Effect of Graphene Oxide on the Crystallization of Calcium Carbonate by C3S Carbonation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CaCO3 by the Carbonation Method
2.3. Characterization Methods
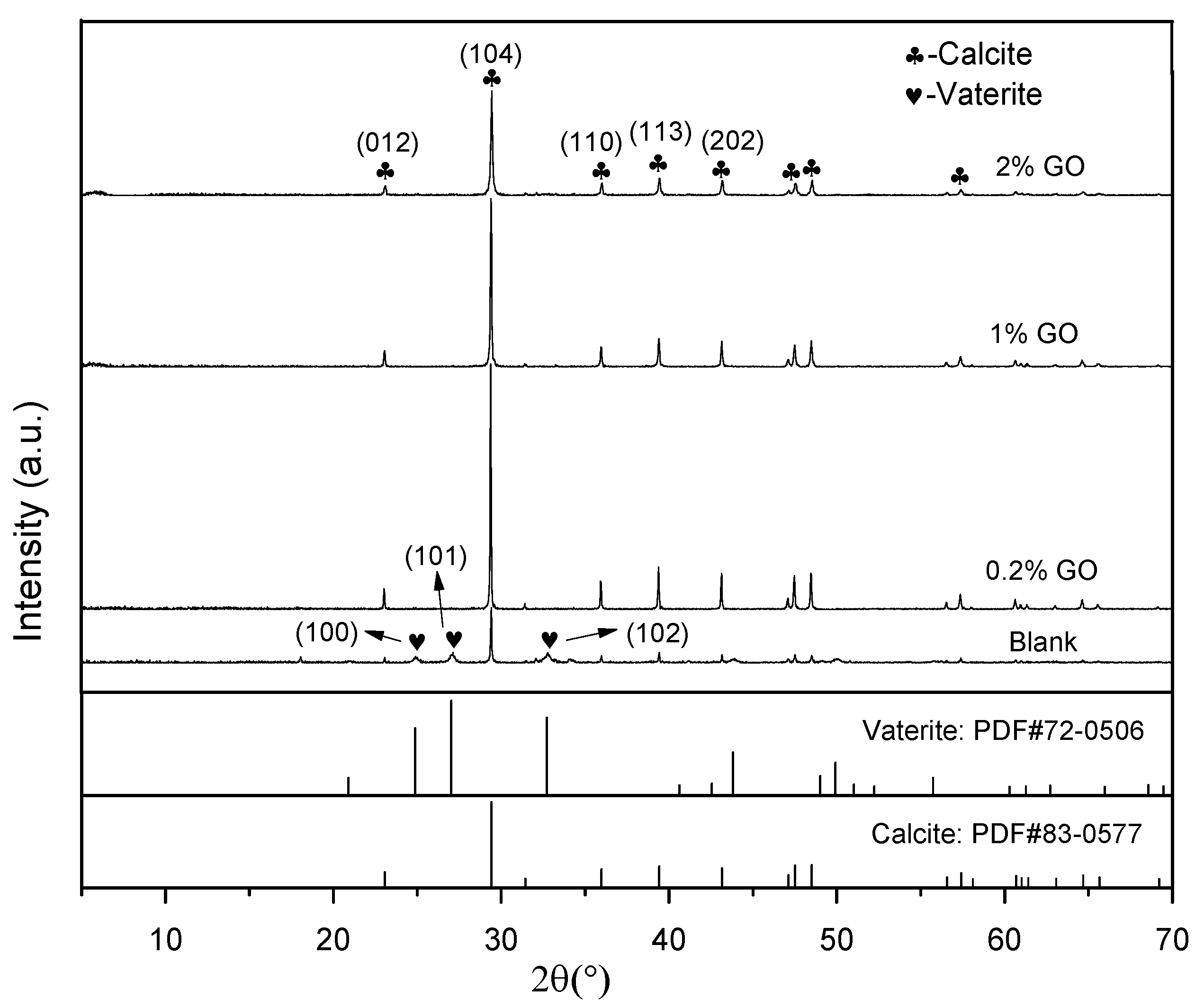
3. Results and Discussion
3.1. Effect of GO on the Crystal Polymorph of CaCO3
3.2. Effect of GO on the Crystal Size of CaCO3
3.3. Effect of GO on the Morphology of CaCO3
4. Conclusions and Recommendations
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GO | Graphene oxide |
| CaCO3 | Calcium carbonate |
| C3S | Tricalcium silicate (3CaO·SiO2) |
| C-S-H | Calcium silicate hydrate (3CaO·SiO2 3H2O) |
| CO2 | Carbon dioxide |
| ESEM | Environmental scanning electronic microscope |
| XRD | X-ray diffraction |
| CaCl2 | Anhydrous calcium chloride |
| AFt | Ettringite |
| AFm | Monosulfate |
References
- Yao, C.; Xie, A.; Shen, Y.; Zhu, J.; Li, H. Nacre-like calcium carbonate controlled by ionic liquid/graphene oxide composite template. Mater. Sci. Eng. C 2015, 51, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Jiang, L.; Tang, Z. Bioinspired layered materials with superior mechanical performance. Acc. Chem. Res. 2014, 47, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wei, W.; Hu, Y.H. Catalytic behavior of graphene oxide for cement hydration process. J. Phys. Chem. Solids 2016, 89, 128–133. [Google Scholar] [CrossRef]
- Tong, T.; Fan, Z.; Liu, Q.; Wang, S.; Tan, S.; Yu, Q. Investigation of the effects of graphene and graphene oxide nanoplatelets on the micro- and macro-properties of cementitious materials. Constr. Build. Mater. 2016, 106, 102–114. [Google Scholar] [CrossRef]
- Wille, K.; Naaman, A.E.; El-Tawil, S.; Parra-Montesinos, G.J. Ultra-high performance concrete and fiber reinforced concrete: Achieving strength and ductility without heat curing. Mater. Struct. 2011, 45, 309–324. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Yang, H.; Monasterio, M.; Cui, H.; Han, N. Experimental study of the effects of graphene oxide on microstructure and properties of cement paste composite. Compos. Part A Appl. Sci. Manuf. 2017, 102, 263–272. [Google Scholar] [CrossRef]
- Yang, H.; Cui, H.; Tang, W.; Li, Z.; Han, N.; Xing, F. A critical review on research progress of graphene/cement based composites. Compos. Part A Appl. Sci. Manuf. 2017, 102, 273–296. [Google Scholar] [CrossRef]
- Yu, J.; Lei, M.; Cheng, B.; Zhao, X. Facile preparation of calcium carbonate particles with unusual morphologies by precipitation reaction. J. Cryst. Growth 2004, 261, 566–570. [Google Scholar] [CrossRef]
- Dalas, E.; Klepetsanis, P.; Koutsoukos, P. The overgrowth of calcium carbonate on poly (vinyl chloride-co-vinyl acetate-co-maleic acid). Langmuir ACS J. Surf. Colloids 1999, 15, 8322–8327. [Google Scholar] [CrossRef]
- Kitamura, M.; Konno, H.; Yasui, A.; Masuoka, H. Controlling factors and mechanism of reactive crystallization of calcium carbonate polymorphs from calcium hydroxide suspensions. J. Cryst. Growth 2002, 236, 323–332. [Google Scholar] [CrossRef]
- He, M.; Forssberg, E.; Wang, Y.; Han, Y. Ultrasonication-Assisted Synthesis of Calcium Carbonate Nanoparticles. Chem. Eng. Commun. 2005, 192, 1468–1481. [Google Scholar] [CrossRef]
- Mishra, S.; Sonawane, S.H.; Singh, R.P. Studies on characterization of nano CaCO3 prepared by thein situ deposition technique and its application in PP-nano CaCO3 composites. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 107–113. [Google Scholar] [CrossRef]
- Nitta, I.; Kida, A.; Fujibayashi, Y.; Katayama, H.; Sugimura, Y. Calcium carbonate deposition in a cell wall sac formed in mulberry idioblasts. Protoplasma 2006, 228, 201–208. [Google Scholar] [CrossRef]
- Robbins, L.; Blackwelder, P. Biochemical and ultrastructural evidence for the origin of whitings: A biologically induced calcium carbonate precipitation mechanism. Geology 1992, 20, 464–468. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, P.; Zhao, J.; Zhao, X.; Liu, Y.; Wang, Z. Biomimetic synthesis of hydrophobic calcium carbonate nanoparticles via a carbonation route. Powder Technol. 2006, 170, 31–35. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, X.; Zhao, G.; Wang, X. Facile preparation of cubic calcium carbonate nanoparticles with hydrophobic properties via a carbonation route. Powder Technol. 2010, 200, 144–148. [Google Scholar] [CrossRef]
- Dalas, E.; Malkaj, P. Calcium Carbonate Crystallization in the Presence of Aspartic Acid. Cryst. Growth Des. 2004, 4, 721–723. [Google Scholar]
- Hashim, A.; Al-Hosney, V.H.G. Carbonic Acid an Important Intermediate in the Surface Chemistry of Calcium Carbonate. J. Am. Chem. Soc. 2004, 126, 8068–8069. [Google Scholar]
- Chen, A.; Ma, P.; Fu, Z.; Wu, Y.; Kong, W. Crystallization and assembling behavior of calcium carbonate controlled by Ca-organic fibers. J. Cryst. Growth 2013, 377, 136–142. [Google Scholar] [CrossRef]
- Guo, H.; Qin, Z.; Qian, P.; Yu, P.; Cui, S.; Wang, W. Crystallization of aragonite CaCO3 with complex structures. Adv. Powder Technol. 2011, 22, 777–783. [Google Scholar] [CrossRef]
- Nassar, M.M.; Farrag, T.E.; Mahmoud, M.S.; Abdelmonem, S.; Khalil, K.A.; Barakat, N.A.M. Influence of the operating conditions on the morphology of CaCO3 nanoparticles prepared by modified co-precipitation with pulse mode feeding. Adv. Powder Technol. 2015, 26, 914–919. [Google Scholar] [CrossRef]
- Xu, A.-W.; Dong, W.-F.; Antonietti, M.; Cölfen, H. Polymorph Switching of Calcium Carbonate Crystals by Polymer-Controlled Crystallization. Adv. Funct. Mater. 2008, 18, 1307–1313. [Google Scholar] [CrossRef]
- Ji, X.; Li, G.; Huang, X. The synthesis of hollow CaCO3 microspheres in mixed solutions of surfactant and polymer. Mater. Lett. 2008, 62, 751–754. [Google Scholar] [CrossRef]
- Nishino, Y.; Oaki, Y.; Imai, H. Magnesium-Mediated Nanocrystalline Mosaics of Calcite. Cryst. Growth Des. 2009, 9, 223–226. [Google Scholar] [CrossRef]
- Cui, J.; Kennedy, J.F.; Nie, J.; Ma, G. Co-effects of amines molecules and chitosan films on in vitro calcium carbonate mineralization. Carbohydr. Polyme. 2015, 133, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Chen, X.; Li, X.; Liang, Q.; Miao, G.; Yuan, B. The synthesis of calcium carbonate microparticles with multiple morphologies through self-assembly method. Powder Technol. 2015, 284, 253–256. [Google Scholar] [CrossRef]
- Yaseen, S.A.; Yiseen, G.A.; Li, Z. Synthesis of calcium carbonate in alkali solution based on graphene oxide and reduced graphene oxide. J. Sol. State Chem. 2018, 262, 127–134. [Google Scholar] [CrossRef]
- Sun, J.; Lu, L. Coupled effect of axially distributed load and carbonization on permeability of concrete. Constr. Build. Mater. 2015, 79, 9–13. [Google Scholar] [CrossRef]
- Wu, B.; Ye, G. Development of porosity of cement paste blended with supplementary cementitious materials after carbonation. Constr. Build. Mater. 2017, 145, 52–61. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y. Effect of early carbonation curing on chloride penetration and weathering carbonation in concrete. Constr. Build. Mater. 2016, 123, 516–526. [Google Scholar] [CrossRef]
- Zelić, J.; Krstulović, R.; Tkalčec, E.; Krolo, P. Durability of the hydrated limestone-silica fume Portland cement mortars under sulphate attack. Cement Concr. Res. 1999, 29, 819–826. [Google Scholar] [CrossRef]
- Vuk, T.; Tinta, V.; Gabrovšek, R.; Kaučič, V. The effects of limestone addition, clinker type and fineness on properties of Portland cement. Cement Concr. Res. 2001, 31, 135–139. [Google Scholar] [CrossRef]
- Cao, M.; Ming, X.; He, K.; Li, L.; Shen, S. Effect of Macro-, Micro- and Nano-Calcium Carbonate on Properties of Cementitious Composites—A Review. Materials 2019, 12, 781. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Unluer, C. Unluer, Influence of mix design on the carbonation, mechanical properties and microstructure of reactive MgO cement-based concrete. Cem. Concr. Compos. 2017, 80, 104–114. [Google Scholar] [CrossRef]
- Jeong, Y.; Hargis, C.W.; Chun, S.; Moon, J. Effect of Calcium Carbonate Fineness on Calcium Sulfoaluminate-Belite Cement. Materials 2017, 10, 900. [Google Scholar] [CrossRef] [PubMed]
- Kudzma, A.; Skamat, J.; Stonys, R.; Krasnikovs, A.; Kuznetsov, D.; Girskas, G.; Antonovic, V. Study on the Effect of Graphene Oxide with Low Oxygen Content on Portland Cement Based Composites. Materials 2019, 12, 802. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; He, L.; Qiu, L.; Korayem, A.H.; Li, G.; Zhu, J.W.; Collins, F.; Li, D.; Duan, W.H.; Wang, M.C. Mechanical properties and microstructure of a graphene oxide–cement composite. Cement Concr. Compos. 2015, 58, 140–147. [Google Scholar] [CrossRef]
- Krystek, M.; Pakulski, D.; Patroniak, V.; Gorski, M.; Szojda, L.; Ciesielski, A.; Samori, P. High-Performance Graphene-Based Cementitious Composites. Adv. Sci. 2019, 6, 1801195. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.H.; Deng, L.J.; Yang, W.Q.; Zhou, Q.F.; Cui, Y.Y. Fabrication of polycarboxylate/graphene oxide nanosheet composites by copolymerization for reinforcing and toughening cement composites. Cement Concr. Compos. 2016, 66, 1–9. [Google Scholar] [CrossRef]
- Cui, H.; Yan, X.; Tang, L.; Xing, F. Possible pitfall in sample preparation for SEM analysis—A discussion of the paper “Fabrication of polycarboxylate/graphene oxide nanosheet composites by copolymerization for reinforcing and toughening cement composites” by Lv et al. Cement Concr. Compos. 2017, 77, 81–85. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhou, B.; Zhao, J.; Tao, N.; Yu, K.; Tian, Y.; Wang, Z. Influence of octadecyl dihydrogen phosphate on the formation of active super-fine calcium carbonate. J. Colloid Interface Sci. 2004, 272, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Monasterio, M.; Gaitero, J.J.; Erkizia, E.; Guerrero Bustos, A.M.; Miccio, L.A.; Dolado, J.S.; Cerveny, S. Effect of addition of silica- and amine functionalized silica-nanoparticles on the microstructure of calcium silicate hydrate (C-S-H) gel. J. Colloid Interface Sci. 2015, 450, 109–118. [Google Scholar] [CrossRef] [PubMed]
- de Sena Costaa, B.L.; de Oliveira Freitas, J.C.; Santos, P.H.S.; de Araújo Melo, D.M.; de Oliveira, Y.H. Effects of carbon dioxide in Portland cement: A relation between static sedimentation and carbonation. Constr. Build. Mater. 2017, 150, 450–458. [Google Scholar] [CrossRef]
- Kojima, Y.; Yamaguchi, K.; Nishimiya, N. Effect of amplitude and frequency of ultrasonic irradiation on morphological characteristics control of calcium carbonate. Ultrason. Sonochem. 2010, 17, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Kirboga, S.; Oner, M.; Akyol, E. The effect of ultrasonication on calcium carbonate crystallization in the presence of biopolymer. J. Cryst. Growth 2014, 401, 266–270. [Google Scholar] [CrossRef]
- Kato, T.; Sugawara, A.; Hosoda, N. Calcium carbonate–organic hybrid materials. Adv. Mater. 2002, 14, 869–877. [Google Scholar] [CrossRef]
- Kamali, M.; Ghahremaninezhad, A. A Study of Calcium-Silicate-Hydrate/Polymer Nanocomposites Fabricated Using the Layer-By-Layer Method. Materials 2018, 11, 527. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.J.; Sakai, E.; Daimon, M. Effect of particle size distribution of fly ash–cement system on the fluidity of cement pastes. Cement Concr. Res. 2003, 33, 763–768. [Google Scholar] [CrossRef]
- Konopacka-Łyskawa, D.; Kościelska, B.; Karczewski, J.; Gołąbiewska, A. The influence of ammonia and selected amines on the characteristics of calcium carbonate precipitated from calcium chloride solutions via carbonation. Mater. Chem. Phys. 2017, 193, 13–18. [Google Scholar] [CrossRef]



| h, k, l | GO% | 2θ(°) | Peak Height | Crystal Size (nm) |
|---|---|---|---|---|
| 012 | 0 | 23.031 | 178 | 92.38 |
| 0.2 | 23.053 | 697 | 87.62 | |
| 1 | 23.055 | 549 | 81.72 | |
| 2 | 23.055 | 225 | 60.71 | |
| 104 | 0 | 29.369 | 1860 | 90.63 |
| 0.2 | 29.399 | 8302 | 85.65 | |
| 1 | 29.400 | 5695 | 79.41 | |
| 2 | 29.400 | 3541 | 68.33 | |
| 110 | 0 | 35.936 | 227 | 84.43 |
| 0.2 | 35.968 | 954 | 79.34 | |
| 1 | 35.973 | 674 | 74.83 | |
| 2 | 35.973 | 417 | 67.35 | |
| 113 | 0 | 39.369 | 332 | 93.11 |
| 0.2 | 39.408 | 1441 | 83.44 | |
| 1 | 39.411 | 964 | 80.41 | |
| 2 | 39.411 | 588 | 59.37 | |
| 202 | 0 | 43.116 | 265 | 93.28 |
| 0.2 | 43.157 | 1221 | 89.72 | |
| 1 | 43.162 | 875 | 81.41 | |
| 2 | 43.162 | 501 | 60.11 |
| GO% | n Value | De Value (μm) |
|---|---|---|
| 0 | 1.847 | 15.705 |
| 0.2 | 1.999 | 13.002 |
| 1 | 2.159 | 9.540 |
| 2 | 2.256 | 6.591 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, D.; Yang, H.; Yu, F.; Zhang, B.; Cui, H. Effect of Graphene Oxide on the Crystallization of Calcium Carbonate by C3S Carbonation. Materials 2019, 12, 2045. https://doi.org/10.3390/ma12132045
Zheng D, Yang H, Yu F, Zhang B, Cui H. Effect of Graphene Oxide on the Crystallization of Calcium Carbonate by C3S Carbonation. Materials. 2019; 12(13):2045. https://doi.org/10.3390/ma12132045
Chicago/Turabian StyleZheng, Dapeng, Haibin Yang, Feng Yu, Bo Zhang, and Hongzhi Cui. 2019. "Effect of Graphene Oxide on the Crystallization of Calcium Carbonate by C3S Carbonation" Materials 12, no. 13: 2045. https://doi.org/10.3390/ma12132045
APA StyleZheng, D., Yang, H., Yu, F., Zhang, B., & Cui, H. (2019). Effect of Graphene Oxide on the Crystallization of Calcium Carbonate by C3S Carbonation. Materials, 12(13), 2045. https://doi.org/10.3390/ma12132045





