aggregate (MM-MTA). Int. Endod. J. 2016, 48, 757–767. [Google Scholar] [CrossRef]
- Chen, I.; Karabucak, B.; Wang, C.; Wang, H.G.; Koyama, E.; Kohli, M.R.; Kim, S. Healing after root-end microsurgery by using mineral trioxide aggregate and a new calcium silicate based bioceramic material as root-end filling materials in dogs. J. Endod. 2015, 41, 389–399. [Google Scholar] [CrossRef]
- Ma, J.; Shen, Y.; Stojicic, S.; Haapasalo, M. Biocompatibility of two novel root repair materials. J. Endod. 2011, 37, 793–798. [Google Scholar] [CrossRef]
- Hirschman, W.R.; Wheater, M.A.; Bringas, J.S.; Hoen, M.M. Cytotoxicity comparison of three current direct pulp-capping agents with a new bioceramic root repair putty. J. Endod. 2012, 38, 385–388. [Google Scholar] [CrossRef]
- Modareszadeh, M.R.; Di Fiore, P.M.; Tipton, D.A.; Salamat, N. Cytotoxicity and alkaline phosphatase activity evaluation of endosequence root repair material. J. Endod. 2012, 38, 1101–1105. [Google Scholar] [CrossRef]
- Damas, B.A.; Wheater, M.A.; Bringas, J.S.; Hoen, M.M. Cytotoxicity comparison of mineral trioxide aggregates and EndoSequence bioceramic root repair materials. J. Endod. 2011, 37, 372–375. [Google Scholar] [CrossRef]
- Braga, J.M.; Oliveira, R.R.; Martins, R.C.; Ribeiro Sobrinho, A.P. The effects of a mineral trioxide aggregate-based sealer on the production of reactive oxygen species, nitrogen species and cytokines by two macrophage subtypes. Int. Endod. J. 2014, 47, 909–919. [Google Scholar] [CrossRef]
- Lee, B.N.; Lee, K.N.; Koh, J.T.; Min, K.S.; Chang, H.S.; Hwang, I.N.; Oh, W.M. Effects of 3 Endodontic Bioactive Cements on Osteogenic Differentiation in Mesenchymal Stem Cells. J. Endod. 2014, 40, 1217–1222. [Google Scholar] [CrossRef]
- Faidah, M.; Noorwali, A.; Atta, H.; Ahmed, N.; Habib, H.; Damiati, L.; Khabaz, M.N. Mesenchymal stem cell therapy of hepatocellular carcinoma in rats: Detection of cell homing and tumor mass by magnetic resonance imaging using iron oxide nanoparticles. Adv. Clin. Exp. Med. 2017, 26, 1171–1178. [Google Scholar] [CrossRef]
- Chen, I.; Salhab, I.; Setzer, F.C.; Kim, S.; Nah, H.D. A new calcium silicate-based bioceramic material promotes human osteo-and odontogenic stem cell proliferation and survival via the extracellular signal-regulated kinase signaling pathway. J. Endod. 2016, 42, 480–486. [Google Scholar] [CrossRef]
- Shen, J.; Hovhannisyan, H.; Lian, J.B.; Montecino, M.A.; Stein, G.S.; Stein, J.L.; Van Wijnen, A.J. Transcriptional induction of the osteocalcin gene during osteoblast differentiation involves acetylation of histones h3 and h4. Mol. Endocrinol. 2003, 17, 743–756. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Fan, M. BioAggregate and iRoot BP Plus optimize the proliferation and mineralization ability of human dental pulp cells. Int. Endod. J. 2013, 46, 923–929. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Shah, S.N.; Feng, R.; Prati, C.; Akintoye, S.O. Biomimetic calcium-silicate cements support differentiation of human orofacial mesenchymal stem cells. J. Endod. 2011, 37, 1102–1108. [Google Scholar] [CrossRef]
- Maxim, M.A.; Soritau, O.; Baciut, M.; Bran, S.; Baciut, G. The role of dental stem cells in regeneration. Clujul. Med. 2015, 88, 479. [Google Scholar] [CrossRef]
- Abu Zeid, S.T.H.; Mokeem Saleh, A.A.Y. Solubility, pH Changes and Releasing Elements of Different Bioceramic and Mineral Trioxide Aggregate Root Canal Sealers Comparative Study. J. Trauma Treat. 2015, 4, 1–4. [Google Scholar]
- Abu Zeid, S.T.; Alothmani, O.S.; Yousef, M.K. Biodentine and mineral trioxide Aggregate: An analysis of solubility, pH changes and leaching elements. Life Sci. J. 2015, 12, 18–23. [Google Scholar]
- Machado, J.; Johnson, J.D.; Paranjpe, A. The effects of endosequence root repair material on differentiation of dental pulp cells. J. Endod. 2016, 42, 101–105. [Google Scholar] [CrossRef]
- Öncel Torun, Z.; Torun, D.; Demirkaya, K.; Yavuz, S.; Elçi, M.; Sarper, M.; Avcu, F. Effects of iRoot BP and white mineral trioxide aggregate on cell viability and the expression of genes associated with mineralization. Int. Endod. J. 2015, 48, 986–993. [Google Scholar] [CrossRef]
- Yang, J.; Shi, P.; Tu, M.; Wang, Y.; Liu, M.; Fan, F.; Du, M. Bone morphogenetic proteins: Relationship between molecular structure and their osteogenic activity. Food Sci. Hum. Well. 2014, 3, 127–135. [Google Scholar] [CrossRef]
- Yun, H.M.; Chang, S.W.; Park, K.R.; Herr, L.; Kim, E.C. Combined effects of growth hormone and mineral trioxide aggregate on growth, differentiation, and angiogenesis in human dental pulp cells. J. Endod. 2016, 42, 269–275. [Google Scholar] [CrossRef]
- Yasuda, Y.; Ogawa, M.; Arakawa, T.; Kadowaki, T.; Saito, T. The effect of mineral trioxide aggregate on the mineralization ability of rat dental pulp cells: An in vitro study. J. Endod. 2008, 34, 1057–1060. [Google Scholar] [CrossRef]
- Jang, W.G.; Kim, E.J.; Kim, D.K.; Ryoo, H.M.; Lee, K.B.; Kim, S.H.; Koh, J.T. BMP2 protein regulates osteocalcin expression via Runx2-mediated Atf6 gene transcription. J. Biol. Chem. 2012, 287, 905–915. [Google Scholar] [CrossRef]
- Reddi, S.; Shanmugam, V.P.; Tanedjeu, K.S.; Kapila, S.; Kapila, R. Effect of buffalo casein-derived novel bioactive peptides on osteoblast differentiation. Eur. J. Nutr. 2018, 57, 593–605. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, T.; Shen, Y.; Haapasalo, M.; Zou, L.; Liu, J. Effect of iRoot Fast Set root repair material on the proliferation, migration and differentiation of human dental pulp stem cells in vitro. PLoS ONE 2017, 12, e0186848. [Google Scholar] [CrossRef]
- Bonson, S.; Jeansonne, B.G.; Lallier, T.E. Root-end filling materials alter fibroblast differentiation. J. Dent. Res. 2004, 83, 408–413. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Leyhausen, G.; Volk, J.; Tsiftsoglou, A.; Garefis, P.; Koidis, P.; Geurtsen, W. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch. Oral Biol. 2011, 56, 709–721. [Google Scholar] [CrossRef]
- Rashid, F.; Shiba, H.; Mizuno, N.; Mouri, Y.; Fujita, T.; Shinohara, H.; Kurihara, H. The effect of extracellular calcium ion on gene expression of bone-related proteins in human pulp cells. J. Endod. 2003, 29, 104–107. [Google Scholar] [CrossRef]
- Han, L.; Okiji, T. Bioactivity evaluation of three calcium silicate-based endodontic materials. Int. Endod. J. 2013, 46, 808–814. [Google Scholar] [CrossRef]
- Patel, N.; Best, S.M.; Bonfield, W.; Gibson, I.R.; Hing, K.A.; Revell, P.A. A comparative study on the in vivo behavior of hydroxyapatite and silicon substituted hydroxyapatite granules. J. Mater. Sci. Mater. Med. 2002, 13, 1199–1206. [Google Scholar] [CrossRef]
- Chen, J.; McCulloch, C.A.G.; Sodek, J. Bone sialoprotein in developing porcine dental tissues: Cellular expression and comparison of tissue localization with osteopontin and osteonectin. Arch. Oral Biol. 1993, 38, 241–249. [Google Scholar] [CrossRef]
- Min, K.S.; Yang, S.H.; Kim, E.C. The combined effect of mineral trioxide aggregate and enamel matrix derivative on odontoblastic differentiation in human dental pulp cells. J. Endod. 2009, 35, 847–851. [Google Scholar] [CrossRef]
- Zhao, X.; He, W.; Song, Z.; Tong, Z.; Li, S.; Ni, L. Mineral trioxide aggregate promotes odontoblastic differentiation via mitogen-activated protein kinase pathway in human dental pulp stem cells. Mol. Biol. Rep. 2012, 39, 215–220. [Google Scholar] [CrossRef]
- Chang, S.W.; Lee, S.Y.; Kum, K.Y.; Kim, E.C. Effects of ProRoot MTA, Bioaggregate, and Micromega MTA on odontoblastic differentiation in human dental pulp cells. J. Endod. 2014, 40, 113–118. [Google Scholar] [CrossRef]
- Damlar, I.; Ozcan, E.; Yula, E.; Yalcin, M.; Celik, S. Antimicrobial effects of several calcium silicatebased root-end filling materials. Dent Mater. J. 2014, 33, 453–457. [Google Scholar] [CrossRef]
- Kohli, M.R.; Yamaguchi, M.; Setzer, F.C.; Karabucak, B. Spectrophotometric analysis of coronal tooth discoloration induced by various bioceramic cements and other endodontic materials. J. Endod. 2015, 41, 1862–1866. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edrees, H.Y.; Abu Zeid, S.T.H.; Atta, H.M.; AlQriqri, M.A. Induction of Osteogenic Differentiation of Mesenchymal Stem Cells by Bioceramic Root Repair Material. Materials 2019, 12, 2311. https://doi.org/10.3390/ma12142311
Edrees HY, Abu Zeid STH, Atta HM, AlQriqri MA. Induction of Osteogenic Differentiation of Mesenchymal Stem Cells by Bioceramic Root Repair Material. Materials. 2019; 12(14):2311. https://doi.org/10.3390/ma12142311
Chicago/Turabian StyleEdrees, Hadeel Y., Sawsan T.H. Abu Zeid, Hazem M. Atta, and Mehal A. AlQriqri. 2019. "Induction of Osteogenic Differentiation of Mesenchymal Stem Cells by Bioceramic Root Repair Material" Materials 12, no. 14: 2311. https://doi.org/10.3390/ma12142311
APA StyleEdrees, H. Y., Abu Zeid, S. T. H., Atta, H. M., & AlQriqri, M. A. (2019). Induction of Osteogenic Differentiation of Mesenchymal Stem Cells by Bioceramic Root Repair Material. Materials, 12(14), 2311. https://doi.org/10.3390/ma12142311




