Improvement of Thermal Cycling Resistance of AlxSi1−xN Coatings on Cu Substrates by Optimizing Al/Si Ratio
Abstract
1. Introduction
2. Experimental Part
3. Results
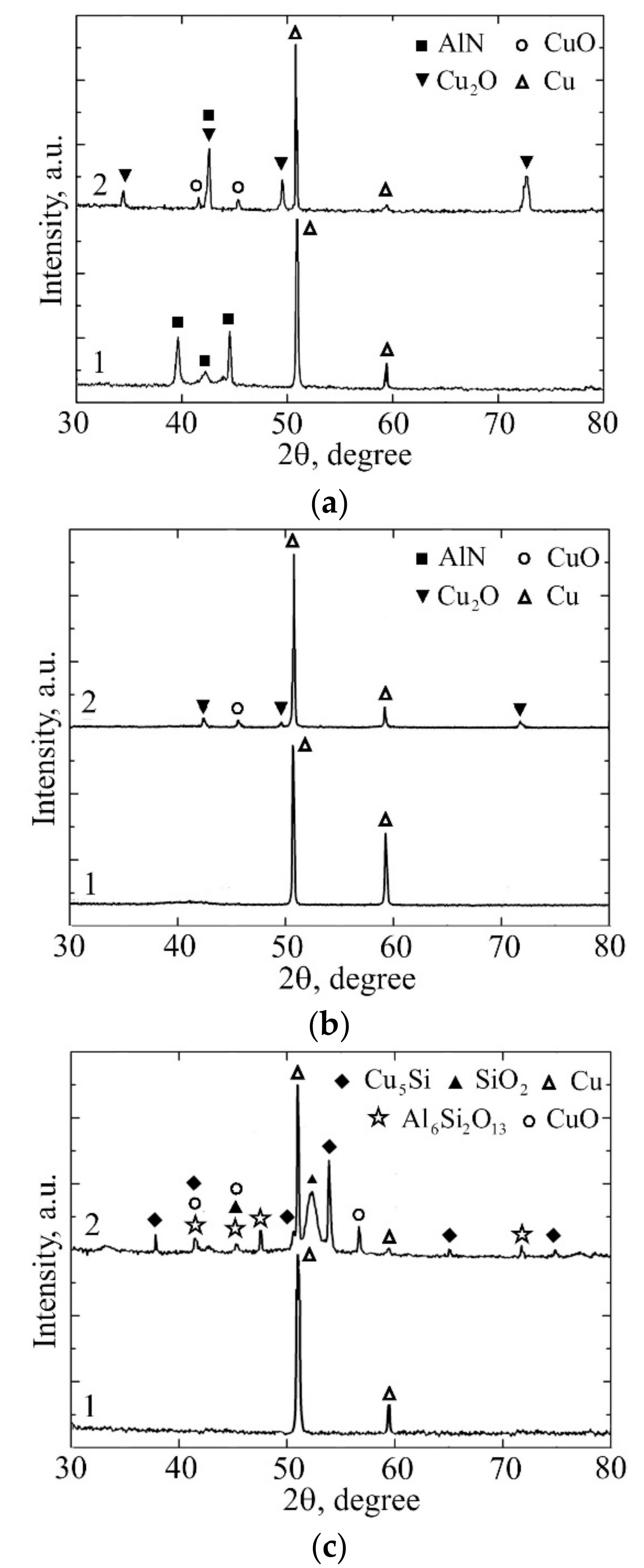
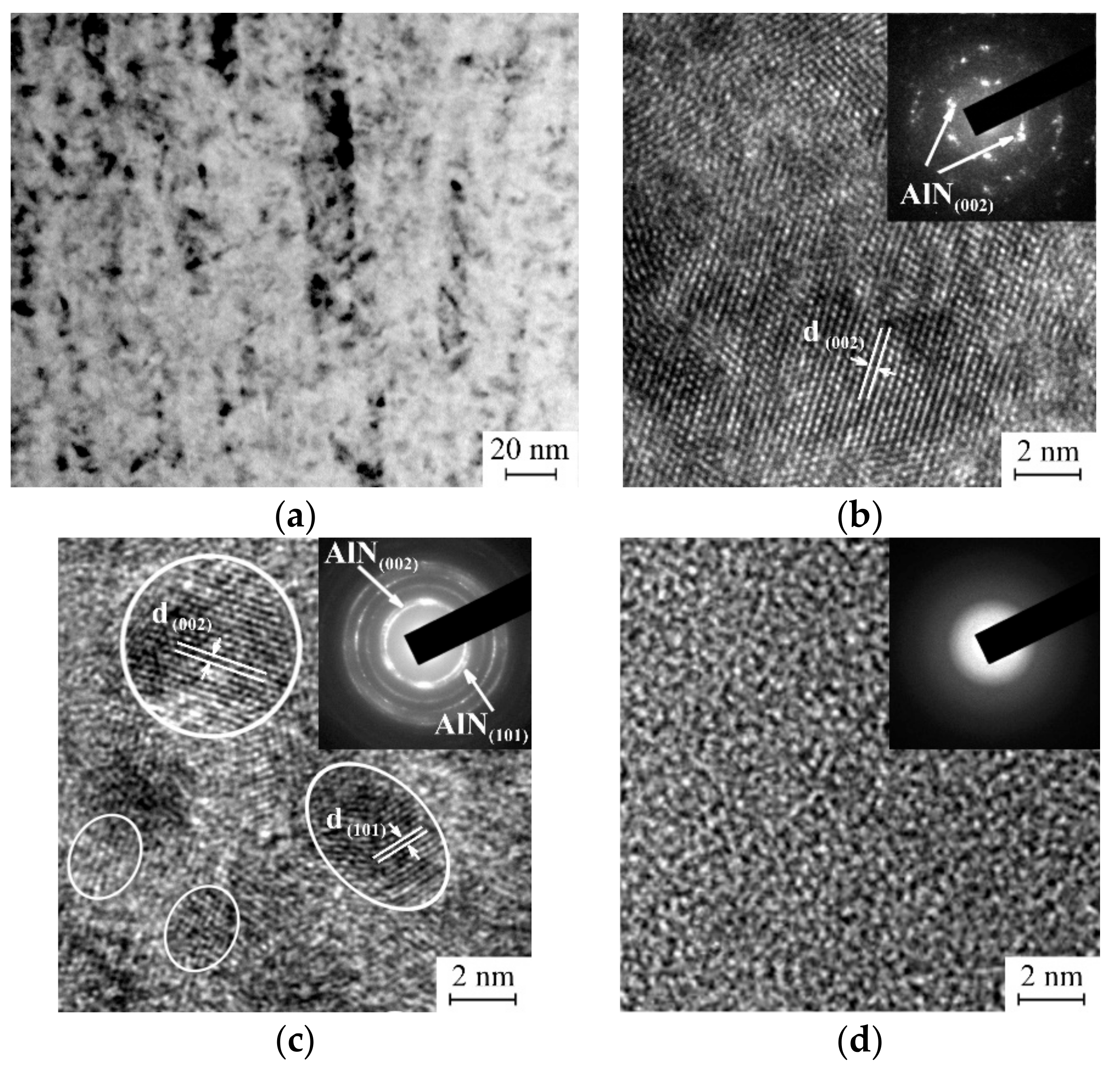
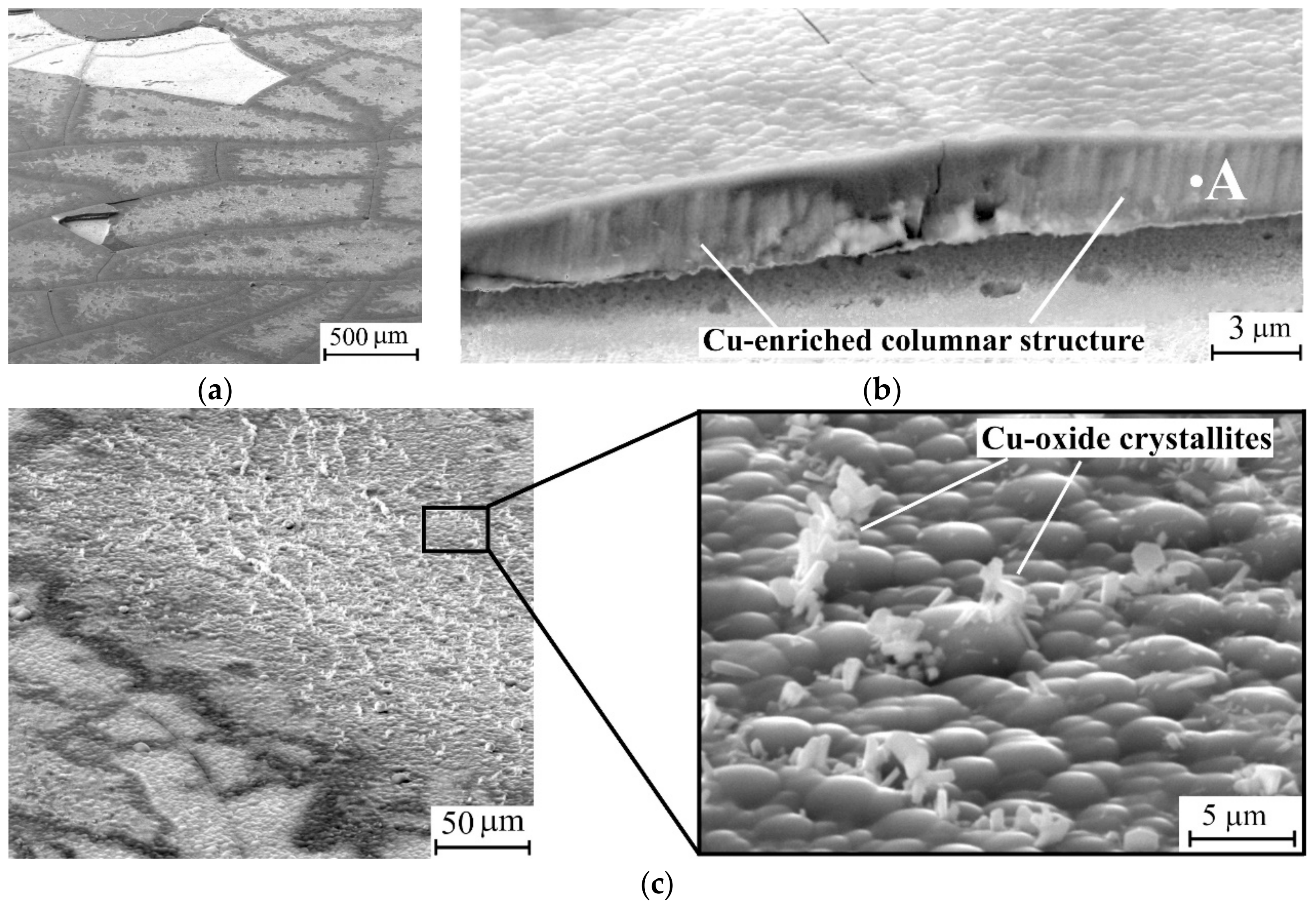
3.1. Characterization of the As-Deposited Coatings
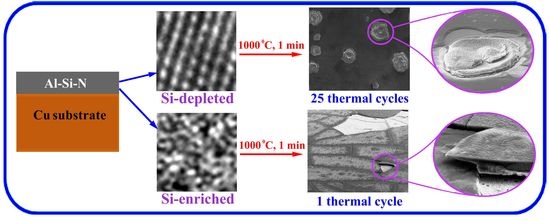
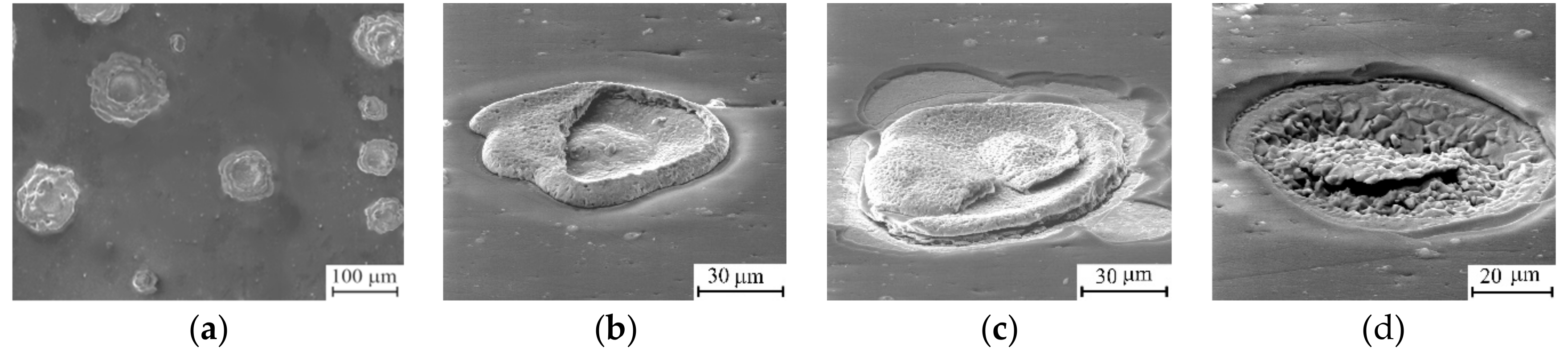
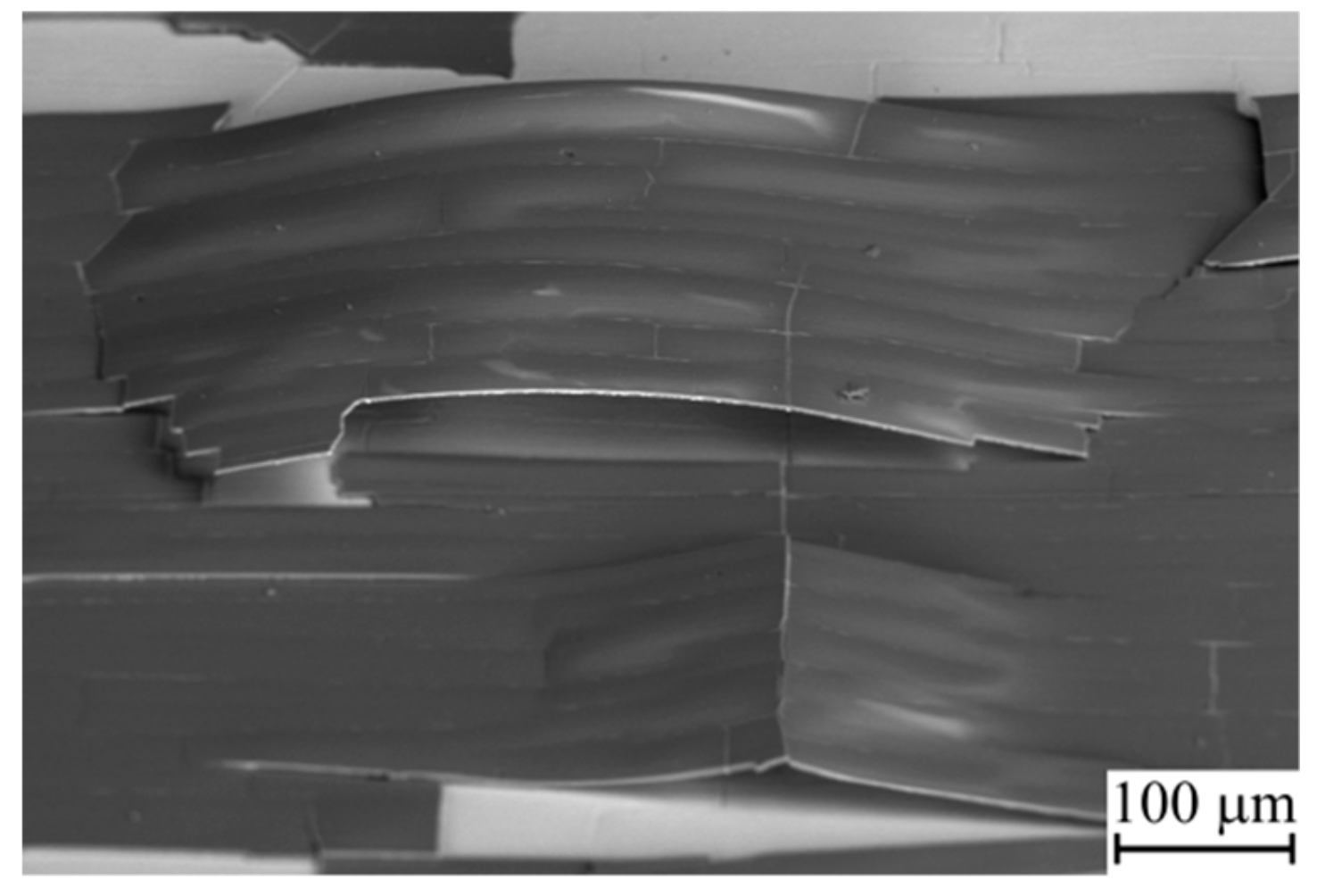
3.2. Thermal Cycling of the Coatings
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kotilainen, M.; Honkanen, M.; Mizohata, K.; Vuoristo, P. Influence of temperature-induced copper diffusion on degradation of selective chromium oxy-nitride solar absorber coatings. Sol. Energy Mater. Sol. Cells 2016, 145, 323–332. [Google Scholar] [CrossRef]
- Kotilainen, M.; Krumpolec, R.; Franta, D.; Souček, P.; Homola, T.; Cameron, D.C.; Vuoristo, P. Hafnium oxide thin films as a barrier against copper diffusion in solar absorbers. Sol. Energy Mater. Sol. Cells 2017, 166, 140–146. [Google Scholar] [CrossRef]
- Wadel, M.; Meyer, M. Validation of High Aspect Ratio Cooling in a 89 kN (20,000 lbf) Thrust Combustion Chamber. In 32nd Joint Propulsion Conference and Exhibit; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 1996. [Google Scholar]
- Sedighi, M.; Padilla, R.; Taylor, R.; Lake, M.; Izadgoshasb, I.; Rose, A. High-temperature, point-focus, pressurised gas-phase solar receivers: A comprehensive review. Energy Convers. Manag. 2019, 185, 678–717. [Google Scholar] [CrossRef]
- Raj, S.V.; Ghosn, L.J.; Robinson, C.; Humphrey, D. High heat flux exposures of coated GRCop-84 substrates. Mater. Sci. Eng. A 2007, 457, 300–312. [Google Scholar] [CrossRef]
- Raynaud, G.M.; Clark, W.A.T.; Rapp, R.A. In Situ observation of copper oxidation at high temperatures. MTA 1984, 15, 573–586. [Google Scholar] [CrossRef]
- Zhu, Y.; Mimura, K.; Isshiki, M. Oxidation Mechanism of Copper at 623-1073 K. Mater. Trans. 2002, 43, 2173–2176. [Google Scholar] [CrossRef]
- Riccius, J.; Haidn, O.; Zametaev, E. Influence of Time Dependent Effects on the Estimated Life Time of Liquid Rocket Combustion Chamber Walls. In 40th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit; Joint Propulsion Conferences; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2004. [Google Scholar]
- Sarius, N.G.; Lauridsen, J.; Lewin, E.; Lu, J.; Högberg, H.; Öberg, Å.; Ljungcrantz, H.; Leisner, P.; Eklund, P.; Hultman, L. Ni and Ti diffusion barrier layers between Ti–Si–C and Ti–Si–C–Ag nanocomposite coatings and Cu-based substrates. Surf. Coat. Technol. 2012, 206, 2558–2565. [Google Scholar] [CrossRef]
- Schloesser, J.; Bäker, M.; Rösler, J. Laser cycling and thermal cycling exposure of thermal barrier coatings on copper substrates. Surf. Coat. Technol. 2011, 206, 1605–1608. [Google Scholar] [CrossRef]
- Mayrhofer, P.; Rachbauer, R.; Holec, D.; Rovere, F.; Schneider, J. Protective Transition Metal Nitride Coatings. Compr. Mater. Process. 2014, 4, 355–388. [Google Scholar]
- Rebouta, L.; Sousa, A.; Andritschky, M.; Cerqueira, F.; Tavares, C.J.; Santilli, P.; Pischow, K. Solar selective absorbing coatings based on AlSiN/AlSiON/AlSiOy layers. Appl. Surf. Sci. 2015, 356, 203–212. [Google Scholar] [CrossRef]
- Sui, X.; Li, G.; Qin, X.; Yu, H.; Zhou, X.; Wang, K.; Wang, Q. Relationship of microstructure, mechanical properties and titanium cutting performance of TiAlN/TiAlSiN composite coated tool. Ceram. Int. 2016, 42. [Google Scholar] [CrossRef]
- Tsai, D.-C.; Chang, Z.-C.; Kuo, B.-H.; Liu, Y.-C.; Chen, E.-C.; Shieu, F.-S. Structural, electro-optical, and mechanical properties of reactively sputtered (TiZrHf)N coatings. Ceram. Int. 2016, 42, 14257–14265. [Google Scholar] [CrossRef]
- Faga, M.G.; Gautier, G.; Cartasegna, F.; Priarone, P.C.; Settineri, L. AlSiTiN and AlSiCrN multilayer coatings: Effects of structure and surface composition on tribological behavior under dry and lubricated conditions. Appl. Surf. Sci. 2016, 365, 218–226. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Bondar, O.V.; Borba, S.O.; Abadias, G.; Konarski, P.; Plotnikov, S.V.; Beresnev, V.M.; Kassenova, L.G.; Drodziel, P. Nanostructured multielement (TiHfZrNbVTa)N coatings before and after implantation of N+ ions (1018 cm−2): Their structure and mechanical properties. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2016, 385, 74–83. [Google Scholar] [CrossRef]
- Bagdasaryan, A.A.; Pshyk, A.V.; Coy, L.E.; Kempiński, M.; Pogrebnjak, A.D.; Beresnev, V.M.; Jurga, S. Structural and mechanical characterization of (TiZrNbHfTa)N/WN multilayered nitride coatings. Mater. Lett. 2018, 229, 364–367. [Google Scholar] [CrossRef]
- McIntyre, D.; Greene, J.E.; Håkansson, G.; Sundgren, J.-E.; Münz, W.-D. Oxidation of metastable single-phase polycrystalline Ti0.5Al0.5N films: Kinetics and mechanisms. J. Appl. Phys. 1990, 67, 1542–1553. [Google Scholar] [CrossRef]
- Kawate, M.; Kimura Hashimoto, A.; Suzuki, T. Oxidation resistance of Cr1−xAlxN and Ti1−xAlxN films. Surf. Coat. Technol. 2003, 165, 163–167. [Google Scholar] [CrossRef]
- Vepřek, S.; Reiprich, S. A concept for the design of novel superhard coatings. Thin Solid Film. 1995, 268, 64–71. [Google Scholar] [CrossRef]
- Carvalho, S.; Rebouta, L.; Ribeiro, E.; Vaz, F.; Denannot, M.F.; Pacaud, J.; Rivière, J.P.; Paumier, F.; Gaboriaud, R.J.; Alves, E. Microstructure of (Ti,Si,Al)N nanocomposite coatings. Surf. Coat. Technol. 2004, 177, 369–375. [Google Scholar] [CrossRef]
- Korotaev, D.A.; Borisov, D.; Moshkov, V.; Ovchinnikov, S.; Pinzhin, Y.; Tyumentsev, A. Elastic stress state in superhard multielement coatings. Phys. Mesomech. Phys. Mesomech. 2009, 12, 269–279. [Google Scholar] [CrossRef]
- Musil, J.; Daniel, R.; Soldán, J.; Zeman, P. Properties of reactively sputtered W–Si–N films. Surf. Coat. Technol. 2006, 200, 3886–3895. [Google Scholar] [CrossRef]
- Zeman, P.; Musil, J.; Daniel, R. High-temperature oxidation resistance of Ta–Si–N films with a high Si content. Surf. Coat. Technol. 2006, 200, 4091–4096. [Google Scholar] [CrossRef]
- Daniel, R.; Musil, J.; Zeman, P.; Mitterer, C. Thermal stability of magnetron sputtered Zr–Si–N films. Surf. Coat. Technol. 2006, 201, 3368–3376. [Google Scholar] [CrossRef]
- Musil, J.; Vlček, J.; Zeman, P. Hard amorphous nanocomposite coatings with oxidation resistance above 1000 °C. Adv. Appl. Ceram. 2008, 107, 148–154. [Google Scholar] [CrossRef]
- Shizhi, L.; Yulong, S.; Hongrui, P. Ti-Si-N films prepared by plasma-enhanced chemical vapor deposition. Plasma Chem. Plasma Process. 1992, 12, 287–297. [Google Scholar] [CrossRef]
- Mazel, A.; Marti, P.; Henry, F.; Armas, B.; Bonnet, R.; Loubradou, M. Nanostructure and local chemical composition of AlNSi3N4 layers grown by LPCVD. Thin Solid Film. 1997, 304, 256–266. [Google Scholar] [CrossRef]
- Bendavid, A.; Martin, P.J.; Takikawa, H. The properties of nanocomposite aluminium–silicon based thin films deposited by filtered arc deposition. Thin Solid Film. 2002, 420, 83–88. [Google Scholar] [CrossRef]
- Pélisson, A.; Parlinska-Wojtan, M.; Hug, H.J.; Patscheider, J. Microstructure and mechanical properties of Al–Si–N transparent hard coatings deposited by magnetron sputtering. Surf. Coat. Technol. 2007, 202, 884–889. [Google Scholar] [CrossRef]
- Musil, J.; Šašek, M.; Zeman, P.; Čerstvý, R.; Heřman, D.; Han, J.G.; Šatava, V. Properties of magnetron sputtered Al–Si–N thin films with a low and high Si content. Surf. Coat. Technol. 2008, 202, 3485–3493. [Google Scholar] [CrossRef]
- Musil, J.; Remnev, G.; Legostaev, V.; Uglov, V.; Lebedynskiy, A.; Lauk, A.; Procházka, J.; Haviar, S.; Smolyanskiy, E. Flexible hard Al-Si-N films for high temperature operation. Surf. Coat. Technol. 2016, 307, 1112–1118. [Google Scholar] [CrossRef]
- Liu, H.; Tang, W.; Hui, D.; Hei, L.; Lu, F. Characterization of (Al, Si) N films deposited by balanced magnetron sputtering. Thin Solid Film. 2009, 517, 5988–5993. [Google Scholar] [CrossRef]
- Lewin, E.; Loch, D.; Montagne, A.; Ehiasarian, A.P.; Patscheider, J. Comparison of Al–Si–N nanocomposite coatings deposited by HIPIMS and DC magnetron sputtering. Surf. Coat. Technol. 2013, 232, 680–689. [Google Scholar] [CrossRef]
- Chang, C.-L.; Huang, C.-S. Effect of bias voltage on microstructure, mechanical and wear properties of Al–Si–N coatings deposited by cathodic arc evaporation. Thin Solid Film. 2011, 519, 4923–4927. [Google Scholar] [CrossRef]
- Slack, G.A.; Tanzilli, R.A.; Pohl, R.O.; Vandersande, J.W. The intrinsic thermal conductivity of AIN. J. Phys. Chem. Solids 1987, 48, 641–647. [Google Scholar] [CrossRef]
- Weitzer, F.; Remschnig, K.; Schuster, J.C.; Rogl, P. Phase equilibria and structural chemistry in the ternary systems M–Si–N and M–B–N (M = Al, Cu, Zn, Ag, Cd, In, Sn, Sb, Au, Tl, Pb, Bi). J. Mater. Res. 1990, 5, 2152–2159. [Google Scholar] [CrossRef]
- Rogl, P.; Schuster, C.J. Phase Diagrams of Ternary Boron Nitride and Silicon Nitride Systems; ASM International: Russell Township, OH, USA, 1992. [Google Scholar]
- Taniyasu, Y.; Kasu, M.; Kobayashi, N. Lattice parameters of wurtzite Al1−xSixN ternary alloys. Appl. Phys. Lett. 2001, 79, 4351–4353. [Google Scholar] [CrossRef]
- Pélisson-Schecker, A.; Hug, H.J.; Patscheider, J. Morphology, microstructure evolution and optical properties of Al–Si–N nanocomposite coatings. Surf. Coat. Technol. 2014, 257, 114–120. [Google Scholar] [CrossRef]
- Goyenola, C.; Gueorguiev, G.K.; Stafström, S.; Hultman, L. Fullerene-like CSx: A first-principles study of synthetic growth. Chem. Phys. Lett. 2011, 506, 86–91. [Google Scholar] [CrossRef]
- Sergeev, P.V.; Fedorischeva, M.; Kalashnikov, M.; Bozhko, I.; Voronov, A.; Ribalko, V.E.; Mironov, P.Y. Structural Phase State and Mechanical Properties of the Si-Al-N Coatings with Different Concentration Ratio of Al and Si. Adv. Mater. Res. 2015, 1085, 289–293. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Pignedoli, C.A.; Passerone, D.; Hug, H.J.; Pélisson-Schecker, A.; Patscheider, J. Role of negatively charged defects in the lattice contraction of Al–Si–N. Appl. Phys. Lett. 2010, 96, 071908. [Google Scholar] [CrossRef]
- Choudhary, R.K.; Mishra, S.C.; Mishra, P.; Limaye, P.K.; Singh, K. Mechanical and tribological properties of crystalline aluminum nitride coatings deposited on stainless steel by magnetron sputtering. J. Nucl. Mater. 2015, 466, 69–79. [Google Scholar] [CrossRef]
- Kumar Chakraborty, A. Phase Transformation of Kaolinite Clay; Springer India: New Dehli, India, 2014. [Google Scholar]
- Ko, Y.K.; Jang, J.H.; Lee, S.; Yang, H.J.; Lee, W.H.; Reucroft, P.J.; Lee, J.G. Effects of molybdenum, silver dopants and a titanium substrate layer on copper film metallization. J. Mater. Sci. 2003, 38, 217–222. [Google Scholar] [CrossRef]
- Miyazaki, H.; Kojima, H.; Hinode, K. Passivation effect of silicon nitride against copper diffusion. J. Appl. Phys. 1997, 81, 7746–7750. [Google Scholar] [CrossRef]
- Qin, W.; Mo, Z.Q.; Tang, L.J.; Yu, B.; Wang, S.R.; Xie, J. Effect of ammonia plasma pretreatment on silicon–nitride barriers for Cu metallization systems. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2001, 19, 1942–1947. [Google Scholar] [CrossRef]
- Freund, L.B.; Suresh, S. Thin Film Materials: Stress, Defect Formation and Surface Evolution; Cambridge University Press: Cambridge, UK, 2004; ISBN 978-0-511-75471-5. [Google Scholar]
- Lee, Y.K.; Latt, K.M.; Osipowicz, T.; Sher-Yi, C. Study of diffusion barrier properties of ternary alloy (TixAlyNz) in Cu/TixAlyNz/SiO2/Si thin film structure. Mater. Sci. Semicond. Process. 2000, 3, 191–194. [Google Scholar] [CrossRef]
- Pelisson, A. Al-Si-N Transparent Hard Nanostructured Coatings. Ph.D. Thesis, University of Basel, Basel, Switzerland, 2009; p. 250. [Google Scholar]
- Musil, J. Hard nanocomposite coatings: Thermal stability, oxidation resistance and toughness. Surf. Coat. Technol. 2012, 207, 50–65. [Google Scholar] [CrossRef]

| Coating | H, GPa | E, GPa |
|---|---|---|
| Al0.88Si0.12N | 10.9 ± 1.8 | 135 ± 12 |
| Al0.74Si0.26N | 24.6 ± 2.1 | 228 ± 11 |
| Al0.11Si0.89N | 20.2 ± 2.2 | 180 ± 9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panin, A.; Shugurov, A.; Kazachenok, M.; Sergeev, V. Improvement of Thermal Cycling Resistance of AlxSi1−xN Coatings on Cu Substrates by Optimizing Al/Si Ratio. Materials 2019, 12, 2249. https://doi.org/10.3390/ma12142249
Panin A, Shugurov A, Kazachenok M, Sergeev V. Improvement of Thermal Cycling Resistance of AlxSi1−xN Coatings on Cu Substrates by Optimizing Al/Si Ratio. Materials. 2019; 12(14):2249. https://doi.org/10.3390/ma12142249
Chicago/Turabian StylePanin, Alexey, Artur Shugurov, Marina Kazachenok, and Victor Sergeev. 2019. "Improvement of Thermal Cycling Resistance of AlxSi1−xN Coatings on Cu Substrates by Optimizing Al/Si Ratio" Materials 12, no. 14: 2249. https://doi.org/10.3390/ma12142249
APA StylePanin, A., Shugurov, A., Kazachenok, M., & Sergeev, V. (2019). Improvement of Thermal Cycling Resistance of AlxSi1−xN Coatings on Cu Substrates by Optimizing Al/Si Ratio. Materials, 12(14), 2249. https://doi.org/10.3390/ma12142249