Reactive Melt Mixing of Poly(3-Hydroxybutyrate)/Rice Husk Flour Composites with Purified Biosustainably Produced Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of RHF
2.3. Melt Mixing
2.4. Characterization
2.4.1. Morphology
2.4.2. Transparency
2.4.3. Color Measurements
2.4.4. Thermal Analysis
2.4.5. Mechanical Tests
2.4.6. Permeability Tests
2.4.7. Statistical Analysis
3. Results
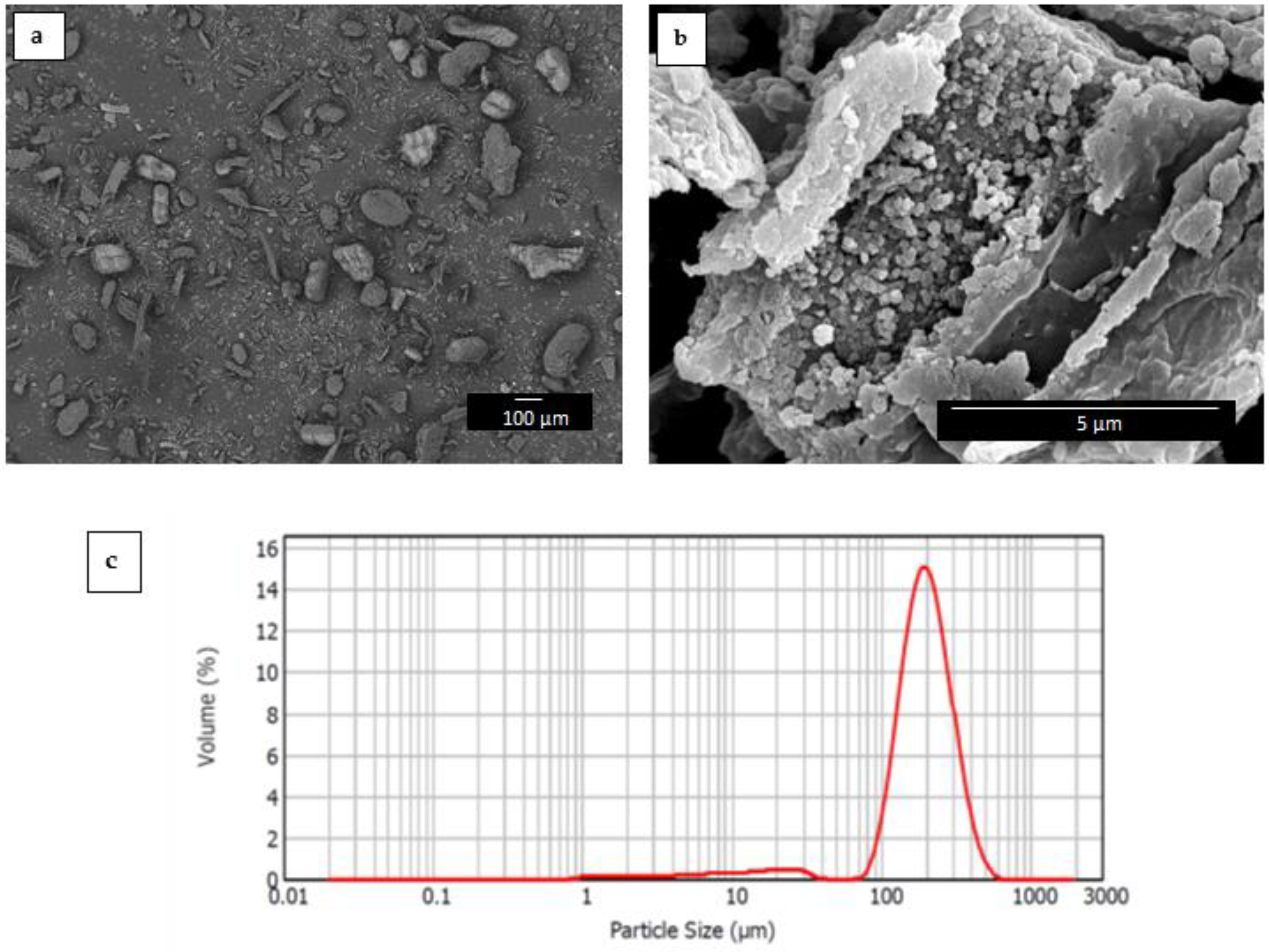
3.1. Morphology of RHF Particles
3.2. Morphology of PHB/PHBV/RHF Films
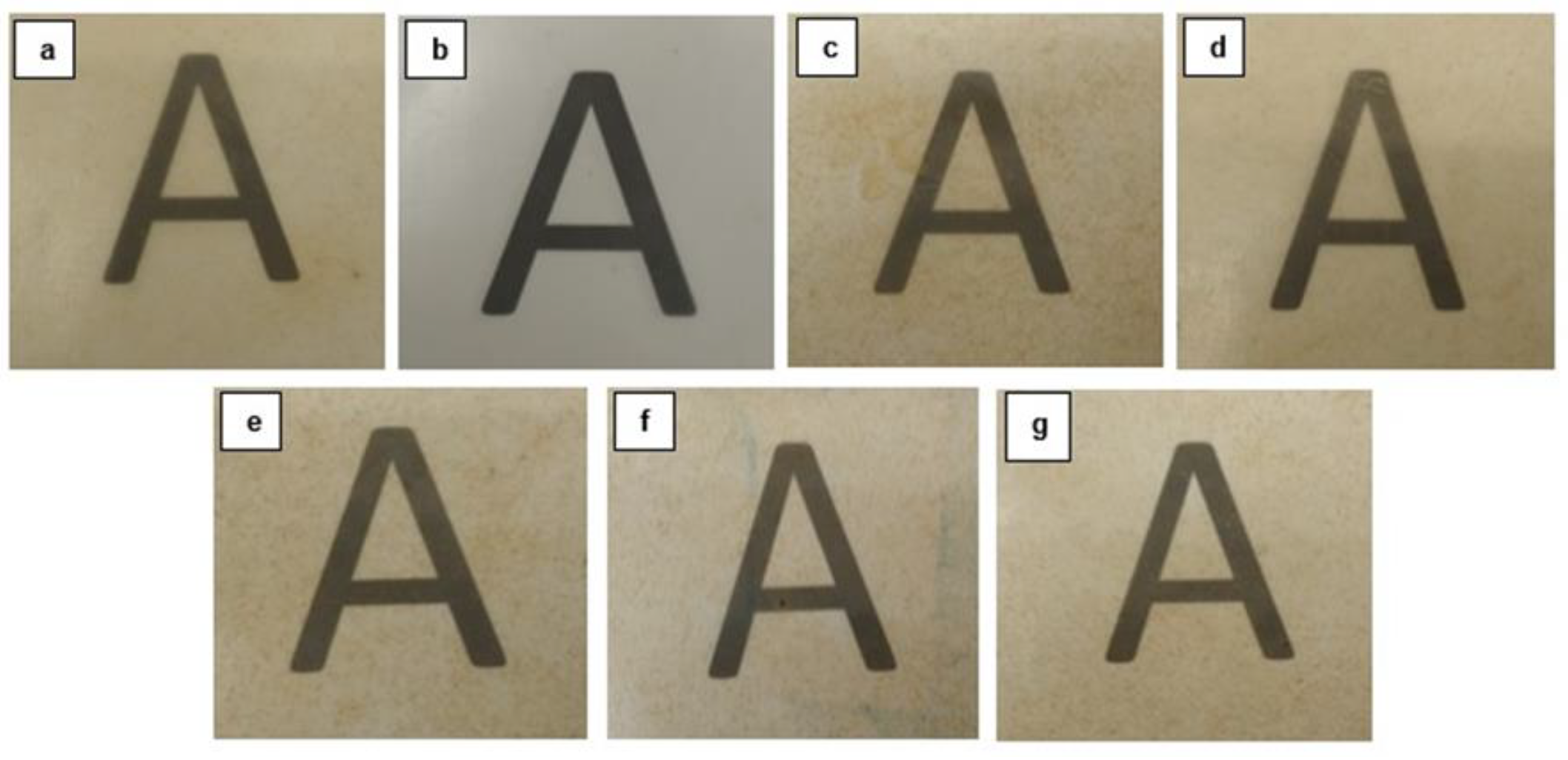
3.3. Optical Properties of PHB/PHBV/RHF Films
3.4. Crystallinity of PHB/PHBV/RHF Films
3.5. Thermal Stability of PHB/PHBV/RHF Films
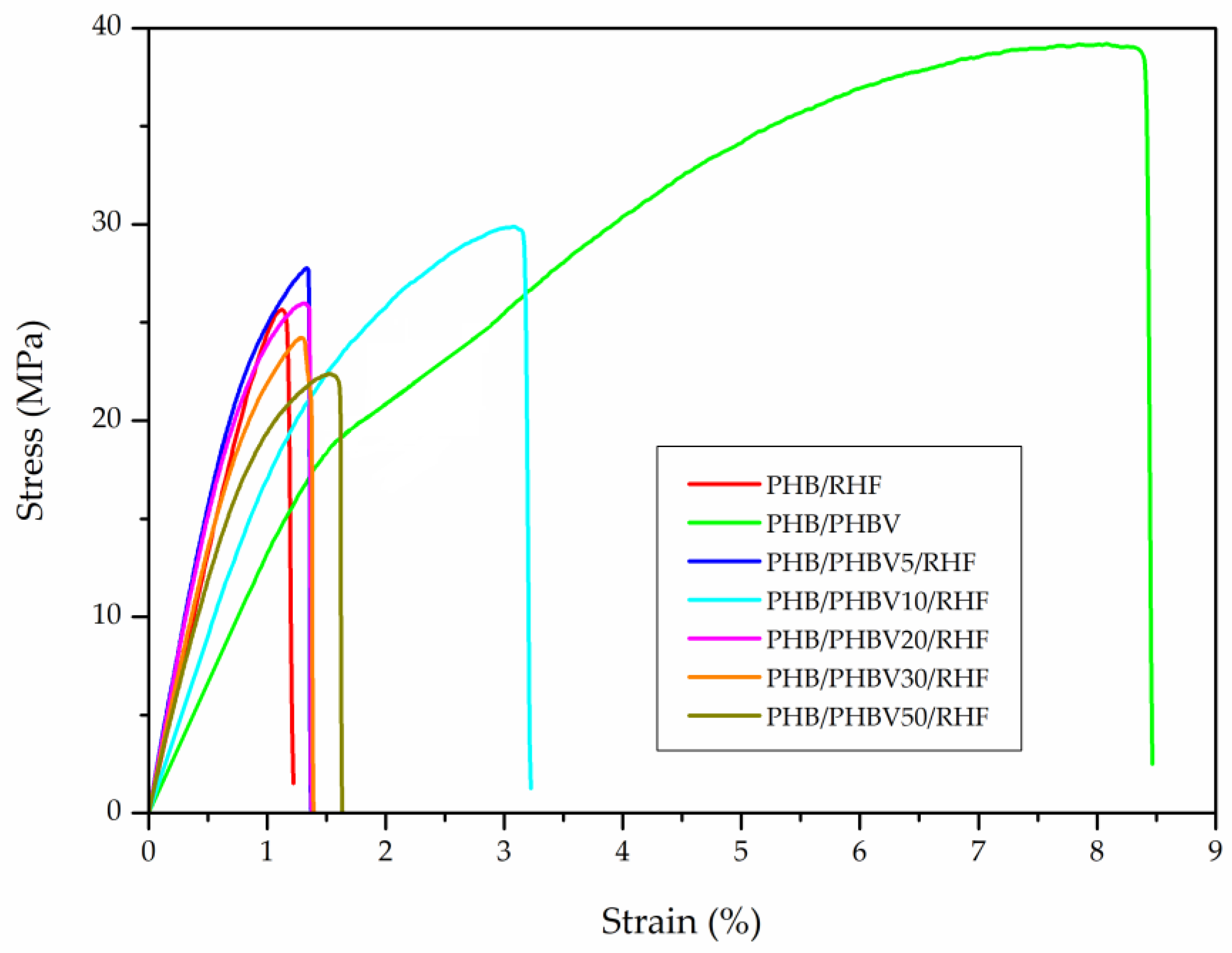
3.6. Mechanical Properties of PHB/PHBV/RHF Films
3.7. Barrier Performance of PHB/PHBV/RHF Films
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rehm, B.H.A. Polyester synthases: Natural catalysts for plastics. Biochem. J. 2003, 376, 15–33. [Google Scholar] [CrossRef]
- Alaerts, L.; Augustinus, M.; VanAcker, K. Impact of bio-based plastics on current recycling of plastics. Sustainability 2018, 10, 1487. [Google Scholar] [CrossRef]
- Smith, R. Biodegradable Polymers for Industrial Applications; Woodhead: Lisle, IL, USA, 2005. [Google Scholar]
- Cava, D.; Giménez, E.; Gavara, R.; Lagaron, J.M. Comparative performance and barrier properties of biodegradable thermoplastics and nanobiocomposites versus PET for food packaging applications. J. Plast. Film. Sheeting 2006, 22, 265–274. [Google Scholar] [CrossRef]
- Reis, K.; Pereira, J.; Smith, A.; Carvalho, C.; Wellner, N.; Yakimets, I. Characterization of polyhydroxybutyrate-hydroxyvalerate (PHB-HV)/maize starch blend films. J. Food Eng. 2008, 89, 361–369. [Google Scholar] [CrossRef]
- Nduko, J.M.; Matsumoto, K.; Taguchi, S. Biological lactate-polymers synthesized by one-pot microbial factory: Enzyme and metabolic engineering. Biobased Monomers Polym. Mater. 2012, 1105, 213–235. [Google Scholar]
- Philip, S.; Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Biodegradable polymers with a range of applications. J. Chem. Technol. Biotechnol. 2007, 82, 233–247. [Google Scholar] [CrossRef]
- Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Bioplastics with a green agenda. Curr. Opin. Microbiol. 2010, 13, 321–326. [Google Scholar] [CrossRef]
- Blunt, W.; Levin, D.B.; Cicek, N. Bioreactor Operating Strategies for Improved Polyhydroxyalkanoate (PHA) Productivity. Polymers 2018, 10, 1197. [Google Scholar] [CrossRef]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A. Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef]
- Jacquel, N.; Lo, C.W.; Wu, H.S.; Wei, Y.H.; Wang, S.S. Solubility of Solubility of polyhydroxyalkanoates by experiment and thermodynamic correlations. AIChE J. 2007, 53, 2704–2714. [Google Scholar] [CrossRef]
- Domingos, J.M.; Puccio, S.; Martinez, G.A.; Amaral, N.; Reis, M.A.; Bandini, S.; Fava, F.; Bertin, L. Cheese whey integrated valorisation: Production, concentration and exploitation of carboxylic acids for the production of polyhydroxyalkanoates by a fed-batch culture. Chem. Eng. J. 2018, 336, 47–53. [Google Scholar] [CrossRef]
- Samorì, C.; Abbondanzi, F.; Galletti, P.; Giorgini, L.; Mazzocchetti, L.; Torri, C.; Tagliavini, E. Extraction of polyhydroxyalkanoates from mixed microbial cultures: Impact on polymer quality and recovery. Bioresour. Technol. 2015, 189, 195–202. [Google Scholar] [CrossRef]
- Lee, S.Y. Plastic bacteria? Progress and prospects for polyhydroxyalkanoate production in bacteria. Trends Biotechnol. 1996, 14, 431–438. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Montanes, N.; Fombuena, V.; Boronat, T.; Sanchez-Nacher, L. Preparation and characterization of compression-molded green composite sheets made of poly(3-hydroxybutyrate) reinforced with long pita fibers. Adv. Polym. Technol. 2018, 37, 1305–1315. [Google Scholar] [CrossRef]
- Zini, E.; Scandola, M. Green composites: An overview. Polym. Compos. 2011, 32, 1905–1915. [Google Scholar] [CrossRef]
- Saheb, D.N.; Jog, J.P. Natural fiber polymer composites: A review. Adv. Polym. Technol. 1999, 18, 351–363. [Google Scholar] [CrossRef]
- Joshi, S.V.; Drzal, L.T.; Mohanty, A.K.; Arora, S. Are natural fiber composites environmentally superior to glass fiber reinforced composites? Compos. Part A Appl. Sci. Manuf. 2004, 35, 371–376. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Morreale, M. Green composites: A brief review. Compos. Part A Appl. Sci. Manuf. 2011, 42, 579–588. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Bhat, A.H.; Yusra, A.F.I. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Ndazi, B.S.; Karlsson, S. Characterization of hydrolytic degradation of polylactic acid/rice hulls composites in water at different temperatures. Express Polym. Lett. 2011, 5, 119–131. [Google Scholar] [CrossRef]
- Yussuf, A.A.; Massoumi, I.; Hassan, A. Comparison of polylactic acid/kenaf and polylactic acid/rice husk composites: The influence of the natural fibers on the mechanical, thermal and biodegradability properties. J. Polym. Environ. 2010, 18, 422–429. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Garcia-Garcia, D.; Carbonell-Verdu, A.; Balart, R.; Torres-Giner, S. Effect of different compatibilizers on injection-molded green composite pieces based on polylactide filled with almond shell flour. Compos. Part B Eng. 2018, 147, 76–85. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Sammon, C.; Balart, R.; Torres-Giner, S. Compatibilization of highly sustainable polylactide/almond shell flour composites by reactive extrusion with maleinized linseed oil. Ind. Crop. Prod. 2018, 111, 878–888. [Google Scholar] [CrossRef]
- Liminana, P.; García-Sanoguera, D.; Quiles-Carrillo, L.; Balart, R.; Montanes, N. Development and characterization of environmentally friendly composites from poly(butylene succinate) (PBS) and almond shell flour with different compatibilizers. Compos. Part B Eng. 2018, 144, 153–162. [Google Scholar] [CrossRef]
- Montava-Jordà, S.; Quiles-Carrillo, L.; Richart, N.; Torres-Giner, S.; Montanes, N. Enhanced Interfacial Adhesion of Polylactide/Poly(ε-caprolactone)/Walnut Shell Flour Composites by Reactive Extrusion with Maleinized Linseed Oil. Polymers 2019, 11, 758. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Carbonell-Verdu, A.; Jordá-Vilaplana, A.; Balart, R.; Garcia-Sanoguera, D.; Garcia-Garcia, D.; Carbonell-Verdu, A.; Jordá-Vilaplana, A.; Garcia-Sanoguera, D. Development and characterization of green composites from bio-based polyethylene and peanut shell. J. Appl. Polym. Sci. 2016, 133, 43940. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Hilliou, L.; Melendez-Rodriguez, B.; Figueroa-Lopez, K.; Madalena, D.; Cabedo, L.; Covas, J.; Vicente, A.; Lagaron, J. Melt processability, characterization, and antibacterial activity of compression-molded green composite sheets made of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) reinforced with coconut fibers impregnated with oregano essential oil. Food Packag. Shelf Life 2018, 17, 39–49. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Lagaron, J.M.; Balart, R.; Torres-Giner, S. On the use of acrylated epoxidized soybean oil as a reactive compatibilizer in injection-molded compostable pieces consisting of polylactide filled with orange peel flour. Polym. Int. 2018, 67, 1341–1351. [Google Scholar] [CrossRef]
- Montava-Jordà, S.; Torres-Giner, S.; Ferrandiz-Bou, S.; Quiles-Carrillo, L.; Montanes, N. Development of Sustainable and Cost-Competitive Injection-Molded Pieces of Partially Bio-Based Polyethylene Terephthalate through the Valorization of Cotton Textile Waste. Int. J. Mol. Sci. 2019, 20, 1378. [Google Scholar] [CrossRef]
- Ferrero, B.; Fombuena, V.; Fenollar, O.; Boronat, T.; Balart, R. Development of natural fiber-reinforced plastics (NFRP) based on biobased polyethylene and waste fibers from Posidonia oceanica seaweed. Polym. Compos. 2015, 36, 1378–1385. [Google Scholar] [CrossRef]
- Aprianti, E.; Shafigh, P.; Bahri, S.; Farahani, J.N. Supplementary cementitious materials origin from agricultural wastes—A review. Constr. Build. Mater. 2015, 74, 176–187. [Google Scholar] [CrossRef]
- Adam, F.; Appaturi, J.N.; Iqbal, A. The utilization of rice husk silica as a catalyst: Review and recent progress. Catal. Today 2012, 190, 2–14. [Google Scholar] [CrossRef]
- Adam, F.; Kandasamy, K.; Balakrishnan, S. Iron incorporated heterogeneous catalyst from rice husk ash. J. Colloid Interface Sci. 2006, 304, 137–143. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, B.; Quan, H.; Yam, R.C.; Yuen, R.K.; Li, R.K.; Yuen, K.K.R. Flame retardancy of rice husk-filled high-density polyethylene ecocomposites. Compos. Sci. Technol. 2009, 69, 2675–2681. [Google Scholar] [CrossRef]
- Panthapulakkal, S.; Law, S.; Sain, M. Enhancement of processability of rice husk filled high-density polyethylene composite profiles. J. Thermoplast. Compos. Mater. 2005, 18, 445–458. [Google Scholar] [CrossRef]
- Nascimento, G.; Cechinel, D.; Piletti, R.; Mendes, E.; Paula, M.; Riella, H.G.; Fiori, M. Effect of Different Concentrations and Sizes of Particles of Rice Husk Ash-RHS in the Mechanical Properties of Polypropylene. Mater. Sci. Forum 2010, 660, 23–28. [Google Scholar] [CrossRef]
- Verheyen, S.; Blaton, N.; Kinget, R.; Kim, H.-S. Thermogravimetric analysis of rice husk flour filled thermoplastic polymer composites. J. Therm. Anal. Calorim. 2004, 76, 395–404. [Google Scholar] [CrossRef]
- Battegazzore, D.; Bocchini, S.; Alongi, J.; Frache, A.; Marino, F. Cellulose extracted from rice husk as filler for poly(lactic acid): Preparation and characterization. Cellulose 2014, 21, 1813–1821. [Google Scholar] [CrossRef]
- Bertini, F.; Canetti, M.; Cacciamani, A.; Elegir, G.; Orlandi, M.; Zoia, L. Effect of ligno-derivatives on thermal properties and degradation behavior of poly(3-hydroxybutyrate)-based biocomposites. Polym. Degrad. Stab. 2012, 97, 1979–1987. [Google Scholar] [CrossRef]
- Boitt, A.P.W.; Barcellos, I.O.; Alberti, L.D.; Bucci, D.Z. Evaluation of the Influence of the Use of Waste from the Processing of Rice in Physicochemical Properties and Biodegradability of PHB in Composites. Polimeros 2014, 24, 640–645. [Google Scholar] [CrossRef]
- Moura, A.; Bolba, C.; Demori, R.; Lima, L.P.F.C.; Santana, R.M. Effect of Rice Husk Treatment with Hot Water on Mechanical Performance in Poly(hydroxybutyrate)/Rice Husk Biocomposite. J. Polym. Environ. 2018, 26, 2632–2639. [Google Scholar] [CrossRef]
- Sánchez-Safont, E.L.; Aldureid, A.; Lagarón, J.M.; Gamez-Perez, J.; Cabedo, L. Biocomposites of different lignocellulosic wastes for sustainable food packaging applications. Compos. Part B Eng. 2018, 145, 215–225. [Google Scholar] [CrossRef]
- Borah, J.S.; Kim, D.S. Recent development in thermoplastic/wood composites and nanocomposites: A review. Korean J. Chem. Eng. 2016, 33, 3035–3049. [Google Scholar] [CrossRef]
- Rowell, R.M.; Young, R.A.; Rowell, J.K.I. Paper and Composites from Agro-Based Resources; CRC press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Lu, J.Z.; Wu, Q.; McNabb, H.S. Chemical coupling in wood fiber and polymer composites: A review of coupling agents and treatments. Wood Fiber Sci. 2000, 32, 88–104. [Google Scholar]
- George, J.; Sreekala, M.S.; Thomas, S. A review on interface modification and characterization of natural fiber reinforced plastic composites. Polym. Eng. Sci. 2001, 41, 1471–1485. [Google Scholar] [CrossRef]
- Chan, C.M.; Vandi, L.-J.; Pratt, S.; Halley, P.; Richardson, D.; Werker, A.; Laycock, B. Mechanical properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/wood flour composites: Effect of interface modifiers. J. Appl. Polym. Sci. 2018, 135, 46828. [Google Scholar] [CrossRef]
- Hao, M.; Wu, H. Effect of in situ reactive interfacial compatibilization on structure and properties of polylactide/sisal fiber biocomposites. Polym. Compos. 2018, 39, E174–E187. [Google Scholar] [CrossRef]
- Dhavalikar, R.; Xanthos, M. Parameters affecting the chain extension and branching of PET in the melt state by polyepoxides. J. Appl. Polym. Sci. 2003, 87, 643–652. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Jorda-Vilaplana, A.; Balart, R.; Torres-Giner, S. A comparative study on the effect of different reactive compatibilizers on injection-molded pieces of bio-based high-density polyethylene/polylactide blends. J. Appl. Polym. Sci. 2019, 136, 47396. [Google Scholar] [CrossRef]
- Wei, L.; McDonald, A.G.; Stark, N.M. Grafting of bacterial polyhydroxybutyrate (PHB) onto cellulose via in situ reactive extrusion with dicumyl peroxide. Biomacromolecules 2015, 16, 1040–1049. [Google Scholar] [CrossRef]
- Ahmad, E.E.M.; Luyt, A.S. Effects of organic peroxide and polymer chain structure on mechanical and dynamic mechanical properties of sisal fiber reinforced polyethylene composites. J. Appl. Polym. Sci. 2012, 125, 2216–2222. [Google Scholar] [CrossRef]
- Nogellova, Z.; Kokta, B.V.; Chodak, I. A composite LDPE/wood flour crosslinked by peroxide. J. Macromol. Sci. Part A 1998, 35, 1069–1077. [Google Scholar] [CrossRef]
- Gu, R.; Sain, M.; Kokta, B.V. Evaluation of wood composite additives in the mechanical property changes of PE blends. Polym. Compos. 2015, 36, 287–293. [Google Scholar] [CrossRef]
- Joseph, K.; Thomas, S.; Pavithran, C. Effect of chemical treatment on the tensile properties of short sisal fibre-reinforced polyethylene composites. Polymer 1996, 37, 5139–5149. [Google Scholar] [CrossRef]
- Mokoena, M.A.; Djoković, V.; Luyt, A.S. Composites of linear low density polyethylene and short sisal fibres: The effects of peroxide treatment. J. Mater. Sci. 2004, 39, 3403–3412. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Castro-Mayorga, J.L.; Reis, M.A.M.; Sammon, C.; Cabedo, L.; Torres-Giner, S.; Lagaron, J.M. Preparation and characterization of electrospun food biopackaging films of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) derived from fruit pulp biowaste. Front. Sustain. Food Syst. 2018, 2, 38. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.; Andrade-Mahecha, M.; Torres-Vargas, O. Development of antimicrobial biocomposite films to preserve the quality of bread. Molecules 2018, 23, 212. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Rao, M.; Chawla, S.; Sharma, A. Active chitosan–polyvinyl alcohol films with natural extracts. Food Hydrocoll. 2012, 29, 290–297. [Google Scholar] [CrossRef]
- Scaglioni, P.T.; Badiale-Furlong, E. Rice husk as an adsorbent: A new analytical approach to determine aflatoxins in milk. Talanta 2016, 152, 423–431. [Google Scholar] [CrossRef]
- Schneider, L.T.; Bonassa, G.; Alves, H.J.; Meier, T.R.W.; Frigo, E.P.; Teleken, J.G. Use of rice husk in waste cooking oil pretreatment. Environ. Technol. 2019, 40, 594–604. [Google Scholar] [CrossRef]
- Rosa, S.M.L.; Santos, E.F.; Ferreira, C.A.; Nachtigall, S.M.B. Studies on the properties of rice-husk-filled-PP composites: Effect of maleated PP. Mater. Res. 2009, 12, 333–338. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Montanes, N.; Boronat, T.; Quiles-Carrillo, L.; Balart, R. Melt grafting of sepiolite nanoclay onto poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by reactive extrusion with multi-functional epoxy-based styrene-acrylic oligomer. Eur. Polym. J. 2016, 84, 693–707. [Google Scholar] [CrossRef]
- Formela, K.; Zedler, Ł.; Hejna, A.; Tercjak, A. Reactive extrusion of bio-based polymer blends and composites—Current trends and future developments. Express Polym. Lett. 2018, 12, 24–57. [Google Scholar] [CrossRef]
- Wei, L.; Stark, N.M.; McDonald, A.G. Interfacial improvements in biocomposites based on poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) bioplastics reinforced and grafted with α-cellulose fibers. Green Chem. 2015, 17, 4800–4814. [Google Scholar] [CrossRef]
- Martínez-Abad, A.; Cabedo, L.; Oliveira, C.S.; Hilliou, L.; Reis, M.; Lagarón, J.M. Characterization of polyhydroxyalkanoate blends incorporating unpurified biosustainably produced poly(3-hydroxybutyrate-co-3-hydroxyvalerate). J. Appl. Polym. Sci. 2016, 133, 42633. [Google Scholar] [CrossRef]
- Maruhashi, Y.; Iida, S. Transparency of polymer blends. Polym. Eng. Sci. 2001, 41, 1987–1995. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Vicente, A.A.; Reis, M.A.M.; Torres-Giner, S.; Lagaron, J.M. Antimicrobial and antioxidant performance of various essential oils and natural extracts and their incorporation into biowaste derived poly(3-hydroxybutyrate-co-3-hydroxyvalerate) layers made from electrospun ultrathin fibers. Nanomaterials 2019, 9, 144. [Google Scholar] [CrossRef]
- Ollier, R.P.; D’Amico, D.A.; Schroeder, W.F.; Cyras, V.P.; Alvarez, V.A. Effect of clay treatment on the thermal degradation of PHB based nanocomposites. Appl. Clay Sci. 2018, 163, 146–152. [Google Scholar] [CrossRef]
- De Matos Costa, A.R.; Santos, R.M.; Ito, E.N.; de Carvalho, L.H.; Canedo, E.L. Melt and cold crystallization in a poly(3-hydroxybutyrate) poly(butylene adipate-co-terephthalate) blend. J. Therm. Anal. Calorim. 2019. [Google Scholar] [CrossRef]
- Yoshie, N.; Asaka, A.; Inoue, Y. Cocrystallization and phase segregation in crystalline/crystalline polymer blends of bacterial copolyesters. Macromolecules 2004, 37, 3770–3779. [Google Scholar] [CrossRef]
- Organ, S.J. Phase separation in blends of poly(hydroxybutyrate) with poly(hydroxybutyrate-co-hydroxyvalerate): Variation with blend components. Polymer 1994, 35, 86–92. [Google Scholar] [CrossRef]
- Kumagai, Y.; Doi, Y. Enzymatic degradation of poly(3-hydroxybutyrate)-based blends: Poly(3-hydroxybutyrate)/poly(ethylene oxide) blend. Polym. Degrad. Stab. 1992, 35, 87–93. [Google Scholar] [CrossRef]
- Saito, M.; Inoue, Y.; Yoshie, N. Cocrystallization and phase segregation of blends of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Polymer 2001, 42, 5573–5580. [Google Scholar] [CrossRef]
- Mansaray, K.G.; Ghaly, A.E. Thermogravimetric analysis of rice husks in an air atmosphere. Energy Sources 1998, 20, 653–663. [Google Scholar] [CrossRef]
- Bugnicourt, E. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Li, S.D.; He, J.D.; Yu, P.H.; Cheung, M.K. Thermal degradation of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) as studied by TG, TG–FTIR, and Py–GC/MS. J. Appl. Polym. Sci. 2003, 89, 1530–1536. [Google Scholar] [CrossRef]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The chemomechanical properties of microbial polyhydroxyalkanoates. Prog. Polym. Sci. 2013, 38, 536–583. [Google Scholar] [CrossRef]
- Orts, W.J.; Marchessault, R.H.; Bluhm, T.L.; Hamer, G.K. Observation of strain-induced β form in poly(β-hydroxyalkanoates). Macromolecules 1990, 23, 5368–5370. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.D.; Gimenez, E.; Lagaron, J.M. Novel PET nanocomposites of interest in food packaging applications and comparative barrier performance with biopolyester nanocomposites. J. Plast. Film Sheeting 2007, 23, 133–148. [Google Scholar] [CrossRef]
- Cherpinski, A.; Torres-Giner, S.; Cabedo, L.; Lagaron, J.M. Post-processing optimization of electrospun submicron poly(3-hydroxybutyrate) fibers to obtain continuous films of interest in food packaging applications. Food Addit. Contam. Part A 2017, 34, 1817–1830. [Google Scholar] [CrossRef]
- Razumovskii, L.; Iordanskii, A.; Zaikov, G.; Zagreba, E.; McNeill, I. Sorption and diffusion of water and organic solvents in poly(β-hydroxybutyrate) films. Polym. Degrad. Stab. 1994, 44, 171–175. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.D.; Gimenez, E.; Lagarón, J.M. Morphology and barrier properties of solvent cast composites of thermoplastic biopolymers and purified cellulose fibers. Carbohydr. Polym. 2008, 71, 235–244. [Google Scholar] [CrossRef]
- Lagarón, J.M. Multifunctional and Nanoreinforced Polymers for Food Packaging; Woodhead Publishing: Lisle, IL, USA, 2011; pp. 1–28. [Google Scholar]




| Sample | PHB (wt %) | PHBV (wt %) | RHF (wt %) | TGIC (phr) | DCP (phr) |
|---|---|---|---|---|---|
| PHB/RHF | 90 | 0 | 10 | 1 | 0.25 |
| PHB/PHBV | 90 | 10 | 0 | 1 | 0.25 |
| PHB/PHBV5/RHF | 85 | 5 | 10 | 1 | 0.25 |
| PHB/PHBV10/RHF | 80 | 10 | 10 | 1 | 0.25 |
| PHB/PHBV20/RHF | 70 | 20 | 10 | 1 | 0.25 |
| PHB/PHBV30/RHF | 60 | 30 | 10 | 1 | 0.25 |
| PHB/PHBV50/RHF | 40 | 50 | 10 | 1 | 0.25 |
| Film | a* | b* | L* | ∆E* | T | O |
|---|---|---|---|---|---|---|
| PHB/RHF | −0.04 ± 0.01 a | 11.82 ± 0.03 a | 80.20 ± 0.02 a | - | 9.08 ± 0.07 a | 0.107 ± 0.03 a |
| PHB/PHBV | −0.25 ± 0.03 b | 1.94 ± 0.02 b | 86.40 ± 0.04 b | - | 8.48 ± 0.03 b | 0.099 ± 0.02 a |
| PHB/PHBV5/RHF | 0.44 ± 0.05 c | 14.60 ± 0.02 c | 78.05 ± 0.03 c | 3.55 ± 0.05 a | 9.06 ± 0.05 a | 0.111 ± 0.03 a |
| PHB/PHBV10/RHF | 0.52 ± 0.04 c | 14.84 ± 0.07 c | 77.62 ± 0.03 d | 4.01 ± 0.06 b | 9.91 ± 0.04 c | 0.116 ± 0.05 a |
| PHB/PHBV20/RHF | 0.56 ± 0.05 c | 15.28 ± 0.06 d | 77.56 ± 0.05 d | 4.39 ± 0.04 c | 10.05 ± 0.03 d | 0.116 ± 0.02 a |
| PHB/PHBV30/RHF | 0.70 ± 0.07 d | 15.36 ± 0.05 d | 77.29 ± 0.06 e | 4.64 ± 0.03 d | 10.18 ± 0.08 e | 0.117 ± 0.04 a |
| PHB/PHBV50/RHF | 1.12 ± 0.08 e | 16.17 ± 0.03 e | 76.36 ± 0.04 f | 5.92 ± 0.06 e | 10.17 ± 0.05 e | 0.113 ± 0.04 a |
| Film | Tc (°C) | ∆Hc (J/g) | Tm1 (°C) | Tm2 (°C) | ∆Hm (J/g) |
|---|---|---|---|---|---|
| PHB/RHF | 113.1 ± 0.6 a | 70.2 ± 0.2 a | 160.5 ± 0.8 a | 167.2 ± 0.9 a | 71.8 ± 2.3 a |
| PHB/PHBV | 113.3 ± 0.3 a | 73.3 ± 0.4 b | 165.1 ± 0.3 b | - | 76.9 ± 1.4 b |
| PHB/PHBV5/RHF | 118.9 ± 0.6 b | 59.8 ± 1.8 c | 171.9 ± 1.5 c | - | 63.7 ± 4.8 c |
| PHB/PHBV10/RHF | 110.9 ± 0.2 c | 60.1 ± 3.9 c | 162.6 ± 1.1 d | 168.3 ± 0.4 a | 63.4 ± 1.6 c |
| PHB/PHBV20/RHF | 115.4 ± 0.6 d | 78.0 ± 4.1 d | 166.0 ± 1.5 b | 173.3 ± 0.3 b | 61.4 ± 0.3 c |
| PHB/PHBV30/RHF | 113.2 ± 0.6 a | 72.7 ± 4.7 d | 162.6 ± 0.7 d | 173.9 ± 0.1 c | 59.4 ± 2.2 c |
| PHB/PHBV50/RHF | 107.7 ± 0.6 e | 64.8 ± 0.8 c | 158.4 ± 1.1 e | 171.0 ± 0.8 d | 53.8 ± 0.1 d |
| Film | T5% (°C) | Tdeg (°C) | Mass Loss (%) | Residual Mass (%) |
|---|---|---|---|---|
| RHF | 228.9 ± 1.5 a | 335.2 ± 0.7 a | 28.5 ± 0.8 a | 33.1 ± 0.3 a |
| PHB/RHF | 242.5 ± 1.2 b | 270.0 ± 0.5 b | 67.4 ± 0.7 b | 3.1 ± 1.3 b |
| PHB/PHBV | 252.6 ± 1.9 c | 282.8 ± 0.4 c | 69.4 ± 1.5 b | 0.6 ± 0.1 c |
| PHB/PHBV5/RHF | 242.5 ± 1.5 b | 268.2 ± 0.5 d | 65.4 ± 0.3 c | 3.5 ± 0.2 b |
| PHB/PHBV10/RHF | 247.1 ± 1.3 d | 270.0 ± 0.5 b | 63.9 ± 0.4 d | 3.1 ± 0.8 b |
| PHB/PHBV20/RHF | 247.5 ± 1.4 d | 274.6 ± 0.6 e | 69.8 ± 1.2 b | 3.3 ± 0.9 b |
| PHB/PHBV30/RHF | 253.5 ± 1.7 c | 275.5 ± 0.6 e | 61.7 ± 0.2 e | 3.2 ± 0.2 b |
| PHB/PHBV50/RHF | 257.2 ± 1.6 e | 275.5 ± 0.6 e | 61.5 ± 0.3 e | 3.8 ± 0.4 b |
| Film | E (MPa) | σy (MPa) | εb (%) | Toughness (mJ/m3) |
|---|---|---|---|---|
| PHB * | ~2900 a | ~37 a | ~4 a | - |
| PHB/RHF | 3025 ± 101 b | 26.7 ± 2.7 b | 1.1 ± 0.1 b | 0.1 ± 0.1 a |
| PHB/PHBV | 1785 ± 129 c | 38.9 ± 2.0 a | 8.4 ± 1.1 c | 1.9 ± 0.3 b |
| PHB/PHBV5/RHF | 2985 ± 119 a,b | 27.2 ± 1.1 b | 1.4 ± 0.1 d | 0.3 ± 0.1 a |
| PHB/PHBV10/RHF | 2508 ± 207 d | 30.4 ± 2.7 b | 3.3 ± 1.3 a | 0.8 ± 0.3 c |
| PHB/PHBV20/RHF | 2830 ± 193 a,b,d | 26.5 ± 2.5 b | 1.2 ± 0.3 b,d | 0.1 ± 0.1 a |
| PHB/PHBV30/RHF | 2765 ± 201 a,b,d | 23.9 ± 0.7 b | 1.3 ± 0.2 b,d | 0.2 ± 0.1 a |
| PHB/PHBV50/RHF | 2649 ± 104 d | 19.5 ± 3.0 c | 1.3 ± 0.4 b,d | 0.2 ± 0.1 a |
| Film | WVP × 1015 (kg·m/m2·Pa·s) | LP × 1015 (kg·m/m2·Pa·s) |
|---|---|---|
| PHB * | 1.75 a | 1.95 a |
| PHB/RHF | 4.52 ± 0.38 b | 2.58 ± 1.35 a |
| PHB/PHBV | 3.27 ± 0.15 c | 2.04 ± 0.19 a |
| PHB/PHBV5/RHF | 4.55 ± 0.75 b | 2.16 ± 0.54 a |
| PHB/PHBV10/RHF | 5.03 ± 0.64 b | 3.10 ± 0.65 a |
| PHB/PHBV20/RHF | 5.36 ± 0.69 b | 3.38 ± 0.63 a |
| PHB/PHBV30/RHF | 6.01 ± 0.60 b | 3.72 ± 0.32 a |
| PHB/PHBV50/RHF | 7.46 ± 0.90 b | 5.04 ± 1.50 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melendez-Rodriguez, B.; Torres-Giner, S.; Aldureid, A.; Cabedo, L.; Lagaron, J.M. Reactive Melt Mixing of Poly(3-Hydroxybutyrate)/Rice Husk Flour Composites with Purified Biosustainably Produced Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate). Materials 2019, 12, 2152. https://doi.org/10.3390/ma12132152
Melendez-Rodriguez B, Torres-Giner S, Aldureid A, Cabedo L, Lagaron JM. Reactive Melt Mixing of Poly(3-Hydroxybutyrate)/Rice Husk Flour Composites with Purified Biosustainably Produced Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate). Materials. 2019; 12(13):2152. https://doi.org/10.3390/ma12132152
Chicago/Turabian StyleMelendez-Rodriguez, Beatriz, Sergio Torres-Giner, Abdulaziz Aldureid, Luis Cabedo, and Jose M. Lagaron. 2019. "Reactive Melt Mixing of Poly(3-Hydroxybutyrate)/Rice Husk Flour Composites with Purified Biosustainably Produced Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate)" Materials 12, no. 13: 2152. https://doi.org/10.3390/ma12132152
APA StyleMelendez-Rodriguez, B., Torres-Giner, S., Aldureid, A., Cabedo, L., & Lagaron, J. M. (2019). Reactive Melt Mixing of Poly(3-Hydroxybutyrate)/Rice Husk Flour Composites with Purified Biosustainably Produced Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate). Materials, 12(13), 2152. https://doi.org/10.3390/ma12132152








