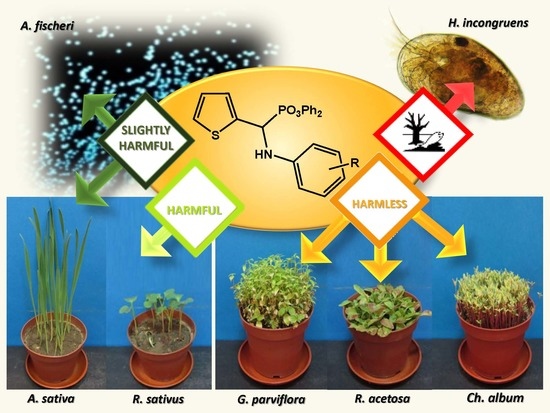
The Effect of New Thiophene-Derived Diphenyl Aminophosphonates on Growth of Terrestrial Plants
Abstract
:1. Introduction
2. Materials and Methods
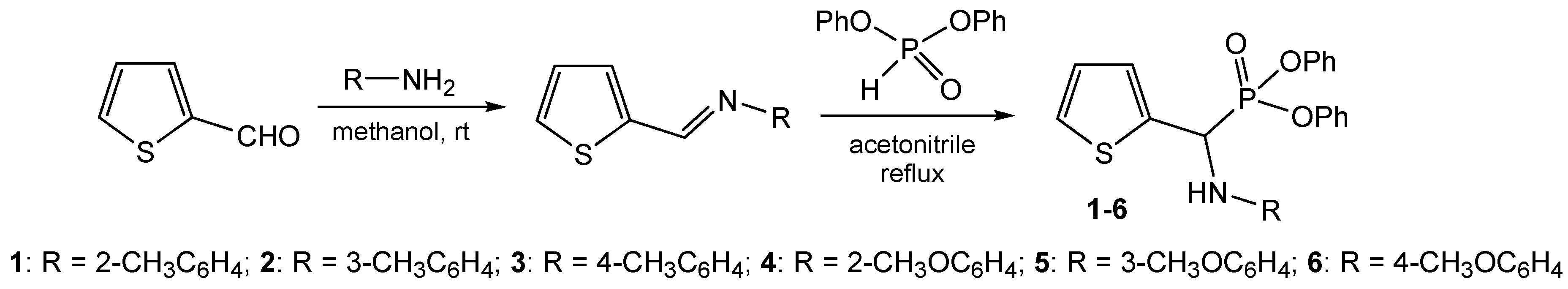
2.1. Preparation of Aminophosphonates 1–6
2.1.1. General Information
2.1.2. Procedure for Preparation of Diphenyl Amino(2-thienyl)methylphosphonates 1–6
2.2. Plant Growth Test of Aminophosphonates 1–6
2.3. Pigment Assay
2.4. Determination of Herbicidal Activity
2.5. Microtox® Toxicity Assay
2.6. Ostracod Test Kit
2.7. Statistical Analysis
3. Results and Discussion
3.1. Preparation of Thiophene-Derived Aminophosphonates 1–6
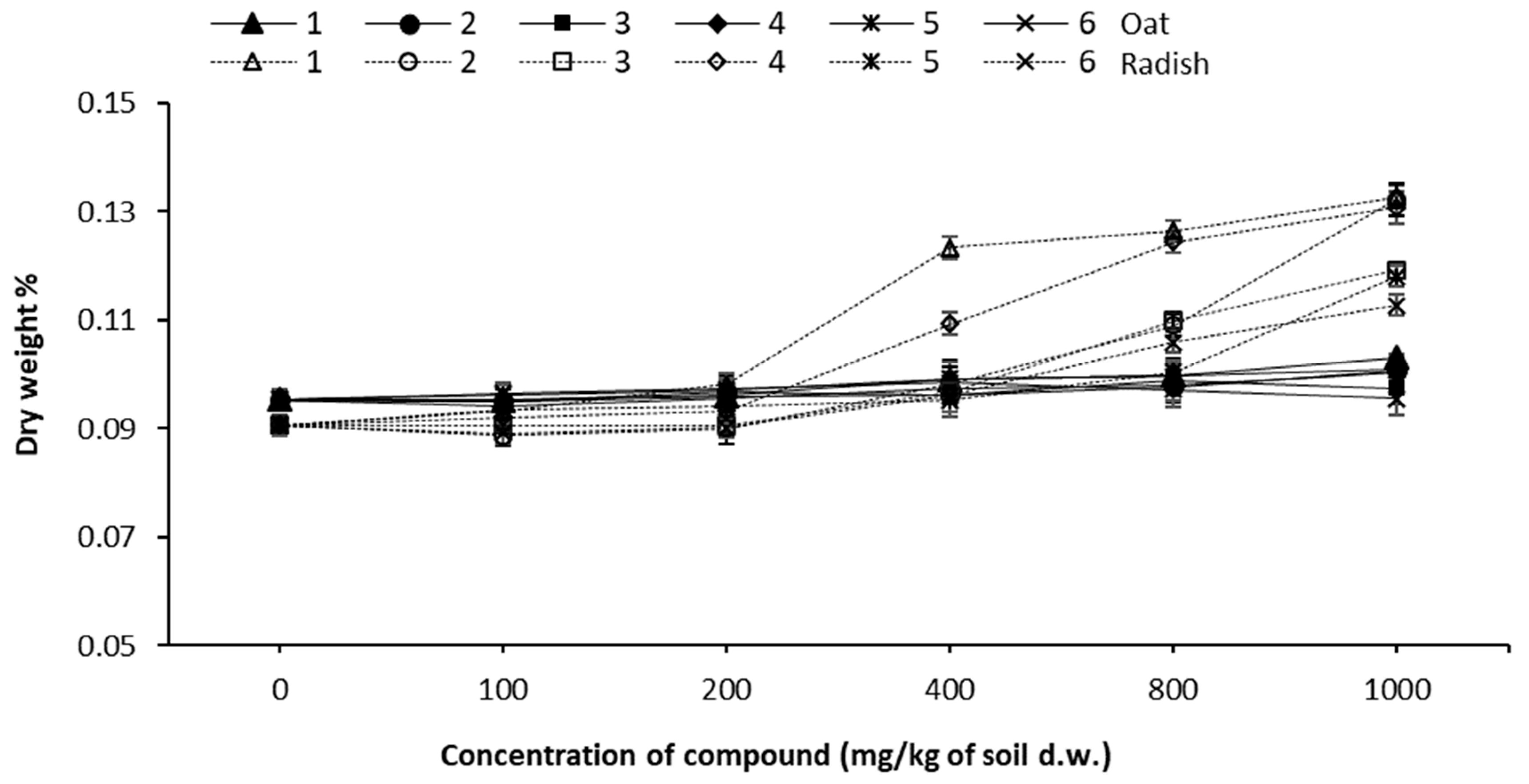
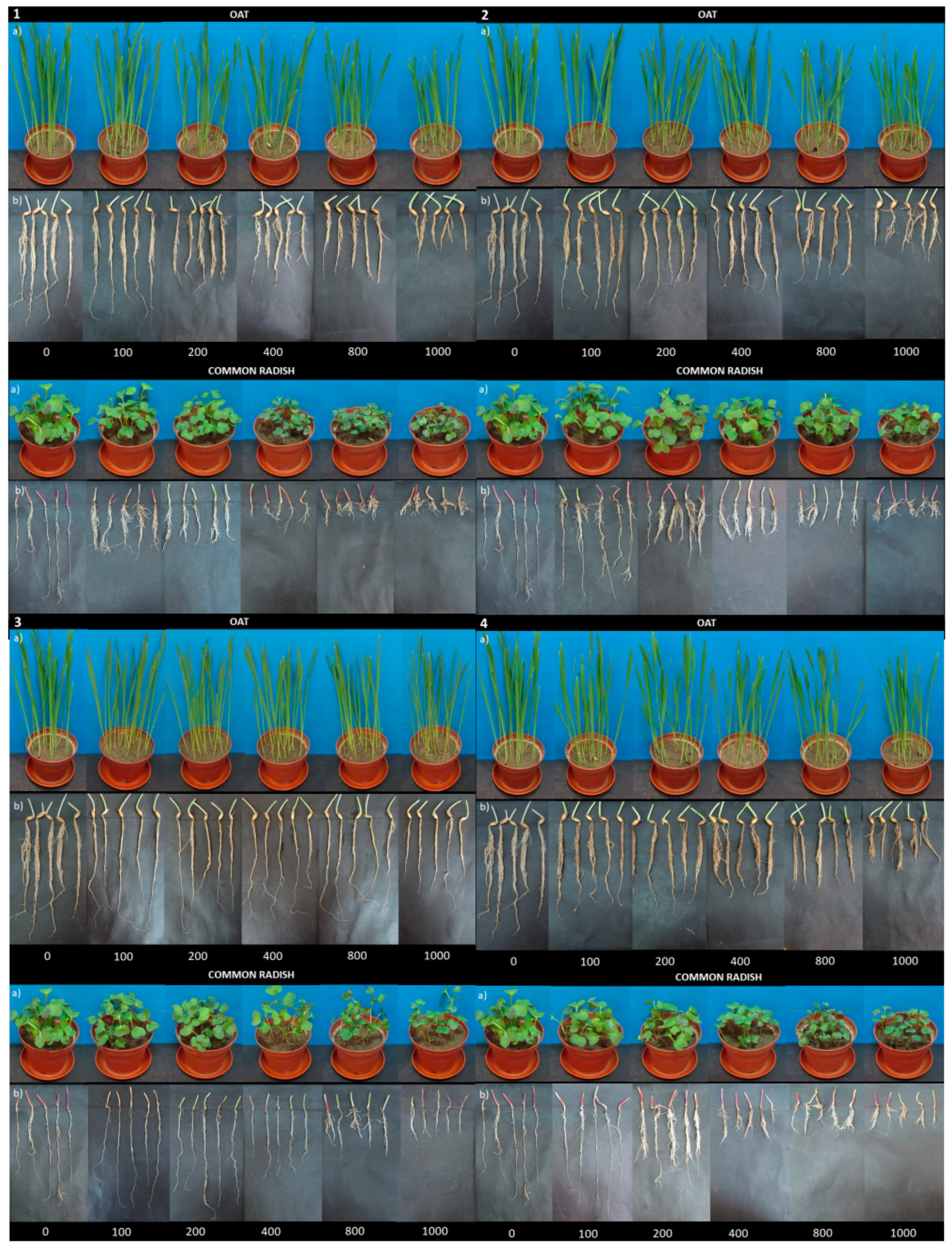
3.2. Growth Inhibition of Shoot Height, Root Length, and Fresh Matter
3.2.1. Yield (Fresh Matter) and Shoot Height Changes
3.2.2. Root Length Changes
3.3. Germination of Seeds
3.4. Values EC50, NOEC, LOEC
3.4.1. EC50Values for Fresh Matter and Shoot Height
3.4.2. EC50 Values for Root Length
3.4.3. NOEC and LOEC Values
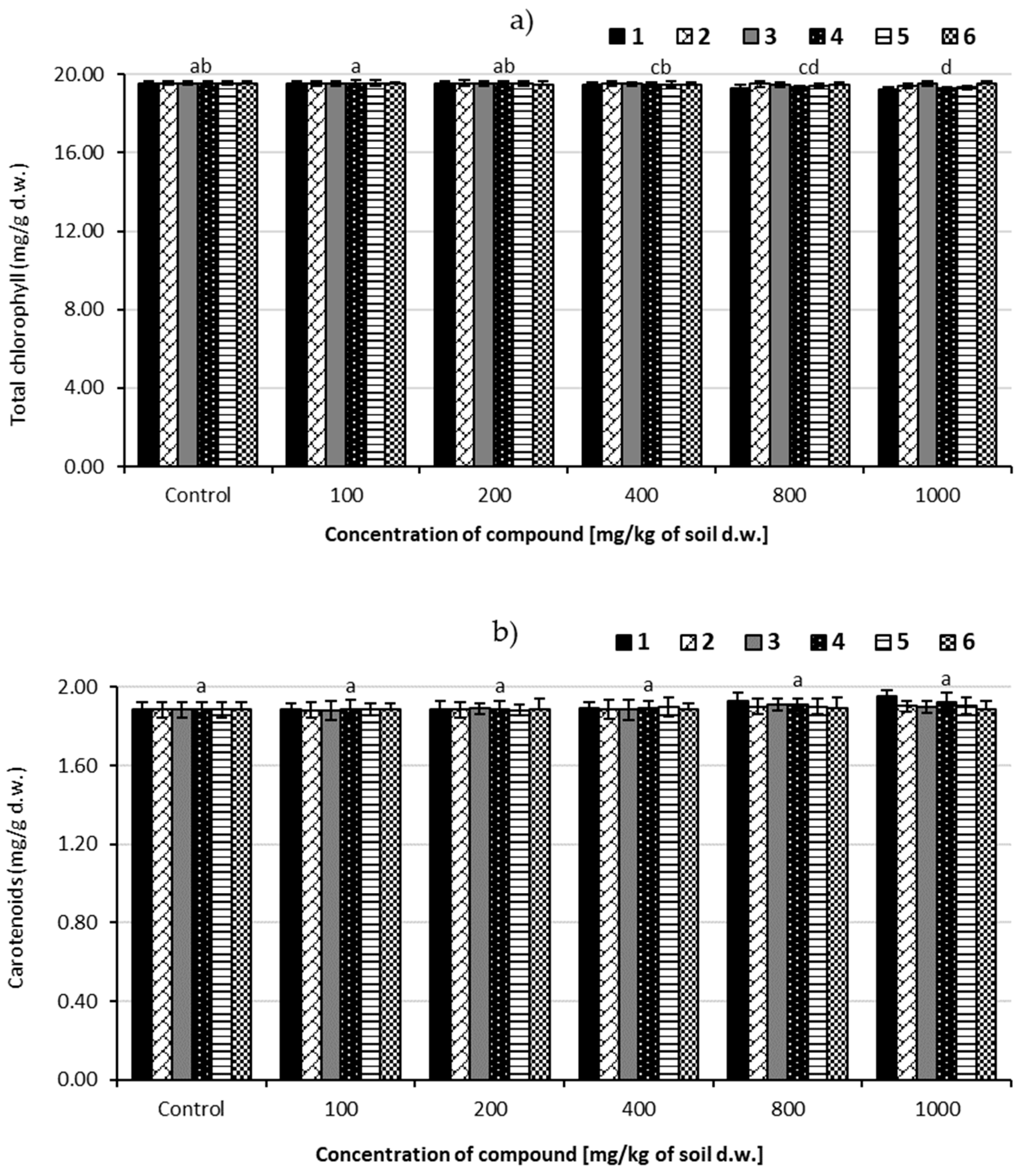
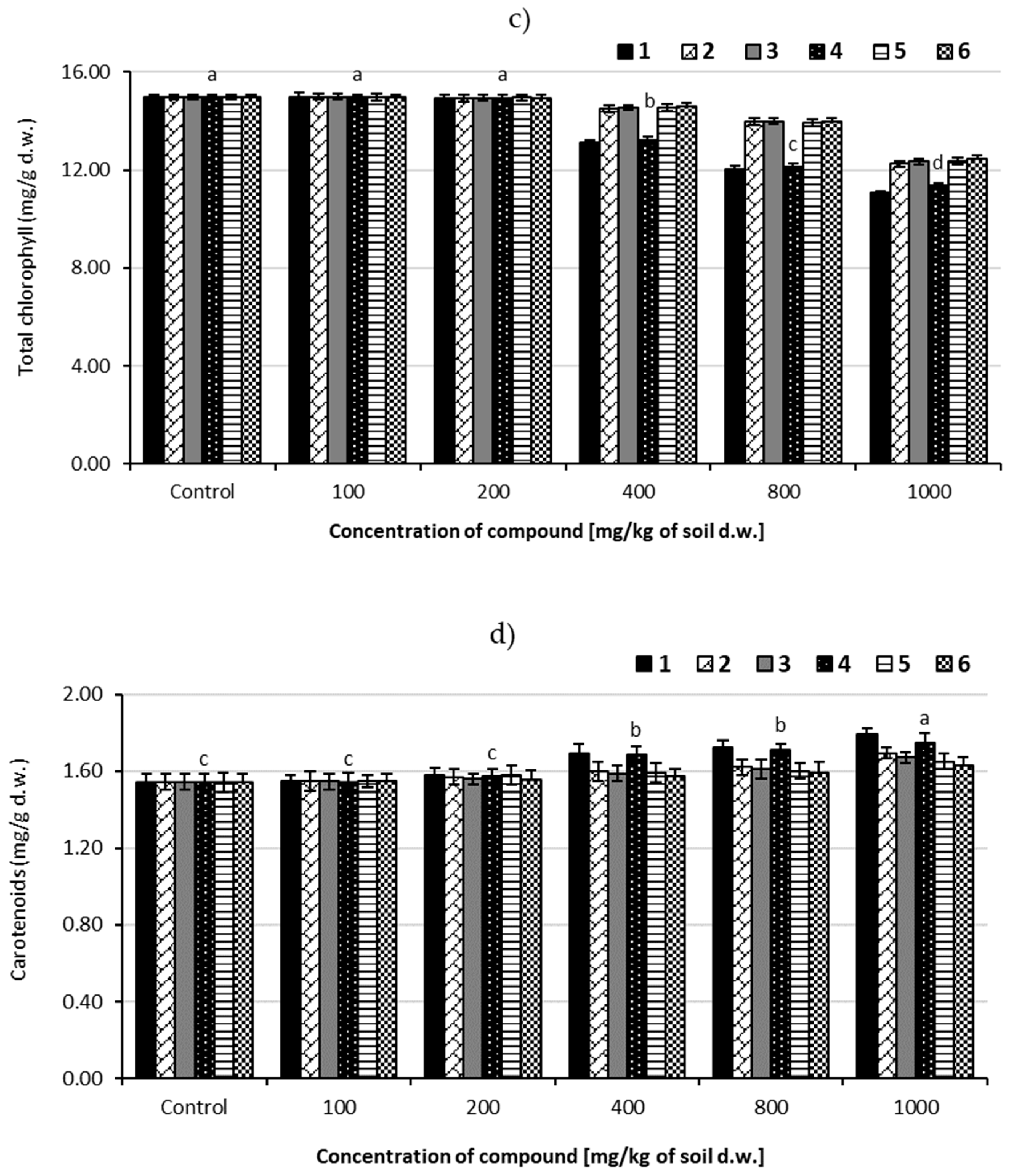
3.5. Pigment Assay
3.5.1. Changes of Total Chlorophyll Content
3.5.2. Changes of Carotenoids Content
3.6. Weed Test
3.6.1. Growth Inhibition of Shoot Height
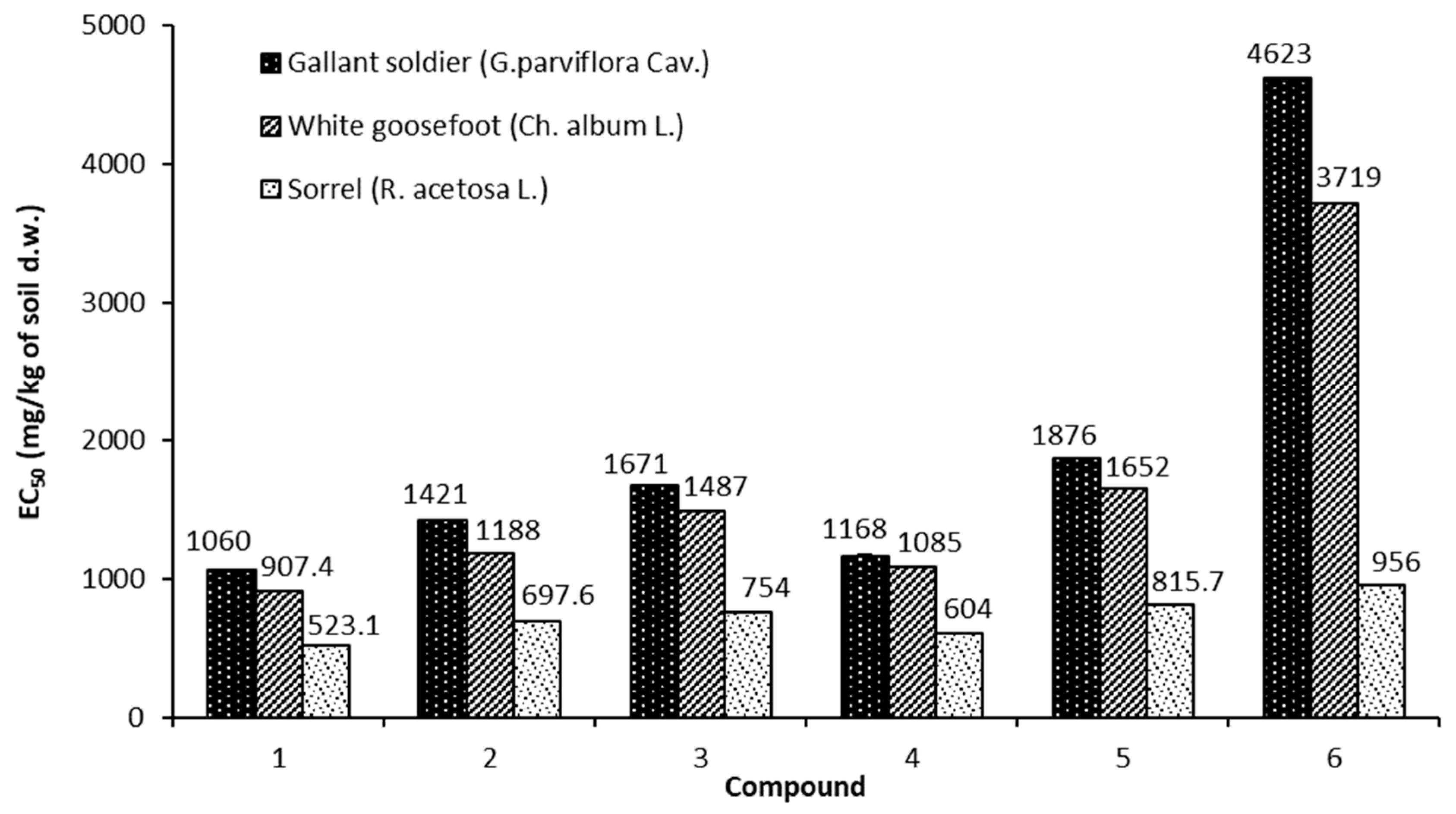
3.6.2. EC50 for Shoot Height
3.6.3. EWRC Rating Scale
3.7. Microtox® Toxicity Assay
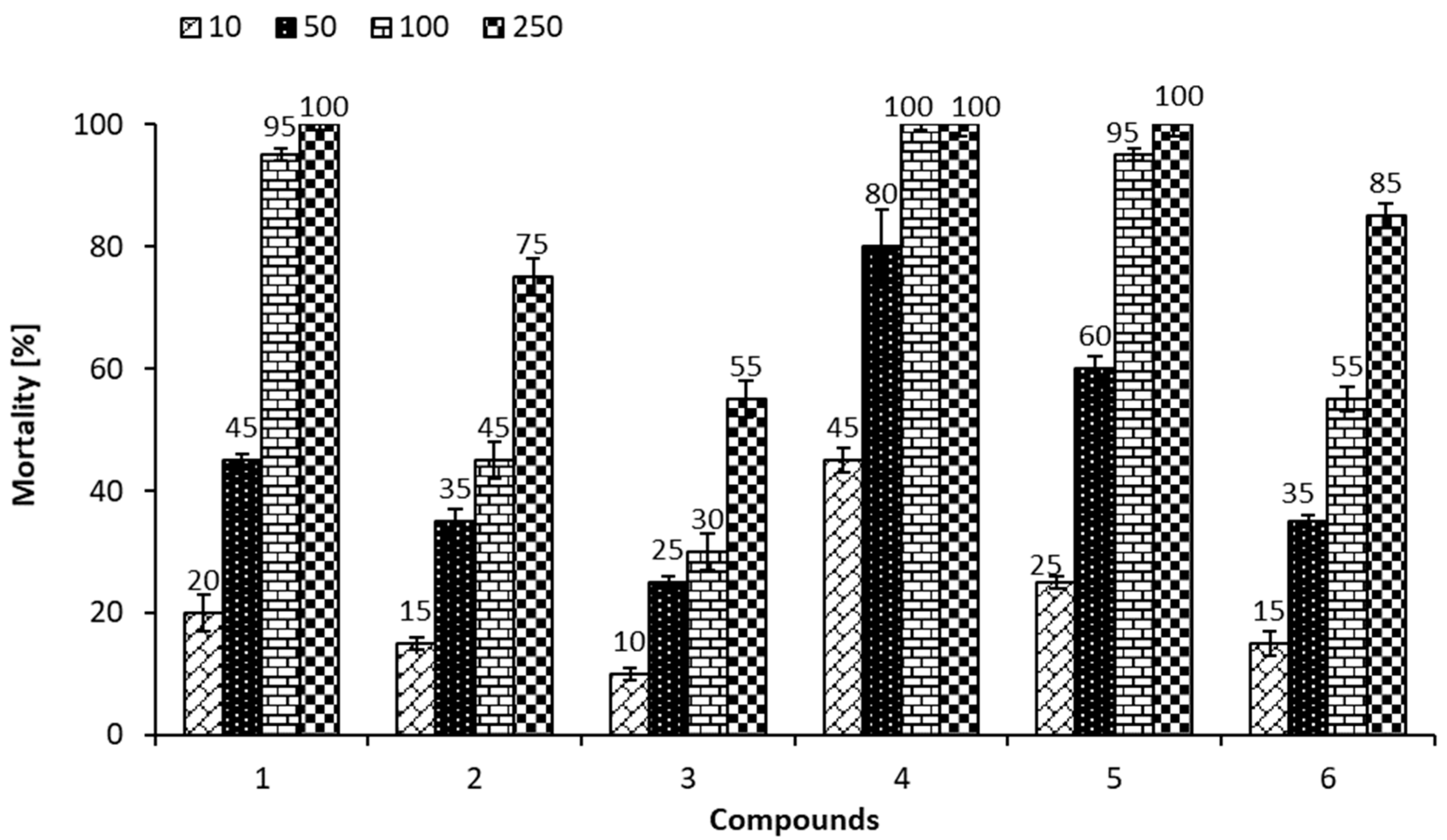
3.8. Ostracod Test Kit
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Barbarella, G.; Zangoli, M.; Di Maria, F. Chapter Three—Synthesis and Applications of Thiophene Derivatives as Organic Materials. Adv. Heterocycl. Chem. 2017, 123, 105–167. [Google Scholar] [CrossRef]
- Printz, A.D.; Lipomi, D.J. Competition between deformability and charge transport in semiconducting polymers for flexible and stretchable electronics. Appl. Phys. Rev. 2016, 3, 021302. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, P.; Hu, W. Organic Light-Emitting Transistors: Materials, Device Configurations, and Operations. Small 2016, 12, 1252–1294. [Google Scholar] [CrossRef] [PubMed]
- Pisignano, D.; Persano, L.; Mele, E.; Visconti, P.; Cingolani, R.; Gigli, G.; Barbarella, G.; Favaretto, L. Emission properties of printed organic semiconductor lasers. Opt. Lett. 2005, 30, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Coskun, Y.; Cirpan, A.; Toppare, L.J. Construction of electrochromic devices using thiophene based conducting polymers. J. Mater. Sci. 2007, 42, 368–372. [Google Scholar] [CrossRef]
- Huynh, T.P.; Sharma, P.S.; Sosnowska, M.; D’Souza, F.; Kutner, W. Functionalized polythiophenes: Recognition materials for chemosensors and biosensors of superior sensitivity, selectivity, and detectability. Prog. Polym. Sci. 2015, 47, 1–25. [Google Scholar] [CrossRef]
- Rasmussen, S.C.; Evenson, S.J.; McCausland, C.B. Fluorescent thiophene-based materials and their outlook for emissive applications. Chem. Commun. 2015, 51, 4528–4543. [Google Scholar] [CrossRef]
- Zambianchi, M.; Di Maria, F.; Cazzato, A.; Gigli, G.; Piacenza, M.; Della Sala, F.; Barbarella, G. Microwave-Assisted Synthesis of Thiophene Fluorophores, Labeling and Multilabeling of Monoclonal Antibodies, and Long Lasting Staining of Fixed Cells. J. Am. Chem. Soc. 2009, 131, 10892–10900. [Google Scholar] [CrossRef]
- Gramec, D.; Peterlin Mašič, L.; Sollner Dolenc, M. Bioactivation Potential of Thiophene-Containing Drugs. Chem. Res. Toxicol. 2014, 27, 1344–1358. [Google Scholar] [CrossRef]
- Dansette, P.M.; Rosi, J.; Bertho, G.; Mansuy, D. Cytochromes P450 Catalyze Both Steps of the Major Pathway of Clopidogrel Bioactivation, whereas Paraoxonase Catalyzes the Formation of a Minor Thiol Metabolite Isomer. Chem. Res. Toxicol. 2012, 25, 348–356. [Google Scholar] [CrossRef]
- Montuschi, P.; Ciabattoni, G. Bronchodilating Drugs for Chronic Obstructive Pulmonary Disease: Current Status and Future Trends. J. Med. Chem. 2015, 58, 4131–4164. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Vashishtha, S.C.; Erve, J.C.L. Characterization of Glutathione Conjugates of Duloxetine by Mass Spectrometry and Evaluation of in Silico Approaches to Rationalize the Site of Conjugation for Thiophene Containing Drugs. Chem. Res. Toxicol. 2010, 23, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Perzborn, E.; Roehrig, S.; Straub, A.; Kubitza, D.; Misselwitz, F. The discovery and development of rivaroxaban, an oral, direct factor Xa inhibitor. Nat. Rev. Drug Discov. 2011, 10, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Filipe, O.M.S.; Santos, S.A.O.; Domingues, M.R.M.; Vidal, M.M.; Silvestre, A.J.D.; Neto, C.P.; Santos, E.B.H. Photodegradation of the fungicide thiram in aqueous solutions. Kinetic studies and identification of the photodegradation products by HPLC–MS/MS. Chemosphere 2013, 91, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Sanchirico, R.; Pinto, G.; Pollio, A.; Cordella, M.; Cozzani, V. Thermal degradation of Fenitrothion: Identification and eco-toxicity of decomposition products. J. Hazard. Mater. 2012, 199–200, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Baghestani, M.A.; Zand, E.; Soufizadeh, S.; Jamali, M.; Mighany, F. Evaluation of sulfosulfuron for broadleaved and grass weed control in wheat (Triticum aestivum L.) in Iran. Crop Prot. 2007, 26, 1385–1389. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, L.; Li, D.; Li, X.; Wang, C.; Zheng, M.; Pan, C.; Qiu, L. Biodegradation of thifensulfuron-methyl by Ochrobactrum sp. in liquid medium and soil. Biotechnol. Lett. 2015, 37, 1385–1392. [Google Scholar] [CrossRef]
- Cessna, A.J.; Donald, D.B.; Bailey, J.; Waiser, M. Persistence of the Sulfonylurea Herbicides Sulfosulfuron, Rimsulfuron, and Nicosulfuron in Farm Dugouts (Ponds). J. Environ. Qual. 2015, 44, 1948–1955. [Google Scholar] [CrossRef]
- Elliott, J.A.; Cessna, A.J. Variability in the distribution and dissipation of the herbicide thifensulfuron-methyl in a prairie wetland. J. Soil Water Conserv. 2014, 69, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Hudson, H. Aminophosphonic and Aminophosphinic Acids and their Derivatives as Agrochemicals. In Aminophosphonic and Aminophosphinic Acids: Chemistry and Biological Activity; Hudson, H.R., Kukhar, V.P., Eds.; Wiley: Chichester, UK, 2000; pp. 443–482. [Google Scholar]
- Tajti, Á.; Keglevich, G. The importance of organophosphorus compounds as biologically active agents. In Organophosphorus Chemistry—Novel Developments; Keglevich, G., Ed.; Walter de Gruyter GmbH: Berlin, Germany, 2018; pp. 53–65. [Google Scholar]
- Bálint, E.; Tripolszky, A.; Tajti, Á. Synthesis of α-aminophosphonates by the Kabachnik–Fields reaction and by the Pudovik reaction. In Organophosphorus Chemistry—Novel Developments; Keglevich, G., Ed.; Walter de Gruyter GmbH: Berlin, Germany, 2018; pp. 108–147. [Google Scholar]
- Lewkowski, J.; Malinowski, Z.; Matusiak, A.; Morawska, M.; Rogacz, D.; Rychter, P. The effect of new thiophene-derived aminophosphonic derivatives on growth of terrestrial plants: A seedling emergence and growth test. Molecules 2016, 21, 694. [Google Scholar] [CrossRef]
- Rogacz, D.; Lewkowski, J.; Malinowski, Z.; Matusiak, A.; Morawska, M.; Rychter, P. Effect of New Thiophene-Derived Aminophosphonic Derivatives on Growth of Terrestrial Plants. Part 2. Their Ecotoxicological Impact and Phytotoxicity Test Toward Herbicidal Application in Agriculture. Molecules 2018, 23, 3173. [Google Scholar] [CrossRef] [PubMed]
- Terrestrial plant test: Seedling emergence and seedling growth test. In OECD/OCDE Guidelines for the Testing of Chemicals. Section 2. Effects on Biotic Systems; Test No. 208; Organization for Economic and Cooperation Development (OECD) Publishing: Paris, France, 2006. [CrossRef]
- Karpowicz, R.; Lewkowski, J.; Stasiak, M.; Czopor, A.; Tokarz, P.; Król, A.; Rychter, P. Synthesis of novel N-(p-toluenesulfonyl) aminophosphonates and evaluation of their biological properties. Phosphorus Sulfur Silicon Rel. Elem. 2018, 193, 423–436. [Google Scholar] [CrossRef]
- Rychter, P.; Rogacz, D.; Lewicka, K.; Kollár, J.; Kawalec, M.; Mosnáček, J. Ecotoxicological Properties of Tulipalin A-Based Superabsorbents versus Conventional Superabsorbent Hydrogels. Adv. Polym. Technol. 2019, 2947152. [Google Scholar] [CrossRef]
- Lewkowski, J.; Morawska, M.; Karpowicz, R.; Rychter, P.; Rogacz, D.; Lewicka, K.; Dobrzyński, P. Evaluation of Ecotoxicological Impact of New Pyrrole-derived Aminophosphonates Using Selected Bioassay Battery. Ecotoxicology 2017, 26, 914–929. [Google Scholar] [CrossRef] [PubMed]
- Lewkowski, J.; Morawska, M.; Karpowicz, R.; Rychter, P.; Rogacz, D.; Lewicka, K. Novel (5-nitrofurfuryl)-substituted esters of phosphonoglycine—Their synthesis and phyto- and ecotoxicological properties. Chemosphere 2017, 188, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-S.; Wang, L.; Wang, L.; Wang, G.; Li, Z.-H.; Wang, J.-J. Effect of 1-butyl-3-methylimidazolium tetrafluoroborate on the wheat (Triticum aestivum L.) seedlings. Environ. Toxicol. 2009, 24, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska, B.; Biczak, R. Evaluation of the effect of tetraethylammonium bromide and chloride on the growth and development of terrestrial plants. Chemosphere 2016, 149, 24–33. [Google Scholar] [CrossRef]
- Oren, A.; Kuehl, M.; Karsten, U. An endoevaporitic microbial mat within a gypsum crust: Zonation of phototrophs, photopigments and light penetration. Mar. Ecol. Prog. Ser. 1995, 128, 151–159. [Google Scholar] [CrossRef]
- Johannes, H. Report of the Third and Fourth Meetings of the European Weed Research Council Committee on Methods. Weed Res. 1964, 4, 79. [Google Scholar] [CrossRef]
- Costa, A.G.F.; Sofiatti, V.; Maciel, C.D.G.; Lira, A.J.S.; Cordeiro, A.F., Jr.; Silva, R.L.M. Weed management with herbicides applied in pre and postemergence on castor crop. Planta Daninha 2015, 33, 551–559. [Google Scholar] [CrossRef]
- Parsons, D.; Lane, P.; Hall, E.; Galloway, A.; Pham Van, B. Effective Weed Control for Talish and Hairy Canary Clover Seed Crops; Rural Industries Research and Development Corporation: Barton, Australia, 2013; pp. 4–5. [Google Scholar]
- Lewkowski, J.; Rodriguez Moya, M.; Chmielak, M.; Rogacz, D.; Lewicka, K.; Rychter, P. Synthesis, spectral characterization of several novel pyrene-derived aminophosphonates and their ecotoxicological evaluation using Heterocypris incongruens and Vibrio fischeri tests. Molecules 2016, 21, 936. [Google Scholar] [CrossRef] [PubMed]
- Doe, K.; Scroggins, R.; Mcleay, D.; Wohlgeschaffen, G. Solid-phase test for sediment toxicity using the luminescent bacterium Vibrio fischeri. In Small-Scale Freshwater Toxicity Investigations; Blaise, C., Férard, J.F., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume 1, pp. 107–136. [Google Scholar]
- Martínez-Sánchez, M.J.; Pérez-Sirvent, C.; García-Lorenzo, M.L.; Martínez-López, S.; Bech, J.; García-Tenorio, R.; Bolívar, J.P. Use of bioassays for the assessment of areas affected by phosphate industry wastes. J. Geochem. Explor. 2014, 147, 130–138. [Google Scholar] [CrossRef]
- Radosevic, K.; Cvjetko, M.; Srcek, V.G.; Grgas, D.; Dragicevic, T.L.; Radojcic Redovnikovic, I. Evaluation of toxicity and biodegradability of choline chloride based deep eutectic solvents. Ecotox. Environ. Saf. 2015, 112, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, J.; Gu, R.; Yue, L.; Zhan, X.; Xing, B. Phenanthrene-triggered chlorosis is caused by elevated chlorophyll degradation and leaf moisture. Environ. Pollut. 2017, 220 Pt B, 1311–1321. [Google Scholar] [CrossRef]
- Hannoufa, A.; Hossain, Z. Regulation of carotenoid accumulation in plants. Biocatal. Agric. Biotechnol. 2012, 1, 198–202. [Google Scholar] [CrossRef]
- Feierabend, J.; Winkelhüsener, T.W.; Kemmerich, P.; Schulz, U. Mechanism of bleaching in leaves treated with chlorosis-inducing herbicides. Z. Naturforsch. 1982, 37, 898–907. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.H.; Pogson, B.J. Carotenoid metabolism in plants. Mol. Plant 2015, 1, 198–202. [Google Scholar] [CrossRef]
- Cazzonelli Cl Cuttriss, A.J.; Cossetto, S.B.; Pye, W.; Crisp, P.; Whelan, J.; Finnegan, E.J.; Turnbull, C.; Pogson, B.J. Regulation of carotenoid composition and shoot branching in Arabidopsis by a chromatin modifying histone methyltransferase, SDG8. Plant Cell 2009, 21, 39–53. [Google Scholar] [CrossRef]
- Jarvis, P.; Lopez-Juez, E. Biogenesis and homeostasis of chloroplasts and other plastids. Nat. Rev. Mol. Cell Biol. 2013, 14, 787–802. [Google Scholar] [CrossRef]
- Gomathi, R.; Rakkiyapan, P. Comparative lipid peroxidation, leaf membranę thermostability, and antioxidant system in four sugarcane speciess differing in salt tolerance. Int. J. Plant Physiol. Biochem. 2011, 3, 67–74. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Gopi, R.; Alagu Lakshmanan, G.M.; Panneerselvam, R. Triadimefon induced changes in the antioxidant metabolism and ajmalicine production in paclobutrazol enhances photosynthesis and ajmalicine production in Catharanthus roseus (L.). Plant Sci. 2006, 171, 271–276. [Google Scholar] [CrossRef]
- Li, F.; Vallabhaneni, R.; Jane Yu Rocheford, T.; Wurtzel, T. The maize phytoene synthase gene family: Overlapping roles for carotenogenesis in endosperm, photomorphogenesis, and thermal stress tolerance. Plant Physiol. 2008, 147, 1334–1346. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.E.; Fletcher, R.A. Paclobutrazol protects wheat seedlings from heat and paraquat injury. Is detoxification of active oxygen involved? Plant Cell Physiol. 1994, 35, 45–52. [Google Scholar] [CrossRef]
- Hernando, M.D.; De Vettori, S.; Martinez Bueno, M.J.; Fernandez-Alba, A.R. Toxicity evaluation with Vibrio fischeri test of organic chemicals used in aquaculture. Chemosphere 2007, 68, 724–730. [Google Scholar] [CrossRef] [PubMed]

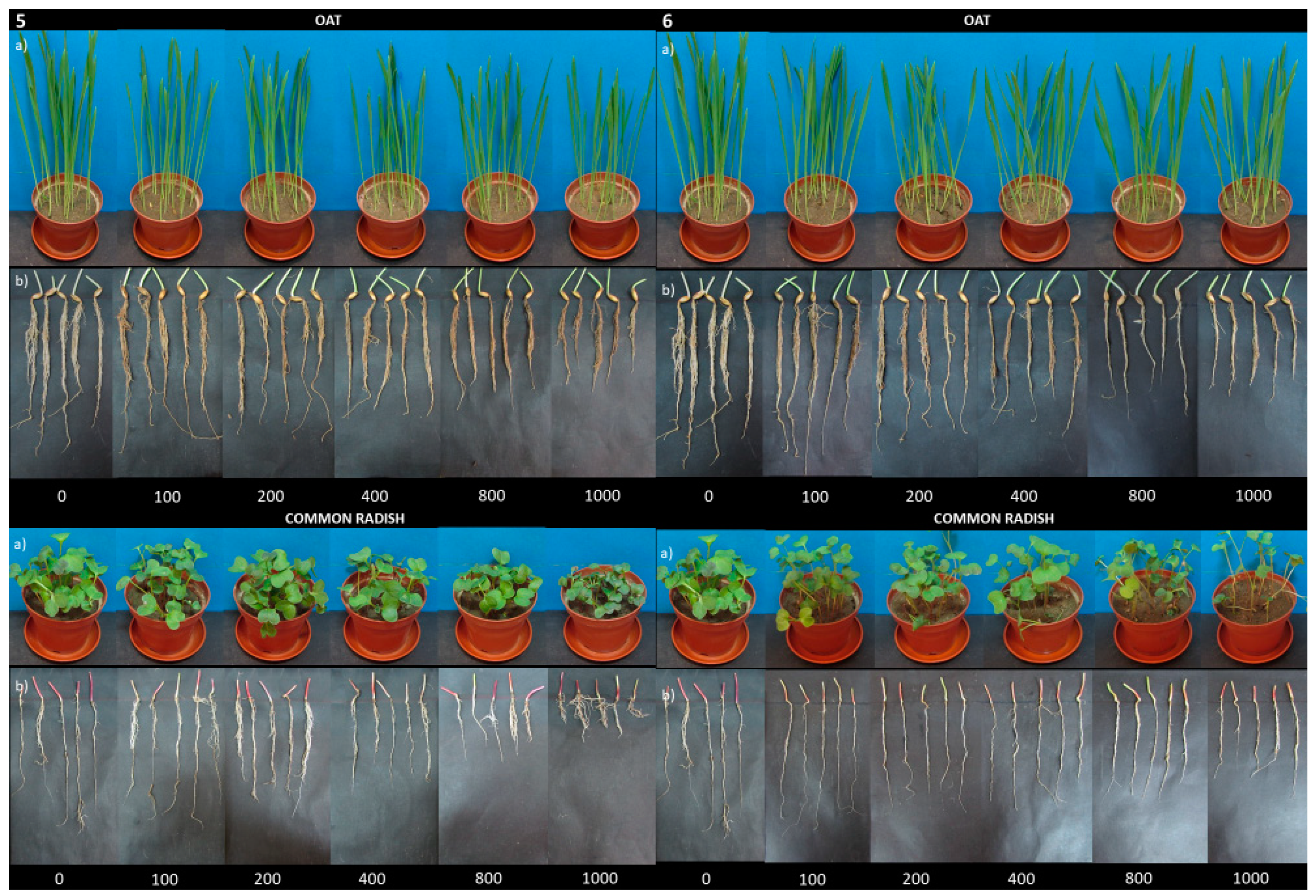


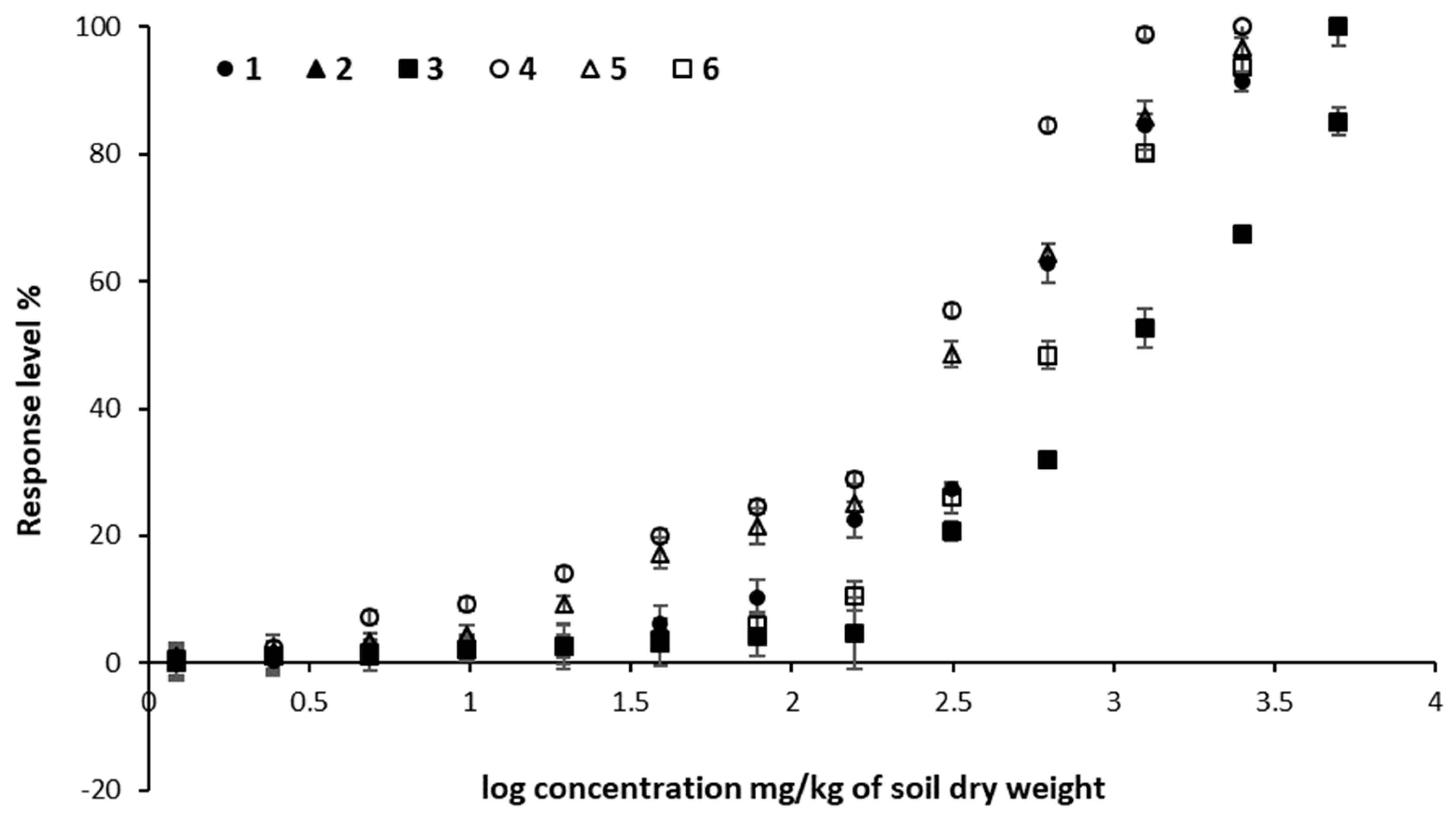
| Compound Concentration (mg/kg s.d.w.) | Inhibition Biomarkers (%) | |||||
|---|---|---|---|---|---|---|
| Fresh matter | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 100 | −0.4 ± 0.2 | 0.4 ± 0.2 | −0.4 ± 0.4 | 0.2 ± 0.1 | 0.2 ± 0.0 | 1.6 ± 0.4 |
| 200 | 2.9 ± 0.1 | −0.5 ± 0.1 | 0.6 ± 0.4 | 0.5 ± 0.1 | 2.3 ± 0.2 | 2.9 ± 0.2 |
| 400 | 5.0 ± 0.1 | 0.6 ± 0.1 | 1.2 ± 0.1 | 2.2 ± 0.2 | 1.5 ± 0.2 | 3.4 ± 0.2 |
| 800 | 5.7 ± 0.4 | 3.5 ± 0.2 | 4.1 ± 0.1 | 3.7 ± 0.1 | 2.6 ± 0.1 | 1.8 ± 0.1 |
| 1000 | 8.0 ± 0.1 | 4.7 ± 0.3 | 1.6 ± 0.3 | 6.1 ± 0.1 | 3.7 ± 0.1 | 1.5 ± 0.1 |
| Shoot height | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 100 | 0.4 ± 0.6 | 0.4 ± 0.5 | −0.4 ± 0.6 | 0.5 ± 1.0 | 0.5 ± 0.2 | 1.3 ± 0.6 |
| 200 | −0.5 ± 1.2 | −0.2 ± 0.7 | 0.5 ± 0.8 | 0.9 ± 0.6 | 2.9 ± 0.3 | 2.9 ± 0.3 |
| 400 | 1.6 ± 1.0 | 1.8 ± 0.9 | 1.8 ± 0.8 | 3.8 ± 0.5 | 1.8 ± 0.4 | 3.1 ± 0.5 |
| 800 | 3.1 ± 0.6 | 3.3 ± 1.4 | 3.8 ± 0.8 | 4.6 ± 0.6 | 2.4 ± 0.2 | 1.3 ± 0.2 |
| 1000 | 5.8 ± 1.1 | 4.4 ± 0.5 | 2.0 ± 1.0 | 6.0 ± 1.0 | 5.5 ± 1.0 | 0.5 ± 0.5 |
| Root length | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 100 | 3.5 ± 0.1 | 2.4 ± 0.2 | 4.0 ± 0.5 | 6.7 ± 0.8 | 5.9 ± 0.8 | 0.5 ± 0.7 |
| 200 | 17.4 ± 0.6 | 21.1 ± 0.1 | 7.8 ± 0.6 | 9.6 ± 0.3 | 5.3 ± 0.7 | 4.3 ± 0.3 |
| 400 | 43.9 ± 0.7 | 29.4 ± 0.3 | 8.8 ± 0.7 | 18.2 ± 0.2 | 9.6 ± 0.3 | 9.9 ± 0.6 |
| 800 | 51.1 ± 0.7 | 42.0 ± 0.8 | 11.8 ± 0.6 | 28.1 ± 0.4 | 16.8 ± 0.7 | 18.4 ± 0.1 |
| 1000 | 68.7 ± 0.4 | 63.4 ± 4.8 | 30.5 ± 0.6 | 53.7 ± 0.3 | 28.6 ± 0.2 | 21.7 ± 0.7 |
| Compound Concentration (mg/kg s.d.w.) | Inhibition Biomarkers (%) | |||||
|---|---|---|---|---|---|---|
| Fresh matter | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 100 | 3.9 ± 0.2 | −0.8 ± 0.1 | 0.1 ± 0.1 | 2.9 ± 0.2 | 4.4 ± 0.2 | −1.5 ± 0.2 |
| 200 | 10.3 ± 0.1 | 0.9 ± 0.1 | 1.3 ± 0.2 | 5.6 ± 0.1 | 5.8 ± 0.1 | 1.1 ± 0.1 |
| 400 | 48.2 ± 0.1 | 8.5 ± 0.1 | 9.3 ± 0.1 | 41.5 ± 0.1 | 5.1 ± 0.2 | 8.0 ± 0.2 |
| 800 | 53.8 ± 0.0 | 26.2 ± 0.0 | 21.8 ± 0.1 | 49.0 ± 0.1 | 14.3 ± 0.2 | 19.8 ± 0.2 |
| 1000 | 63.4 ± 0.1 | 48.9 ± 0.0 | 47.4 ± 0.1 | 55.6 ± 0.0 | 43.8 ± 0.1 | 41.0 ± 0.1 |
| Shoot height | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 100 | 3.6 ± 0.7 | −2.8 ± 0.2 | 0.6 ± 0.5 | 3.5 ± 0.2 | 3.4 ± 0.7 | −0.9 ± 0.4 |
| 200 | 12.3 ± 0.2 | 0.3 ± 0.5 | 0.9 ± 0.5 | 5.4 ± 0.6 | 5.4 ± 0.6 | −1.3 ± 0.3 |
| 400 | 49.8 ± 0.5 | 9.5 ± 0.7 | 2.2 ± 0.9 | 38.2 ± 0.4 | 5.7 ± 0.6 | −1.9 ± 0.2 |
| 800 | 56.5 ± 0.4 | 21.5 ± 0.2 | −0.6 ± 0.3 | 46.7 ± 0.4 | 13.2 ± 0.3 | −3.2 ± 0.7 |
| 1000 | 66.9 ± 0.4 | 55.2 ± 0.3 | −1.6 ± 0.3 | 52.4 ± 0.4 | 42.6 ± 0.5 | −4.1 ± 0.2 |
| Root length | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 100 | 48.6 ± 0.2 | 0.8 ± 0.6 | −0.6 ± 0.2 | 2.0 ± 0.3 | 2.2 ± 0.6 | −0.8 ± 0.2 |
| 200 | 53.7 ± 0.4 | 43.8 ± 0.3 | −1.7 ± 0.5 | 35.7 ± 0.6 | 15.7 ± 0.6 | 4.5 ± 0.7 |
| 400 | 72.8 ± 0.7 | 64.9 ± 0.6 | 27.0 ± 0.7 | 68.8 ± 0.4 | 30.6 ± 0.4 | 9.5 ± 0.7 |
| 800 | 86.2 ± 0.1 | 71.1 ± 0.2 | 69.1 ± 0.4 | 73.9 ± 1.0 | 70.2 ± 0.3 | 16.4 ± 0.5 |
| 1000 | 89.0 ± 0.8 | 76.1 ± 0.5 | 76.1 ± 0.6 | 79.2 ± 1.0 | 74.0 ± 0.5 | 22.9 ± 0.6 |
| Sample Concentration (mg/kg of s.d.w.) | Number of Emerged Seedlings | Germination% | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oat | ||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Control | 20 | 20 | 20 | 20 | 20 | 20 | 100 | 100 | 100 | 100 | 100 | 100 |
| 100 | 20 | 20 | 20 | 20 | 20 | 20 | 100 | 100 | 100 | 100 | 100 | 100 |
| 200 | 19 | 20 | 20 | 20 | 20 | 20 | 98 | 100 | 100 | 100 | 100 | 100 |
| 400 | 19 | 20 | 19 | 19 | 20 | 20 | 98 | 100 | 98 | 98 | 100 | 100 |
| 800 | 19 | 19 | 19 | 19 | 20 | 20 | 97 | 98 | 98 | 98 | 100 | 100 |
| 1000 | 19 | 19 | 19 | 19 | 19 | 19 | 97 | 97 | 97 | 98 | 97 | 98 |
| LSDS = 1 LSDC = 1 | ||||||||||||
| Radish | ||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Control | 19 | 19 | 19 | 19 | 19 | 19 | 100 | 100 | 100 | 100 | 100 | 100 |
| 100 | 19 | 19 | 19 | 19 | 19 | 19 | 100 | 100 | 100 | 100 | 100 | 100 |
| 200 | 19 | 19 | 19 | 19 | 19 | 19 | 100 | 98 | 100 | 100 | 98 | 100 |
| 400 | 19 | 18 | 18 | 19 | 19 | 18 | 98 | 96 | 96 | 98 | 98 | 96 |
| 800 | 18 | 18 | 17 | 18 | 19 | 16 | 96 | 96 | 89 | 96 | 98 | 84 |
| 1000 | 17 | 18 | 14 | 17 | 18 | 13 | 89 | 95 | 74 | 89 | 96 | 70 |
| LSDS = 1 LSDC = 2 | ||||||||||||
| Compound Concentration (mg/kg s.d.w.) | Inhibition (%) | |||||
|---|---|---|---|---|---|---|
| Gallant soldier (Galinsoga parviflora Cav.) | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 100 | 8.9 ± 0.6 | 8.7 ± 0.6 | 8.1 ± 0.5 | 7.9 ± 0.3 | 7.6 ± 0.3 | 7.7 ± 0.8 |
| 400 | 22.1 ± 1.1 | 13.0 ± 0.8 | 9.7 ± 0.3 | 8.2 ± 0.5 | 8.9 ± 0.5 | 6.9 ± 0.9 |
| 1000 | 55.3 ± 0.6 | 40.0 ± 1.1 | 34.1 ± 0.45 | 42.1 ± 0.4 | 34.1 ± 1.2 | 18.3 ± 0.6 |
| White goosefoot (Chenopodium album L.) | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 100 | 23.3 ± 0.5 | 21.0 ± 1.1 | 18.1 ± 0.4 | 21.9 ± 0.3 | 7.6 ± 0.3 | 3.9 ± 0.8 |
| 400 | 41.7 ± 0.5 | 33.3 ± 0.7 | 26.3 ± 1.5 | 35.1 ± 0.6 | 27.5 ± 0.8 | 10.6 ± 0.7 |
| 1000 | 56.1 ± 1.2 | 42.2 ± 0.8 | 39.3 ± 0.4 | 47.0 ± 0.6 | 35.1 ± 0.7 | 26.3 ± 0.2 |
| Sorrel (Rumex acetosa L.) | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 100 | 50.9 ± 0.3 | 47.3 ± 0.7 | 26.4 ± 0.4 | 28.7 ± 0.3 | 27.4 ± 0.3 | 24.4 ± 0.6 |
| 400 | 53.2 ± 1.1 | 49.4 ± 0.4 | 31.4 ± 0.8 | 33.7 ± 1.3 | 32.5 ± 0.6 | 29.6 ± 0.9 |
| 1000 | 59.4 ± 0.5 | 51.2 ± 1.1 | 51.6 ± 0.4 | 51.5 ± 0.4 | 49.0 ± 0.2 | 42.4 ± 0.5 |
| Weeds | Compounds | ||||||
|---|---|---|---|---|---|---|---|
| Control | 1 | 2 | 3 | 4 | 5 | 6 | |
| Gallant soldier (G. parviflora Cav.) | − | 7 | 8 | 8 | 8 | 8 | 9 |
| White goosefoot (Ch. album L.) | − | 7 | 8 | 8 | 8 | 8 | 9 |
| Common sorrel (R. acetosa L.) | − | 7 | 8 | 8 | 8 | 8 | 8 |
| Compound | EC50 (Lower Limit; Upper Limit (mg/L)) | EC50 (Lower Limit; Upper Limit (mg/kg s.d.w.)) | Coefficient of Determination (R2) |
|---|---|---|---|
| 1 | 410.3 (356.1;472.7) | 541.6 (470;623.9) | 0.9746 |
| 2 | 422.5 (365.9;487.9) | 557.7 (219.6;644) | 0.9750 |
| 3 | 1201 (1065;1354) | 1585.3 (1405.8;1787.3) | 0.0979 |
| 4 | 105.8 (69.99;159.9) | 139.6 (92.4;211.1) | 0.8167 |
| 5 | 232.9 (182.7;296.9) | 307.4 (241.2;391.9) | 0.9290 |
| 6 | 538.9 (483.7;600.6) | 710.9 (638.5;792.7) | 0.9836 |
| Compound Concentration (mg/kg s.d.w.) | Growth Inhibition (%) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 10 | 11 ± 2 | 9 ± 1 | 4 ± 1 | NM | 12 ± 1 | 6 ± 1 |
| 50 | NM | NM | 14 ± 1 | NM | NM | NM |
| 100 | NM | NM | 26 ± 2 | NM | NM | NM |
| 250 | NM | NM | NM | NM | NM | NM |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogacz, D.; Lewkowski, J.; Siedlarek, M.; Karpowicz, R.; Kowalczyk, A.; Rychter, P. The Effect of New Thiophene-Derived Diphenyl Aminophosphonates on Growth of Terrestrial Plants. Materials 2019, 12, 2018. https://doi.org/10.3390/ma12122018
Rogacz D, Lewkowski J, Siedlarek M, Karpowicz R, Kowalczyk A, Rychter P. The Effect of New Thiophene-Derived Diphenyl Aminophosphonates on Growth of Terrestrial Plants. Materials. 2019; 12(12):2018. https://doi.org/10.3390/ma12122018
Chicago/Turabian StyleRogacz, Diana, Jarosław Lewkowski, Marta Siedlarek, Rafał Karpowicz, Anna Kowalczyk, and Piotr Rychter. 2019. "The Effect of New Thiophene-Derived Diphenyl Aminophosphonates on Growth of Terrestrial Plants" Materials 12, no. 12: 2018. https://doi.org/10.3390/ma12122018