1. Introduction
WC-Co cemented carbide is widely used in cutting, mining, and manufacturing fields, due to its excellent combination of hardness and toughness [
1,
2,
3]. In WC-Co cemented carbide, however, WC grain size strongly affects its mechanical properties. Carbide grain growth inhibitors (GGI), such as VC, NbC, and Cr
3C
2, are usually added to WC-Co systems to restrain the growth of WC grains and improve the hardness and strength [
4,
5,
6,
7,
8]. However, the GGI content must be restricted to small amounts (less than 1%) [
5] because too much will lead to GGI precipitation at the grain boundary and reduce the fracture toughness. In addition, high-temperature performance and corrosion resistance of WC-Co are not good [
9]. The shortage of Co resources and its toxicity to the human body further limit its application [
10].
Ni
3Al-based intermetallics are considered a promising alternative to Co because of their good wettability with WC, high-temperature properties and corrosion resistance [
11]. Li et al. [
12] prepared WC-10 wt.% Ni
3Al cemented carbide with excellent mechanical properties (hardness HV
10 is 17.76 GPA, transverse rupture strength (TRS) is 2092 MPa, and fracture toughness is 21.56 MPa·m
1/2) by using spark plasma sintering (SPS). Long et al. [
13] reported that WC-40 vol.% (Ni
3Al-B) composites could be prepared by the pre-alloy reaction of pure nickel powder, pure aluminum powder and WC powder followed by sintering. The hardness of these composites was 9.7 GPa, the bending strength was 1800 MPa, and the fracture toughness was 18 MPa·m
1/2. Liang et al. [
14] studied the cutting performances of WC-10 wt.% Ni
3Al compared with that of WC-8 wt.% Co. It is demonstrated that WC-10Ni
3Al has higher crater and flank wear resistance than WC-8 wt.% Co, which is attributed to the synergistic mechanism of chemical inertness and superior hardness induced by Ni
3Al binder at high temperature. Liu et al. [
15] also found that WC-8 wt.% Ni
3Al cemented carbide had better wear resistance than WC-8 wt.% Co cemented carbide, because WC-8 wt.% Ni
3Al cemented carbide has higher hardness and Ni
3Al induced subsurface crack resistance. The oxidation resistance of WC-Ni
3Al alloy was improved compared with that of cemented carbide WC-Co, which is due to the formation of a dense oxide layer on the WC-Ni
3Al substrate, and the good combination of the oxide layer with the substrate [
9].
These studies were carried out on WC-Ni3Al cemented carbides using the powder metallurgy (PM) method. Thus, the powder preparation process is crucial, especially the control of oxygen content. However, the powder will inevitably be oxidized in the PM process. The influence of different ball-milling suspension fluids on the oxygen content of WC-Ni3Al cemented carbide has not been reported. In this paper, we will study the effect of two different ball-milling suspensions (ethanol and cyclohexane) on the oxygen content, microstructure, and mechanical properties of WC-Ni3Al cemented carbide.
3. Results and Discussion
Figure 1 shows the XRD patterns of WC-10Ni
3Al composite powders and sintered bulk materials using different ball-milling suspensions. It can be found that the XRD patterns of using two kinds of ball-milling suspensions are the same. The characteristic peaks of the mixed powder were wider and shorter, while the characteristic peaks of the sintered block were narrower and higher, which was due to the grain growth during sintering. In the WC-Co system, according to the Ostwald ripening [
17], WC grain growth can be described as the dissolution of smaller WC particles into binder Co phase during heating. The dissolved WC particles re-precipitated on the surface of the larger undissolved WC particles, which promoted the growth of WC grains. Since both Co and Ni
3Al can dissolve a certain amount of WC near the sintering temperature [
18], the same mechanism should occur in WC-Ni
3Al system. In addition, the results show that the sintered samples are both composed of WC and Ni
3Al.
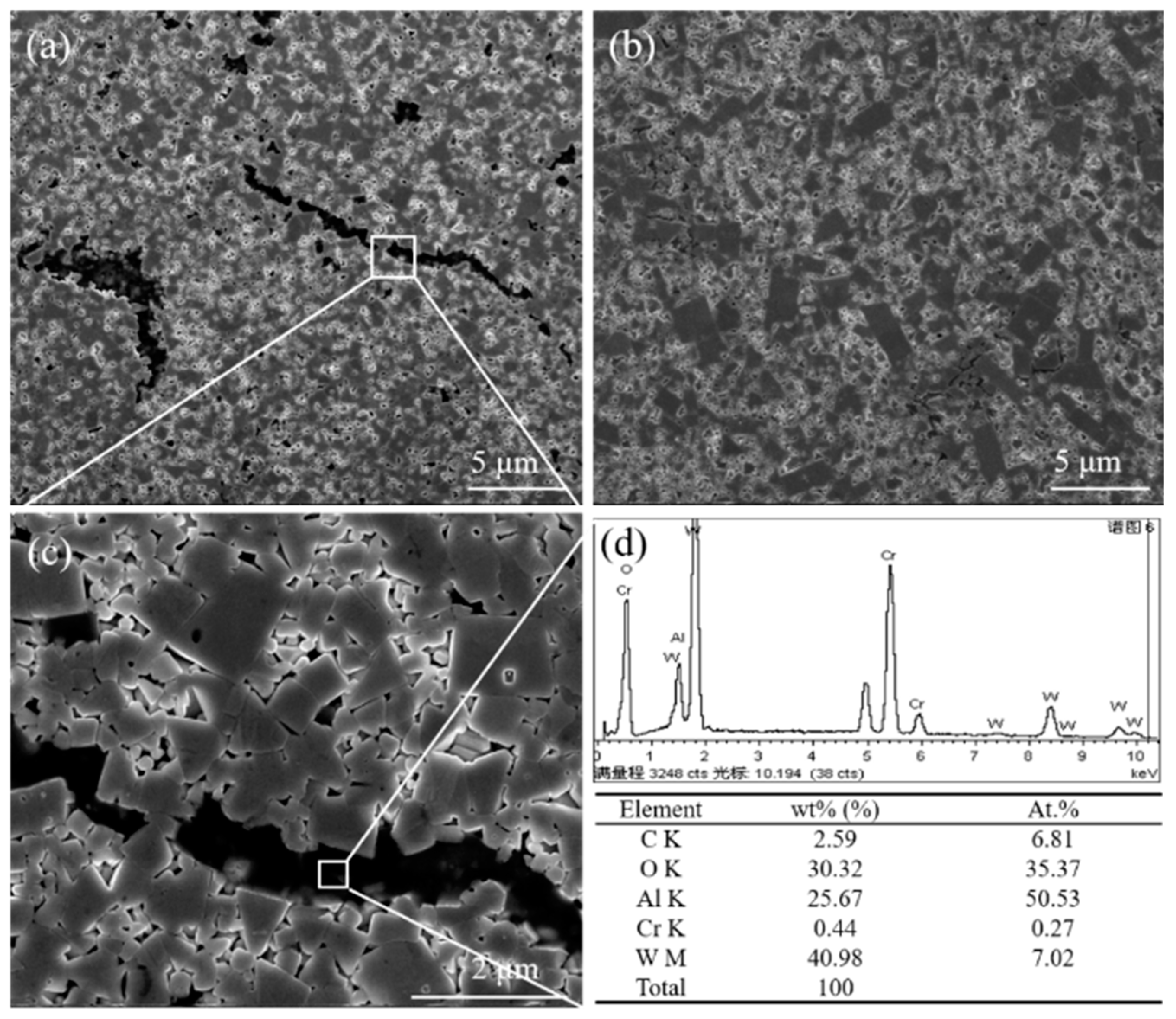
Figure 2 shows the microstructure of WC-10Ni
3Al bulk materials using different ball-milling suspensions. There are noticeable dark regions in the micrographs when CH
3CH
2OH was used as milling suspension.
Figure 2c shows a more detailed micrograph of the boxed region in
Figure 2a, while
Figure 2d is the corresponding energy dispersion X-ray spectroscopy (EDS, X-act one, Oxford, Oxford, UK) analysis. The results show that there was a significant amount of O in the black phase, where the ratio of Al and O atoms was basically in agreement with that of Al
2O
3. We can preliminarily determine that this phase is Al
2O
3. The reason why the existence of Al
2O
3 was not observed in the XRD pattern may be due to the high X-ray absorption coefficient of Al
2O
3 [
19]. Additionally, the Al
2O
3 content in the samples was likely insufficient to be detected by XRD. While in
Figure 2b, there was no black phase distribution, which means that no Al
2O
3 was produced in WC-Ni
3Al cemented carbide while using C
6H
12 as the milling suspension. Therefore, in the mixing process of wet milling, the adsorption of oxygen in the mixed powder is strongly influenced by the organic solvent in the ball-milling suspension. In addition, it should be noted that in the samples containing Al
2O
3, the WC grains were fine and uniform, while using C
6H
12, some grains grew abnormally as plate-like grains.
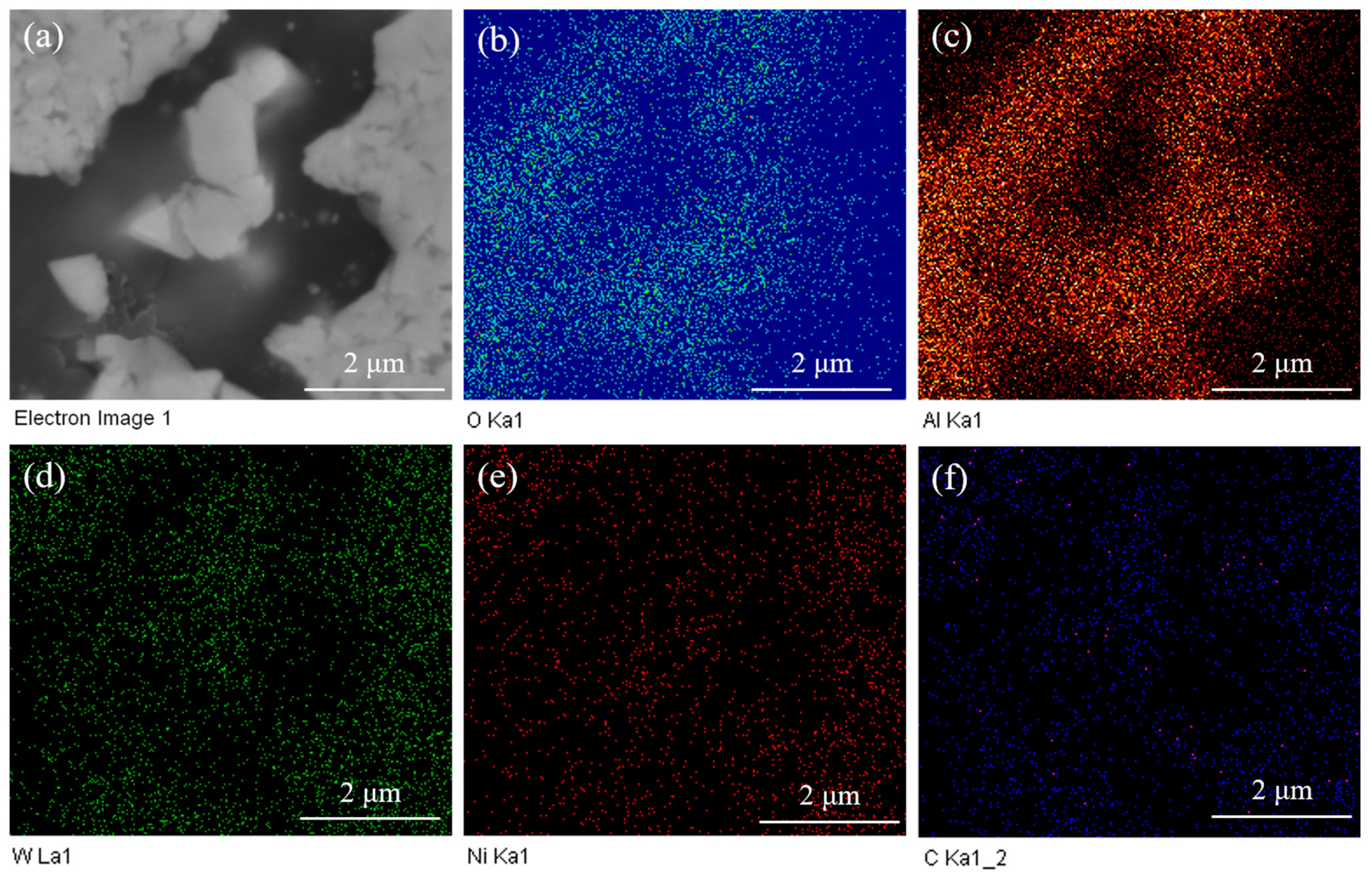
Figure 3 shows the EDS mapping of WC-10Ni
3Al bulk materials using CH
3CH
2OH as ball-milling suspension. The different colors represent the distribution of different elements. The black phase in the electron image was the region where Al and O atoms congregate, W atoms were the opposite, and other elements were evenly distributed. Our results indicate that the black phase is formed by the aggregation of oxide.
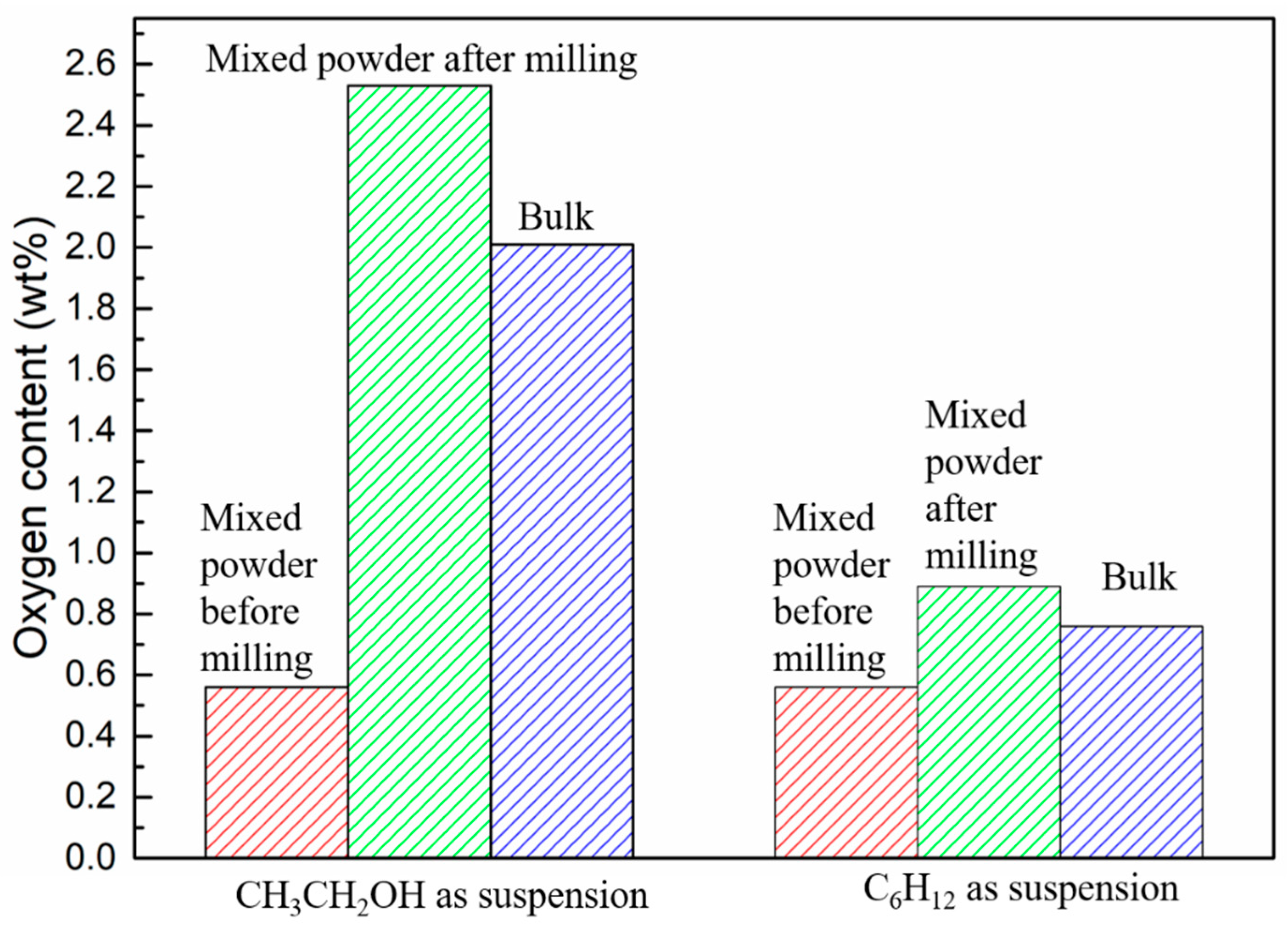
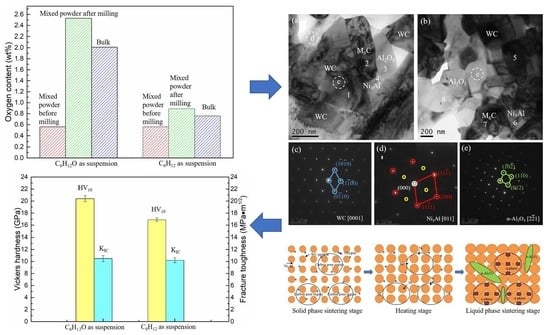
In order to investigate the source of oxygen, the oxygen contents of WC-10Ni
3Al composites before and after ball-milling, and bulk materials were measured. As shown in
Figure 4, there was a certain oxygen content (about 0.5%) in the powder before ball-milling, which should be the oxygen absorbed by the powder in the air or dissolved in the gap between the powder particles. The oxygen content of mixed powder after ball-milling with CH
3CH
2OH was 2.53%, while the oxygen content of mixed powder after ball-milling with C
6H
12 was not significantly increased from 0.56 to 0.89. It is suggested that oxygen atoms in organic solvent CH
3CH
2OH can be adsorbed on the surface of the powder or dissolved in the interspace of the powder particles, resulting in the increase of oxygen content. Although there were no O atoms in C
6H
12 ball-milling suspension, there is a little air in the ball-milling jar. The adsorption of oxygen on the surface of the powder increases with the increase of the specific surface area of the wet-milling powder. In addition, the ball-milling process inevitably leads to the reduction of particle size, and due to surface energy and core-shell constellation, the oxygen content of the powder increases. Therefore, the oxygen in the CH
3CH
2OH ball-milled powder mainly comes from the adsorption and dissolution of O atoms in the organic solvent. In addition, we can see that the oxygen content of the bulk materials decreases after sintering, which indicates that oxygen may participate in the gas reaction and lead to the loss of oxygen content during sintering.
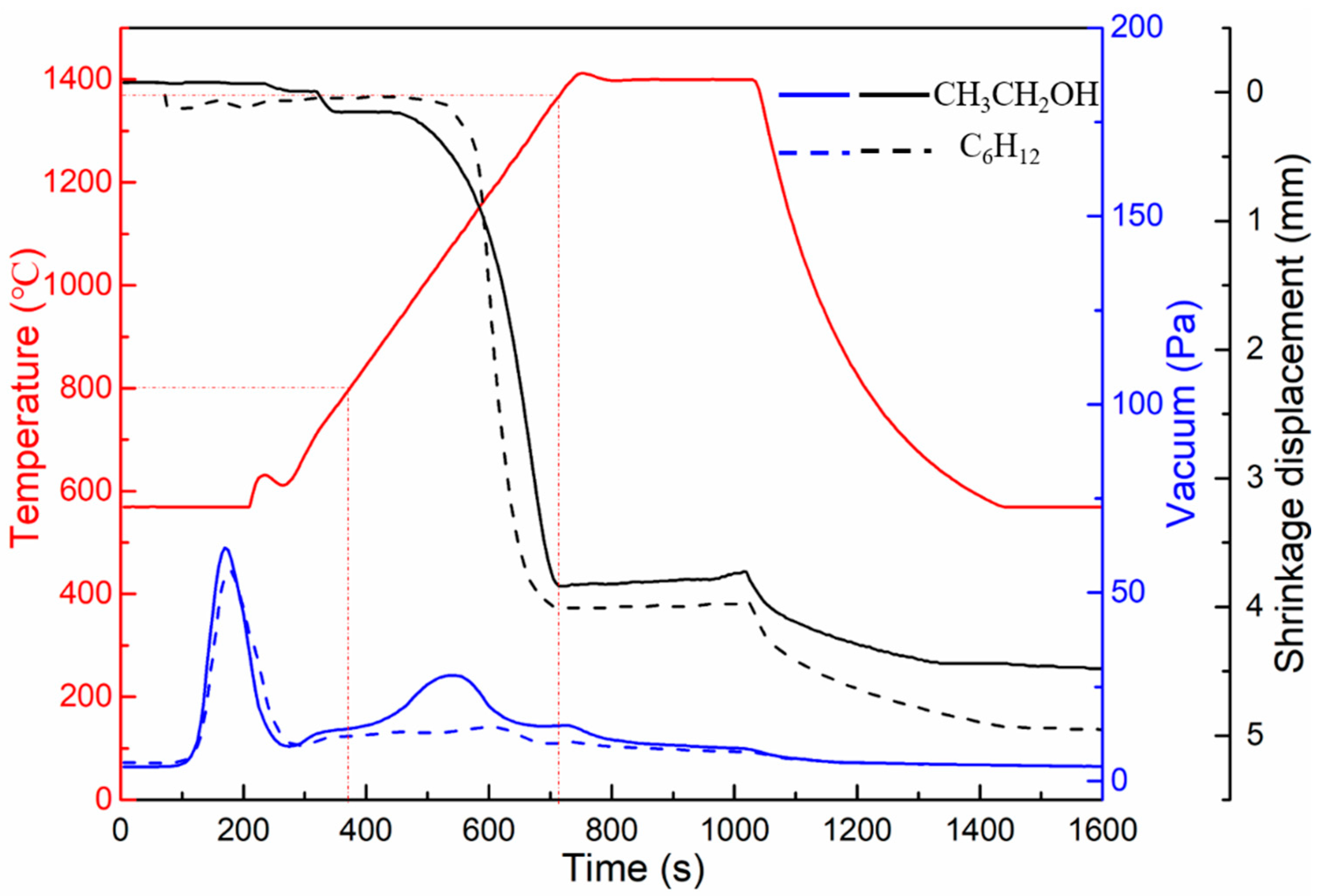
Figure 5 shows the sintering curves of WC-10Ni
3Al composites at 1400 °C for 5 min (including the curves of displacement, vacuum, and temperature with time) in different ball-milling suspensions. Since the temperature range measured by the infrared thermometer was above 570 °C, the temperature appears to be 570 °C before 4 min, and in fact, the temperature rises from room temperature to this temperature during this period. There was a decrease in the vacuum level in both mixed powders at 570 °C, which has been shown to be the gasification process of water and other volatile substances in the powders [
20]. In addition, it can be found that from 800 °C, the vacuum level of the mixed powder using CH
3CH
2OH as the ball-milling suspension decreases with increasing temperature, while the change was not obvious in the mixed powder using C
6H
12 as the ball-milling suspension. This means that gas is produced at this stage in the CH
3CH
2OH milled powders, but not in the C
6H
12 powders. On the basis of the decrease of oxygen content after sintering and the investigation of the Ellingham Diagram [
21], it is suggested that the oxygen adsorbed on the surface of the powder and the dissolved oxygen in the gap between the powder particles may react with carbon to form CO, because the formation free energy of CO is lower than that of CO
2 and the formation temperature of CO is within this temperature range. This explains why the oxygen content of the bulk materials in
Figure 4 is lower than that of the powders. Even though carbon and oxygen were lost from WC-10Ni
3Al as CO gas, the oxygen content in the bulk material milled with CH
3CH
2OH was as high as 2.01%. This remaining oxygen should be mainly in the form of Al
2O
3 oxide, because according to the Ellingham Diagram [
21], the free energy of oxide formation of W, Ni and Cr are high. In addition, according to the shrinkage and displacement of the samples, the samples milled using CH
3CH
2OH start densification at ~800 °C, which is consistent with the temperature at which CO gas is produced. While the samples milled using C
6H
12 start densification at ~1000 °C. Both samples were densified at 1350 °C, so the sintering temperature of 1400 °C was reasonable.
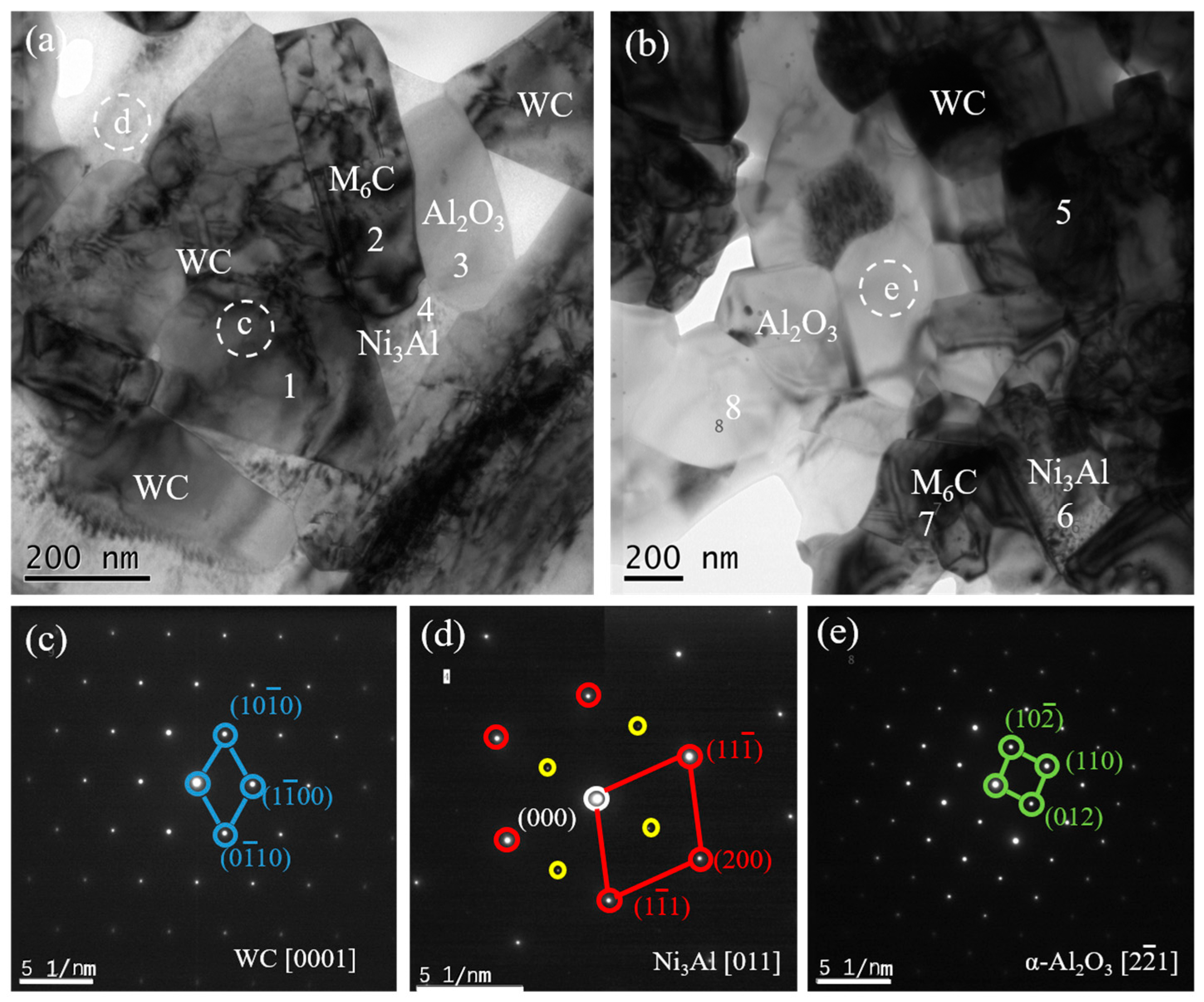
To further confirm the phase composition of WC-10Ni
3Al composites,
Figure 6 shows TEM images of bulk using CH
3CH
2OH as the ball-milling suspension.
Figure 6c,d is the selected area diffraction pattern (SADP) corresponding to the white dotted circles in
Figure 6a,b. After analysis, it can be identified that the black phases with triangular and quadrilateral shape are the WC matrix, and the Ni
3Al phases are distributed among the WC grains. It is worth mentioning that the SADP of Ni
3Al phases has a standard superlattice structure, as shown in
Figure 6d. The white phases in the micrographs are polycrystalline α-Al
2O
3. In addition, EDS was used to analyze the elemental composition of the numbered spots in
Figure 6a,b, as summarized in
Table 1. According to the element atomic ratio, spots 1 and 5 are the WC phase, spots 4 and 6 are the Ni
3Al phase, and spots 3 and 8 are the Al
2O
3 phase. It has been determined that the gas produced during the reaction between oxygen and carbon leads to the carbon deficiency of the matrix. On the other hand, the production of oxide Al
2O
3 must be accompanied by the change of the composition of Ni
3Al phase and the formation of a η-phase (Ni
3W
3C) to maintain the carbon balance. According to EDS results, a small amount of η-phase (Ni
3W
3C) was found around the α-Al
2O
3 phase (spots 2 and 7). The results show that the organic solvent has a great influence on the oxygen content of the WC-Ni
3Al composite, and the absorbed or dissolved oxygen can change the phase composition of the WC-Ni
3Al composite.
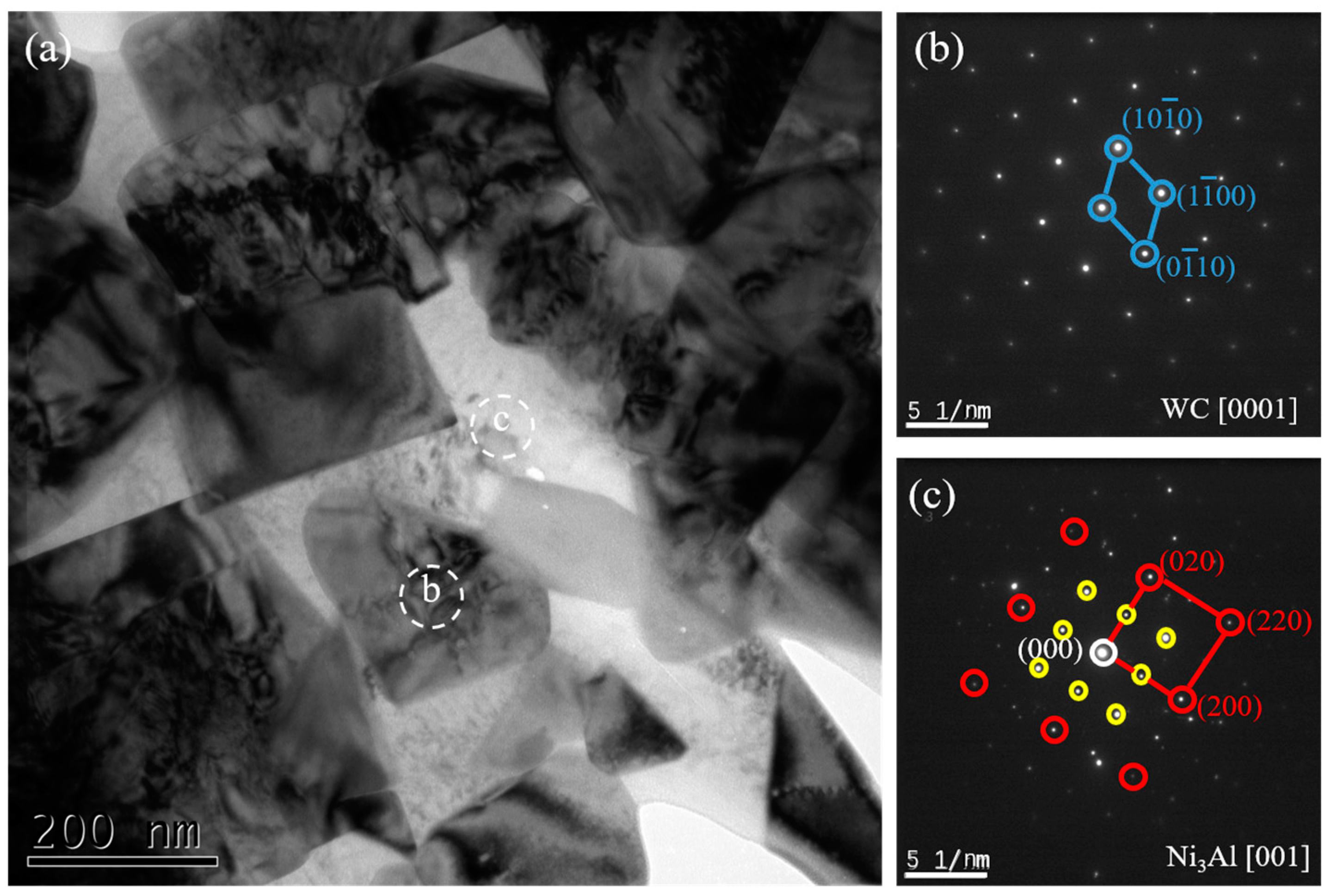
Figure 7 shows TEM images of the WC-10Ni
3Al samples milled by C
6H
12 and the SADP of each phase. Only two phases exist in this sample, WC and Ni
3Al.
Ni
3Al as a bulk material has good oxidation resistance, but it is inevitable that oxygen will be doped in the WC-Ni
3Al composites prepared by the traditional powder metallurgy method. The oxygen in the organic solvent CH
3CH
2OH is easily adsorbed in the mixed powder. The oxygen content of the ball-milling powder is obviously higher than that of the original powder, which indicates that the adsorption of oxygen on the surface of the powder increases with the increase of the specific surface area of the wet-milling powder. In the samples using CH
3CH
2OH as ball-milling suspension, the main relationship between oxygen content and microstructure is the formation of α-Al
2O
3 and the change of Ni
3Al composition.
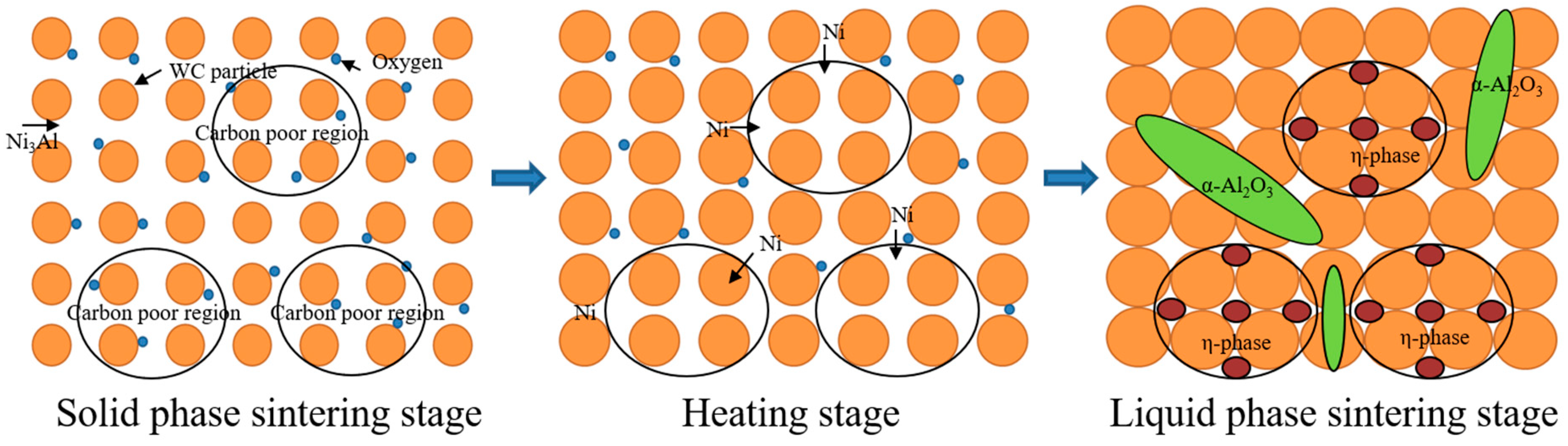
Figure 8 shows a schematic diagram of η-phase and α-Al
2O
3 formation in CH
3CH
2OH ball-milled WC-Ni
3Al composites. Some oxides and carbides are produced by sintering WC-10Ni
3Al mixed powders in the presence of a large amount of oxygen. The decrease of oxygen content after sintering and the decrease of vacuum level during sintering proved that the formation of CO and the loss of carbon lead to a reduction of carbon in the matrix. In the process of liquid phase sintering, the formation of α-Al
2O
3 inevitably leads to changes in the Ni
3Al composition. On the other hand, the diffusion of Ni to the carbon poor regions and the formation of a carbon-poor phase maintain the carbon balance in the material. The η-phase (Ni
3W
3C) and α-Al
2O
3 in CH
3CH
2OH ball-milled WC-Ni
3Al composites were confirmed by TEM and EDS analysis.
In addition, in WC-Co system, the Co
3W
3C produced by lack of carbon is the main factor to restrain WC grain growth, so the controlling factor of WC grain growth rate depends on carbon content [
22]. When the carbon content is low and the η-phase is formed, the self-diffusion of carbon is the controlling factor for the rate of dissolution and precipitation of W and C into WC. In the same way, the formation of the η-phase (Ni
3W
3C) in WC-Ni
3Al composites is also the reason for inhibiting grain growth. As shown in
Figure 2a,b, the grain size of CH
3CH
2OH ball-milled samples is fine and uniform, while there is some abnormal grain growth in the C
6H
12 ball-milled samples. These results show that control of oxygen content is an important factor in the preparation of WC-Ni
3Al composites with controllable structure.
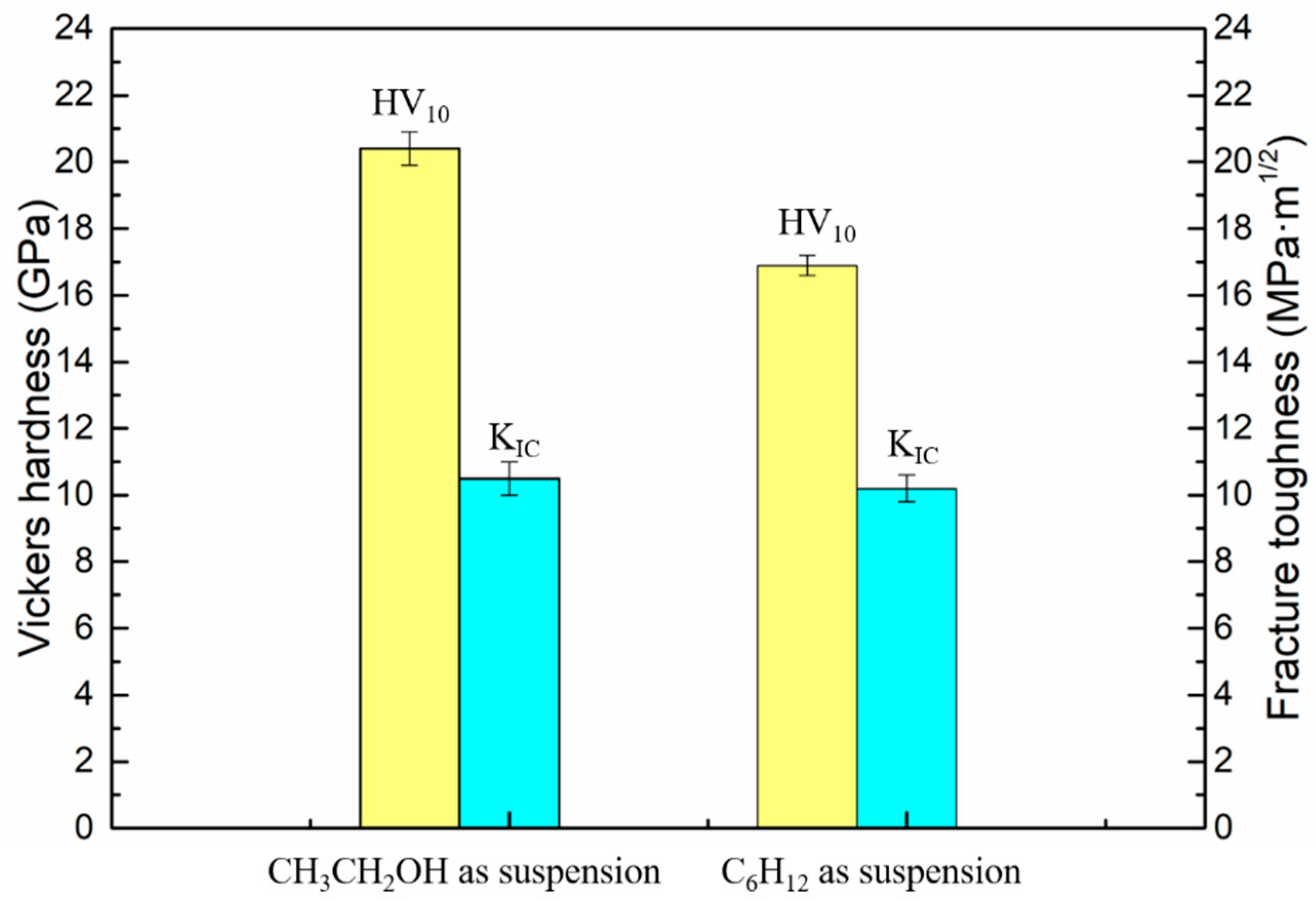
Figure 9 shows the hardness and fracture toughness of WC-Ni
3Al composites using two different ball-milling suspensions. The hardness of CH
3CH
2OH ball-milled samples (20.40 GPa) is higher than that of C
6H
12 ball-milled samples (16.89 GPa). This is because the WC-Ni
3Al composite conforms to the Hall-Petch relationship in the range of submicron grains, and the decrease in grain size leads to the increase of hardness. It is interesting, however, that there is little difference in fracture toughness, 10.5 Mpa·m
1/2, and 10.2 Mpa·m
1/2, respectively.
In order to understand the toughening mechanism of WC-Ni
3Al composites using two different ball-milling suspensions, the Vickers hardness indentation crack on the surface of WC-Ni
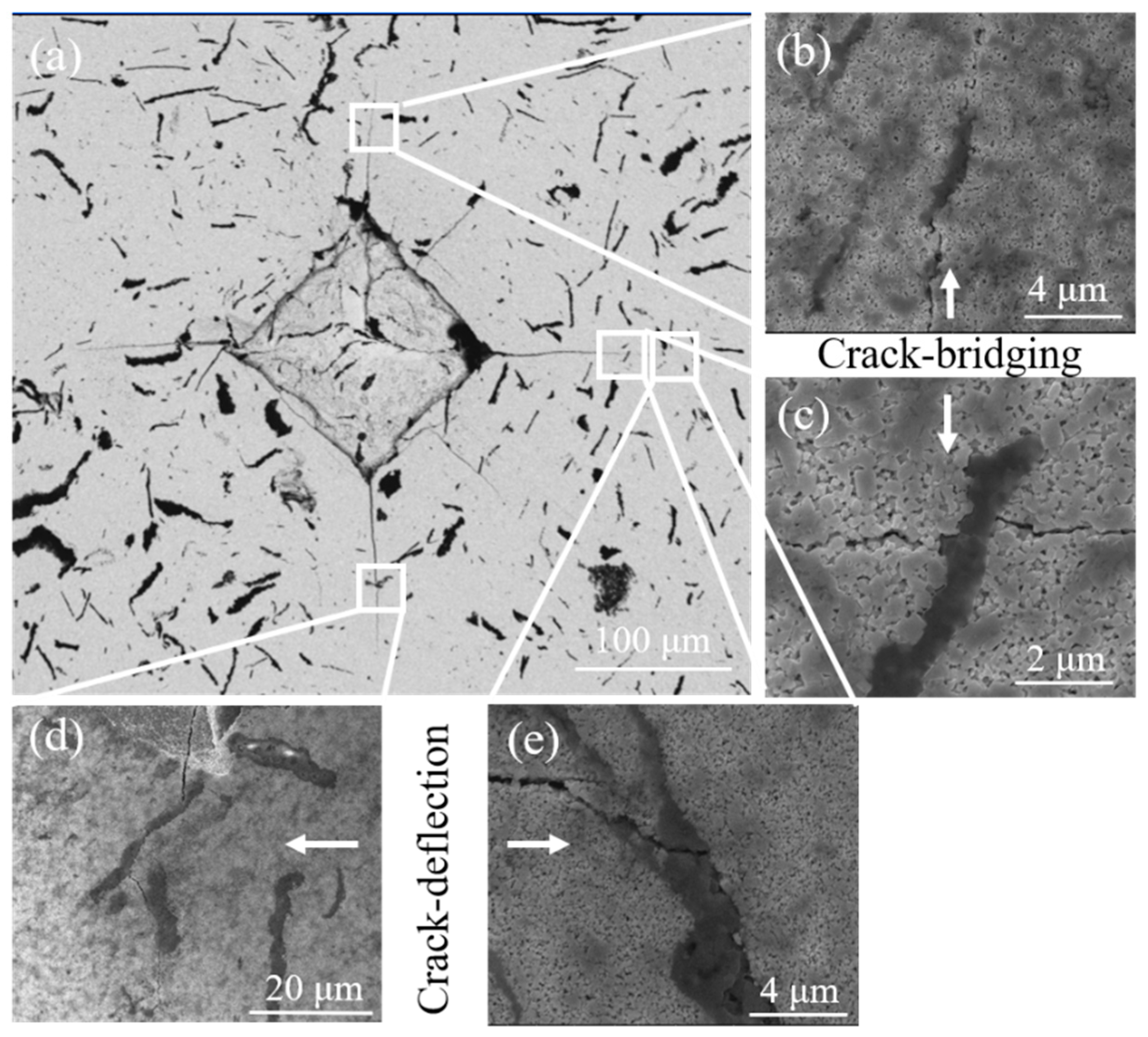
3Al composites were observed. Generally speaking, the crack propagation path in WC-based cemented carbide can be divided into intergranular and transgranular. When the grain size is small, the intergranular fracture is formed at the WC/WC grain boundary and the WC/binder interface. When the grain size is large, there will be a transgranular fracture in the large grains. As shown in
Figure 10, it can be found that intergranular fracture is the dominant mechanism in CH
3CH
2OH ball-milled samples with small grain size. It should be noted that α-Al
2O
3 can deflect and bridge the crack growth and increase the resistance to crack growth. This explains why the grain size of CH
3CH
2OH ball-milled samples is smaller, but the toughness does not decrease.
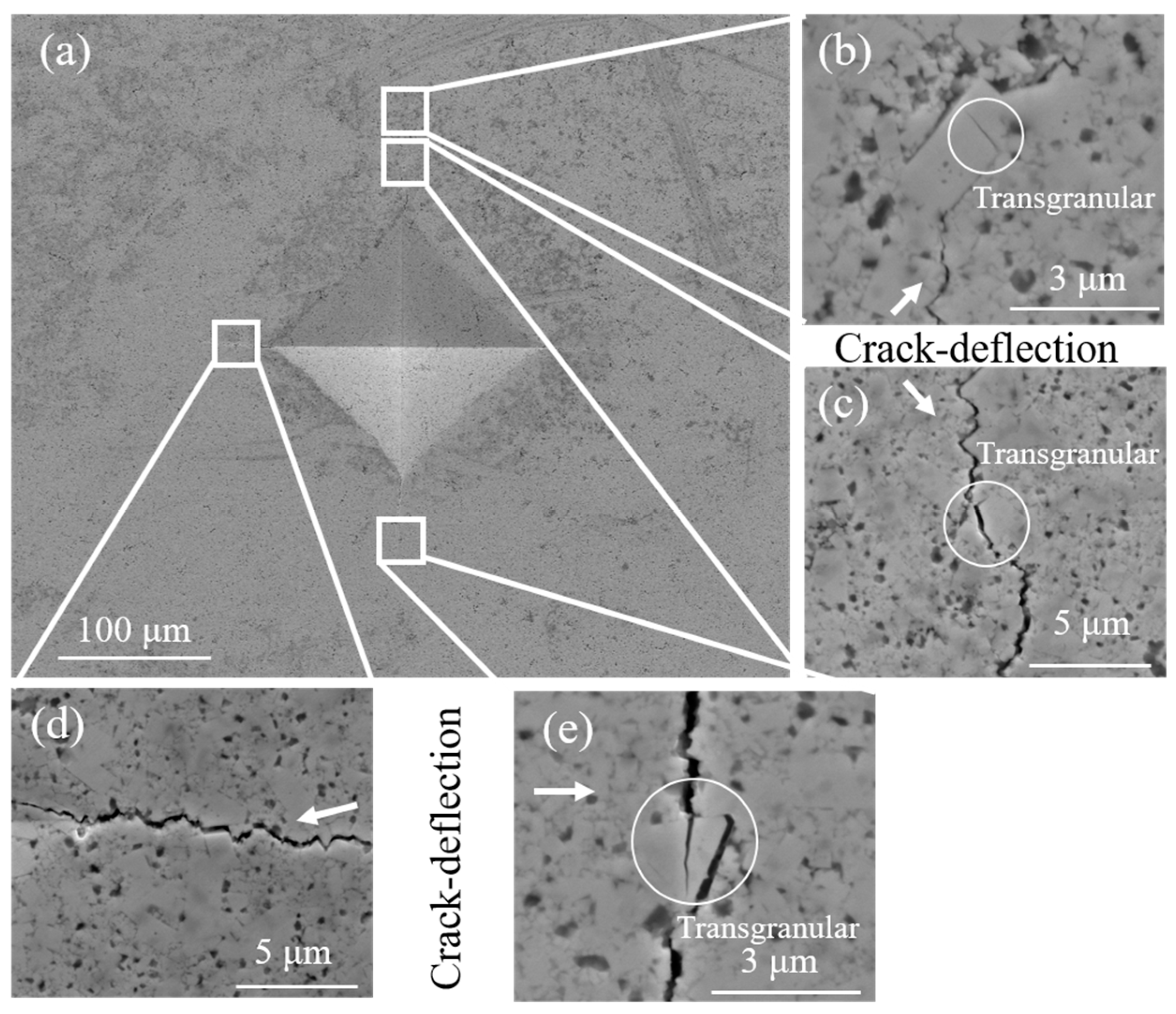
Figure 11 is the micrograph of crack propagation in C
6H
12 ball-milled sample. It can be seen that the abnormally grown plate-like grains have the same deflection effect on the crack, even when the crack passes through the plate-like grains. When large grains are encountered, deflection and transmigration are more likely to occur simultaneously, as shown in
Figure 11e. It should be noted that more energy is needed to form a transgranular crack, which hinders crack propagation. Therefore, the toughness of C
6H
12 ball-milled samples is better due to the existence of plate-like crystals.