Non-Isothermal Oxidation Behaviors and Mechanisms of Ti-Al Intermetallic Compounds
Abstract
:1. Introduction
2. Experimental
2.1. Specimen Preparation
2.2. Non-Isothermal Oxidation Experiment
2.3. Microstructural Characterization
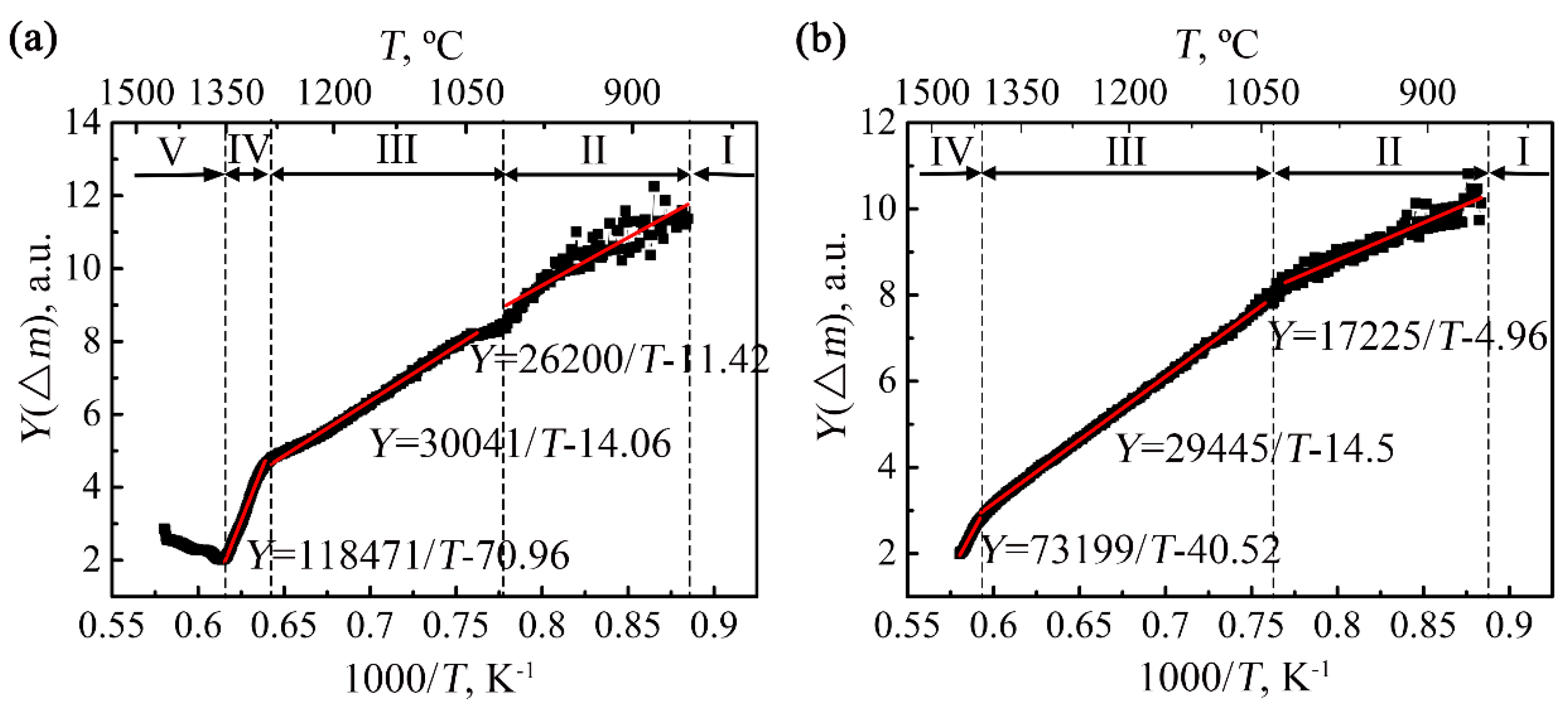
3. Calculation of Oxidation Activation Energy
4. Results and Discussion
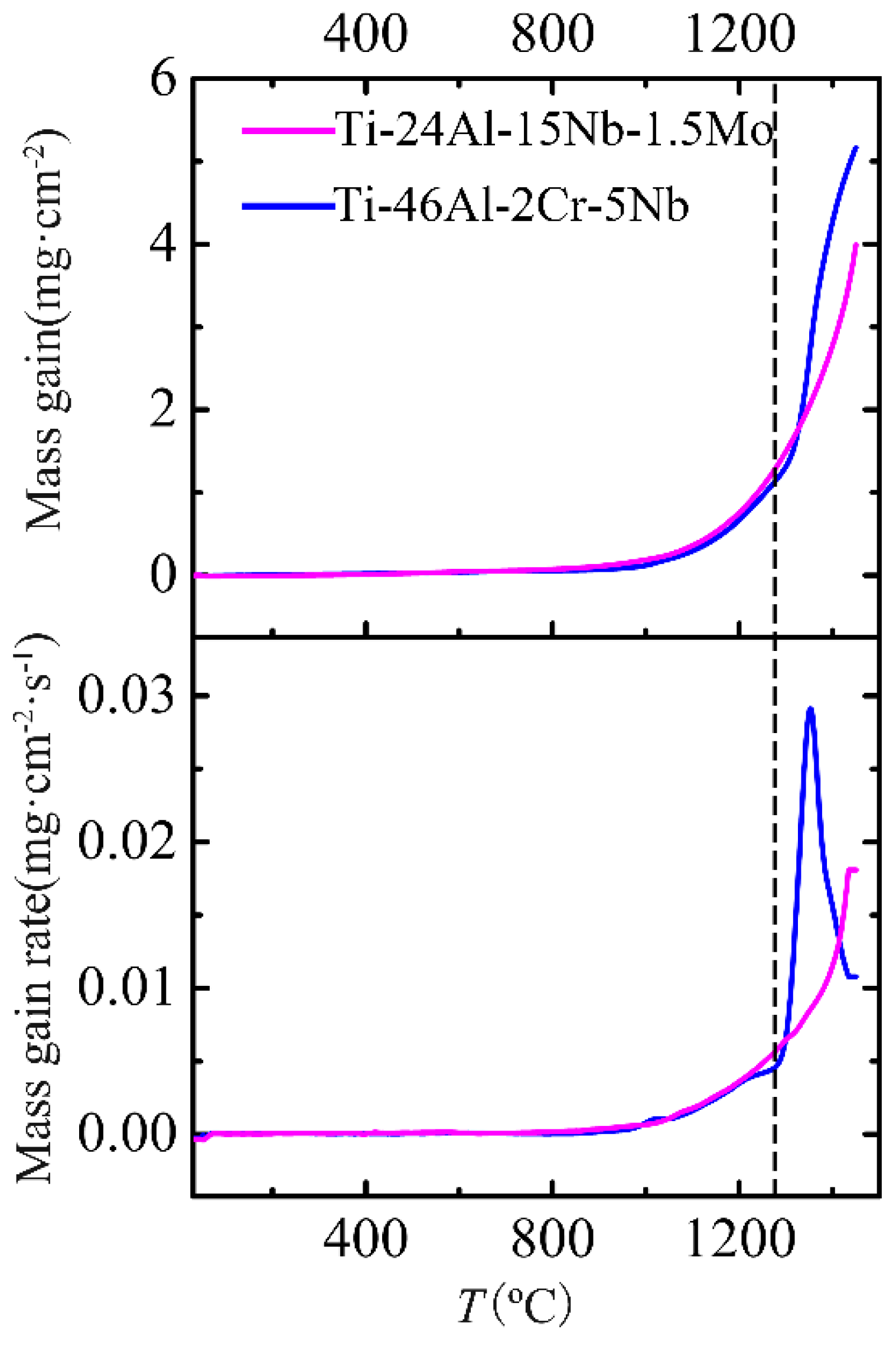
4.1. Oxidation Mass Gain and Activation Energies
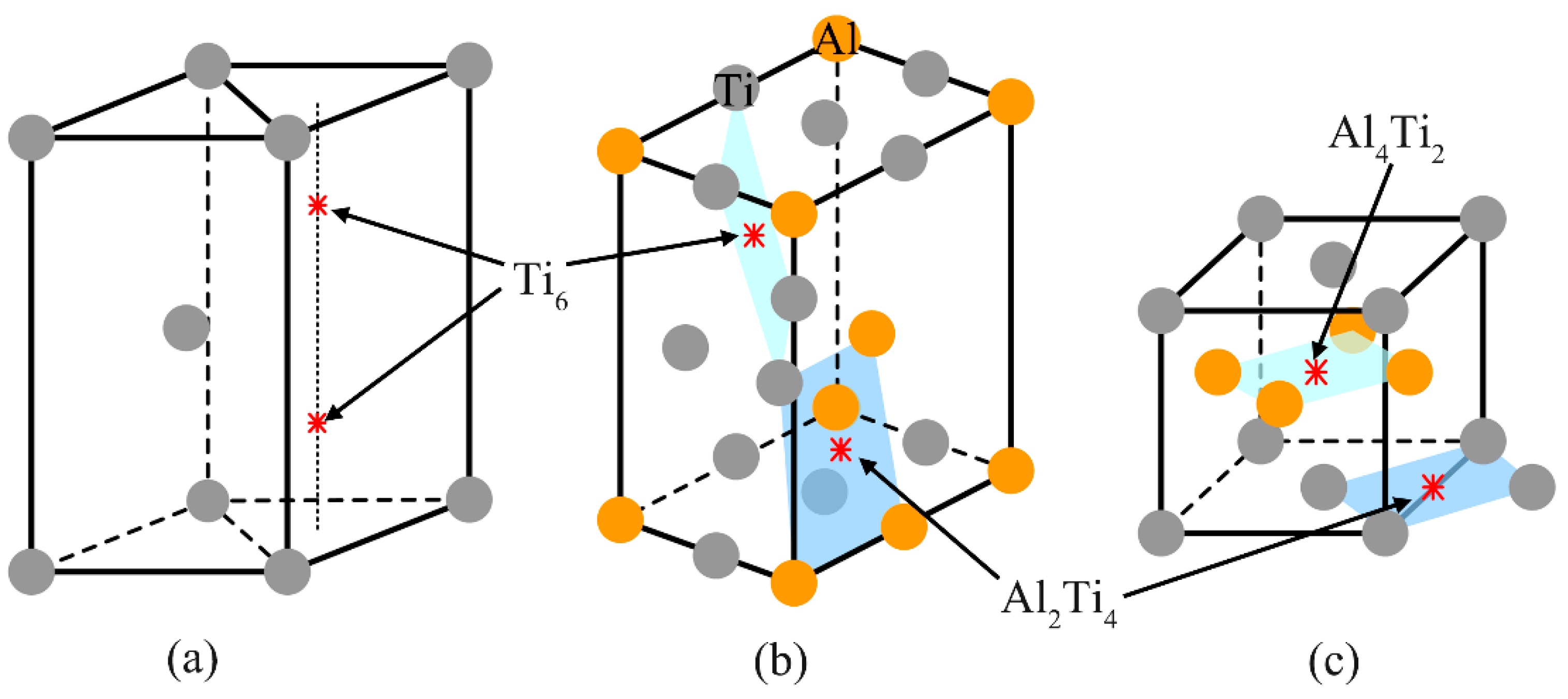
4.2. Matrix Phases
4.3. Non-Isothermal Oxidation Mechanisms
4.3.1. Nearly Non-Oxidation Stage (Stage I)
4.3.2. Slow Oxidation Stage (Stage II)
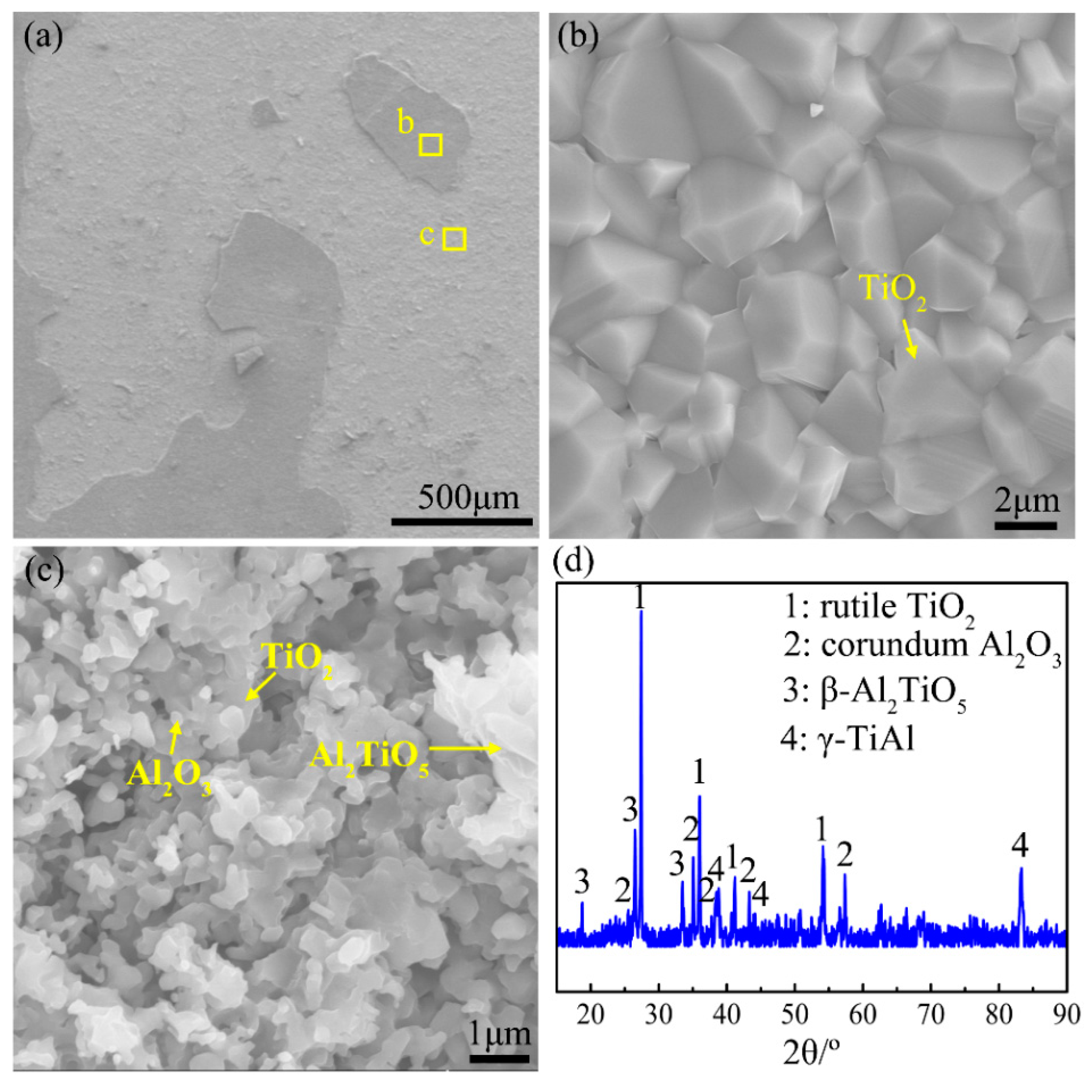
4.3.3. Accelerated Oxidation Stage (Stage III)
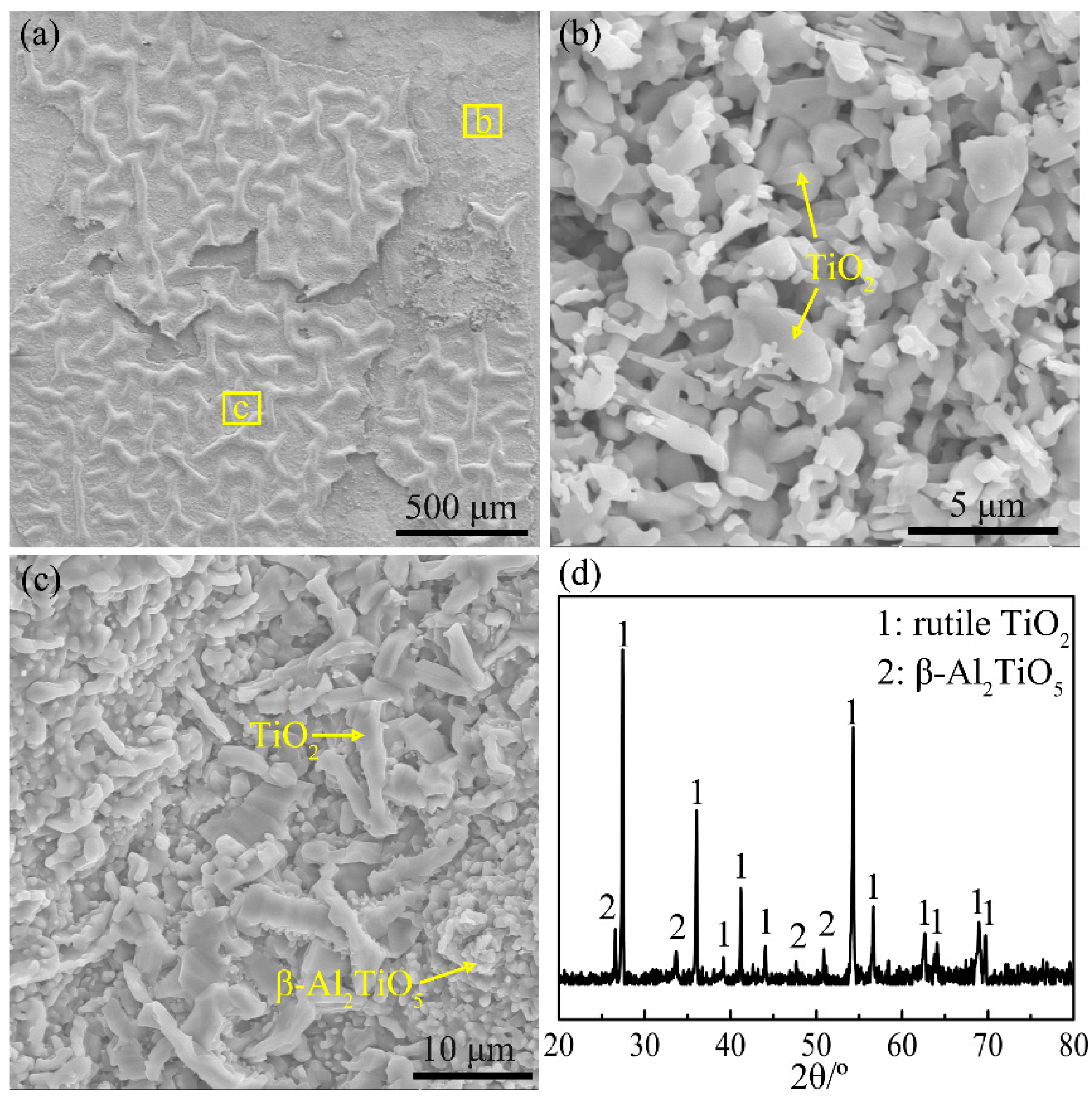
4.3.4. Severe Oxidation Stage (Stage IV)
4.3.5. Decelerated Oxidation Stage (Stage V)
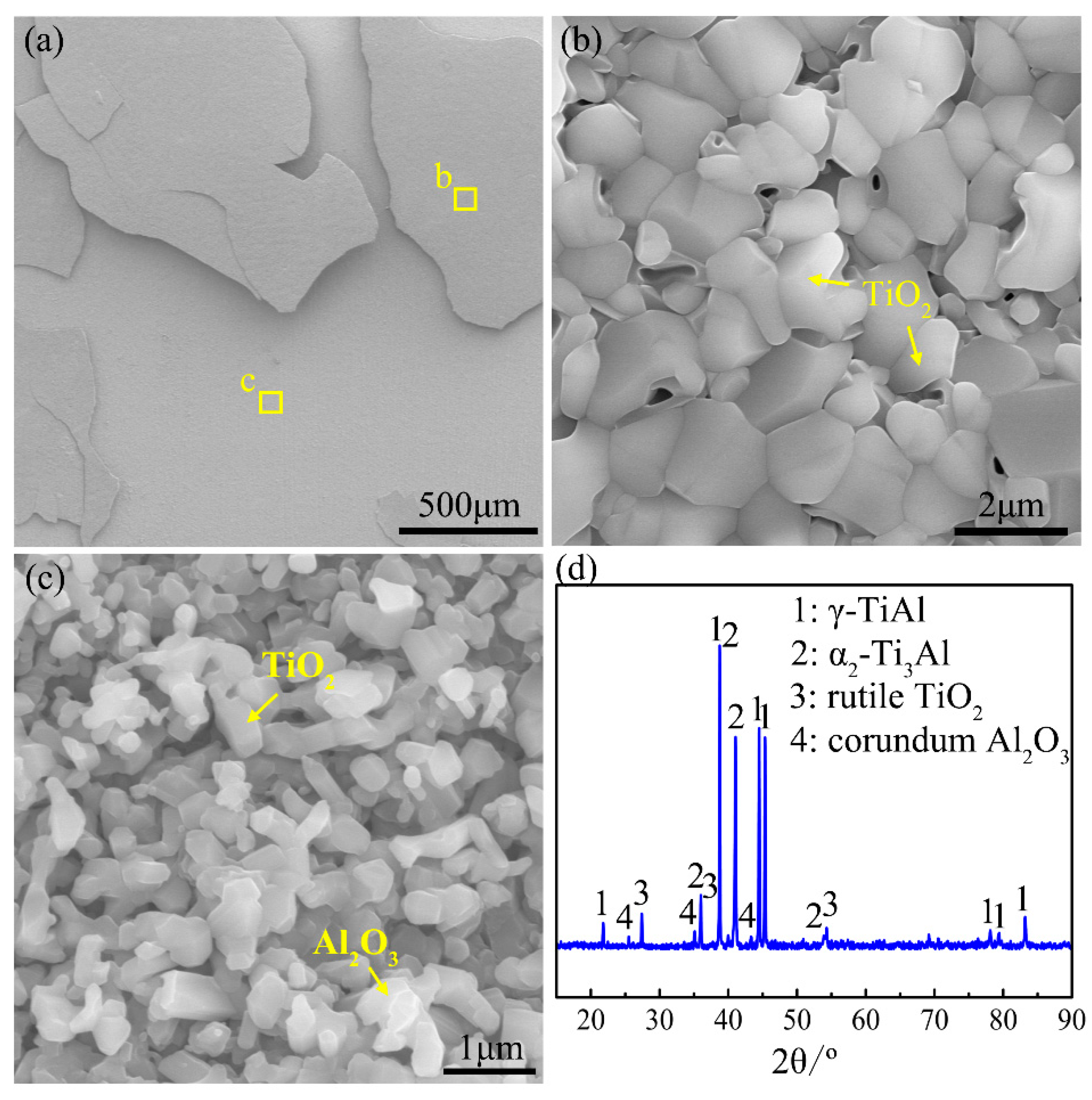
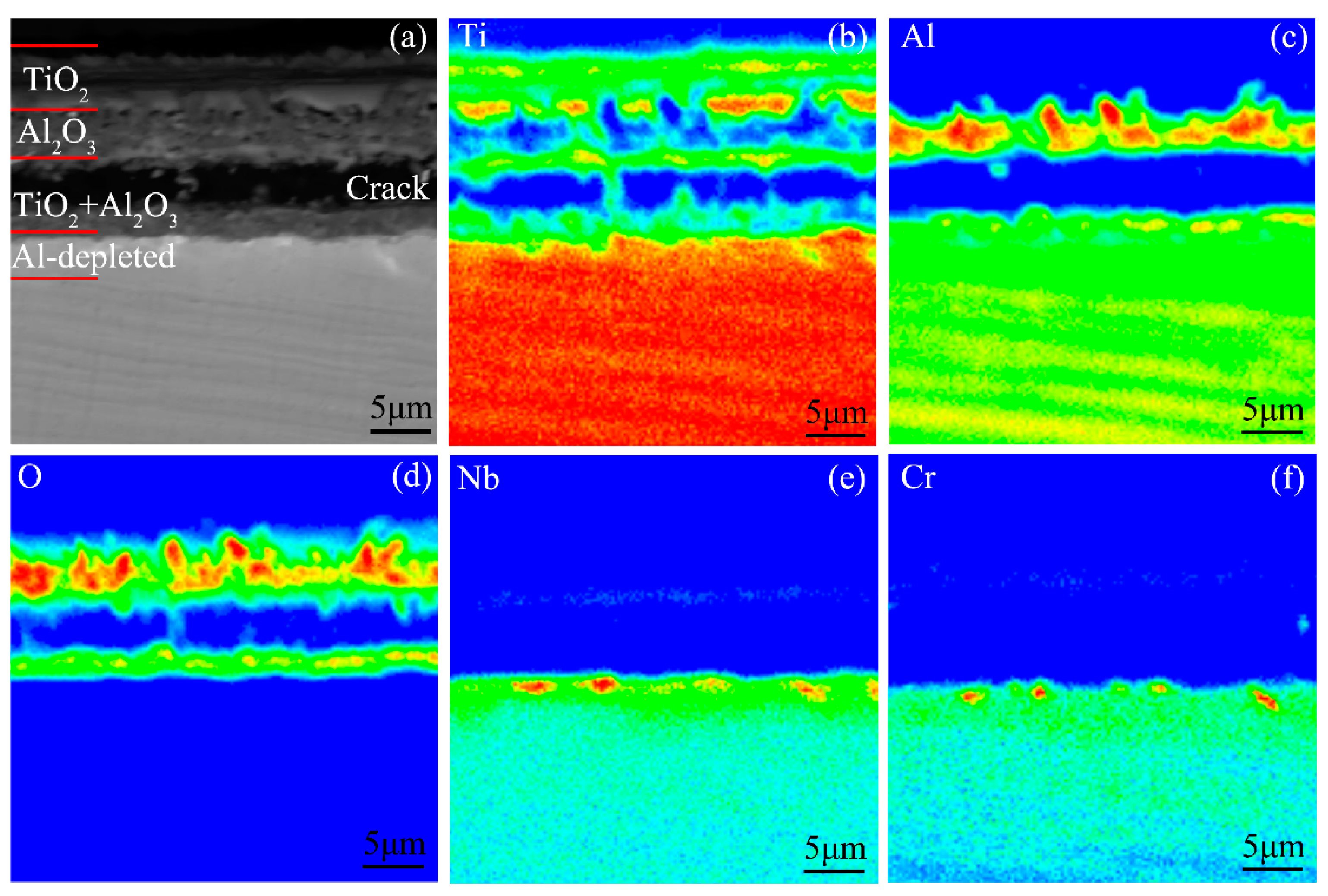
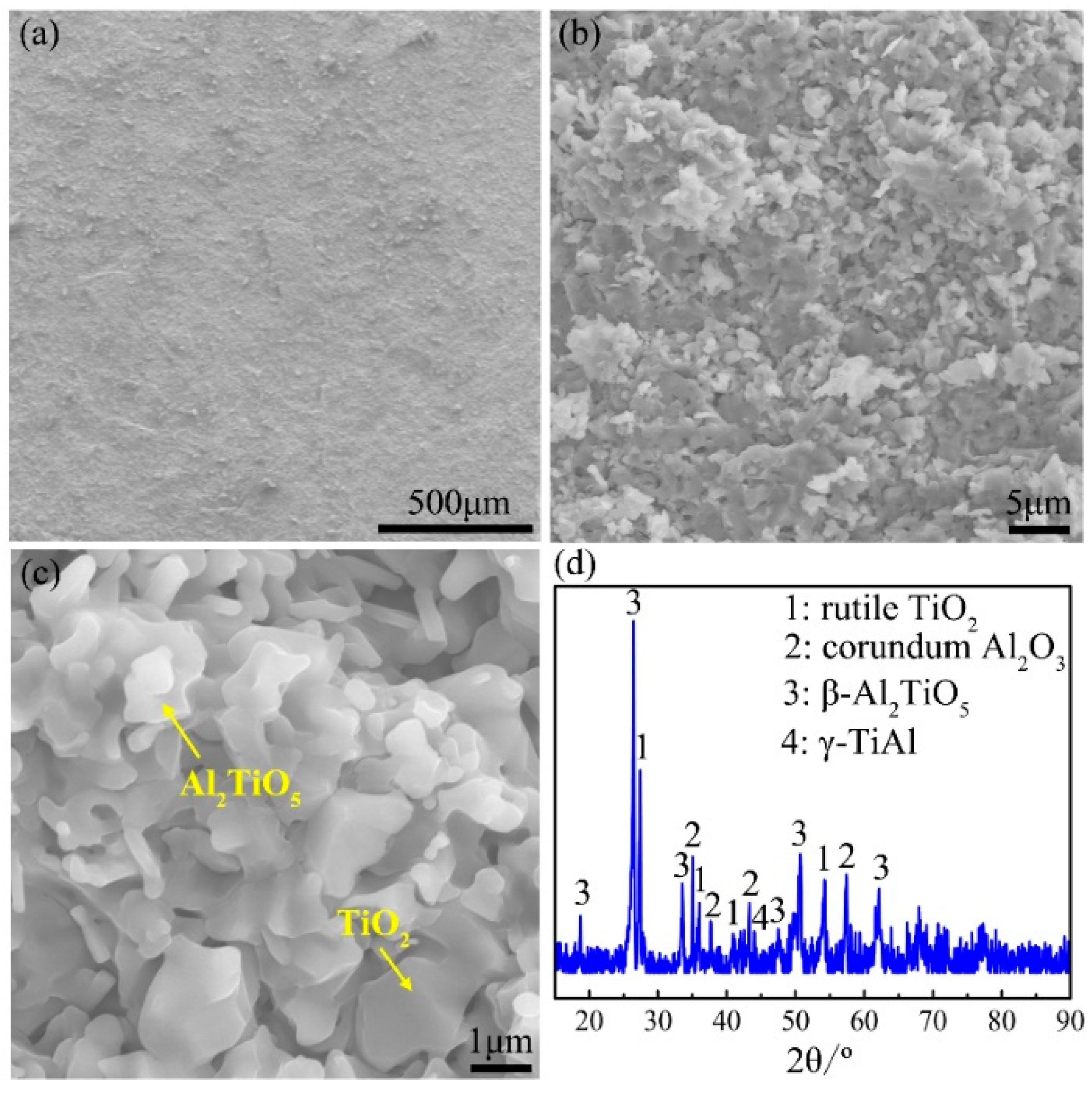
4.4. Reasons for the Occurrence of Internal Oxidation in the Ti-46Al-2Cr-5Nb Alloy
5. Conclusions
- The non-isothermal oxidation behaviors of the Ti-46Al-2Cr-5Nb and Ti-24Al-15Nb-1.5Mo alloys are similar when the temperature is below 1280 °C, while the Ti-46Al-2Cr-5Nb alloy exhibits poorer oxidation resistance than the Ti-24Al-15Nb-1.5Mo alloy when the temperature exceeds 1280 °C, even though the oxidation rate of the Ti-46Al-2Cr-5Nb alloy decreases significantly when the temperature is above 1350 °C.
- There are five stages in the non-isothermal oxidation process of the Ti-46Al-2Cr-5Nb alloy, including nearly non-oxidation (<870 °C), slow oxidation (870–980 °C), accelerated oxidation (980–1280 °C), severe oxidation (1280–1350 °C) and decelerated oxidation (1350–1450 °C) stages. The corresponding oxidation mechanisms are as follows: oxygen-barrier effect of the thin titanium oxide film; oxygen dissolution in the alloy; growth of the oxide scale dominated by TiO2; internal oxidation of Al; formation of an oxygen-barrier Al2TiO5-rich layer by reaction between TiO2 and Al2O3 in the oxide scale.
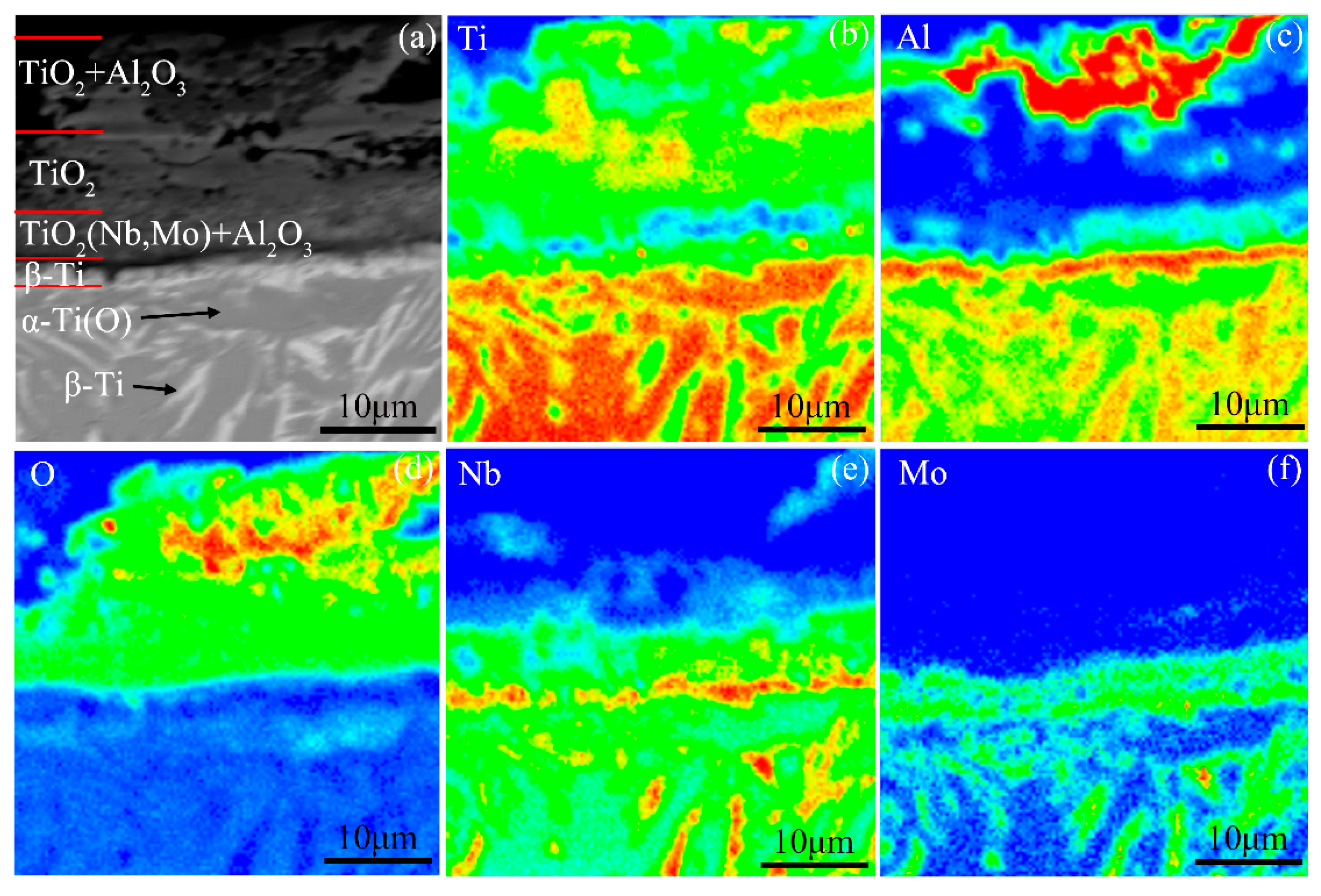
- There are four stages in the non-isothermal oxidation process of the Ti-24Al-15Nb-1.5Mo alloy, including nearly non-oxidation (<800 °C), slow oxidation (800–1020 °C), accelerated oxidation (1020–1400 °C) and severe oxidation (1400–1450 °C) stages. The oxidation mechanisms for the first three stages are the same with that of the Ti-46Al-2Cr-5Nb alloy, while the oxidation mechanism for the last stage is the dissolution, migration and re-precipitation of Al2O3 in the oxide.
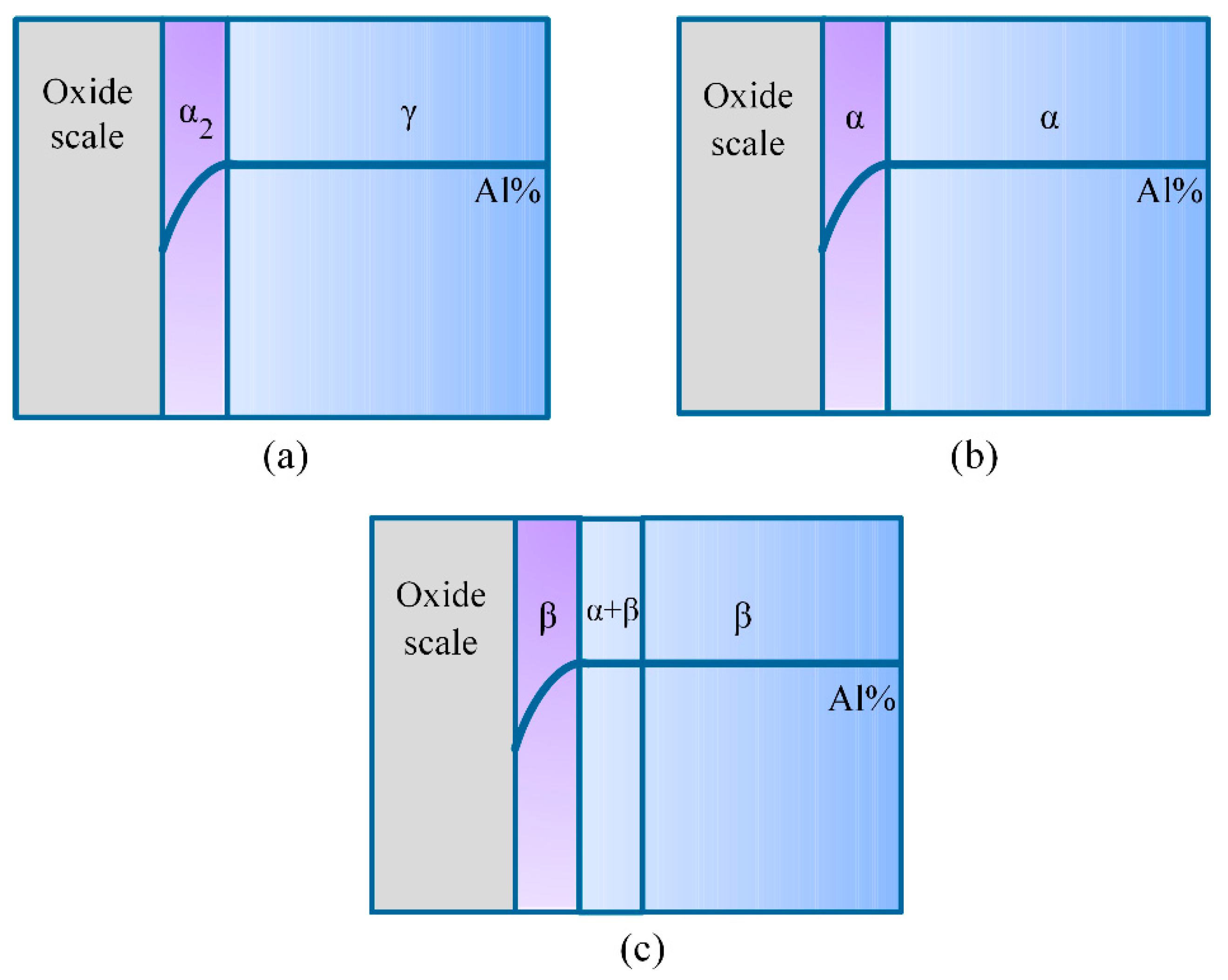
- The tendency of internal oxidation for the different phases in the subsurface of the Ti-46Al-2Cr-5Nb alloy is in the order of α > β > α2 > γ. The formation of α phase in the subsurface is the basic reason for the occurrence of internal oxidation in the Ti-46Al-2Cr-5Nb alloy. The tendency of internal oxidation in the TiAl-based alloys could be reduced through avoiding the formation of α phase by optimizing alloy ingredients such as increasing Al content or adding β-stabilizing elements.
Author Contributions
Funding
Conflicts of Interest
References
- Wu, X. Review of alloy and process development of TiAl alloys. Intermetallics 2006, 14, 1114–1122. [Google Scholar] [CrossRef]
- Clemens, H.; Mayer, S. Design, processing, microstructure, properties, and applications of advanced intermetallic TiAl alloys. Adv. Eng. Mater. 2013, 15, 191–215. [Google Scholar] [CrossRef]
- Bewlay, B.; Weimer, M.; Kelly, T.; Suzuki, A.; Subramanian, P. The science, technology, and implementation of TiAl alloys in commercial aircraft engines. MRS Online Proc. Lib. Arch. 2013, 1516, 49–58. [Google Scholar] [CrossRef]
- Cai, J.; Mi, G.; Gao, F.; Huang, H.; Cao, J.; Huang, X.; Cao, C. Research and development of some advanced high temperature titanium alloys for aero-engine. J. Mater. Eng. 2016, 44, 1–10. [Google Scholar]
- Ouyang, P.; Mi, G.; Cao, J.; Huang, X.; He, L.; Li, P. Microstructure characteristics after combustion and fireproof mechanism of TiAl-based alloys. Mater. Today Commun. 2018, 16, 364–373. [Google Scholar] [CrossRef]
- Kim, D.; Seo, D.; Saari, H.; Sawatzky, T.; Kim, Y.W. Isothermal oxidation behavior of powder metallurgy beta gamma TiAl-2Nb-2Mo alloy. Intermetallics 2011, 19, 1509–1516. [Google Scholar] [CrossRef]
- Kim, S.W.; Hong, J.K.; Na, Y.S.; Yeom, J.T.; Kim, S.E. Development of TiAl alloys with excellent mechanical properties and oxidation resistance. Mater. Design 2014, 54, 814–819. [Google Scholar] [CrossRef]
- Legzdina, D.; Robertson, I.; Birnbaum, H. Oxidation behavior of a single phase γ-TiAl alloy in low-pressure oxygen and hydrogen. Acta Mater. 2005, 53, 601–608. [Google Scholar] [CrossRef]
- Lu, W.; Chen, C.; He, L.; Wang, F.; Lin, J.; Chen, G. (S)TEM study of different stages of Ti-45Al-8Nb-0.2W-0.2B-0.02Y alloy oxidation at 900 °C. Corros. Sci. 2008, 50, 978–988. [Google Scholar] [CrossRef]
- Qu, S.; Tang, S.; Feng, A.; Feng, C.; Shen, J.; Chen, D. Microstructural evolution and high-temperature oxidation mechanisms of a titanium aluminide based alloy. Acta Mater. 2018, 148, 300–310. [Google Scholar] [CrossRef]
- Strobridge, T.R.; Moulder, J.C.; Clark, A.F. Titanium Combustion in Turbine Engines; Report FAA-RD-79-51, NBSIR 79-1616; US National Bureau of Standards: Washington, DC, USA, 1979.
- Li, B.; Chen, G.; Zhang, H.; Sheng, C.D. Development of non-isothermal TGA–DSC for kinetics analysis of low temperature coal oxidation prior to ignition. Fuel 2014, 118, 385–391. [Google Scholar] [CrossRef]
- Hosseini, S.G.; Sheikhpour, A.; Keshavarz, M.H.; Tavangar, S. The effect of metal oxide particle size on the thermal behavior and ignition kinetic of Mg-CuO thermite mixture. Thermochim. Acta 2016, 626, 1–8. [Google Scholar] [CrossRef]
- Beye, R.; Verwerft, M.; Hosson, J.D.; Gronsky, R. Oxidation subscale of γ-titanium aluminide. Acta Mater. 1996, 44, 4225–4231. [Google Scholar] [CrossRef]
- Beye, R.; Gronsky, R. Novel phases in the oxidation of γ-titanium aluminum. Acta Metall. Mater. 1994, 42, 1373–1381. [Google Scholar] [CrossRef]
- Rahmel, A.; Schütze, M.; Quadakkers, W. Fundamentals of TiAl oxidation-a critical review. Mater. Corros. 1995, 46, 271–285. [Google Scholar] [CrossRef]
- Becker, S.; Rahmel, A.; Schorr, M.; Schütze, M. Mechanism of isothermal oxidation of the intermetallic TiAl and of TiAl alloys. Oxid. Met. 1992, 38, 425–464. [Google Scholar] [CrossRef]
- Vaidya, R.U.; Park, Y.S.; Zhe, J.; Gray, G.T.; Butt, D.P. High-temperature oxidation of Ti-48Al-2Nb-2Cr and Ti-25Al-10Nb-3V-1Mo. Oxid. Met. 1998, 50, 215–240. [Google Scholar] [CrossRef]
- Das, S. The Al-O-Ti (aluminum-oxygen-titanium) system. J. Phase Equilib. 2002, 23, 525–536. [Google Scholar] [CrossRef]
- Chattopadhyay, K.; Mitra, R.; Ray, K. Nonisothermal and isothermal oxidation behavior of Nb-Si-Mo alloys. Metall. Mater. Trans. A 2008, 39, 577–592. [Google Scholar] [CrossRef]
- Schulz, O.; Eisenreich, N.; Kelzenberg, S.; Schuppler, H.; Neutz, J.; Kondratenko, E. Non-isothermal and isothermal kinetics of high temperature oxidation of micrometer-sized titanium particles in air. Thermochim. Acta 2011, 517, 98–104. [Google Scholar] [CrossRef]
- Mi, G.B.; Huang, X.S.; Li, P.J.; Cao, J.X.; Huang, X.; Cao, C.X. Non-isothermal oxidation and ignition prediction of Ti-Cr alloys. Trans. Nonferr. Metal Soc. 2012, 22, 2409–2415. [Google Scholar] [CrossRef]
- Ouyang, P.; Mi, G.; Li, P.; He, L.; Cao, J.; Huang, X. Non-isothermal oxidation behavior and mechanism of a high temperature near-α titanium alloy. Materials 2018, 11, 2141. [Google Scholar] [CrossRef] [PubMed]
- Kofstad, P. High-temperature oxidation of titanium. J. Less-Common Metals 1967, 12, 449–464. [Google Scholar] [CrossRef]
- Kofstad, P.; Hauffe, K.; Kjollesdal, H. Investigation on the oxidation mechanism of titanium. Acta Chem. Scand. 1958, 12, 239–266. [Google Scholar] [CrossRef]
- Kofstad, P.; Anderson, P.; Krudtaa, O. Oxidation of titanium in the temperature range 800–1200 °C. J. Less-Common Metals 1961, 3, 89–97. [Google Scholar] [CrossRef]
- Overton, J.M. Thermophysical Property and Phase Transformation Determination of Gamma-TiAl Intermetallics. Master’s Thesis, Carleton University, Ottawa, ON, Canada, May 2006. [Google Scholar]
- Ishikawa, K.; Kazuhiro, R.; Ishida, K. The Ti-Al Binary System. Available online: https://materials.springer.com/msi/phase-diagram/docs/sm_msi_r_10_010909_02_full_LnkDia0 (accessed on 15 May 2019).
- Terner, M.; Biamino, S.; Ugues, D.; Sabbadini, S.; Fino, P.; Pavese, M.; Badini, C. Phase transitions assessment on γ-TiAl by thermo mechanical analysis. Intermetallics 2013, 37, 7–10. [Google Scholar] [CrossRef]
- Malinov, S.; Novoselova, T.; Sha, W. Experimental and modelling studies of the thermodynamics and kinetics of phase and structural transformations in a gamma TiAl-based alloy. Mater. Sci. Eng. A 2004, 386, 344–353. [Google Scholar] [CrossRef]
- Huang, X. Advanced Aeronautical Titanium Alloys and Applications; National Defense Industry Press: Beijing, China, 2012. [Google Scholar]
- Koizumi, Y.; Kishimoto, M.; Minamino, Y.; Nakajima, H. Oxygen diffusion in Ti3Al single crystals. Philos. Mag. 2008, 88, 2991–3010. [Google Scholar] [CrossRef]
- Scotti, L.; Mottura, A. Interstitial diffusion of O, N, and C in α-Ti from first-principles: Analytical model and kinetic Monte Carlo simulations. J. Chem. Phys. 2016, 144, 084701. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.H.; Trinkle, D.R. Direct diffusion through interpenetrating networks: Oxygen in titanium. Phys. Rev. Lett. 2011, 107, 045504. [Google Scholar] [CrossRef] [PubMed]
- Bakulin, A.V.; Latyshev, A.M.; Kulkova, S.E. Absorption and diffusion of oxygen in the Ti3Al alloy. J. Exp. Theor. Phys. 2017, 125, 138–147. [Google Scholar] [CrossRef]
- Jones, C.Y.; Luecke, W.E.; Copland, E. Neutron diffraction study of oxygen dissolution in α2-Ti3Al. Intermetallics 2006, 14, 54–60. [Google Scholar] [CrossRef]
- Unnam, J.; Shenoy, R.; Clark, R. Oxidation of commercial purity titanium. Oxid. Met. 1986, 26, 231–252. [Google Scholar] [CrossRef]
- Venkatu, D.; Poteat, L. Diffusion of titanium of single crystal rutile. Mater. Sci. Eng. 1970, 5, 258–262. [Google Scholar] [CrossRef]
- Maurice, V.; Despert, G.; Zanna, S.; Josso, P.; Bacos, M.P.; Marcus, P. XPS study of the initial stages of oxidation of α2-Ti3Al and γ-TiAl intermetallic alloys. Acta Mater. 2007, 55, 3315–3325. [Google Scholar] [CrossRef]
- Taniguchi, S.; Tachikawa, Y.; Shibata, T. Influence of oxygen partial pressure on the oxidation behaviour of TiAl at 1300 K. Mater. Sci. Eng. A 1997, 232, 47–54. [Google Scholar] [CrossRef]
- Goldberg, D. Contribution to study of systems formed by alumina and some oxides of trivalent and tetravalent metals especially titanium oxide. Rev. Int. Hautes Temp. Refract. 1968, 5, 181. [Google Scholar]
- Lang, C.; Schütze, M. TEM investigations of the early stages of TiAl oxidation. Oxid. Met. 1996, 46, 255–285. [Google Scholar] [CrossRef]
- Swain, M.V. Structure and Properties of Ceramics; Wiley-VCH: Weinheim, Germany, 1994. [Google Scholar]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys: Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Mishin, Y.; Herzig, C. Diffusion in the Ti-Al system. Acta Mater. 2000, 48, 589–623. [Google Scholar] [CrossRef]
- Liu, Z.; Welsch, G. Literature survey on diffusivities of oxygen, aluminum, and vanadium in alpha titanium, beta titanium, and in rutile. Metall. Trans. A 1988, 19, 1121–1125. [Google Scholar] [CrossRef]



| Stage | Temperature Range (°C) | Activation Energy (kJ/mol) | Matrix Phases | |||
|---|---|---|---|---|---|---|
| TiAl 1 | Ti3Al 2 | TiAl 1 | Ti3Al 2 | TiAl 1 | Ti3Al 2 | |
| I | <870 | <800 | - | - | γ + α2 | α2 + O + B2 |
| II | 870–980 | 800–1020 | 217.8 | 143.2 | γ + α2 | α2 + O + B2 → α2 + B2 |
| III | 980–1280 | 1020–1400 | 249.8 | 244.8 | γ + α2 → γ + α→α | α2 + B2 → B2 → β |
| IV | 1280–1350 | 1400–1450 | 985.0 | 608.6 | α | β |
| V | 1350–1450 | - | - | - | α | - |
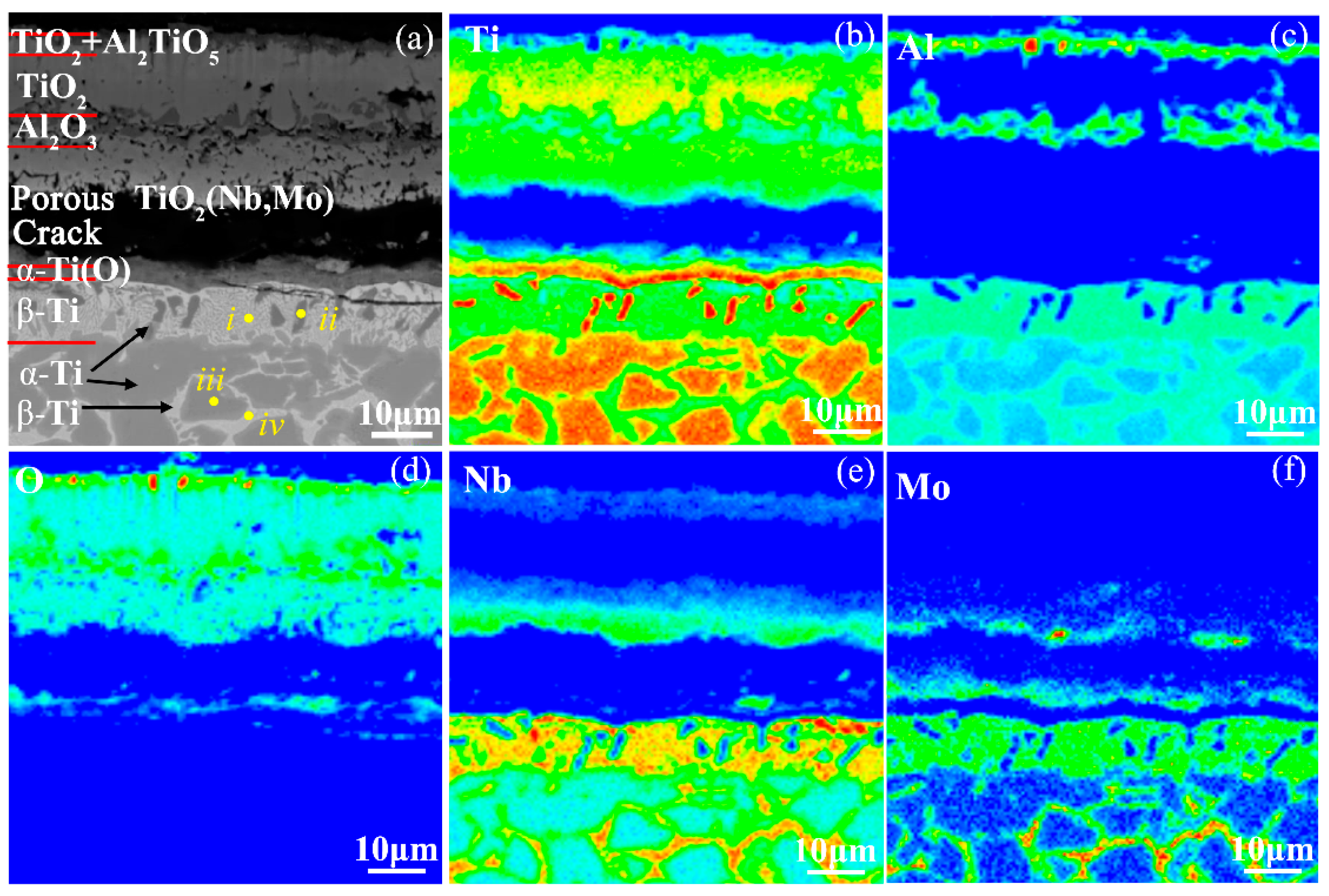
| Microstructures in Figure 12a | Compositions (at.%) | |||
|---|---|---|---|---|
| Ti | Al | Nb | Mo | |
| i | 42.99 | 20.15 | 34.66 | 2.2 |
| ii | 87.35 | 4.95 | 7.7 | - |
| iii | 75.62 | 12.77 | 11.23 | 0.37 |
| iv | 42.67 | 19.57 | 34.77 | 2.99 |
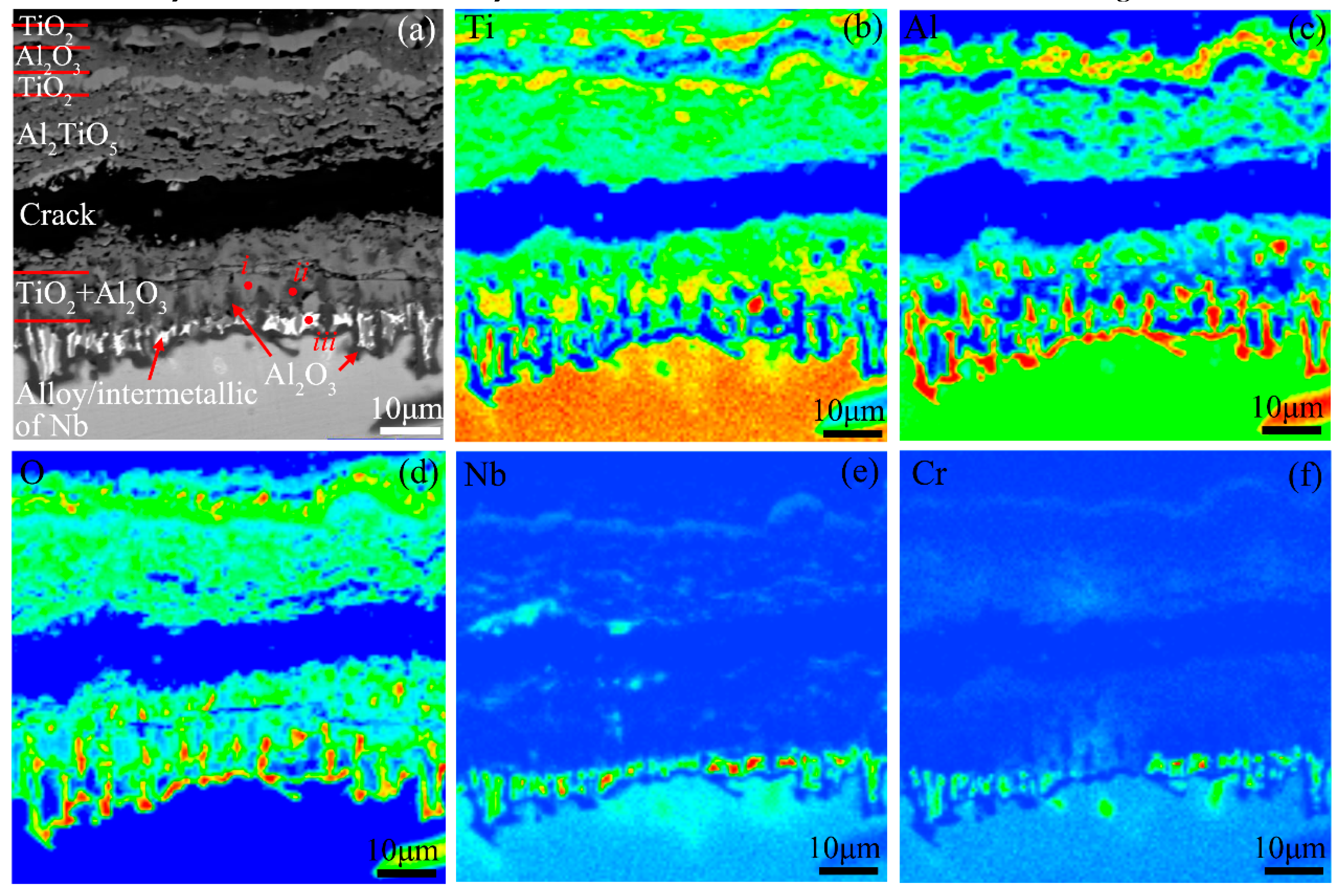
| Microstructures in Figure 14a | Compositions (at.%) | ||||
|---|---|---|---|---|---|
| Ti | Al | Nb | Cr | O | |
| i | 44.61 | 3.96 | 0.24 | - | 51.19 |
| ii | 5.66 | 26.09 | - | - | 68.25 |
| iii | 29.25 | 14.25 | 39.59 | 16.91 | - |
| Phases | Parameters | Results | |||
|---|---|---|---|---|---|
| Vm/(cm3/mol) | DO/(m2/s) | DAl [45]/(m2/s) | DO/DAl | Ncrit(Al) | |
| γ | 19.7 | <3.66 × 10−15 | 1.97 × 10−14 | <0.186 | <0.05 |
| α2 | 40.7 | 3.66 × 10−15 [32] | 1.37 × 10−14 | 0.267 | 0.085 |
| α | 10.6 | 3.54 × 10−11 [46] | 5.80 × 10−14 | 610.3 | 2.08 |
| β | 11.0 | 3.75 × 10−10 [46] | 1.04 × 10−12 | 360.6 | 1.62 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, P.; Mi, G.; Li, P.; He, L.; Cao, J.; Huang, X. Non-Isothermal Oxidation Behaviors and Mechanisms of Ti-Al Intermetallic Compounds. Materials 2019, 12, 2114. https://doi.org/10.3390/ma12132114
Ouyang P, Mi G, Li P, He L, Cao J, Huang X. Non-Isothermal Oxidation Behaviors and Mechanisms of Ti-Al Intermetallic Compounds. Materials. 2019; 12(13):2114. https://doi.org/10.3390/ma12132114
Chicago/Turabian StyleOuyang, Peixuan, Guangbao Mi, Peijie Li, Liangju He, Jingxia Cao, and Xu Huang. 2019. "Non-Isothermal Oxidation Behaviors and Mechanisms of Ti-Al Intermetallic Compounds" Materials 12, no. 13: 2114. https://doi.org/10.3390/ma12132114
APA StyleOuyang, P., Mi, G., Li, P., He, L., Cao, J., & Huang, X. (2019). Non-Isothermal Oxidation Behaviors and Mechanisms of Ti-Al Intermetallic Compounds. Materials, 12(13), 2114. https://doi.org/10.3390/ma12132114





