The mathematics employed in standard dielectric theory indicates an implicit assumption: The nature of the material on the ‘outside’ of a capacitor is irrelevant. A good example is the mathematics of the most ubiquitous capacitor, a parallel plate capacitor. To determine the dielectric constant of a material that fills the space between the electrodes of a parallel plate capacitor three values are required: Measured capacitance, the area of the electrodes, and the distance between them (Equation (2)). There is no mathematical provision made to account for the properties of material not between the electrodes. True also: In standard narrative descriptions of the impact of dielectrics on capacitance there is never consideration given to properties of material outside the volume enclosed by the electrodes. In contrast, in SDM theory the properties of all dielectric materials, both between the plates and outside the plates, must be considered. One notable shortcoming of the SDM theory is that there is no simple equation linking geometric and materials properties equivalent to Equation (2), thus at present the theory is only qualitative.
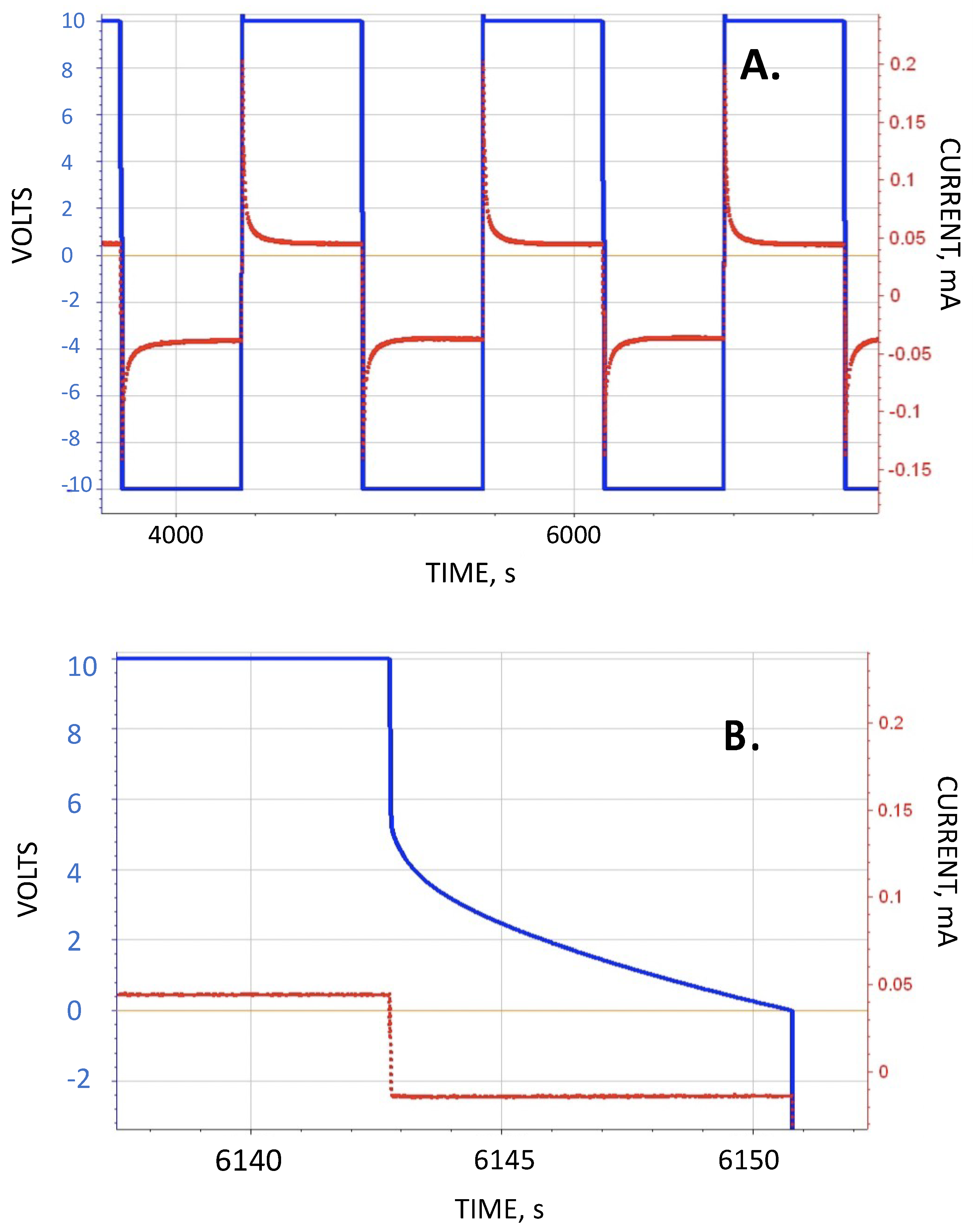
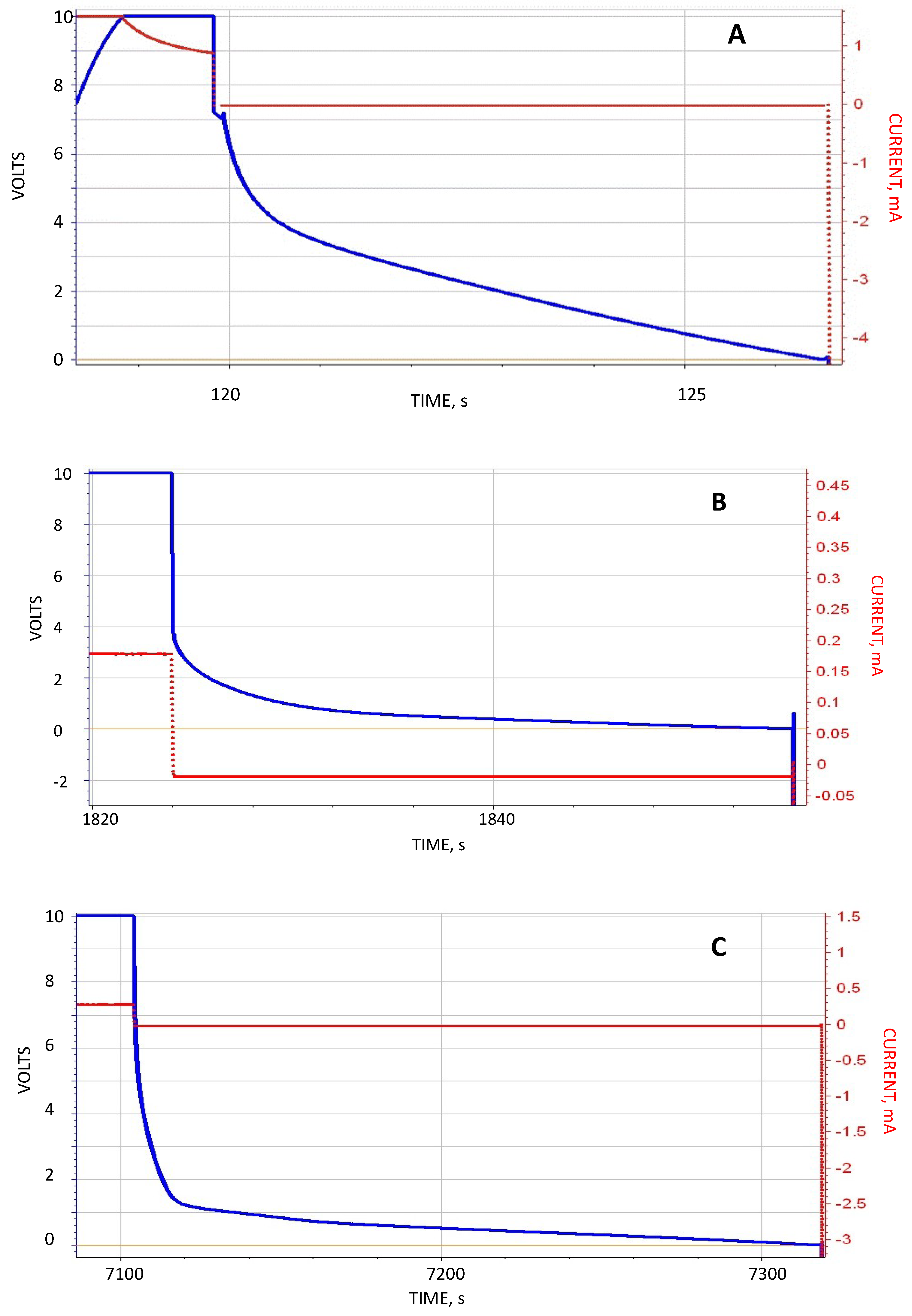
This study regards the use of a very simple test to contrast the predictions of the standard dielectric theory with the SDM theory. In this study parallel plate capacitors were constructed such that in most cases only ambient laboratory air was between the electrodes. The capacitors were then “immersed” in different media (1) ambient laboratory air, (2) DI water, (3) DI water containing 0.5 wt % NaCl, and (4) DI water containing 5.0 wt % NaCl. According to standard theory the impact of the dielectric properties of material outside the region between the plates is irrelevant, hence all four capacitor “immersed” configurations should operate identically. In contrast, according to SDM theory, the measured capacitance of the test capacitors immersed in water or salt water should be substantially higher than those embedded in ambient laboratory atmosphere. The results, in brief, were that those capacitors immersed in water or salt water had a capacitance at least seven orders of magnitude higher than measured for the same capacitors immersed in air. In fact, for the 5 wt % NaCl case the effective dielectric constant below 1 V was spectacular, more than >10,000,000,000× larger than the same capacitor immersed in laboratory air. Thus, the outcome of the experiments was only consistent with the SDM hypothesis.
This was not the first report of dielectric material outside the volume between the electrodes profoundly impacting performance. All the results reported were consistent with an earlier report from our laboratory, on the behavior of parallel plate capacitors covered with an SDM “gel” outside the volume between the electrodes [
2]. As noted earlier, the intent of the present study was to confirm and “generalize” the conclusions reached in the first publication on the topic.
4.1. Secondary Findings
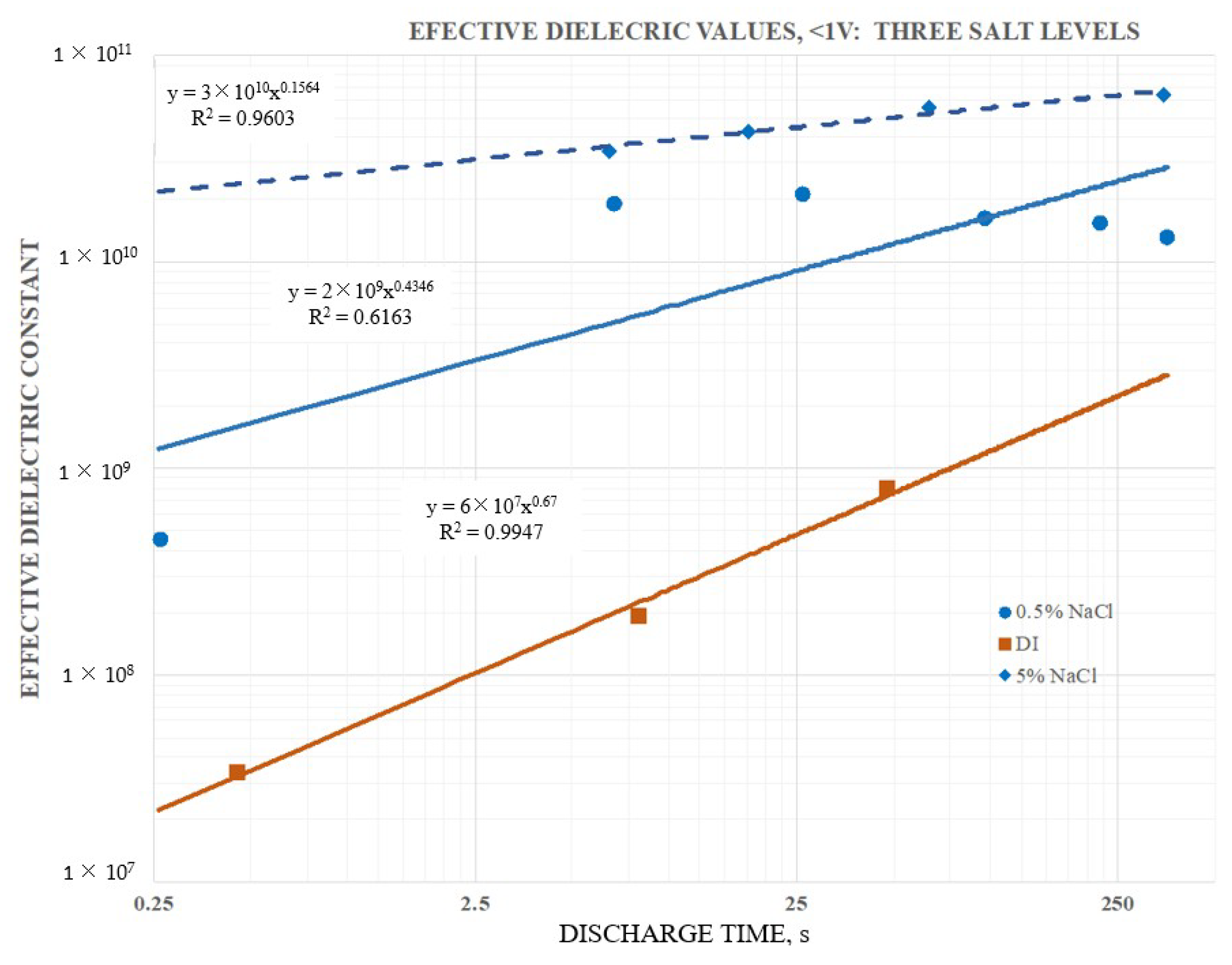
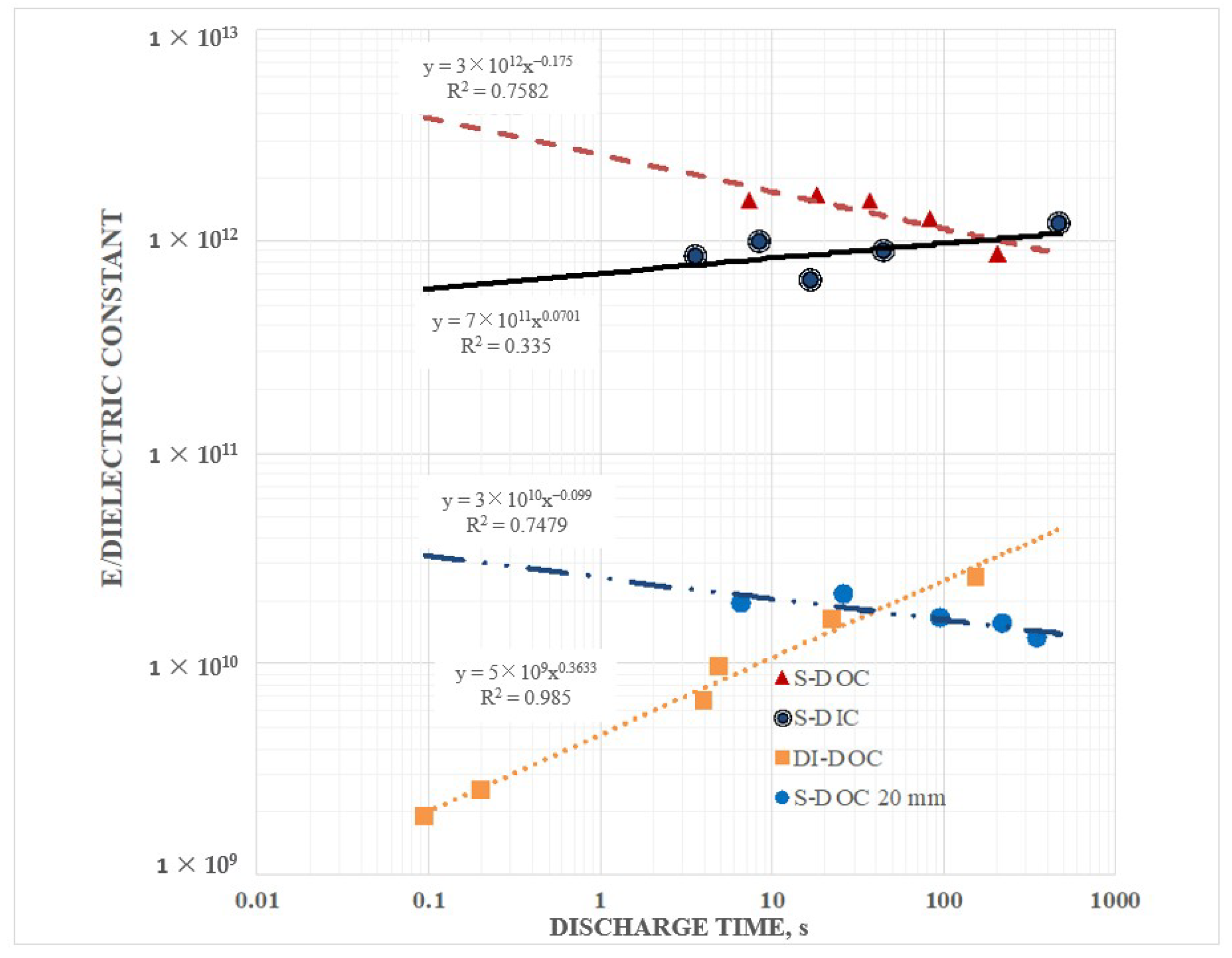
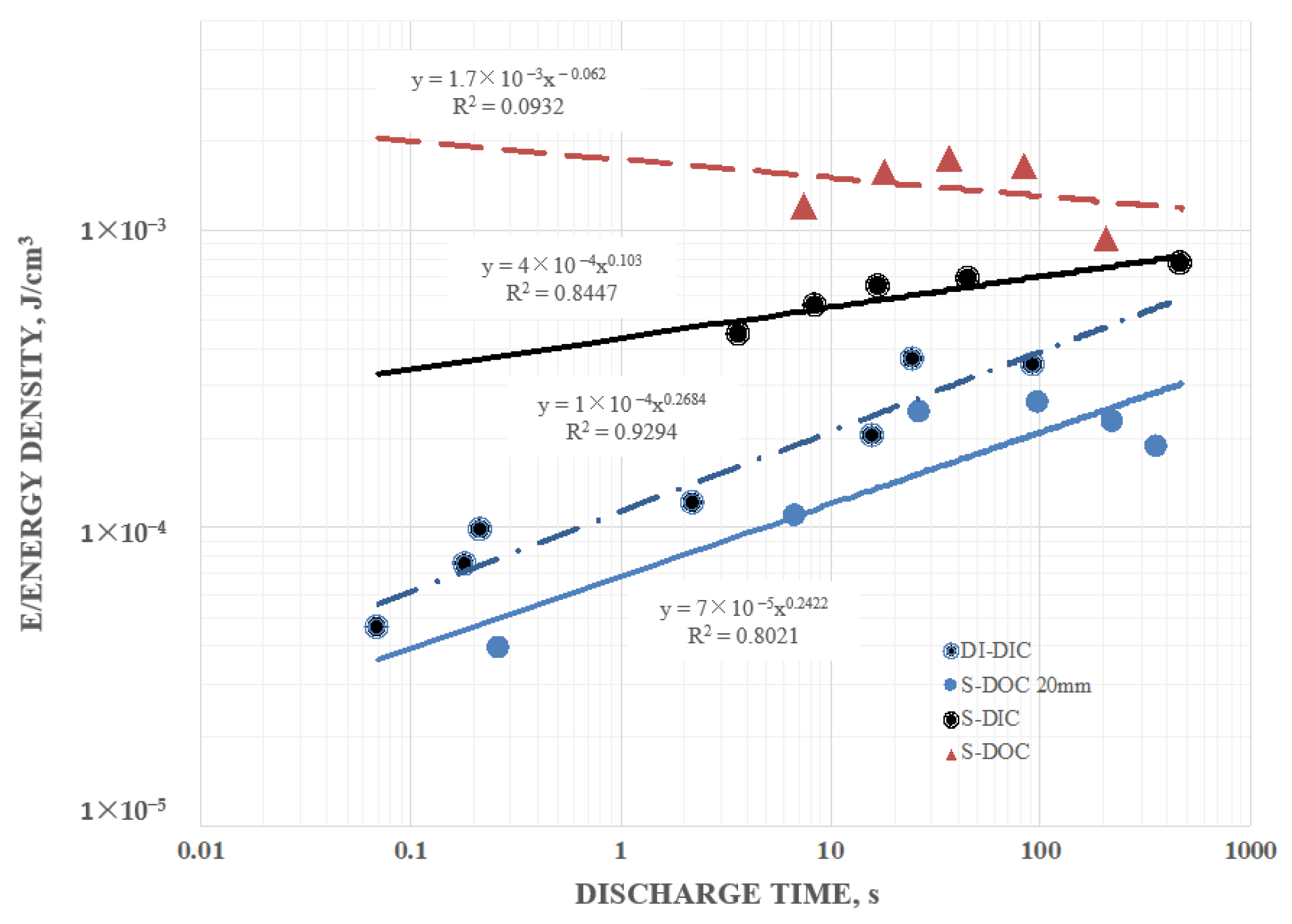
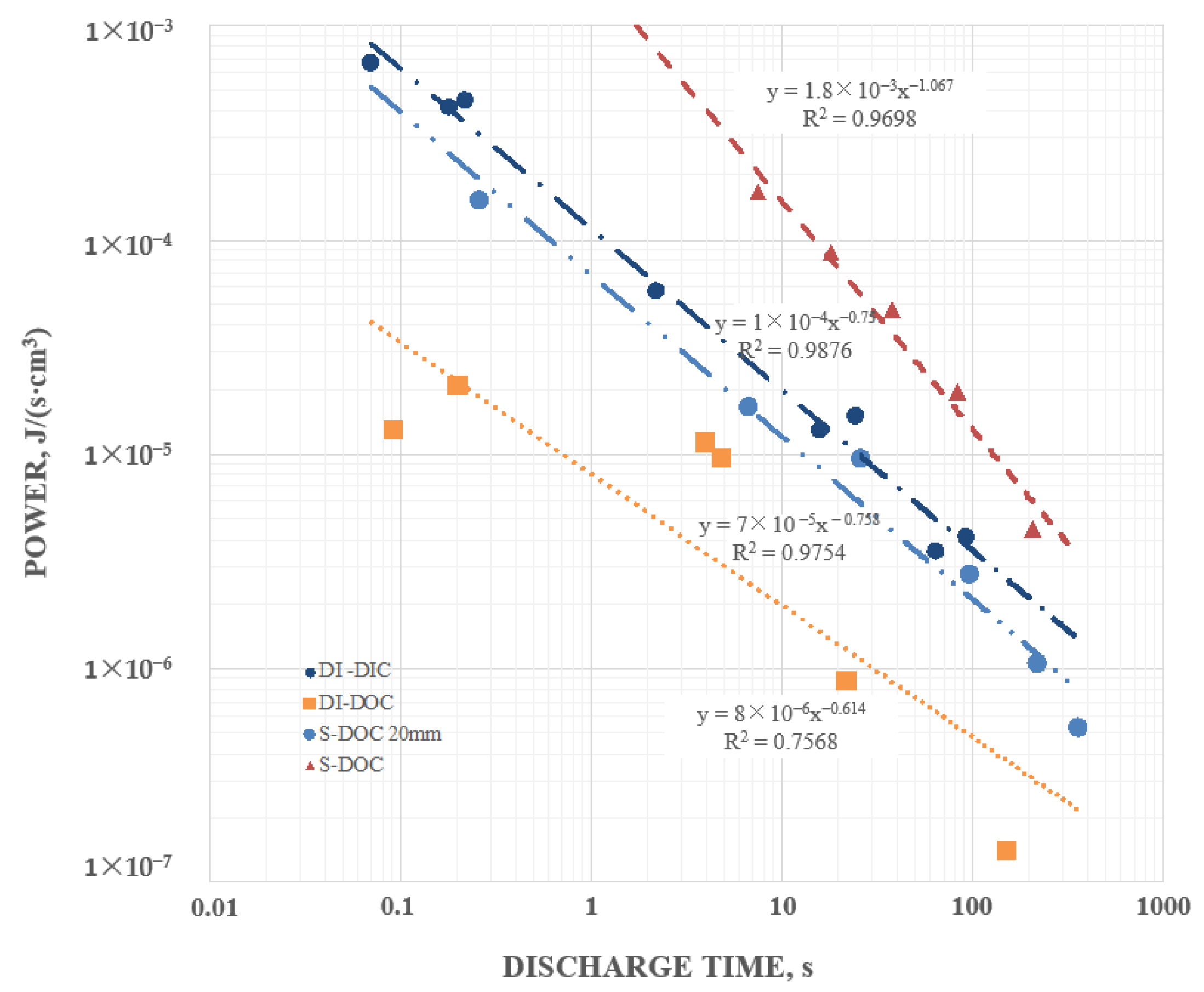
Secondary information found in the data included the following: (1) Pure water at short periods, order 1 s (roughly equivalent to a frequency of 1 Hz), had a dielectric constant in excess of 107, as reported elsewhere. (2) Salt does increase the dielectric constant. DI with even low dissolved salt concentrations (ca. 0.5 wt % NaCl) could have remarkably high dielectric values, >1010, even for a one second hold time at 10 V. At one second discharge time the difference in the effective dielectric constant between DI, and 5 wt % NaCl in DI, was almost three orders of magnitude. (3) Increasing salt concentration did increase effective dielectric constant. Consistently, a bath of salt with 5 wt % NaCl produced higher capacitance, energy density, etc. values than a bath with 0.5 wt % NaCl. (4) There was evidence of a maximum, or “saturation” value to energy density achievable with salt water dielectric. In this study even as the discharge time was increased, effective dielectric constant remained relatively constant over a range of discharge times from about 1 s to 250 s. (5) Effective dielectric constant values were similar in magnitude to the dielectric constants of the same materials “between the plates”. (6) Finally, in this study it was found that the effective dielectric constant of a dielectric material was always measured to be higher if it were outside the region between the electrodes than if it was placed between the electrodes. All of these secondary findings were only semi-quantitative and more detailed investigation is justified.
Most of these findings were consistent with earlier work on SDM, and expectations developed on the basis of those studies. Indeed, the high effective dielectric constant values for salt water were within an order of magnitude of those published previously for SDM gels on the outside of parallel plate capacitors [
2] as well as SDM, in various configurations, “between the electrodes” [
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13].
It is notable that other groups studying the dielectric value of water at low frequency (ca. near 1 Hz) report values of dielectric constant very similar to those reported here [
14,
15]. Moreover; those teams used other methods, not the constant current method employed herein. Thus, the present results further demonstrate the generality and reliability of the results.
4.2. Theory
It is illustrative to compare models of the origin of high dielectric value found in the literature for DI water at low frequency, which is the standard model vs. SDM model. The standard model is that the extremely high dielectric values (ca. 10
7 at 1 Hz) result from charged species in the water (e.g., OH
−, H
3O
+) forming oppositely charged electric double layers at each electrode [
15]. According to the model, at the positive electrode OH
− forms a double layer, and at the negative electrode H
3O
+. For several reasons it is not at all clear how the remarkably high net dielectric values observed are consistent with that model: (1) In standard supercapacitor models it is assumed the dielectric value of the double layer is in the low double digits [
1] at low frequency. (2) This standard model cannot explain why the dielectric constant of water is at least five orders of magnitude greater than solid titanates [
1]. Generally some double layer like feature is proposed to explain the dielectric value of solid dielectrics [
21,
22,
23]. (3) The model is not consistent with the fact that voltage is a state property. Given all paths yield equivalent voltage, how does the double layer reduce the voltage for a charge that travels between the electrodes via a path outside the volume between the electrodes?
The SDM model, it is argued, is consistent with all observations, and all laws of physics [
28,
29]. As explained in more detail elsewhere [
1,
2,
3,
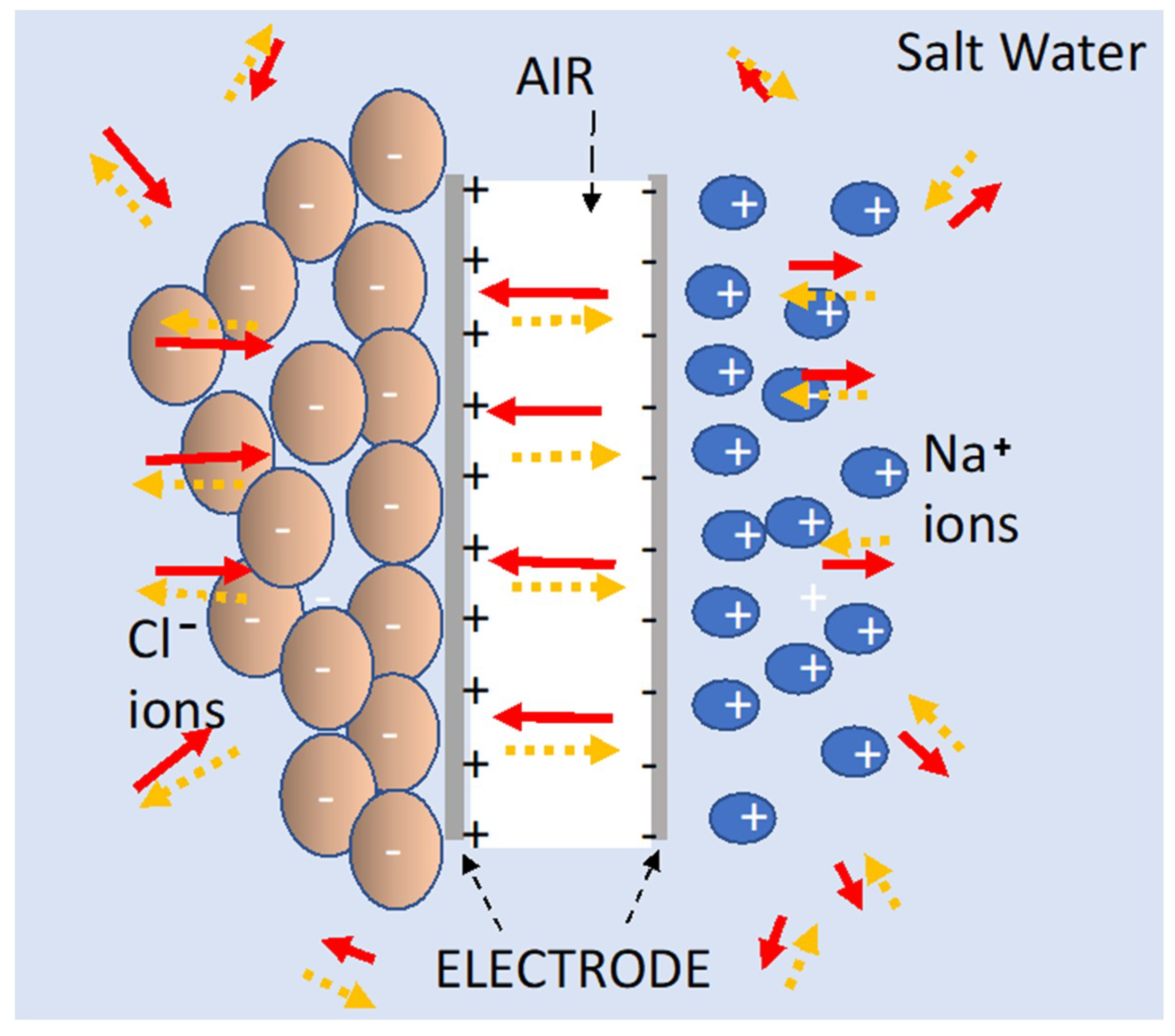
4], and illustrated in
Figure 9, the theory is based on the field strength and direction of the field generated by a dielectric, placed between electrodes or surrounding the electrodes, “partially cancelling” the quasi dipole field produced by charges on the electrodes. The field generated by polarizing the dielectric will also be quasi-dipole, with a vector direction necessarily opposite at every point in space to that of the “dipole” field generated by charges on the electrodes. Thus, the field produced by the dielectric reduces the electric field produced by the charge on the electrode at every point in space, both between the electrodes and outside the volume between electrodes. As the voltage is the line integral of the field, for any given charge density on the electrodes, that line integral, and concomitantly the voltage, is lowered. Hence, given a constant charge, capacitance (charge/voltage) is increased by the presence of the dielectric.
The SDM model predicts, consistent with the data presented herein, and contrary to the standard model, that dipoles outside the volume between the electrodes will increase capacitance. The dipoles formed in the dielectric reduce the field at every point in space whether the dielectric is in the volume between the electrodes, or outside that volume, a concept completely consistent with the standard E/M theory [
30]: The electric field at any point is space is the vector sum of the fields of all charges in the universe. In either geometry the field at all points in space produced by the charges on the electrodes is reduced by the oppositely polarized dipoles of the dielectric. Also, unlike the standard model, there is no need to postulate either a double layer, or a high field region near the electrodes. According to the SDM model the electric field distribution in space is nearly the same for a particular voltage, including the region adjacent to the electrodes, with and without a dielectric [
1].
The SDM model applies to all dielectrics, solids, and liquids. Two factors [
1,
2,
3,
4] should impact the observed dielectric constant at low frequency; (1) the dipole density in the dielectric and, (2) the dipole length in the dielectric. There is no fundamental difference in the “action” of a solid (e.g., barium titanate) or a liquid dielectric. Both reduce the field created by charges on the electrode at all points in space via the formation of dipoles oriented opposite to the dipole orientation of the electrodes. Indeed, according to SDM theory the underlying physical explanation for the enormous difference in a dielectric constant at low frequency (ca. 1 Hz) between salt water and barium titanate is the length of the dipoles. In barium titanate it is well under 10
−10 m, and in the salt water it can clearly be even centimeters long [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13]. Calculations show that NaCl saturated water has about 30% as many dipoles per volume as barium titanate. The longer dipoles of salt water lead to higher dipole fields at all points in space, hence more significant cancellation of electrode charge produced field, and concomitantly higher capacitance. That is, barium titanate, with far shorter dipoles, according to SDM theory should have far lower dielectric constant than salt water, as observed.
Some features of the data collected for the present work can readily be shown consistent with the SDM model. First, water should be an excellent dielectric at low frequencies because, as suggested elsewhere [
15], the dipoles of water molecules align in the presence of an electric field. The structure of water in this condition is not known. Once, aligned, the water molecules will effectively “cancel” the field of the charges on electrodes, leading to extraordinarily high dielectric constants. Second, dissolved ions will further reduce the net field at all points in space by forming an effective large dipole with a length greater than the distance between the electrodes (
Figure 9). The magnitude of this dipole may even explain why it was observed that S-DOC outperformed S-DIC as S-DIC dipoles, restricted by the internal volume, are necessarily shorter than those found in the S-DOC configuration. Third, the effect of ion separation should increase with hold time. That is, the longer the hold time, the more charges can travel from elsewhere in the liquid bath to arrive at the proper electrode. In contrast, hold time has virtually no impact on the capacitive behavior of DI. Indeed, there is no need to provide time for ions to travel, only enough time for the water molecule alignment, clearly a far faster process.
Further study of a variety of related topics is arguably justified. What is the impact of salt type? For example, is KCl or NH3Cl better than NaCl? Is KOH a better ion source than NaCl? How does the pH of salt-free water impact behavior? Is there a trend in the energy density as a function of inter-electrode distance?
4.3. Application
Potential significant applications of the SDM theory supported by these experiments, are: (1) Possible novel energy storage devices, and (2) improved understanding of charge/discharge mechanisms in nerve tissue. Regarding the former: As noted elsewhere, the high dielectric constant value of “salt water” at low frequencies suggest capacitors can be created with higher energy densities than the best batteries. An “ideal” example: A parallel plate capacitor with a gap of 1 micron into which a material of dielectric constant of 1 × 1010 and specific gravity of 2 is placed, then charged to 1 V will have an energy density of about 6000 Wh/kg of dielectric. This compares rather well with a lithium ion battery with an energy density of order 150 Wh/kg. Even a less “ideal” capacitor, same dimensions, but a dielectric of only 109, and assuming the dielectric is only thirty percent of the weight, still yields an energy density as good as the best lithium ion batteries. The present work suggests an interesting variation: The SDM dielectric need not be in the space between the electrodes, but in fact can merely “surround” the electrodes. Regarding the latter: One third of the fluid in the body is interstitial water with a relatively high Na+ ion concentration. The present work suggests the capacitance of any “solvated” circuit, such as a circuit of neurons, will be impacted by the effective dielectric constant of the surrounding fluid. The present results suggest the dielectric constant of the “salt water” in the body is far higher than previously believed. Thus, the capacitance and charge stored in “biological circuits”, even the roll of ions in interstitial media, may need to be reconsidered.