Tissue Constructs with Human Adipose-Derived Mesenchymal Stem Cells to Treat Bone Defects in Rats
Abstract
1. Introduction
2. Materials and Methods
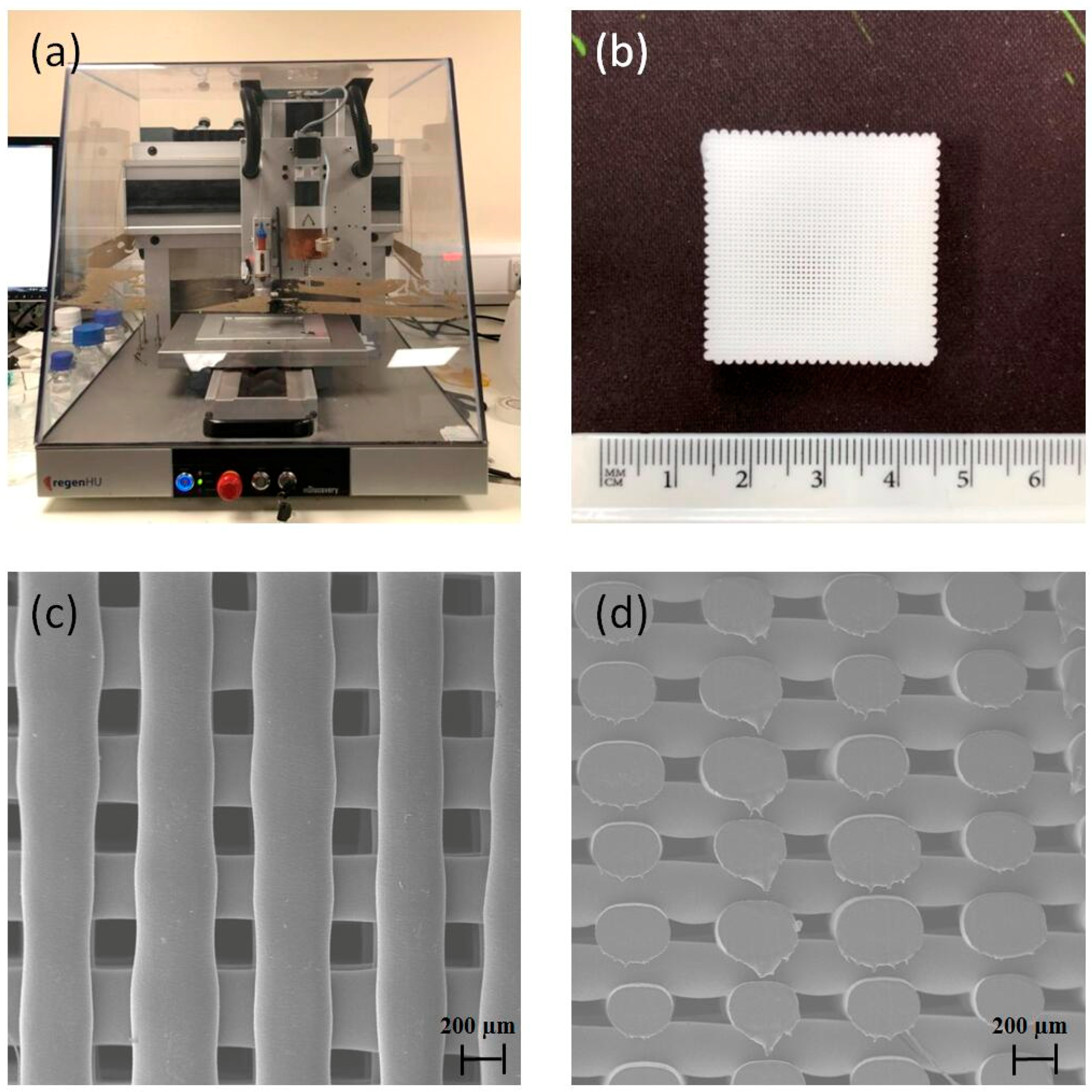
2.1. Scaffold Design and Fabrication
2.2. In Vitro Cell Proliferation, Differentiation and Bioimaging
2.3. Cell Source and Culture for In Vivo Studies
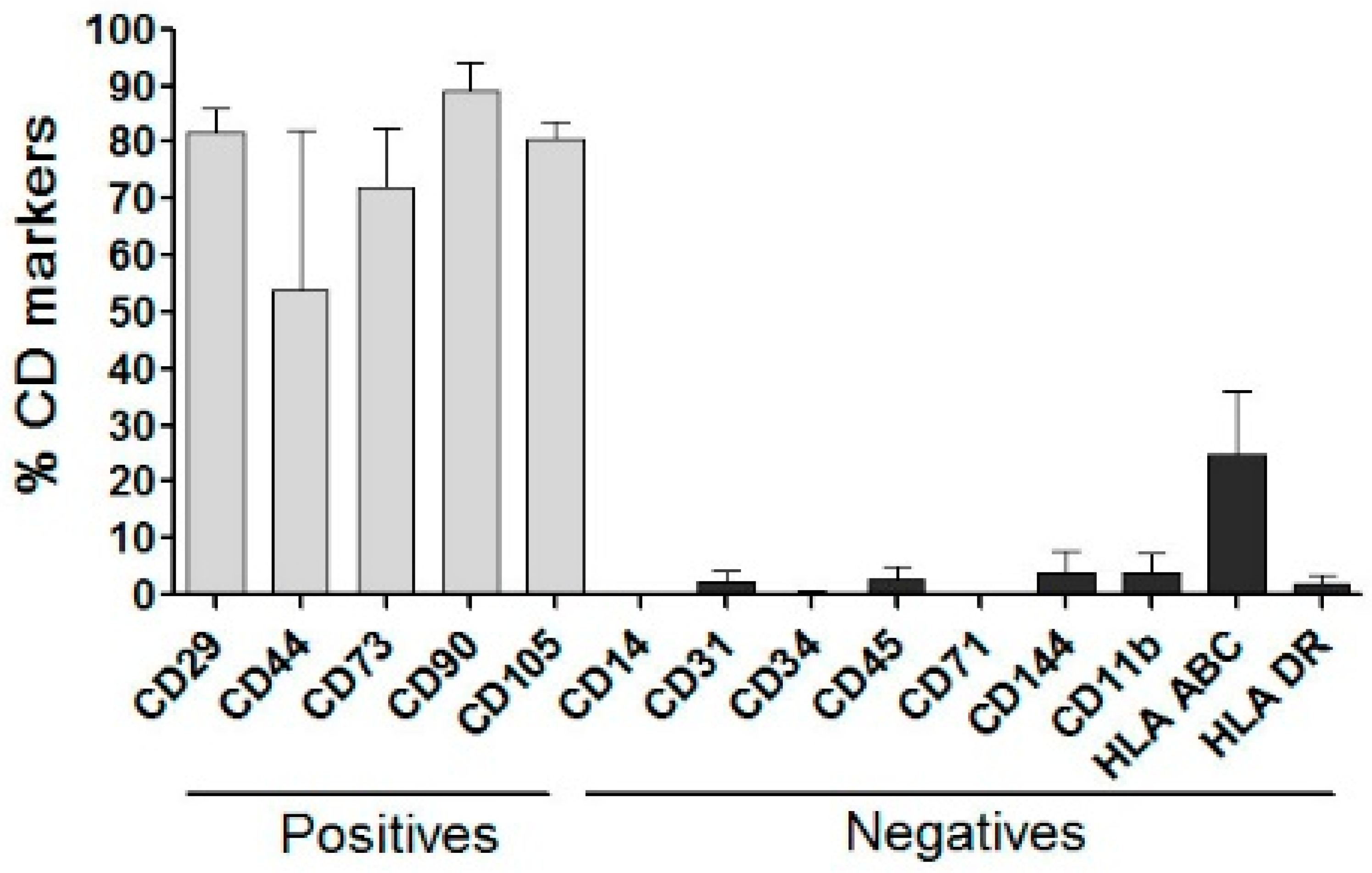
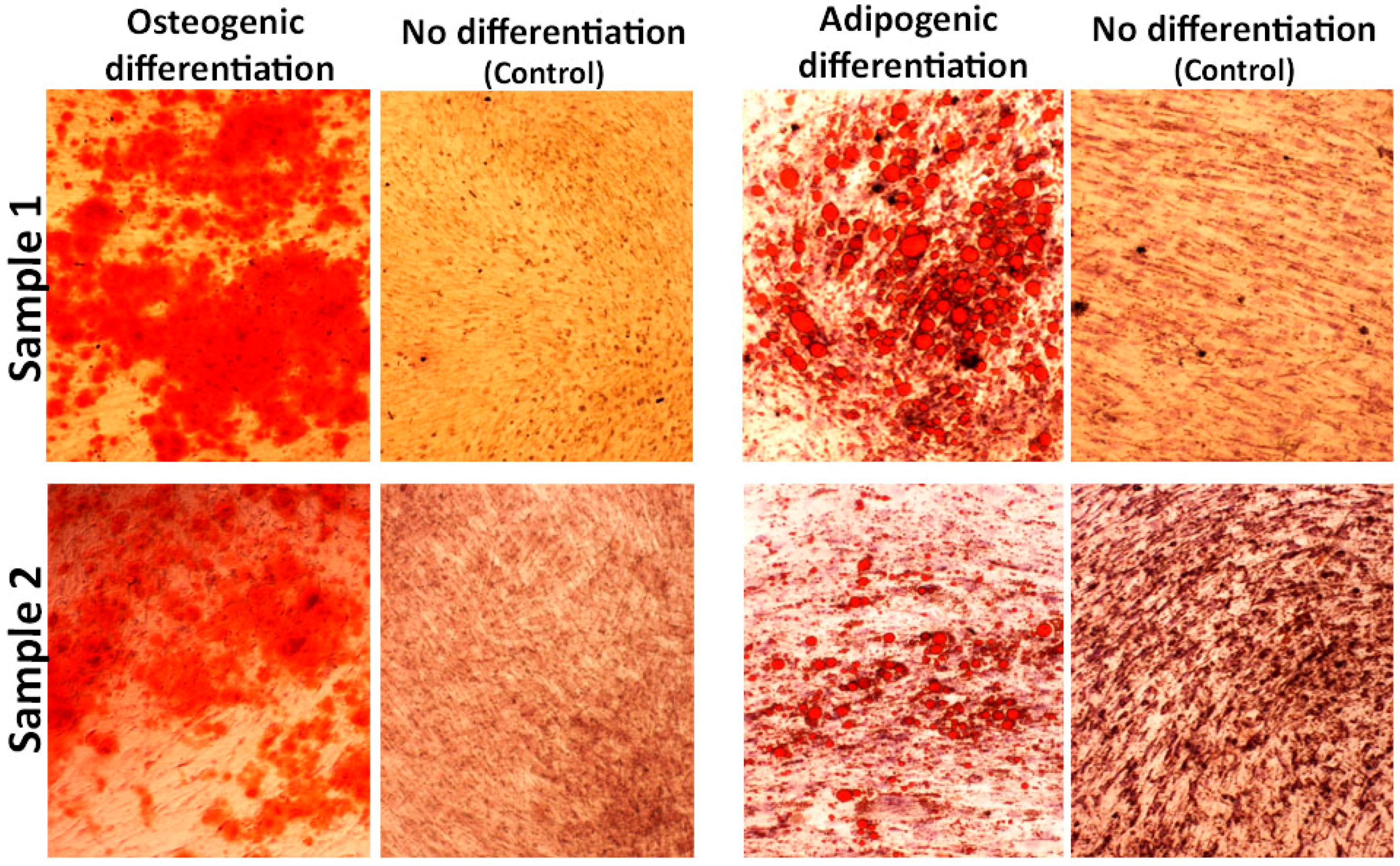
2.4. hADSC Characterization and Multilineage Differentiation
2.5. Cell Seeding for In Vivo Studies
2.6. Bone Defect Creation
2.7. Histology
2.8. Immunohistochemistry
3. Results
3.1. 3D Printed Scaffolds
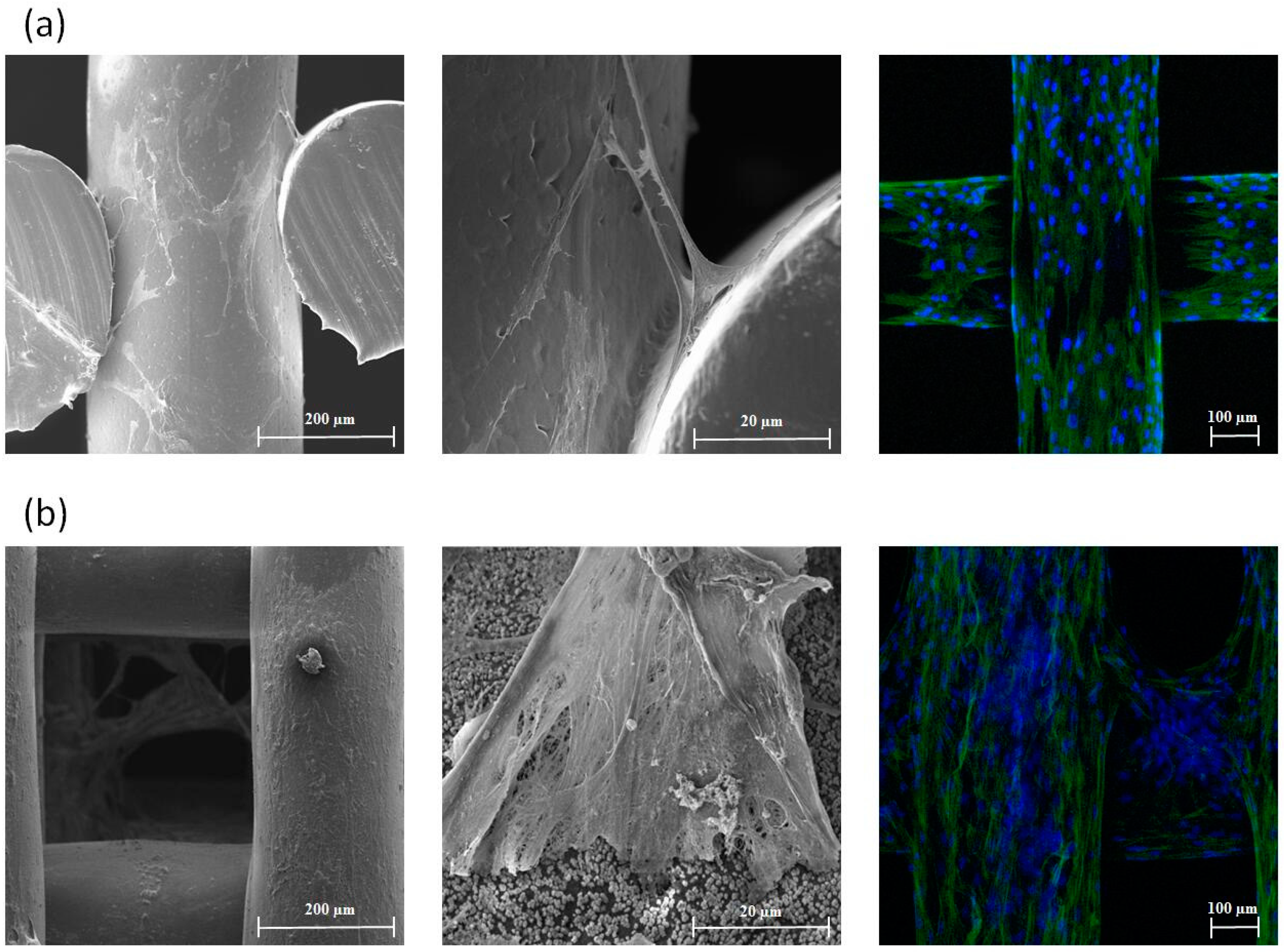
3.2. In Vitro Cell Proliferation, Differentation
3.3. hADSCs Characterization for In Vivo Study
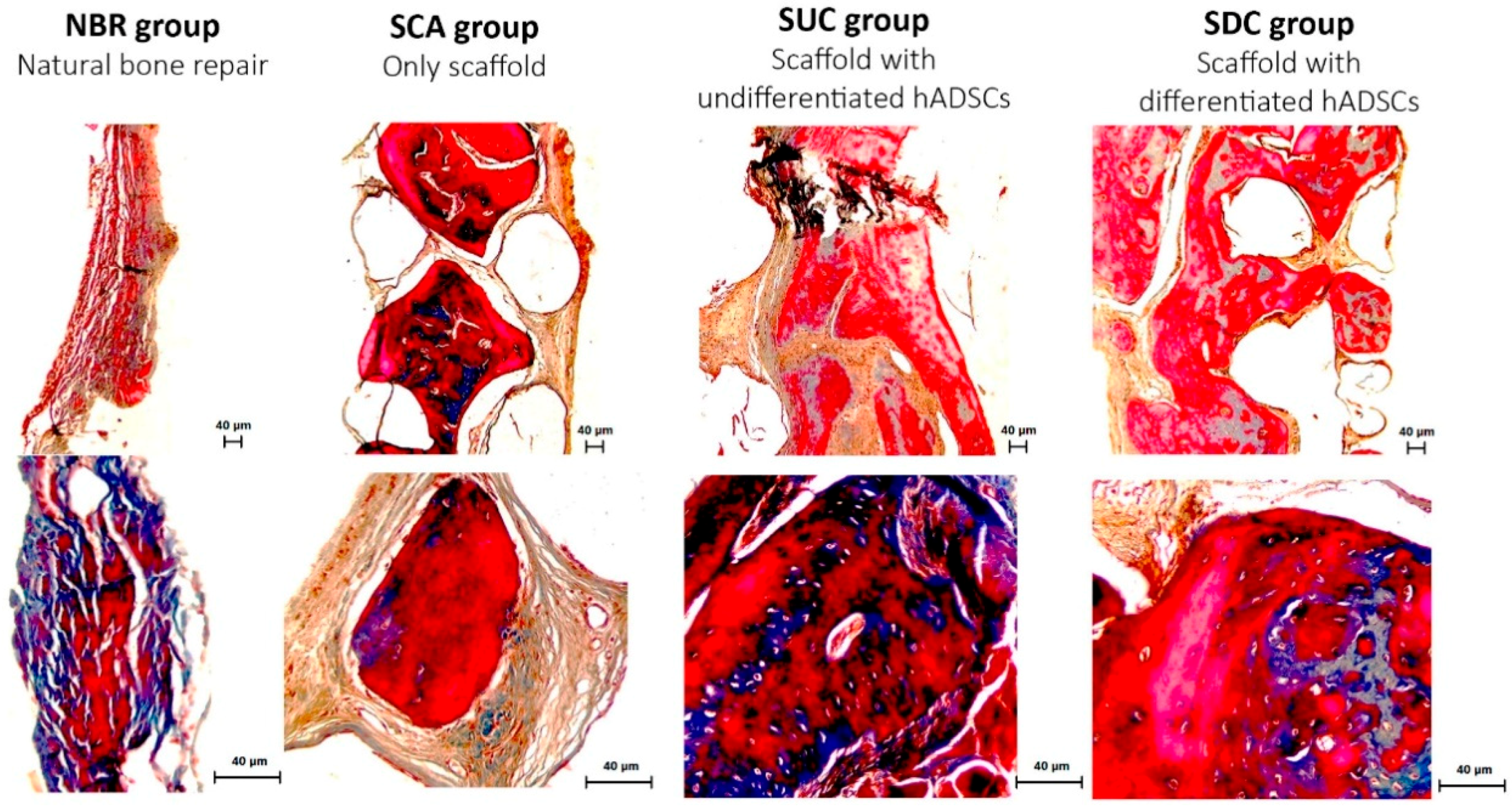
3.4. Histological Assessment
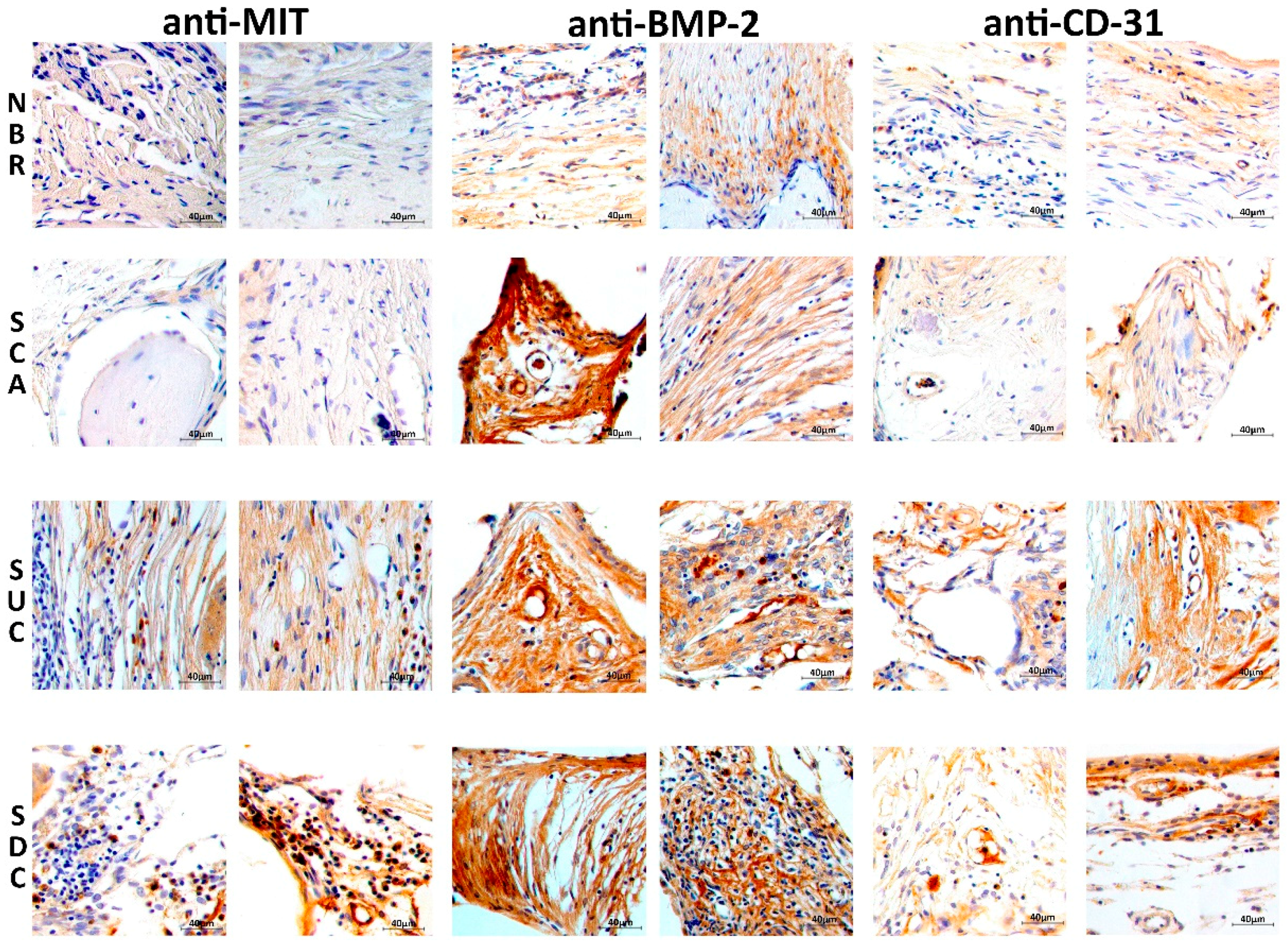
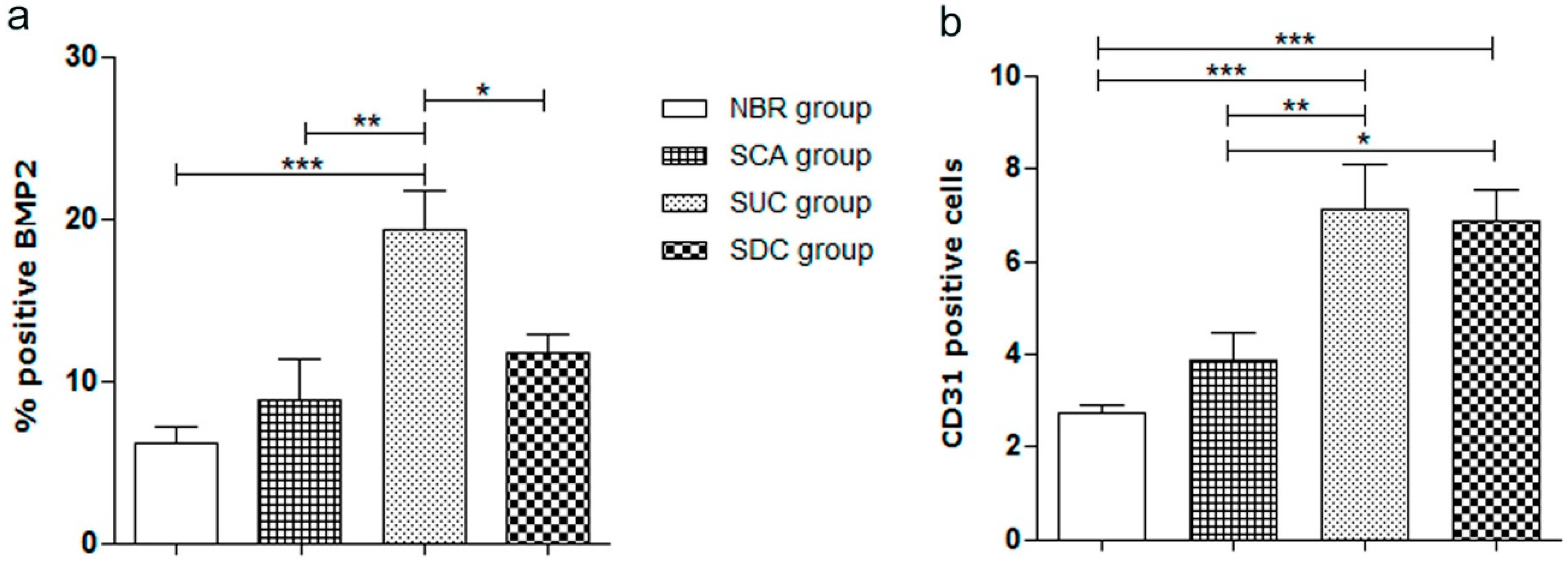
3.5. Immunohistochemical Assessment
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pereira, R.F.; Sousa, A.; Barrias, C.C.; Bayat, A.; Granja, P.L.; Bártolo, P.J. Advances in bioprinted cell-laden hydrogels for skin tissue engineering. Biomanuf. Rev. 2017, 2, 1–26. [Google Scholar] [CrossRef]
- Melchels, F.P.; Domingos, M.A.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive manufacturing of tissues and organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef]
- Bartolo, P.; Kruth, J.P.; Silva, J.; Levy, G.; Malshe, A.; Rajurkar, K.; Mitsuishi, M.; Ciurana, J.; Leu, M. Biomedical production of implants by additive electro-chemical and physical processes. CIRP Ann. 2012, 61, 635–655. [Google Scholar] [CrossRef]
- Bártolo, P.J.; Chua, C.K.; Almeida, H.A.; Chou, S.M.; Lim, A.S. Biomanufacturing for tissue engineering: Present and future trends. Virtual Phys. Prototyp. 2009, 4, 203–216. [Google Scholar] [CrossRef]
- Lee, J.; Farag, M.M.; Park, E.K.; Lim, J.; Yun, H.S. A simultaneous process of 3D magnesium phosphate scaffold fabrication and bioactive substance loading for hard tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 36, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Black, C.R.; Goriainov, V.; Gibbs, D.; Kanczler, J.; Tare, R.S.; Oreffo, R.O. Bone tissue engineering. Curr. Mol. Biol. Rep. 2015, 1, 132–140. [Google Scholar] [CrossRef]
- Huang, S.T.; Huang, C.C.; Sheen, J.M.; Lin, T.K.; Liao, P.L.; Huang, W.L.; Wang, P.W.; Liou, C.W.; Chuang, J.H. Phyllanthus urinaria’s inhibition of human osteosarcoma xenografts growth in mice is associated with modulation of mitochondrial fission/fusion machinery. Am. J. Chin. Med. 2016, 44, 1507–1523. [Google Scholar] [CrossRef]
- Wang, W.; Junior, J.R.; Nalesso, P.R.; Musson, D.; Cornish, J.; Mendonça, F.; Caetano, G.F.; Bártolo, P. Engineered 3D printed poly (ɛ-caprolactone)/graphene scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 759–770. [Google Scholar] [CrossRef]
- Huang, B.; Vyas, C.; Roberts, I.; Poutrel, Q.A.; Chiang, W.H.; Blaker, J.J.; Huang, Z.; Bártolo, P. Fabrication and characterisation of 3D printed MWCNT composite porous scaffolds for bone regeneration. Mater. Sci. Eng. C 2019, 98, 266–278. [Google Scholar] [CrossRef]
- Pereira, R.F.; Sousa, A.; Barrias, C.C.; Bártolo, P.J.; Granja, P.L. A single-component hydrogel bioink for bioprinting of bioengineered 3D constructs for dermal tissue engineering. Mater. Horiz. 2018, 5, 100–111. [Google Scholar] [CrossRef][Green Version]
- Dias, J.R.; Baptista-Silva, S.; Sousa, A.; Oliveira, A.L.; Bártolo, P.J.; Granja, P.L. Biomechanical performance of hybrid electrospun structures for skin regeneration. Mater. Sci. Eng. C 2018, 93, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Caetano, G.F.; Wang, W.; Chiang, W.H.; Cooper, G.; Diver, C.; Blaker, J.J.; Frade, M.A.; Bártolo, P. 3D-Printed Poly (ɛ-caprolactone)/Graphene Scaffolds Activated with P1-Latex Protein for Bone Regeneration. 3D Print. Addit. Manuf. 2018, 5, 127–137. [Google Scholar] [CrossRef]
- Wang, W.; Caetano, G.; Ambler, W.; Blaker, J.; Frade, M.; Mandal, P.; Diver, C.; Bártolo, P. Enhancing the hydrophilicity and cell attachment of 3D printed PCL/graphene scaffolds for bone tissue engineering. Materials 2016, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Caetano, G.F.; Chiang, W.H.; Braz, A.L.; Blaker, J.J.; Frade, M.A.; Bartolo, P.J. Morphological, mechanical and biological assessment of PCL/pristine graphene scaffolds for bone regeneration. Int. J. Bioprint. 2016, 2, 95–104. [Google Scholar] [CrossRef]
- Bártolo, P.J.; Almeida, H.A.; Rezende, R.A.; Laoui, T.; Bidanda, B. Advanced processes to fabricate scaffolds for tissue engineering. In Virtual Prototyping & Bio Manufacturing in Medical Applications; Springer: Boston, MA, USA, 2008; pp. 149–170. [Google Scholar]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 1, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Wang, Z.; Samanipour, R.; Koo, K.I.; Kim, K. Adipose-derived stem cells for tissue engineering and regenerative medicine applications. Stem Cells Int. 2016, 2016, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Patrício, T.; Domingos, M.; Gloria, A.; Amora, U.D.; Coelho, J.F.; Bártolo, P.J. Fabrication and characterisation of PCL and PCL/PLA scaffolds for tissue engineering. Rapid Prototyp. J. 2014, 20, 145–156. [Google Scholar] [CrossRef]
- Sasmazel, H.T. Novel hybrid scaffolds for the cultivation of osteoblast cells. Int. J. Biol. Macromol. 2011, 49, 838–846. [Google Scholar] [CrossRef]
- Caetano, G.F.; Bártolo, P.J.; Domingos, M.; Oliveira, C.C.; Leite, M.N.; Frade, M.A. Osteogenic differentiation of adipose-derived mesenchymal stem cells into Polycaprolactone (PCL) scaffold. Procedia Eng. 2015, 1, 59–66. [Google Scholar] [CrossRef]
- Caetano, G.; Violante, R.; Sant, A.B.; Murashima, A.B.; Domingos, M.; Gibson, A.; Bártolo, P.; Frade, M.A. Cellularized versus decellularized scaffolds for bone regeneration. Mater. Lett. 2016, 1, 318–322. [Google Scholar] [CrossRef]
- Wang, W.; Huang, B.; Byun, J.J.; Bártolo, P. Assessment of PCL/carbon material scaffolds for bone regeneration. J. Mech. Behav. Biomed. Mater. 2019, 93, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Wu, Y.; Su, W.; Tian, M.; Jiang, W.; Cao, L.; Zhao, L.; Zhao, Z. Carbon nanotube reinforced collagen/hydroxyapatite scaffolds improve bone tissue formation in vitro and in vivo. Ann. Biomed. Eng. 2017, 45, 2075–2087. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Ma, S.; Jin, L.; Liu, Z.; Liu, D.; Zhang, X.; Cai, Q.; Yang, X. Repairing a bone defect with a three-dimensional cellular construct composed of a multi-layered cell sheet on electrospun mesh. Biofabrication 2017, 9, 025036. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Sun, J.; Hu, Y.; Gao, Y.; Xiao, C.; Liu, S.; Li, S. Overexpression of PLAP-1 in bone marrow stromal cells inhibits the rat critical-size skull defect repair. J. Mol. Histol. 2015, 46, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Wang, Y.; Zhang, J.; Zhang, T.; Li, S. Healing of periodontal defects and calcitonin gene related peptide expression following inferior alveolar nerve transection in rats. J. Mol. Histol. 2014, 45, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.H.; Bagne, L.; Meneghetti, D.H.; dos Santos, G.M.; Esquisatto, M.A.; Andrade, T.A.; do Amaral, M.E.; Felonato, M.; Caetano, G.F.; Santamaria, M.; et al. Electrical stimulation: Complementary therapy to improve the performance of grafts in bone defects? J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.L.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Kerkis, I.; Caplan, A.I. Stem cells in dental pulp of deciduous teeth. Tissue Eng. Part B Rev. 2012, 18, 129–138. [Google Scholar] [CrossRef]
- Kim, K.I.; Park, S.; Im, G.I. Osteogenic differentiation and angiogenesis with cocultured adipose-derived stromal cells and bone marrow stromal cells. Biomaterials 2014, 35, 4792–4804. [Google Scholar] [CrossRef]
- Abuna, R.P.; Stringhetta-Garcia, C.T.; Fiori, L.P.; Dornelles, R.C.; Rosa, A.L.; Beloti, M.M. Aging impairs osteoblast differentiation of mesenchymal stem cells grown on titanium by favoring adipogenesis. J. Appl. Oral Sci. 2016, 24, 376–382. [Google Scholar] [CrossRef]
- Guan, M.; Yao, W.; Liu, R.; Lam, K.S.; Nolta, J.; Jia, J.; Panganiban, B.; Meng, L.; Zhou, P.; Shahnazari, M.; et al. Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat. Med. 2012, 18, 456. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.M.; Lee, J.H.; Park, C.M.; Song, H.R.; Wang, J.H. Xenotransplantation of human mesenchymal stem cells for repair of osteochondral defects in rabbits using osteochondral biphasic composite constructs. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Koh, Y.G.; Choi, Y.J.; Kim, S.H.; Yoon, D.S.; Lee, M.; Lee, J.W. Characterization of adipose tissue-derived stromal vascular fraction for clinical application to cartilage regeneration. Cell. Dev. Biol. Anim. 2015, 51, 142–150. [Google Scholar] [CrossRef]
- Chuang, C.K.; Lin, K.J.; Lin, C.Y.; Chang, Y.H.; Yen, T.C.; Hwang, S.M.; Sung, L.Y.; Chen, H.C.; Hu, Y.C. Xenotransplantation of human mesenchymal stem cells into immunocompetent rats for calvarial bone repair. Tissue Eng. Part A 2009, 16, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Zong, C.; Xue, D.; Yuan, W.; Wang, W.; Shen, D.; Tong, X.; Shi, D.; Liu, L.; Zheng, Q.; Gao, C.; et al. Reconstruction of rat calvarial defects with human mesenchymal stem cells and osteoblast-like cells in poly-lactic-co-glycolic acid scaffolds. Eur. Cells Mater. 2010, 1, 109–120. [Google Scholar] [CrossRef]
- Almubarak, S.; Nethercott, H.; Freeberg, M.; Beaudon, C.; Jha, A.; Jackson, W.; Marcucio, R.; Miclau, T.; Healy, K.; Bahney, C. Tissue engineering strategies for promoting vascularized bone regeneration. Bone 2016, 1, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.C.; Kim, H.J.; Bae, I.H.; Lee, K.N.; Lee, K.Y.; Oh, W.M.; Kim, S.H.; Kang, I.C.; Lee, S.E.; Koh, G.Y. COMP-Ang1, a chimeric form of Angiopoietin 1, enhances BMP2-induced osteoblast differentiation and bone formation. Bone 2010, 46, 479–486. [Google Scholar] [CrossRef]
- Choi, H.; Jeong, B.C.; Hur, S.W.; Kim, J.W.; Lee, K.B.; Koh, J.T. The Angiopoietin-1 Variant COMP-Ang1 Enhances BMP2-Induced Bone Regeneration with Recruiting Pericytes in Critical Sized Calvarial Defects. PLoS ONE 2015, 10, e0140502. [Google Scholar] [CrossRef] [PubMed]
- Kaigler, D.; Krebsbach, P.H.; West, E.R.; Horger, K.; Huang, Y.C.; Mooney, D.J. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. FASEB J. 2005, 19, 665–667. [Google Scholar] [CrossRef]
- Sartori, M.; Pagani, S.; Ferrari, A.; Costa, V.; Carina, V.; Figallo, E.; Maltarello, M.C.; Martini, L.; Fini, M.; Giavaresi, G. A new bi-layered scaffold for osteochondral tissue regeneration: In vitro and in vivo preclinical investigations. Mater. Sci. Eng. C 2017, 70, 101–111. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caetano, G.; Wang, W.; Murashima, A.; Passarini, J.R., Jr.; Bagne, L.; Leite, M.; Hyppolito, M.; Al-Deyab, S.; El-Newehy, M.; Bártolo, P.; et al. Tissue Constructs with Human Adipose-Derived Mesenchymal Stem Cells to Treat Bone Defects in Rats. Materials 2019, 12, 2268. https://doi.org/10.3390/ma12142268
Caetano G, Wang W, Murashima A, Passarini JR Jr., Bagne L, Leite M, Hyppolito M, Al-Deyab S, El-Newehy M, Bártolo P, et al. Tissue Constructs with Human Adipose-Derived Mesenchymal Stem Cells to Treat Bone Defects in Rats. Materials. 2019; 12(14):2268. https://doi.org/10.3390/ma12142268
Chicago/Turabian StyleCaetano, Guilherme, Weiguang Wang, Adriana Murashima, José Roberto Passarini, Jr., Leonardo Bagne, Marcel Leite, Miguel Hyppolito, Salem Al-Deyab, Mohamed El-Newehy, Paulo Bártolo, and et al. 2019. "Tissue Constructs with Human Adipose-Derived Mesenchymal Stem Cells to Treat Bone Defects in Rats" Materials 12, no. 14: 2268. https://doi.org/10.3390/ma12142268
APA StyleCaetano, G., Wang, W., Murashima, A., Passarini, J. R., Jr., Bagne, L., Leite, M., Hyppolito, M., Al-Deyab, S., El-Newehy, M., Bártolo, P., & Frade, M. A. C. (2019). Tissue Constructs with Human Adipose-Derived Mesenchymal Stem Cells to Treat Bone Defects in Rats. Materials, 12(14), 2268. https://doi.org/10.3390/ma12142268






