Effect of Diffusion Annealing Temperature on the Formation Process and Properties of a Carbon–Aluminum Composite Layer on Pure Titanium
Abstract
:1. Introduction
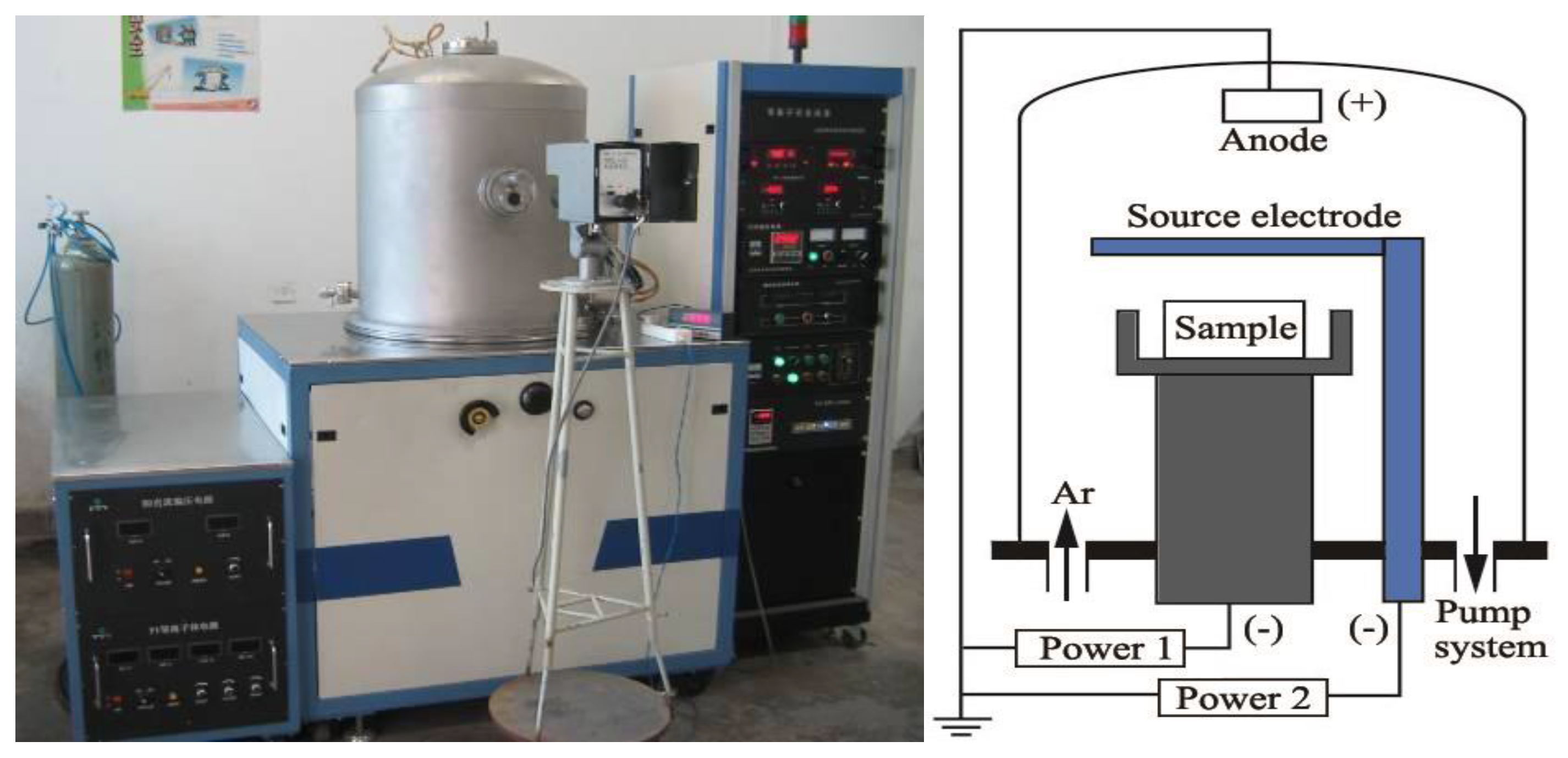
2. Materials and Methods
3. Results
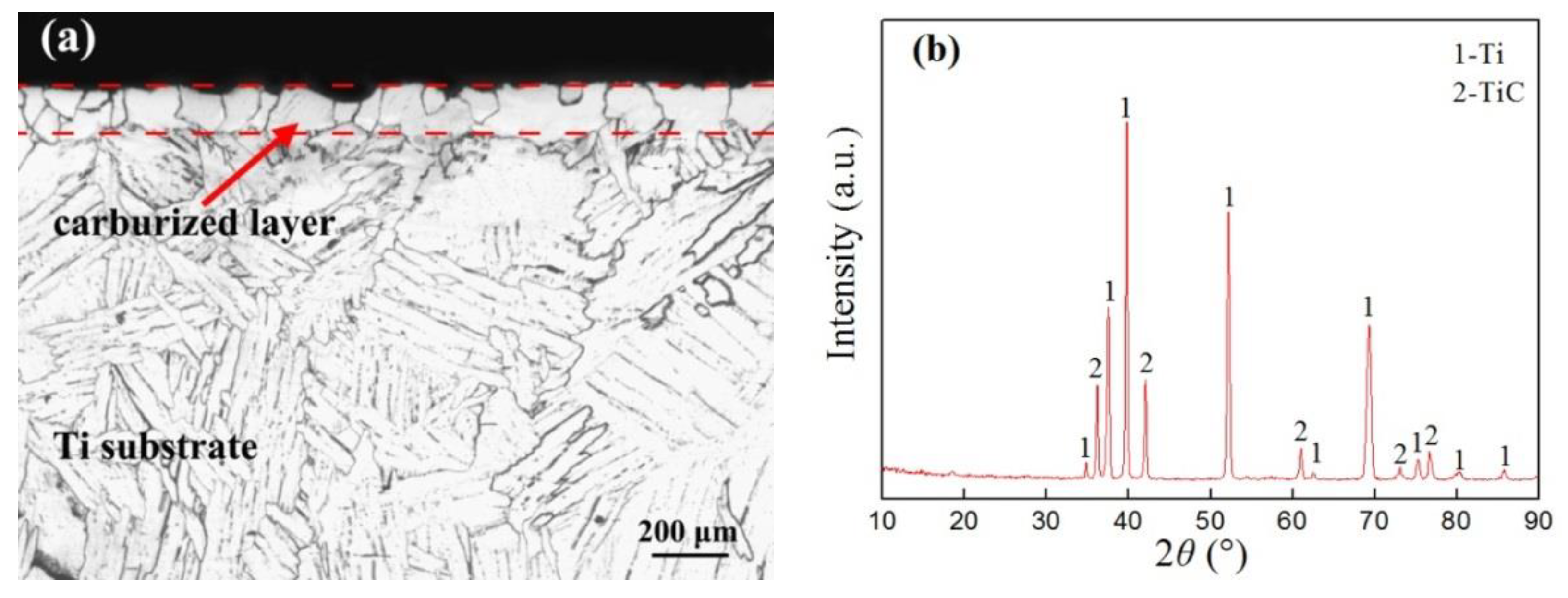
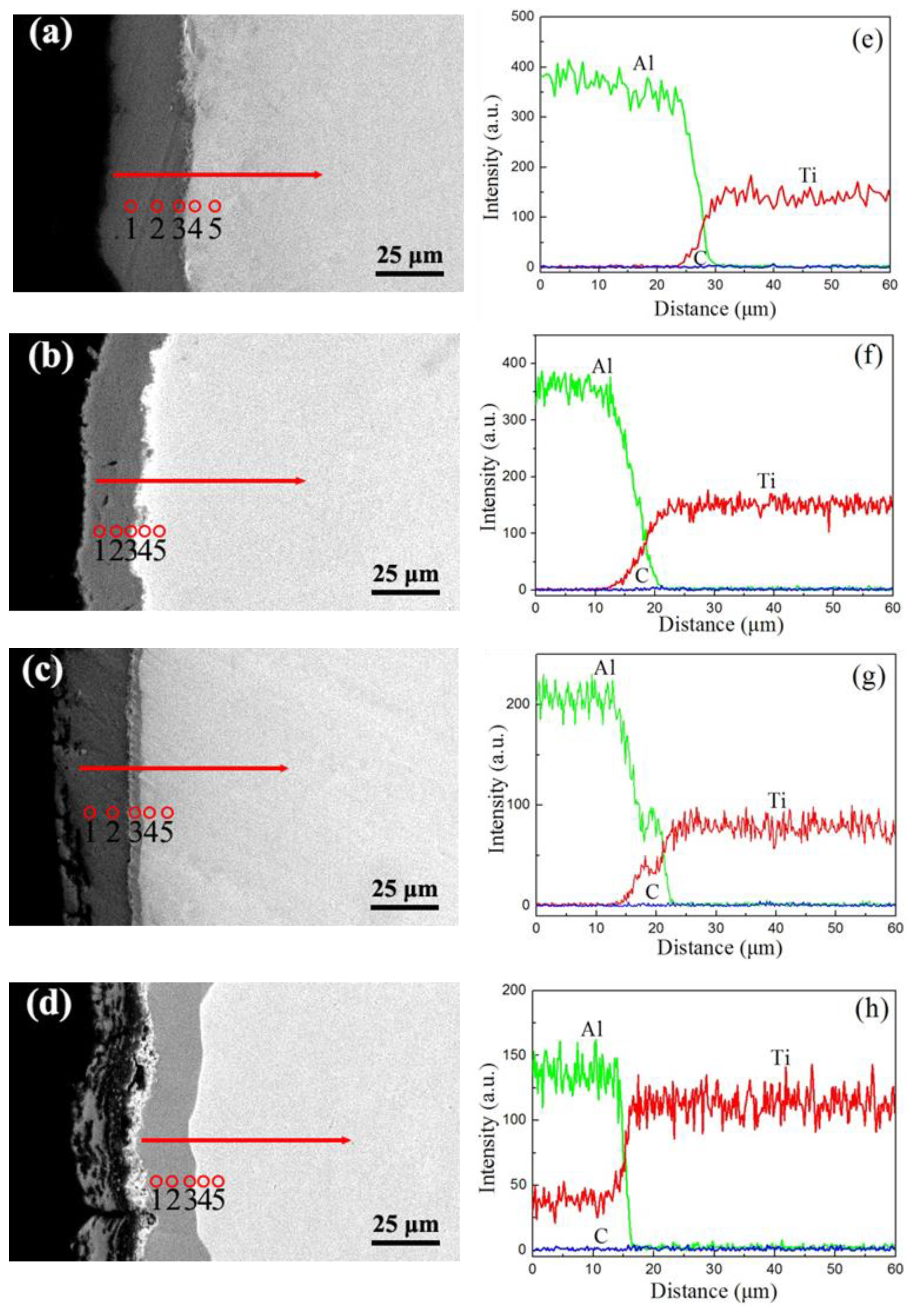
3.1. Microstructure of the Carburized Layer
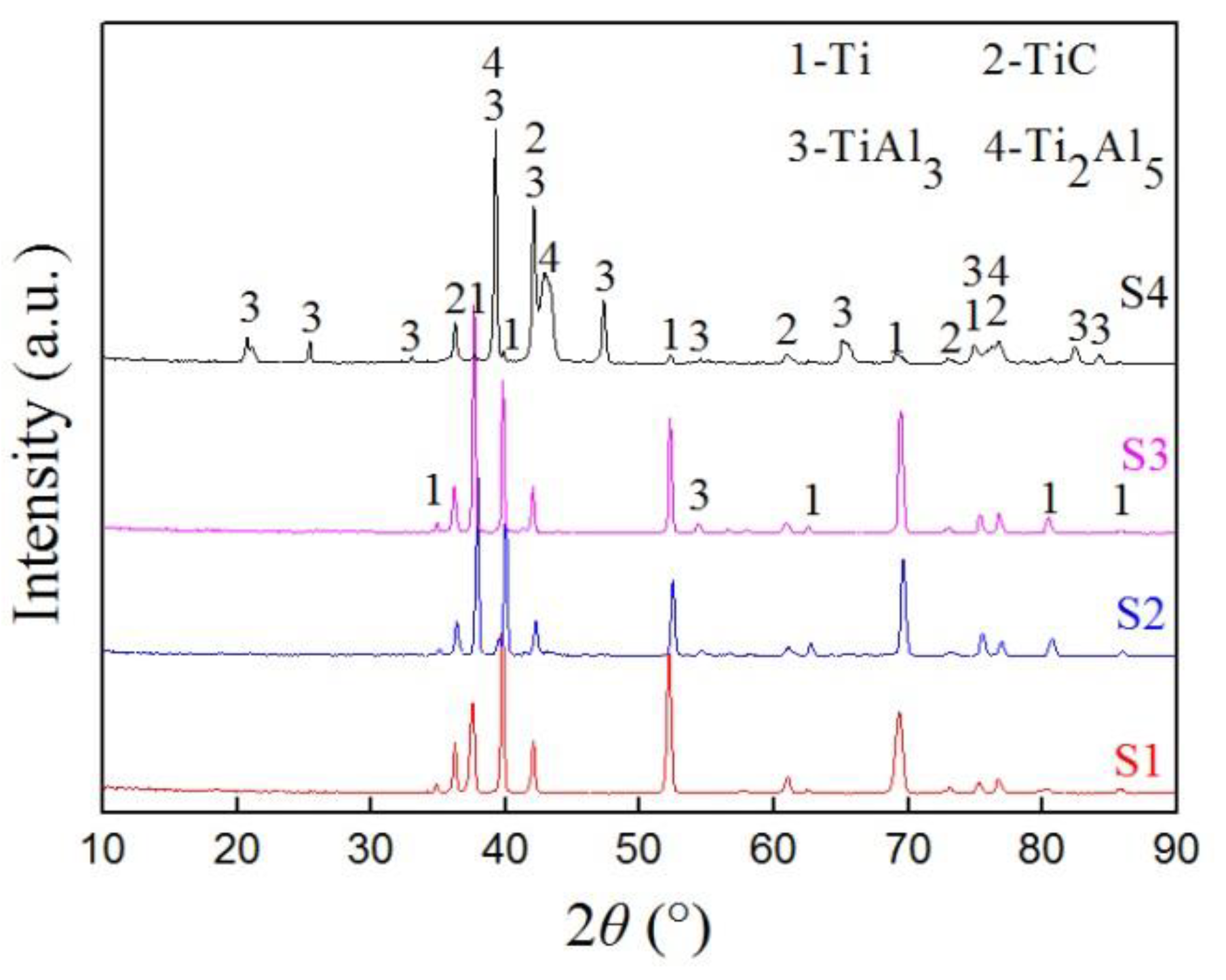
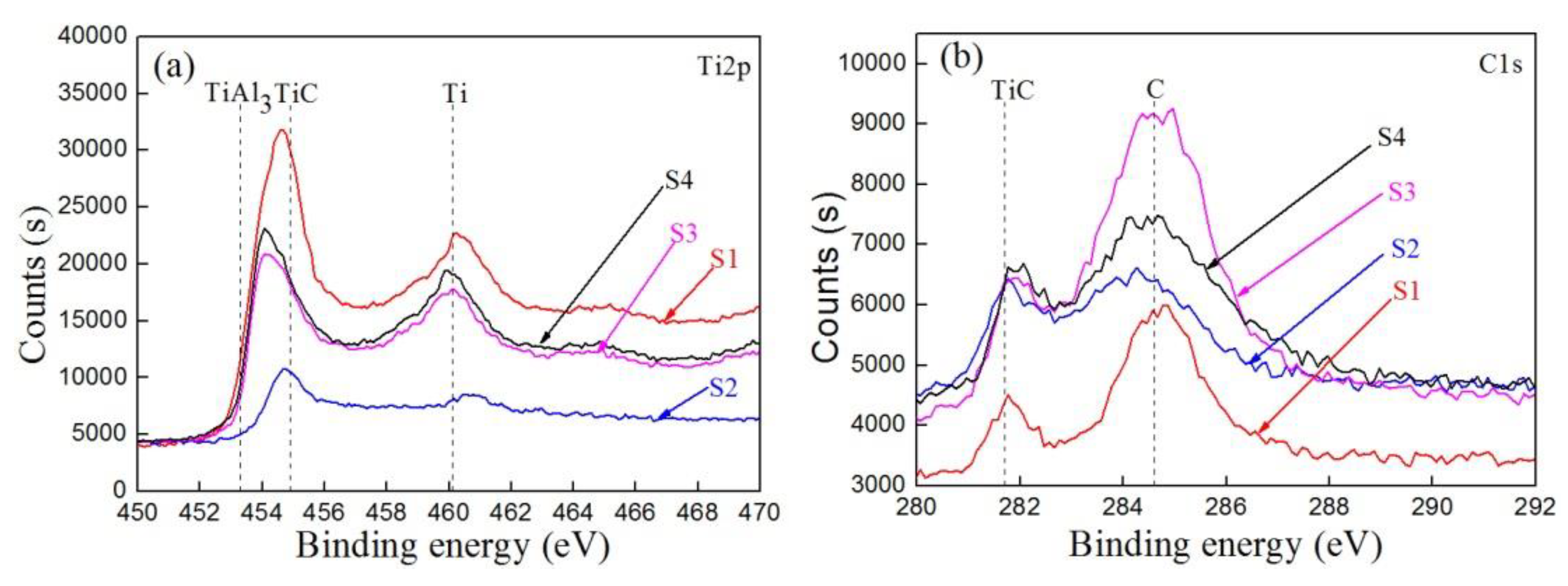
3.2. Composition of the Carbon–Aluminum Composite Layer
3.3. Cross-Sectional Microstructure
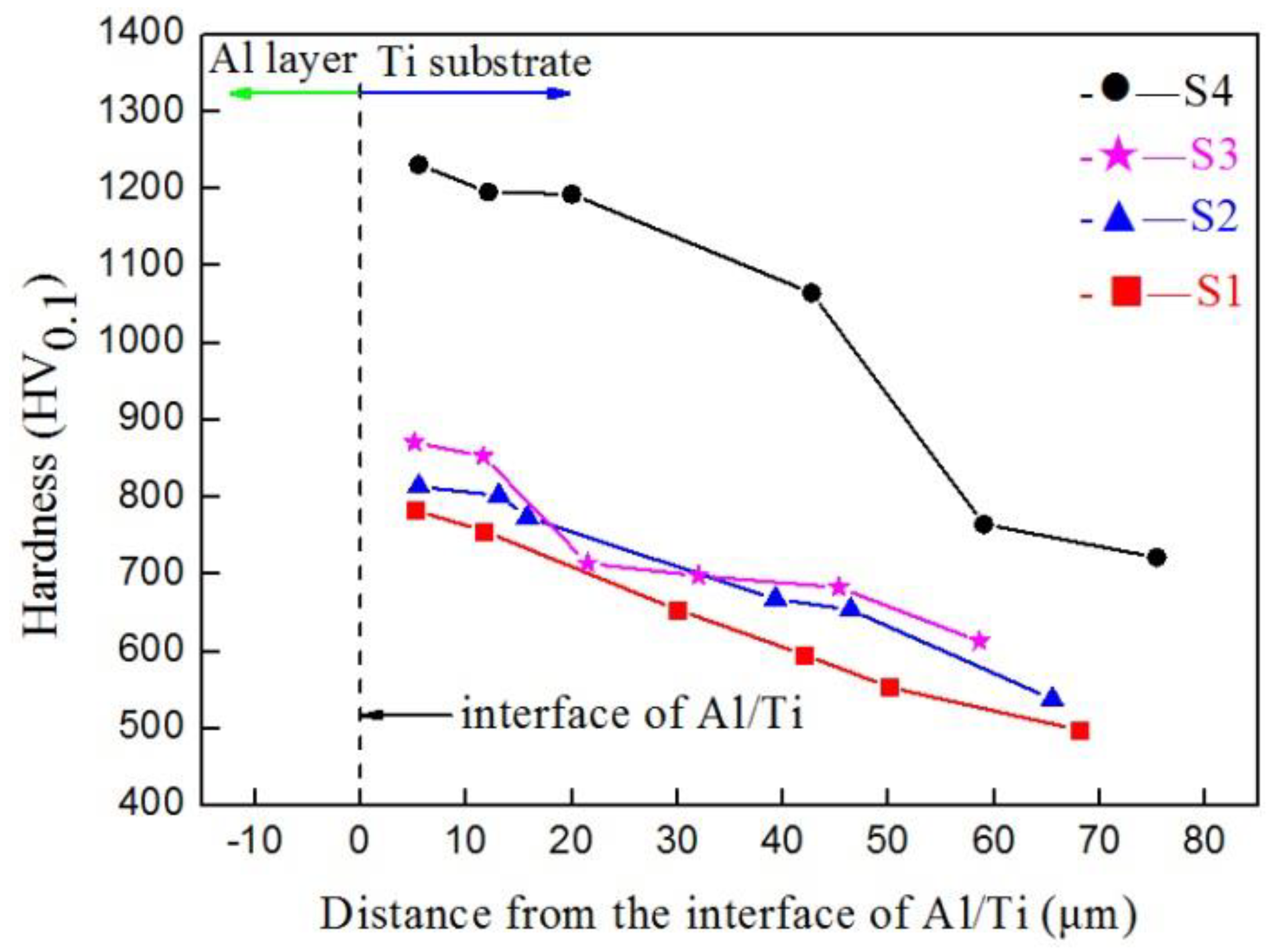
3.4. Hardness
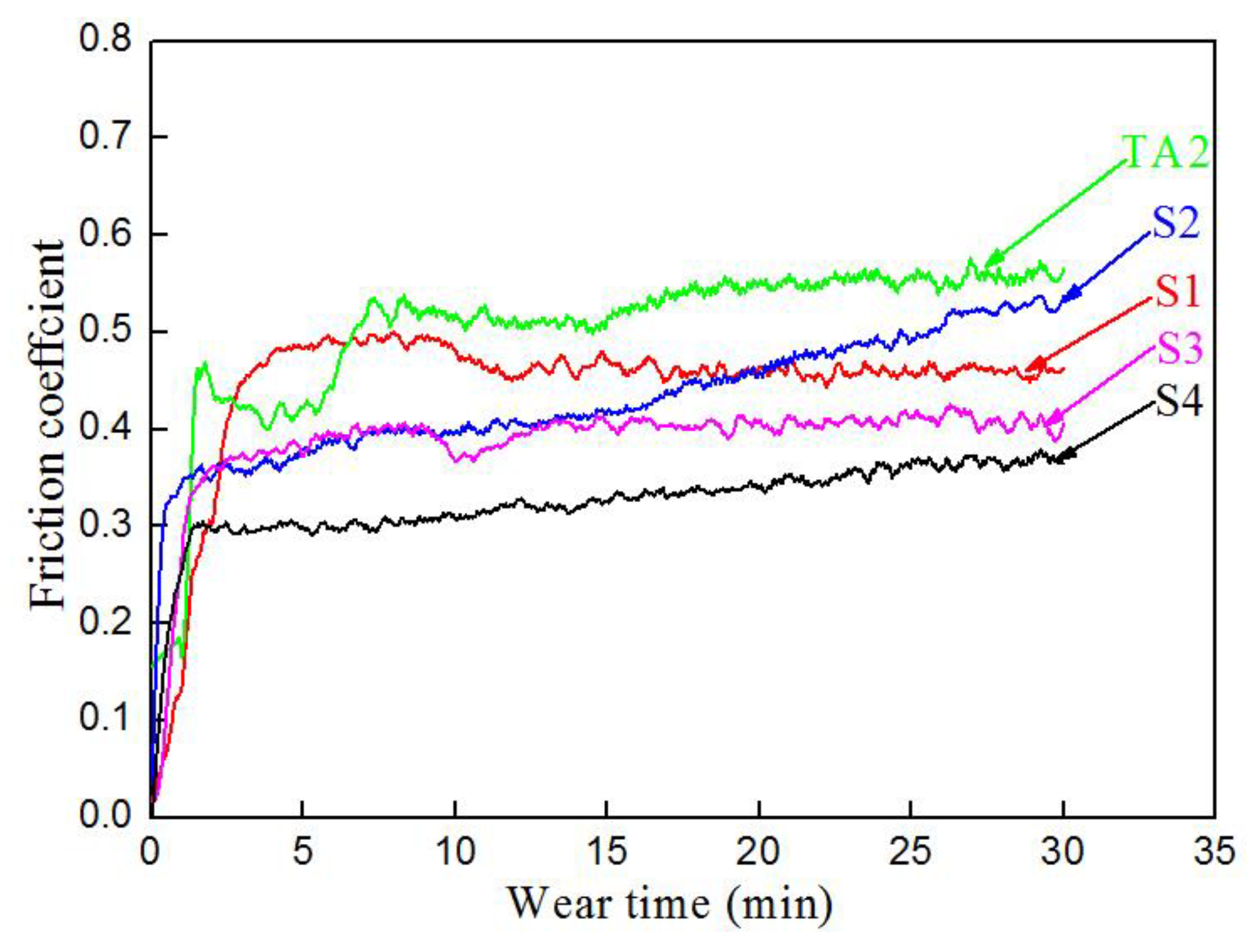
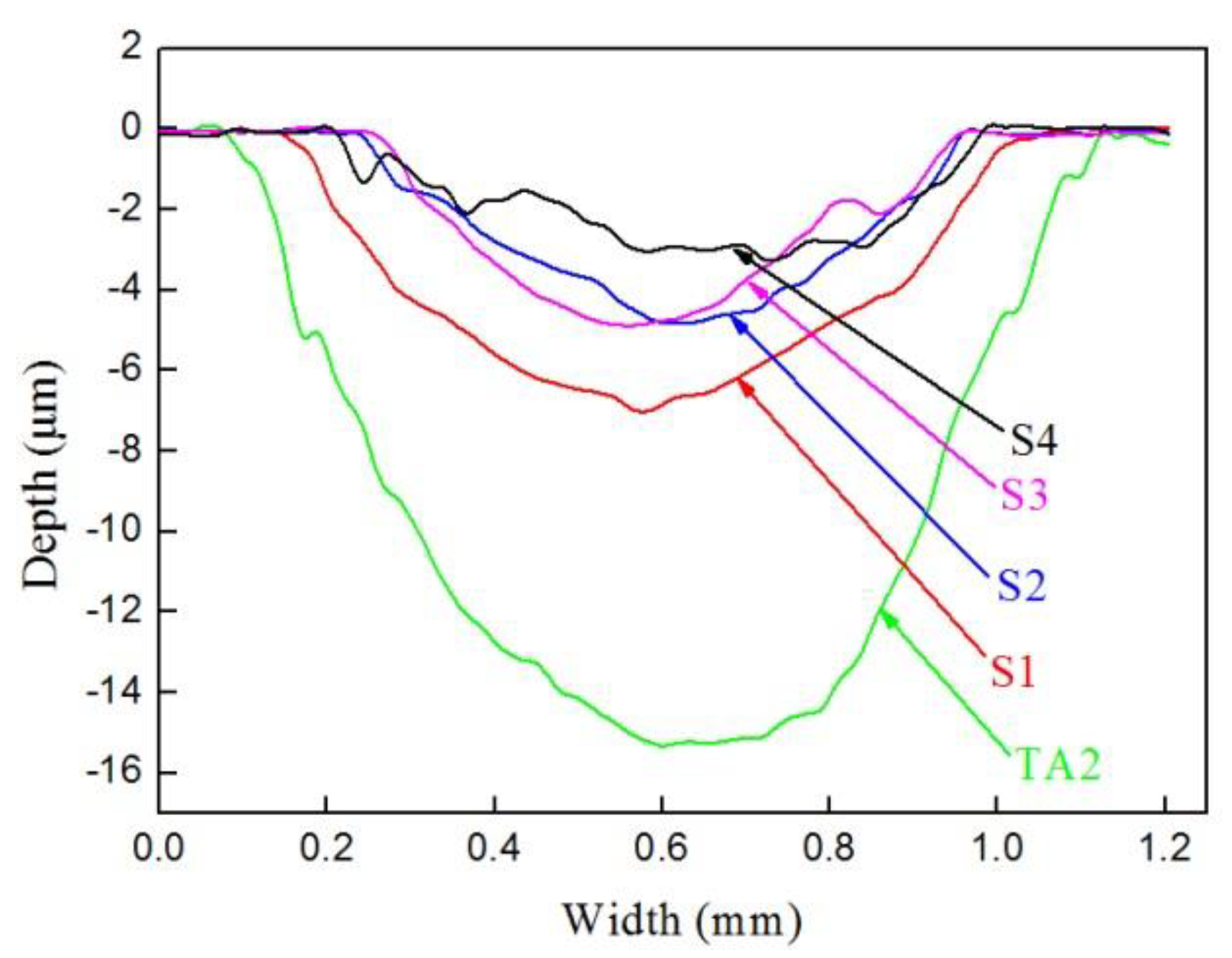
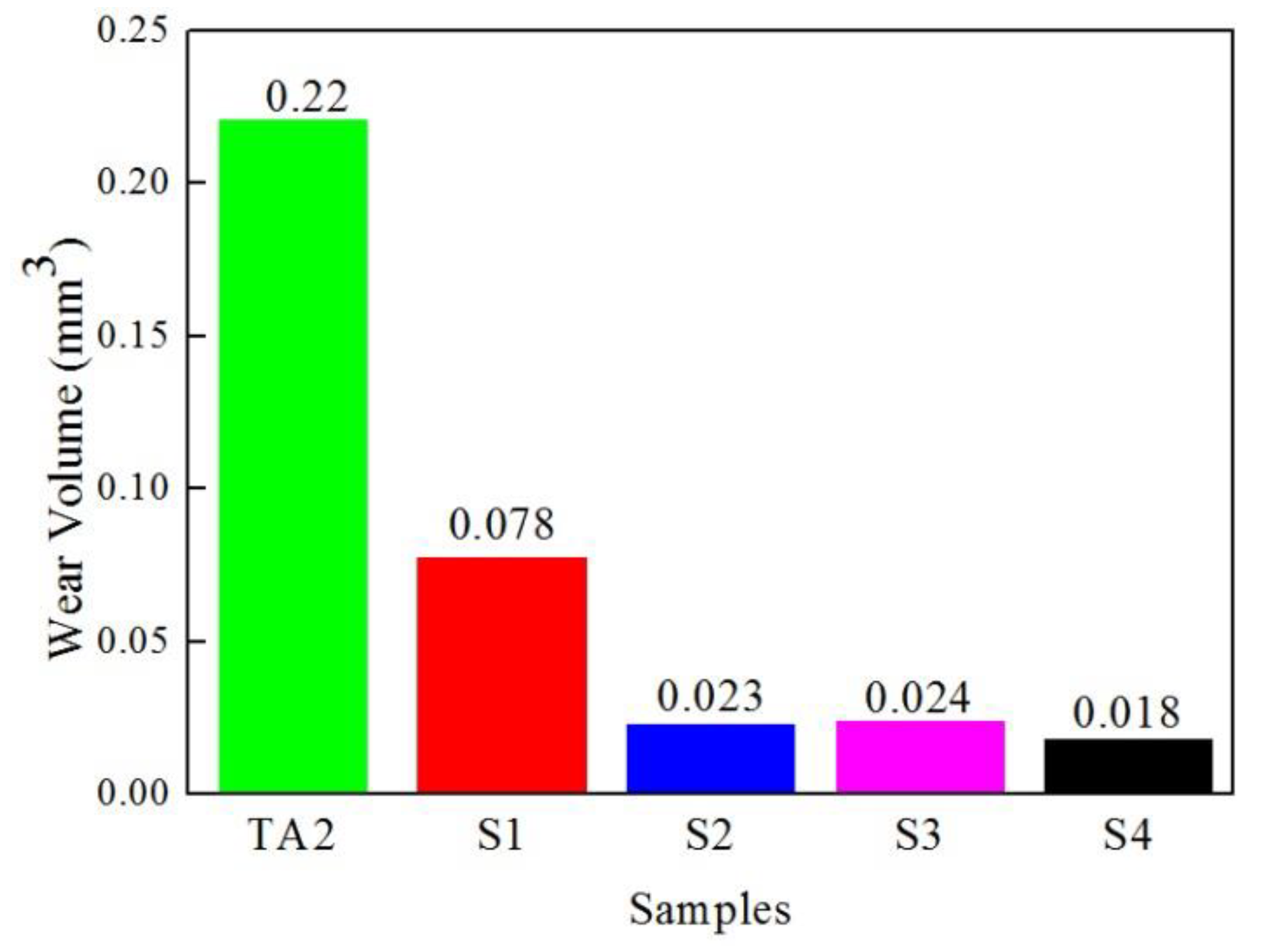
3.5. Friction and Wear Properties
4. Discussion
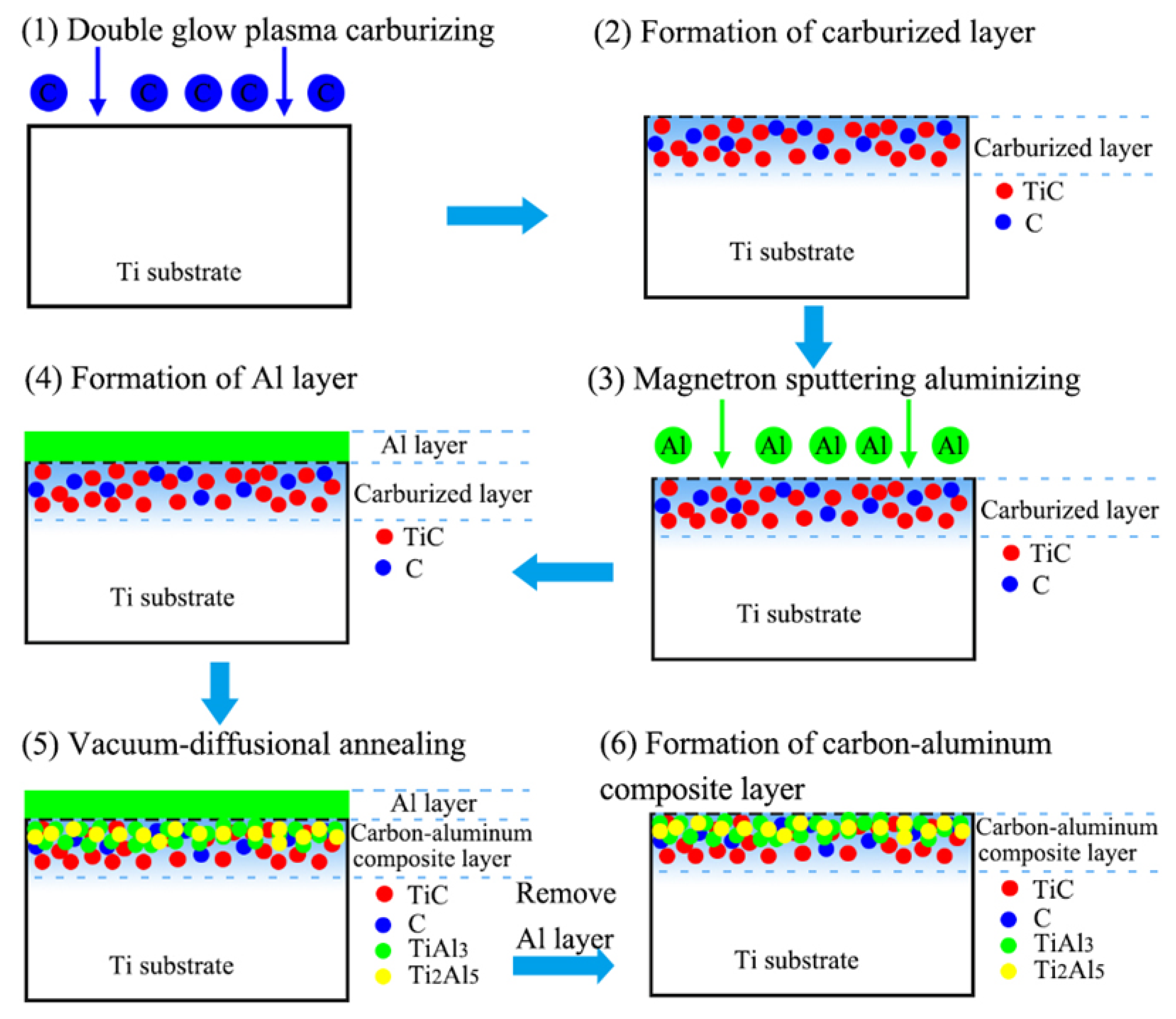
4.1. The Formation Process of the Carbon–Aluminum Composite Layer
4.2. The Diffusion Layer and Composition
4.3. Properties of the Carbon–Aluminum Composite Layer
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, W.; Han, C. Microstructure and Wear Resistance of Ti6Al4V Coating Fabricated by Electro-Spark Deposition. Metals 2018, 9, 23. [Google Scholar] [CrossRef]
- Wiklund, U.; Hutchings, I.M. Investigation of surface treatments for galling protection of titanium alloys. Wear 2001, 251, 1034–1041. [Google Scholar] [CrossRef]
- Kikuchi, S.; Akebono, H.; Ueno, A.; Ameyama, K. Formation of commercially pure titanium with a bimodal nitrogen diffusion phase using plasma nitriding and spark plasma sintering. Powder Technol. 2018, 330, 349–356. [Google Scholar] [CrossRef]
- Yin, M.; Wang, W.; He, W.; Cai, Z. Impact-Sliding Tribology Behavior of TC17 Alloy Treated by Laser Shock Peening. Materials (Basel) 2018, 11, 1229. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, F.; Wang, H.; Liu, D. Laser Cladding In-Situ Ti(C, N) Particles Reinforced Ni-Based Composite Coatings Modified with CeO2 Nanoparticles. Metals 2018, 8, 601. [Google Scholar] [CrossRef]
- Ren, L.; Wang, T.; Chen, Z.; Li, Y.; Qian, L. Self-Lubricating PEO–PTFE Composite Coating on Titanium. Metals 2019, 9, 170. [Google Scholar] [CrossRef]
- Khorasanian, M.; Dehghan, A.; Shariat, M.H.; Bahrololoom, M.E.; Javadpour, S. Microstructure and wear resistance of oxide coatings on Ti–6Al–4V produced by plasma electrolytic oxidation in an inexpensive electrolyte. Surf. Coat. Technol. 2011, 206, 1495–1502. [Google Scholar] [CrossRef]
- Aliasghari, S.; Skeldon, P.; Thompson, G.E. Plasma electrolytic oxidation of titanium in a phosphate/silicate electrolyte and tribological performance of the coatings. Appl. Surf. Sci. 2014, 316, 463–476. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Hagino, H. Formation of titanium carbide layer by laser alloying with a light-transmitting resin. Opt. Lasers Eng. 2017, 88, 13–19. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, A.H.; Zhang, Z.; Xia, H.B.; Wang, Y.N. Microstructure, wear resistance and cell proliferation ability of in situ synthesized Ti–B coating produced by laser alloying. Opt. Lasers Eng. 2015, 67, 176–182. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, W.; Ma, Y.; Liang, C.; Liu, C.; Zhang, C.; Cai, Q. Microstructure and wear resistance of compositionally graded Ti-Al intermetallic coating on Ti6Al4V alloy fabricated by laser powder deposition. Surf. Coat. Technol. 2018, 353, 32–40. [Google Scholar] [CrossRef]
- Utua, I.D.; Marginean, G. Effect of electron beam remelting on the characteristics of HVOF sprayed Al2O3-TiO2 coatings deposited on titanium substrate. Colloids Surf. A Physicochem. Eng. Asp. 2017, 526, 70–75. [Google Scholar] [CrossRef]
- Chen, J.; Geng, M.; Li, Y.; Yang, Z.; Chai, Y.; He, G. Erosion Resistance and Damage Mechanism of TiN/ZrN Nanoscale Multilayer Coating. Coatings 2019, 9, 64. [Google Scholar] [CrossRef]
- Vadiraj, A.; Kamaraj, M.; Gnanamoorthy, R. Fretting wear studies on PVD TiN coated, ion implanted and thermally oxidised biomedical titanium alloys. Surf. Eng. 2013, 23, 209–215. [Google Scholar] [CrossRef]
- Sitek, R.; Kaminski, J.; Borysiuk, J.; Matysiak, H.; Kubiak, K.; Kurzydlowski, K.J. Microstructure and properties of titanium aluminides on Ti6Al4V titanium alloy produced by chemical vapor deposition method. Intermetallics 2013, 36, 36–44. [Google Scholar] [CrossRef]
- Belkin, P.N.; Kusmanov, S.A.; Dyakov, I.G.; Komissarova, M.R.; Parfenyuk, V.I. Anode plasma electrolytic carburizing of commercial pure titanium. Surf. Coat. Technol. 2016, 307, 1303–1309. [Google Scholar] [CrossRef]
- Zhao, Z.; Hui, P.; Wang, T.; Wang, X.; Xu, Y.; Zhong, L.; Zhao, M. New strategy to grow TiC coatings on titanium alloy: Contact solid carburization by cast iron. J. Alloys Compd. 2018, 745, 637–643. [Google Scholar] [CrossRef]
- Afarani, M.S.; Khorshahian, S.; Sharifitabar, M. Synthesis of Ti(C, N) ceramic layer on surface of Ti–6Al–4V alloy via pack nitro-carburizing route. Surf. Coat. Technol. 2013, 219, 94–100. [Google Scholar] [CrossRef]
- Rastkar, A.R.; Parseh, P.; Darvishnia, N.; Hadavi, S.M.M. Microstructural evolution and hardness of TiAl3 and TiAl2 phases on Ti–45Al–2Nb–2Mn–1B by plasma pack aluminizing. Appl. Surf. Sci. 2013, 276, 112–119. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Peng, Y.P.; Mao, Y.L.; Pang, C.J.; Lu, L.Y. Effect of hot-dip aluminizing on the oxidation resistance of Ti–6Al–4V alloy at high temperatures. Corros. Sci. 2012, 55, 187–193. [Google Scholar] [CrossRef]
- Szkliniarz, A.; Moskal, G.; Szkliniarz, W.; Swadźba, R. Improvement of oxidation resistance of Ti–47Al–2W–0.5Si alloy modified by aluminizing method. Surf. Coat. Technol. 2015, 277, 270–277. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, C.; Tao, J. Induction effect of α-Al2O3 seeds on formation of alumina coatings prepared by double glow plasma technique. Surf. Coat. Technol. 2013, 235, 544–551. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, L.; Li, C.; Li, Z.; Li, H. Morphology and Wear Resistance of Composite Coatings Formed on a TA2 Substrate Using Hot-Dip Aluminising and Micro-Arc Oxidation Technologies. Materials (Basel) 2019, 12, 799. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.N.; Chen, J.; Pan, J.D. Double Glow Plasma Hydrogen-Free Carburizing on the Surface of Purity Titanium. Adv. Mater. Res. 2011, 314, 108–111. [Google Scholar] [CrossRef]
- Nishimoto, A.; Nishi, C. Carbide layer coating on titanium by spark plasma sintering technique. Surf. Coat. Technol. 2018, 353, 324–328. [Google Scholar] [CrossRef]
- Xing, Y.; Jiang, C.; Hao, J. Time dependence of microstructure and hardness in plasma carbonized Ti–6Al–4V alloys. Vacuum 2013, 95, 12–17. [Google Scholar] [CrossRef]
- Felten, A.; Suarez-Martinez, I.; Xiaoxing Ke, J.G.; Pireaux, J.-J.; Drube, W.; Bittencourt, C.; Ewels, C.P. The role of oxygen at the interface between titanium and carbon nanotubes. ChemPhysChem 2009, 10, 1799–1804. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xia, L. Structural characterization of TiCx films prepared by plasma based ion implantation. Thin Solid Films 2001, 396, 16–21. [Google Scholar] [CrossRef]
- Tavoosi, M. The Kirkendall void formation in Al/Ti interface during solid-state reactive diffusion between Al and Ti. Surf. Interfaces 2017, 9, 96–200. [Google Scholar] [CrossRef]
- Luo, J.; Acoff, V.L. Using cold roll bonding and annealing to process Ti/Al multi-layered composites from elemental foils. Mater. Sci. Eng. A. 2004, 379, 164–172. [Google Scholar] [CrossRef]
- Assari, A.H.; Eghbali, B. Solid state diffusion bonding characteristics at the interfaces of Ti and Al layers. J. Alloys Compd. 2019, 773, 50–58. [Google Scholar] [CrossRef]
- Sun, Y.; Wan, Z.; Hu, L.; Wu, B.; Deng, T. Characterization on Solid Phase Diffusion Reaction Behavior and Diffusion Reaction Kinetic of Ti/Al. Rare Metal Mater. Eng. 2017, 46, 2080–2086. [Google Scholar] [CrossRef]
- Guo, X.; Fan, M.; Liu, Z.; Ma, F.; Wang, L.; Tao, J. Explosive Cladding and Hot Pressing of Ti/Al/Ti Laminates. Rare Metal Mater. Eng. 2017, 46, 1192–1196. [Google Scholar] [CrossRef]
- Liu, Z.; Rakita, M.; Xu, W.; Wang, X.; Han, Q. Ultrasound assisted combustion synthesis of TiC in Al–Ti–C system. Ultrason. Sonochem. 2015, 27, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yang, H.; Li, H.; Wang, D.; Li, G.-j. Tribological behaviors between commercial pure titanium sheet and tools in warm forming. Trans. Nonferrous Metals Soc. China 2015, 25, 2924–2931. [Google Scholar] [CrossRef]




| Parameter | Value |
|---|---|
| Source voltage/V | 800 |
| Workpiece voltage/V | 450 |
| Ar pressure/Pa | 30 |
| Distance between workpiece and source/mm | 20 |
| Temperature/°C | 900 |
| Time/h | 3 |
| Parameter | Value |
|---|---|
| Base pressure/Pa | 9.0 × 10−4 |
| Target Power/W | 150 |
| Ar flow rate/sccm | 20 |
| Work pressure/Pa | 0.2 |
| Time/h | 3 |
| Sample No. | Temperature (°C) | Degree of Vacuum (Pa) | Time (h) |
|---|---|---|---|
| S1 | - | - | - |
| S2 | 550 | 4 × 10−3 | 6 |
| S3 | 600 | 4 × 10−3 | 6 |
| S4 | 650 | 4 × 10−3 | 6 |
| Sample | Element | Position | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| S1 | Al | 90.52 | 87.21 | 14.92 | 2.04 | - |
| Ti | / | 2.24 | 68.45 | 78.32 | 81.38 | |
| C | 9.48 | 10.55 | 16.63 | 19.64 | 18.62 | |
| S2 | Al | 92.88 | 83.58 | 33.97 | 1.38 | - |
| Ti | / | 8.99 | 53.19 | 78.35 | 84.39 | |
| C | 7.12 | 7.43 | 12.84 | 20.27 | 15.61 | |
| S3 | Al | 93.54 | 89.61 | 56.91 | 2.21 | - |
| Ti | / | 3.13 | 24.45 | 80.2 | 83.86 | |
| C | 6.46 | 7.26 | 18.64 | 17.59 | 16.14 | |
| S4 | Al | 60.05 | 61.39 | 5.05 | - | - |
| Ti | 20.53 | 21.89 | 79.00 | 85.03 | 85.97 | |
| C | 19.42 | 16.72 | 15.95 | 14.97 | 14.03 | |
| Sample | Wear Scar Depth (mm) | Wear Scar Width (mm) | Wear Volume (mm3) |
|---|---|---|---|
| TA2 | 0.015 | 1.054 | 0.220 |
| S1 | 0.007 | 0.958 | 0.078 |
| S2 | 0.005 | 0.725 | 0.023 |
| S3 | 0.005 | 0.729 | 0.024 |
| S4 | 0.003 | 0.792 | 0.018 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Ma, Y.; Li, Z.; Ji, S.; Wang, Y.; Wang, Y. Effect of Diffusion Annealing Temperature on the Formation Process and Properties of a Carbon–Aluminum Composite Layer on Pure Titanium. Materials 2019, 12, 1994. https://doi.org/10.3390/ma12121994
Li H, Ma Y, Li Z, Ji S, Wang Y, Wang Y. Effect of Diffusion Annealing Temperature on the Formation Process and Properties of a Carbon–Aluminum Composite Layer on Pure Titanium. Materials. 2019; 12(12):1994. https://doi.org/10.3390/ma12121994
Chicago/Turabian StyleLi, Hongzhan, Youping Ma, Zhengxian Li, Shouchang Ji, Yanfeng Wang, and Yifei Wang. 2019. "Effect of Diffusion Annealing Temperature on the Formation Process and Properties of a Carbon–Aluminum Composite Layer on Pure Titanium" Materials 12, no. 12: 1994. https://doi.org/10.3390/ma12121994
APA StyleLi, H., Ma, Y., Li, Z., Ji, S., Wang, Y., & Wang, Y. (2019). Effect of Diffusion Annealing Temperature on the Formation Process and Properties of a Carbon–Aluminum Composite Layer on Pure Titanium. Materials, 12(12), 1994. https://doi.org/10.3390/ma12121994




