Improved NOx Storage/Release Properties of Ceria-Based Lean NOx Trap Compositions with MnOx Modification
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sample Characterization
2.3. NOx Storage/Release Tests
3. Results
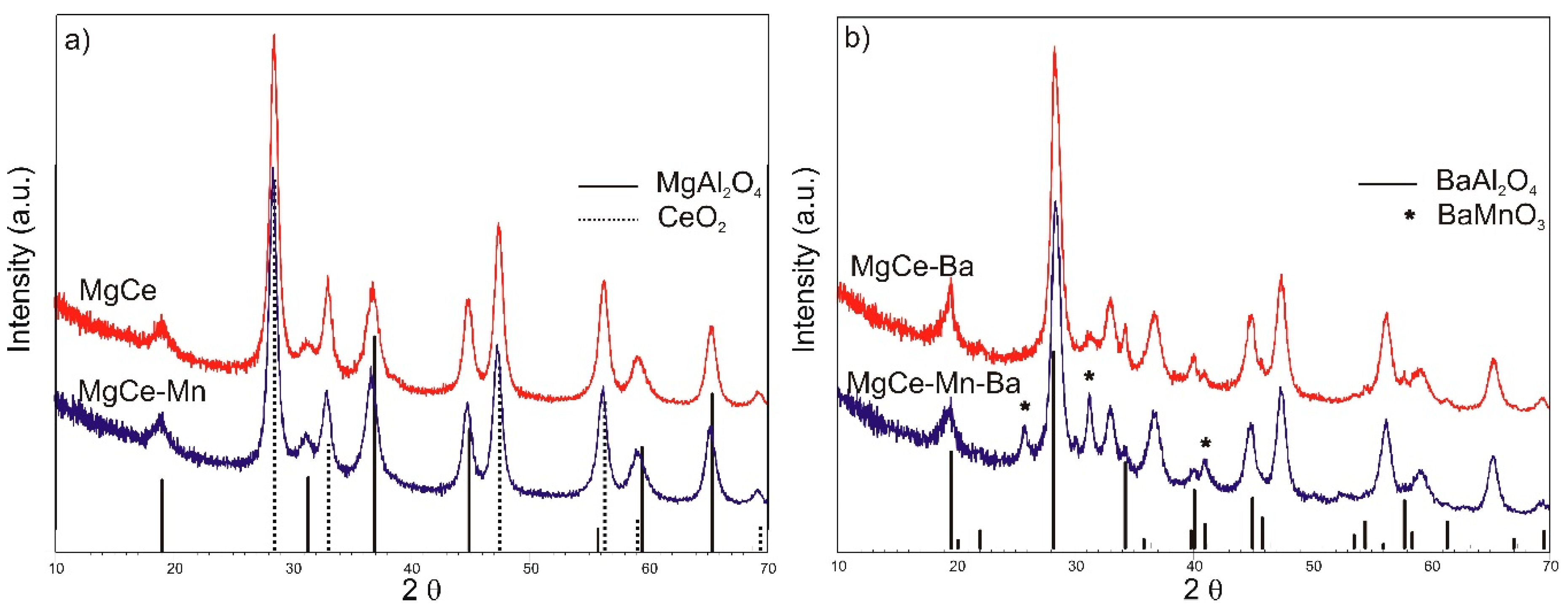
3.1. Structural Properties
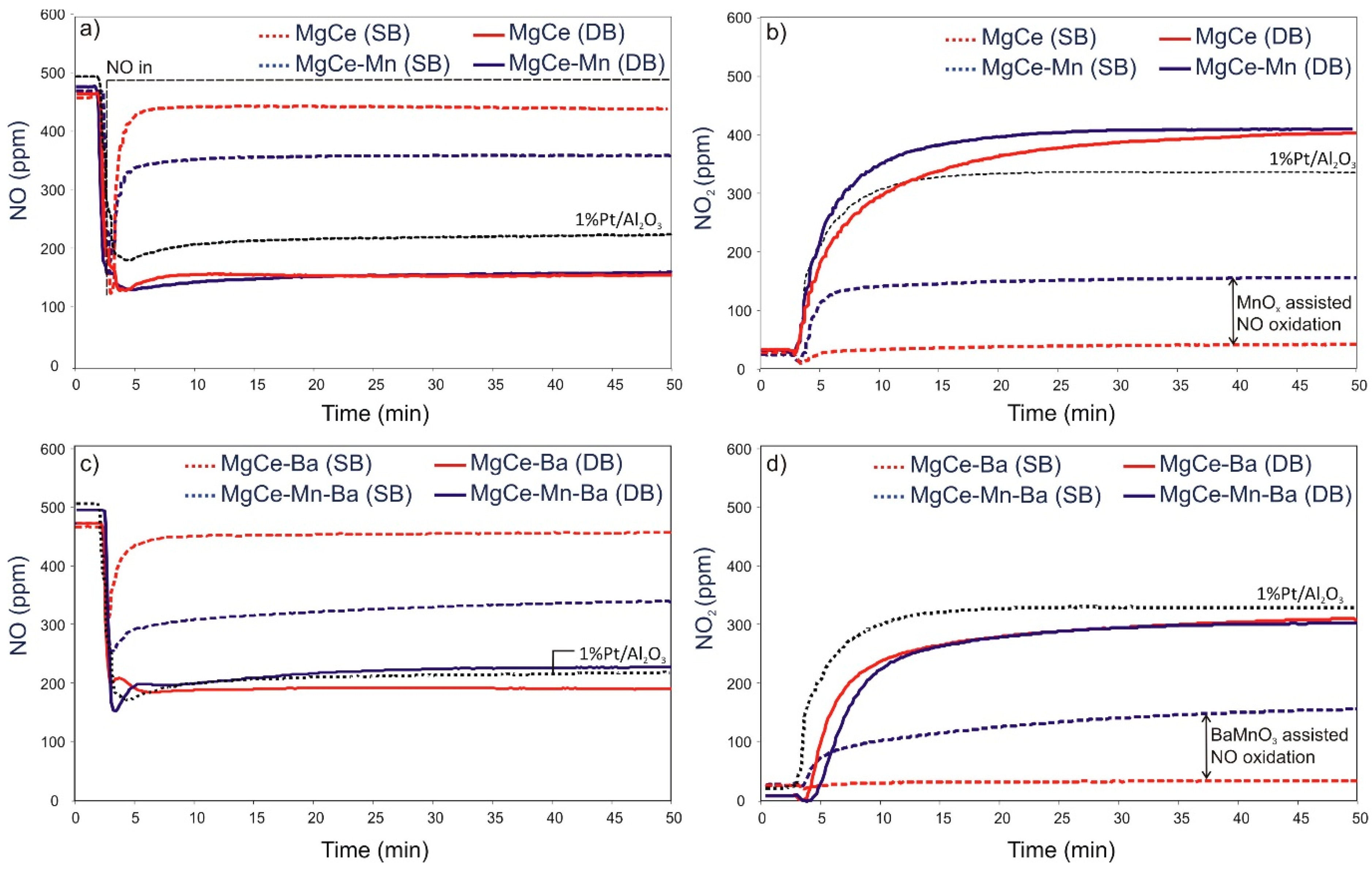
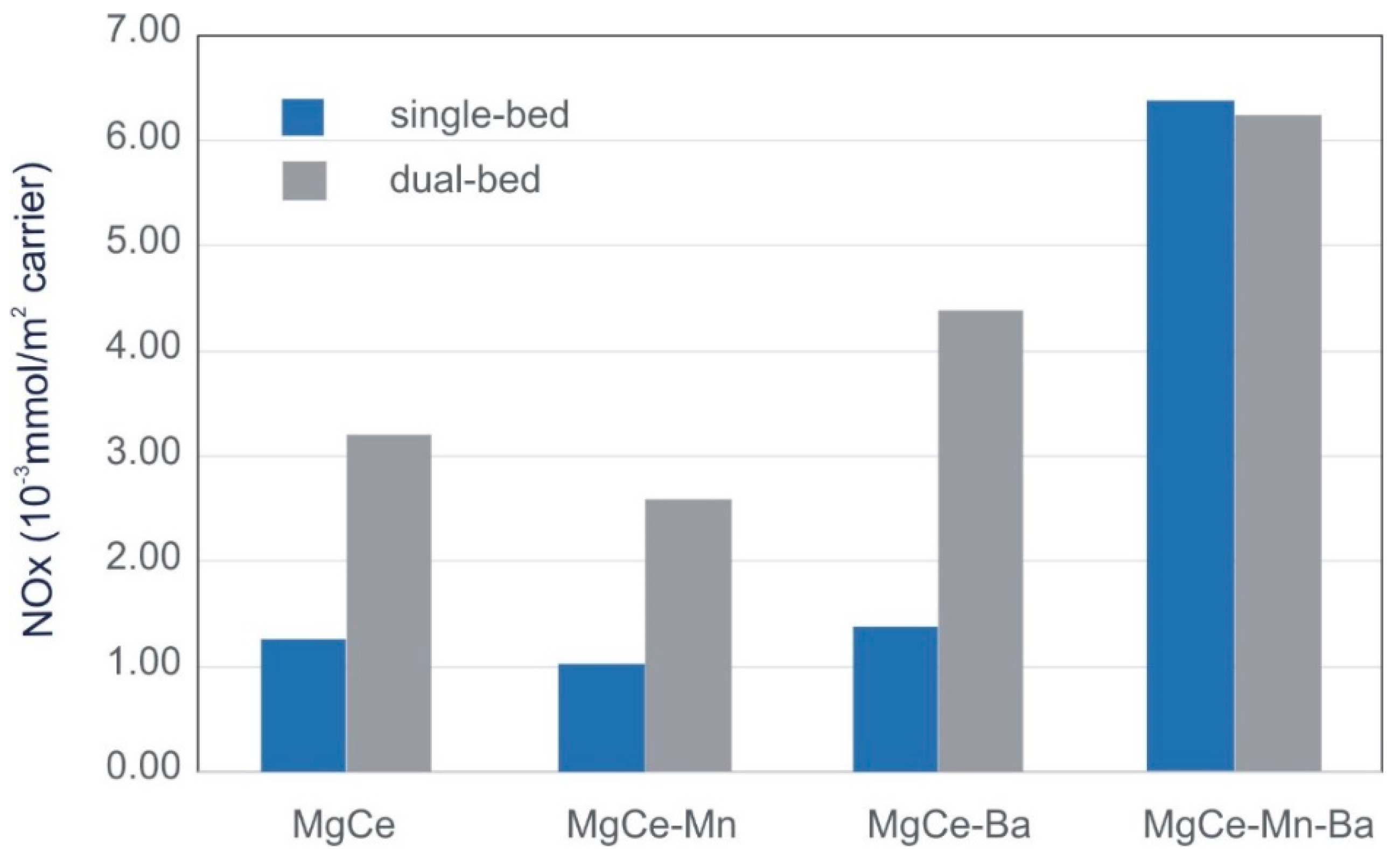
3.2. NOx Storage
3.2.1. Dual-bed Experiments
3.2.2. Single-bed Experiments
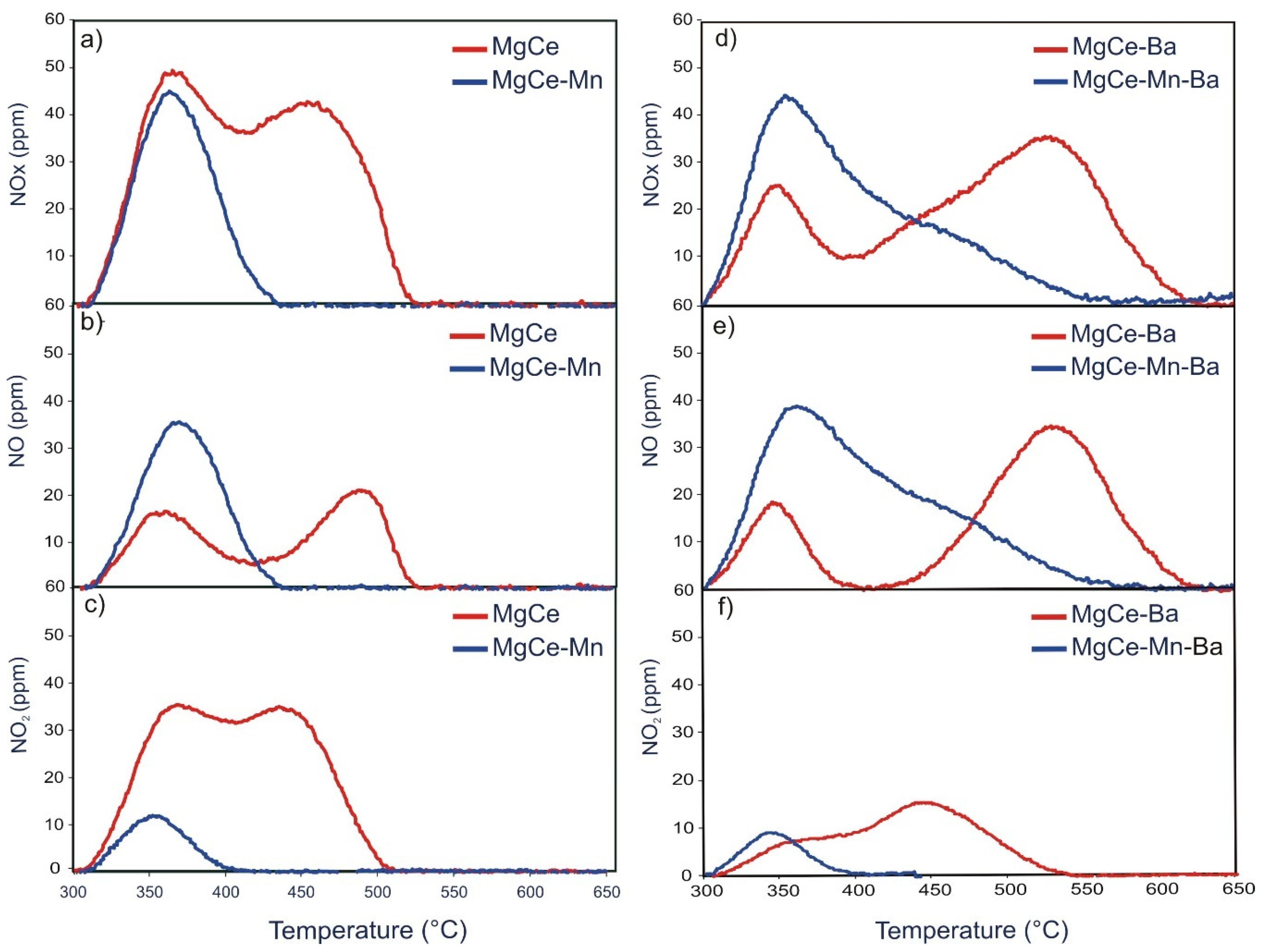
3.3. NOx Release
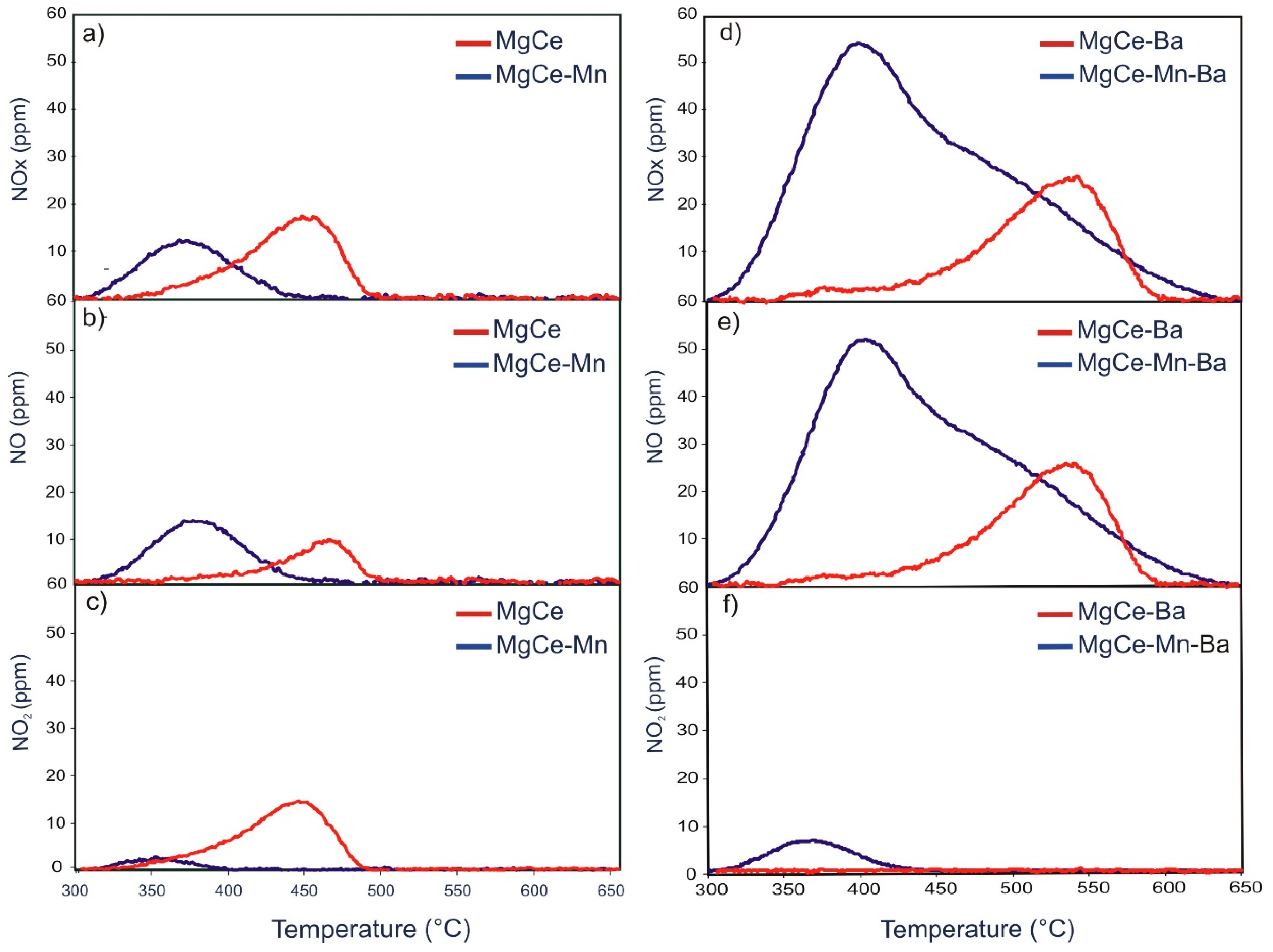
3.3.1. TPD after NOx Adsorption under Dual-bed Configuration
3.3.2. TPD after NOx Adsorption under Single-bed Configuration
4. Discussion
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Walker, J. Johnson Matthey Sector Call—Clean Air. Available online: https://seekingalpha.com/article/4187530-johnson-matthey-jmply-sector-call-clean-air-slideshow (accessed on 2 May 2019).
- Twigg, M.V. Catalytic control of emissions from cars. Catal. Today 2011, 163, 33–41. [Google Scholar] [CrossRef]
- Heck, R.M.; Farrauto, R.J.; Gulati, S.T. Catalytic Air Pollution Control, 3rd ed.; Wiley: Hoboken, NJ, USA, 2009; pp. 158–163. [Google Scholar]
- Metkar, P.S.; Harold, M.P.; Balakotaiah, V. Experimental and kinetic modeling study of NH3-SCR of NOx on Fe-ZSM-5, Cu-chabazite and combined Fe- and Cu-zeolite monolithic catalysts. Chem. Eng. Sci. 2013, 87, 51–66. [Google Scholar] [CrossRef]
- Koebel, M.; Madia, G.; Elsener, M. Selective catalytic reduction of NO and NO2 at low temperatures. Catal. Today 2002, 73, 239–247. [Google Scholar] [CrossRef]
- Stadlbauer, S.; Waschl, H.; Schilling, A.; del Re, L. DOC Temperature Control for Low Temperature Operating Ranges with Post and Main Injection Actuation; SAE International: Warrendale, PA, USA, 2013; Volume 1. [Google Scholar] [CrossRef]
- Epling, W.S.; Campbell, L.E.; Yezerets, A.; Currier, N.W.; Parks, J.E. Overview of the Fundamental Reactions and Degradation Mechanisms of NOx Storage/Reduction Catalysts. Catal. Rev. 2004, 46, 163–245. [Google Scholar] [CrossRef]
- Chan, D.; Gremminger, A.; Deutschmann, O. Effect of Hydrothermal Aging on Physical and Chemical Properties of a Commercial NOx-Storage Catalyst. Top. Catal. 2013, 56, 293–297. [Google Scholar] [CrossRef]
- Kubiak, L.; Castoldi, L.; Lietti, L.; Andonova, S.; Olsson, L. Mechanistic Investigation of the Reduction of NOx over Pt- and Rh-Based LNT Catalysts. Catalysts 2016, 6, 46. [Google Scholar] [CrossRef]
- Fridell, E.; Skoglundh, M.; Westerberg, B.; Johansson, S.; Smedler, G. NOx storage in barium-containing catalysts. J. Catal. 1999, 183, 196–209. [Google Scholar] [CrossRef]
- Lietti, L.; Forzatti, P.; Nova, I.; Tronconi, E. NOx Storage Reduction over Pt@Ba/γ-Al2O3 Catalyst. J. Catal. 2001, 204, 175–191. [Google Scholar] [CrossRef]
- Nova, I.; Castoldi, L.; Lietti, L.; Tronconi, E.; Forzatti, P.; Prinetto, F.; Ghiotti, G. NOx adsorption study over Pt–Ba/alumina catalysts: FT-IR and pulse experiments. J. Catal. 2004, 222, 377–388. [Google Scholar] [CrossRef]
- Kwak, J.H.; Mei, D.; Yi, C.-W.W.; Kim, D.H.; Peden, C.H.F.F.; Allard, L.F.; Szanyi, J. Understanding the nature of surface nitrates in BaO/γ-Al2O3 NOx storage materials: A combined experimental and theoretical study. J. Catal. 2009, 261, 17–22. [Google Scholar] [CrossRef]
- Kumar, A.; Harold, M.P.; Balakotaiah, V. Isotopic studies of NOX storage and reduction on Pt/BaO/Al2O3 catalyst using temporal analysis of products. J. Catal. 2010, 270, 214–223. [Google Scholar] [CrossRef]
- Forzatti, P.; Castoldi, L.; Nova, I.; Lietti, L.; Tronconi, E. NOx removal catalysis under lean conditions. Catal. Today 2006, 117, 316–320. [Google Scholar] [CrossRef]
- Cumaranatunge, L.; Mulla, S.S.; Yezerets, A.; Currier, N.W.; Delgass, W.N.; Ribeiro, F.H. Ammonia is a hydrogen carrier in the regeneration of Pt/BaO/Al2O3 NOx traps with H2. J. Catal. 2007, 246, 29–34. [Google Scholar] [CrossRef]
- Bhatia, D.; Clayton, R.D.; Harold, M.P.; Balakotaiah, V. A global kinetic model for NOx storage and reduction on Pt/BaO/Al2O3 monolithic catalysts. Catal. Today 2009, 147, 250–256. [Google Scholar] [CrossRef]
- Pereda-Ayo, B.; González-Velasco, J.R.; Burch, R.; Hardacre, C.; Chansai, S. Regeneration mechanism of a Lean NOx Trap (LNT) catalyst in the presence of NO investigated using isotope labelling techniques. J. Catal. 2012, 285, 177–186. [Google Scholar] [CrossRef]
- Luo, J.-Y.; Epling, W.S.; Qi, G.; Li, W. Low Temperature Ceria-Based Lean NOx Traps. Catal. Lett. 2012, 142, 946–958. [Google Scholar] [CrossRef]
- Shi, C.; Ji, Y.; Graham, U.M.; Jacobs, G.; Crocker, M.; Zhang, Z.; Wang, Y.; Toops, T.J. NOx storage and reduction properties of model ceria-based lean NOx trap catalysts. Appl. Catal. B Environ. 2012, 119–120, 183–196. [Google Scholar] [CrossRef]
- Hilaire, S.; Wang, X.; Luo, T.; Gorte, R.J.; Wagner, J. A comparative study of water-gas-shift reaction over ceria-supported metallic catalysts. Appl. Catal. A Gen. 2004, 258, 271–276. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Kondarides, D.I. Effect of the nature of the support on the catalytic performance of noble metal catalysts for the water–gas shift reaction. Catal. Today 2006, 112, 49–52. [Google Scholar] [CrossRef]
- Infantes-Molina, A.; Righini, L.; Castoldi, L.; Loricera, C.V.; Fierro, J.L.G.; Sin, A.; Lietti, L. Characterization and reactivity of Ce-promoted PtBa lean NOx trap catalysts. Catal. Today 2012, 197, 178–189. [Google Scholar] [CrossRef]
- Say, Z.; Vovk, E.I.; Bukhtiyarov, V.I.; Ozensoy, E. Influence of ceria on the NOx reduction performance of NOx storage reduction catalysts. Appl. Catal. B Environ. 2013, 142–143, 89–100. [Google Scholar] [CrossRef]
- Philipp, S.; Drochner, A.; Kunert, J.; Vogel, H.; Theis, J.; Lox, E.S. Investigation of NO Adsorption and NO/O2 Co-adsorption on NOx -Storage-Components by DRIFT-Spectroscopy. Top. Catal. 2004, 30, 235–238. [Google Scholar] [CrossRef]
- Ji, Y.; Toops, T.J.; Graham, U.M.; Jacobs, G.; Crocker, M. A kinetic and DRIFTS study of supported Pt catalysts for NO oxidation. Catal. Lett. 2006, 110, 29–37. [Google Scholar] [CrossRef]
- Ryou, Y.; Lee, J.; Lee, H.; Kim, C.H.; Kim, D.H. Low temperature NO adsorption over hydrothermally aged Pd/CeO2 for cold start application. Catal. Today 2018, 307, 93–101. [Google Scholar] [CrossRef]
- Filtschew, A.; Stranz, D.; Hess, C. Mechanism of NO2 storage in ceria studied using combined in situ Raman/FT-IR spectroscopy. Phys. Chem. Chem. Phys. 2013, 15, 9066–9069. [Google Scholar] [CrossRef]
- Filtschew, A.; Hess, C. Unravelling the mechanism of NO and NO2 storage in ceria: The role of defects and Ce-O surface sites. Appl. Catal. B Environ. 2018, 237, 1066–1081. [Google Scholar] [CrossRef]
- AL-Harbi, M.; Epling, W.S. Effects of different regeneration timing protocols on the performance of a model NOx storage/reduction catalyst. Catal. Today 2010, 151, 347–353. [Google Scholar] [CrossRef]
- Corbos, E.C.; Haneda, M.; Courtois, X.; Marecot, P.; Duprez, D.; Hamada, H. Cooperative effect of Pt–Rh/Ba/Al and CuZSM-5 catalysts for NOx reduction during periodic lean-rich atmosphere. Catal. Commun. 2008, 10, 137–141. [Google Scholar] [CrossRef]
- Corbos, E.C.; Haneda, M.; Courtois, X.; Marecot, P.; Duprez, D.; Hamada, H. NOx abatement for lean-burn engines under lean–rich atmosphere over mixed NSR-SCR catalysts: Influences of the addition of a SCR catalyst and of the operational conditions. Appl. Catal. A Gen. 2009, 365, 187–193. [Google Scholar] [CrossRef]
- Weibel, M.; Waldbüer, N.; Wunsch, R.; Chatterjee, D.; Bandl-Konrad, B.; Krutzsch, B. A novel approach to catalysis for NOx reduction in diesel exhaust gas. Top. Catal. 2009, 52, 1702–1708. [Google Scholar] [CrossRef]
- Pereda-Ayo, B.; Duraiswami, D.; González-Velasco, J.R. Control of NOx storage and reduction in NSR bed for designing combined NSR–SCR systems. Catal. Today 2011, 172, 66–72. [Google Scholar] [CrossRef]
- Castoldi, L.; Bonzi, R.; Lietti, L.; Forzatti, P.; Morandi, S.; Ghiotti, G.; Dzwigaj, S. Catalytic behaviour of hybrid LNT/SCR systems: Reactivity and in situ FTIR study. J. Catal. 2011, 282, 128–144. [Google Scholar] [CrossRef]
- Can, F.; Courtois, X.; Royer, S.; Blanchard, G.; Rousseau, S.; Duprez, D. An overview of the production and use of ammonia in NSR+SCR coupled system for NOx reduction from lean exhaust gas. Catal. Today 2012, 197, 144–154. [Google Scholar] [CrossRef]
- Wang, J.; Ji, Y.; Jacobs, G.; Jones, S.; Kim, D.J.; Crocker, M. Effect of aging on NOx reduction in coupled LNT–SCR systems. Appl. Catal. B Environ. 2014, 148–149, 51–61. [Google Scholar] [CrossRef]
- Wittka, T.; Holderbaum, B.; Dittmann, P.; Pischinger, S. Experimental Investigation of Combined LNT + SCR Diesel Exhaust Aftertreatment. Emiss. Control Sci. Technol. 2015, 1, 167–182. [Google Scholar] [CrossRef]
- Eberhardt, M.; Riedel, R.; Göbel, U.; Theis, J.; Lox, E.S. Fundamental investigations of thermal aging phenomena of model NOx storage systems. Top. Catal. 2004, 30, 135–142. [Google Scholar] [CrossRef]
- Kim, D.H.; Chin, Y.-H.; Kwak, J.H.; Szanyi, J.; Peden, C.H.F. Changes in Ba Phases in BaO/Al2O3 upon Thermal Aging and H2O Treatment. Catal. Lett. 2005, 105, 259–268. [Google Scholar] [CrossRef]
- Kwak, J.H.; Kim, D.H.; Szanyi, J.; Cho, S.J.; Peden, C.H.F. Enhanced High Temperature Performance of MgAl2O4-Supported Pt–BaO Lean NOx Trap Catalysts. Top. Catal. 2012, 55, 70–77. [Google Scholar] [CrossRef]
- Jeong, S.; Youn, S.; Kim, D.H. Effect of Mg/Al ratios on the NOx storage activity over Pt-BaO/Mg–Al mixed oxides. Catal. Today 2014, 231, 155–163. [Google Scholar] [CrossRef]
- Roy, S.; van Vegten, N.; Baiker, A. Single-step flame-made Pt/MgAl2O4—A NOx storage-reduction catalyst with unprecedented dynamic behavior and high thermal stability. J. Catal. 2010, 271, 125–131. [Google Scholar] [CrossRef]
- Casapu, M.; Grunwaldt, J.-D.; Maciejewski, M.; Wittrock, M.; Göbel, U.; Baiker, A. Formation and stability of barium aluminate and cerate in NOx storage-reduction catalysts. Appl. Catal. B Environ. 2006, 63, 232–242. [Google Scholar] [CrossRef]
- Wu, X.; Lin, F.; Xu, H.; Weng, D. Effects of adsorbed and gaseous NOx species on catalytic oxidation of diesel soot with MnOx-CeO2 mixed oxides. Appl. Catal. B Environ. 2010, 96, 101–109. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, L.; Sun, N.; Wang, H.; Zhong, L.; He, C.; Wei, W.; Sun, Y. Hollow MnOx-CeO2 mixed oxides as highly efficient catalysts in NO oxidation. Chem. Eng. J. 2017, 322, 46–55. [Google Scholar] [CrossRef]
- Qi, G.; Li, W. NO oxidation to NO2 over manganese-cerium mixed oxides. Catal. Today 2015, 258, 205–213. [Google Scholar] [CrossRef]
- Le Phuc, N.; Courtois, X.; Can, F.; Royer, S.; Marecot, P.; Duprez, D. NOx removal efficiency and ammonia selectivity during the NOx storage-reduction process over Pt/BaO(Fe, Mn, Ce)/Al2O3 model catalysts. Part II: Influence of Ce and Mn–Ce addition. Appl. Catal. B Environ. 2011, 102, 353–361. [Google Scholar] [CrossRef]
- Le-Phuc, N. Removal of NOx in the presence of oxygen over Mn/BaO/Al2O3 catalysts. Mater. Sci. Nanaotechnol. 2017, 1, 37–40. [Google Scholar]
- Zhang, Z.; Chen, B.; Wang, X.; Xu, L.; Au, C.; Shi, C.; Crocker, M. NOx storage and reduction properties of model manganese-based lean NOx trap catalysts. Appl. Catal. B Environ. 2015, 165, 232–244. [Google Scholar] [CrossRef]
- Xiao, J.; Li, X.; Deng, S.; Wang, F.; Wang, L. NOx storage-reduction over combined catalyst Mn/Ba/Al2O3–Pt/Ba/Al2O3. Catal. Commun. 2008, 9, 563–567. [Google Scholar] [CrossRef]
- Schöneborn, M.; Harmening, T.; Niemeyer, D.; Rolfs, S.; Fabian, J. NOx Trap Catalyst Support Material with Improved Stability against BaAl2O4 Formation. Patent No. WO2016142058A1, 15 September 2016. [Google Scholar]
- Adouane, D.; Dutilleul, H.; Guibert, P.; Darcy, P.; Costa, P. Da Effect of thermal ageing on structure and reactivity of commercial Lean NOx trap : From laboratory to engine scale. In Proceedings of the 7th International Conference on Environmental Catalysis, Lyon, France, 2–6 September 2012. [Google Scholar]
- Al-Harbi, M.; Epling, W.S. Investigating the effect of NO versus NO2 on the performance of a model NOx storage/reduction catalyst. Catal. Lett. 2009, 130, 121–129. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, X.; Liu, S.; Weng, D.; Zhang, H.; Ran, R. Formation of BaMnO3 in Ba/MnOx-CeO2 catalyst upon the hydrothermal ageing and its effects on oxide sintering and soot oxidation activity. Catal. Today 2015, 253, 83–88. [Google Scholar] [CrossRef]
- Xiao, J.H.; Li, X.H.; Deng, S.; Xu, J.C.; Wang, L.F. The NOx oxidation-storage and tolerance of SO2 poison of Mn/Ba/Al2O3 catalyst. Acta Phys.-Chim. Sin. 2006, 22, 815–819. [Google Scholar] [CrossRef]
- Kapteijn, F.; Vanlangeveld, A.D.; Moulijn, J.A.; Andreini, A.; Vuurman, M.A.; Turek, A.M.; Jehng, J.M.; Wachs, I.E. Alumina-Supported Manganese Oxide Catalysts: I. Characterization: Effect of Precursor and Loading. J. Catal. 1994, 150, 94–104. [Google Scholar] [CrossRef]
- Guo, L.H.; Guo, L.; Zhao, D.Y.; Gao, Z.N.; Tian, Y.; Ding, T.; Zhang, J.; Zheng, L.R.; Li, X.G. Oxidizing, trapping and releasing NOx over model manganese oxides in alternative lean-burn/fuel-rich atmospheres at low temperatures. Catal. Today 2017, 297, 27–35. [Google Scholar] [CrossRef]
- Feeley, J.S.; Farrauto, R.J.; Deeba, M. Method and Apparatus for NOx Abatement in Lean Gaseous Streams. U.S. Patent No. 6471924B1, 29 October 2002. [Google Scholar]
- Jarvis, M.; Adams, K.M. Method for Converting Exhaust Gases from a Diesel Engine using Nitrogen Oxide Absorbent. U.S. Patent No. 6182443B1, 6 February 2001. [Google Scholar]
- Gu, Y.; Epling, W.S. Passive NOx adsorber: An overview of catalyst performance and reaction chemistry. Appl. Catal. A Gen. 2019, 570, 1–14. [Google Scholar] [CrossRef]
- Jones, S.; Ji, Y.; Bueno-Lopez, A.; Song, Y.; Crocker, M. CeO2-M2O3 Passive NOx Adsorbers for Cold Start Applications. Emiss. Control Sci. Technol. 2017, 3, 59–72. [Google Scholar] [CrossRef]
- Ji, Y.; Xu, D.; Crocker, M.; Theis, J.R.; Lambert, C.; Bueno-Lopez, A.; Harris, D.; Scapens, D. Mn-based mixed oxides for low temperature NOx adsorber applications. Appl. Catal. A Gen. 2018, 567, 90–101. [Google Scholar] [CrossRef]
| Sample | Al2O3 | MgO | CeO2 | MnO2 | BaO |
|---|---|---|---|---|---|
| MgCe | 63.0 | 17.0 | 20.0 | 0 | 0 |
| MgCe-Mn | 58.3 | 14.9 | 17.7 | 9.1 | 0 |
| MgCe-Ba | 52.8 | 14.7 | 17.0 | 0 | 15.5 |
| MgCe-Mn-Ba | 48.4 | 13.3 | 15.5 | 7.3 | 15.5 |
| Sample | SBET (m2/g) | rP (nm) | VP (cm3/g) |
|---|---|---|---|
| MgCe | 96 | 13 | 0.64 |
| MgCe-Mn | 104 | 10 | 0.53 |
| MgCe-Ba | 68 | 11 | 0.37 |
| MgCe-Mn-Ba | 59 | 11 | 0.31 |
| Element | Sample | |||
|---|---|---|---|---|
| MgCe | MgCe-Mn | MgCe-Ba | MgCe-Mn-Ba | |
| C | 33.46 | 43.7 | 50.4 | 58.1 |
| O | 40.0 | 37.7 | 35.5 | 30.1 |
| N | 0.4 | 0.3 | 0.7 | 0.5 |
| Al | 17.3 | 11.1 | 10.5 | 8.5 |
| Ce | 2.9 | 2.5 | 0.7 | 0.5 |
| Mg | 2.3 | 2.3 | 1.1 | 1.0 |
| Mn | 0.0 | 2.5 | 0.0 | 0.7 |
| Ba | 0.0 | 0.0 | 1.2 | 0.8 |
| Al/(Al + Ce + Mg + Mn + Ba) 1 | 0.77 | 0.60 | 0.78 | 0.74 |
| Ce/(Al + Ce + Mg + Mn + Ba) 1 | 0.13 | 0.14 | 0.05 | 0.04 |
| Mg/(Al + Ce + Mg + Mn + Ba) 1 | 0.10 | 0.13 | 0.08 | 0.09 |
| Mn/(Al + Ce + Mg + Mn + Ba) 1 | - | 0.13 | - | 0.06 |
| Ba/(Al + Ce + Mg + Mn + Ba) 1 | - | - | 0.09 | 0.07 |
| Samples without Ba | NOx Stored (10−3 mmol/m2carrier) | Samples with Ba | NOx Stored (10−3 mmol/m2carrier) |
|---|---|---|---|
| MgCe | 3.23 | MgCe-Ba | 4.41 |
| MgCe-Mn | 2.60 | MgCe-Mn-Ba | 6.27 |
| Sample | NO2/NO Ratio | NOx Stored (10−3 mmol/m2carrier) |
|---|---|---|
| MgCe | 0.07 | 1.27 |
| MgCe-Mn | 0.43 | 1.03 |
| MgCe-Ba | 0.08 | 1.40 |
| MgCe-Mn-Ba | 0.48 | 6.39 |
| Sample | NOx Released (10−3 mmol/m2carrier) | NO Released (10−3 mmol/m2carrier) | NO2 Released (10−3 mmol/m2carrier) |
|---|---|---|---|
| MgCe | 2.47 | 0.79 | 1.68 |
| MgCe-Mn | 0.97 | 0.78 | 0.19 |
| MgCe-Ba | 4.69 | 3.15 | 1.54 |
| MgCe-Mn-Ba | 4.76 | 4.39 | 0.37 |
| Sample | NOx Released (10−3 mmol/m2carrier) | NO Released (10−3 mmol/m2carrier) | NO2 Released (10−3 mmol/m2carrier) |
|---|---|---|---|
| MgCe | 0.59 | 0.18 | 0.41 |
| MgCe-Mn | 0.36 | 0.32 | 0.04 |
| MgCe-Ba | 1.34 | 1.34 | 0 |
| MgCe-Mn-Ba | 5.05 | 4.61 | 0.44 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schöneborn, M.; Harmening, T.; Giménez-Mañogil, J.; Martínez-Munuera, J.C.; García-García, A. Improved NOx Storage/Release Properties of Ceria-Based Lean NOx Trap Compositions with MnOx Modification. Materials 2019, 12, 2127. https://doi.org/10.3390/ma12132127
Schöneborn M, Harmening T, Giménez-Mañogil J, Martínez-Munuera JC, García-García A. Improved NOx Storage/Release Properties of Ceria-Based Lean NOx Trap Compositions with MnOx Modification. Materials. 2019; 12(13):2127. https://doi.org/10.3390/ma12132127
Chicago/Turabian StyleSchöneborn, Marcos, Thomas Harmening, Javier Giménez-Mañogil, Juan Carlos Martínez-Munuera, and Avelina García-García. 2019. "Improved NOx Storage/Release Properties of Ceria-Based Lean NOx Trap Compositions with MnOx Modification" Materials 12, no. 13: 2127. https://doi.org/10.3390/ma12132127
APA StyleSchöneborn, M., Harmening, T., Giménez-Mañogil, J., Martínez-Munuera, J. C., & García-García, A. (2019). Improved NOx Storage/Release Properties of Ceria-Based Lean NOx Trap Compositions with MnOx Modification. Materials, 12(13), 2127. https://doi.org/10.3390/ma12132127






