Preparation, Characterization and Application of Multi-Mode Imaging Functional Graphene Au-Fe3O4 Magnetic Nanocomposites
Abstract
:1. Introduction
2. Experimental Reagents and Instruments
2.1. Experimental Reagents
2.2. Experimental Instruments
3. Experimentation
3.1. Preparation of Au-Fe3O4 Nanoparticles
3.2. Preparation of 1a
3.3. Preparation of 1b
3.4. Preparation of 1c
3.5. Preparation of 1d
3.6. Drug Release Test
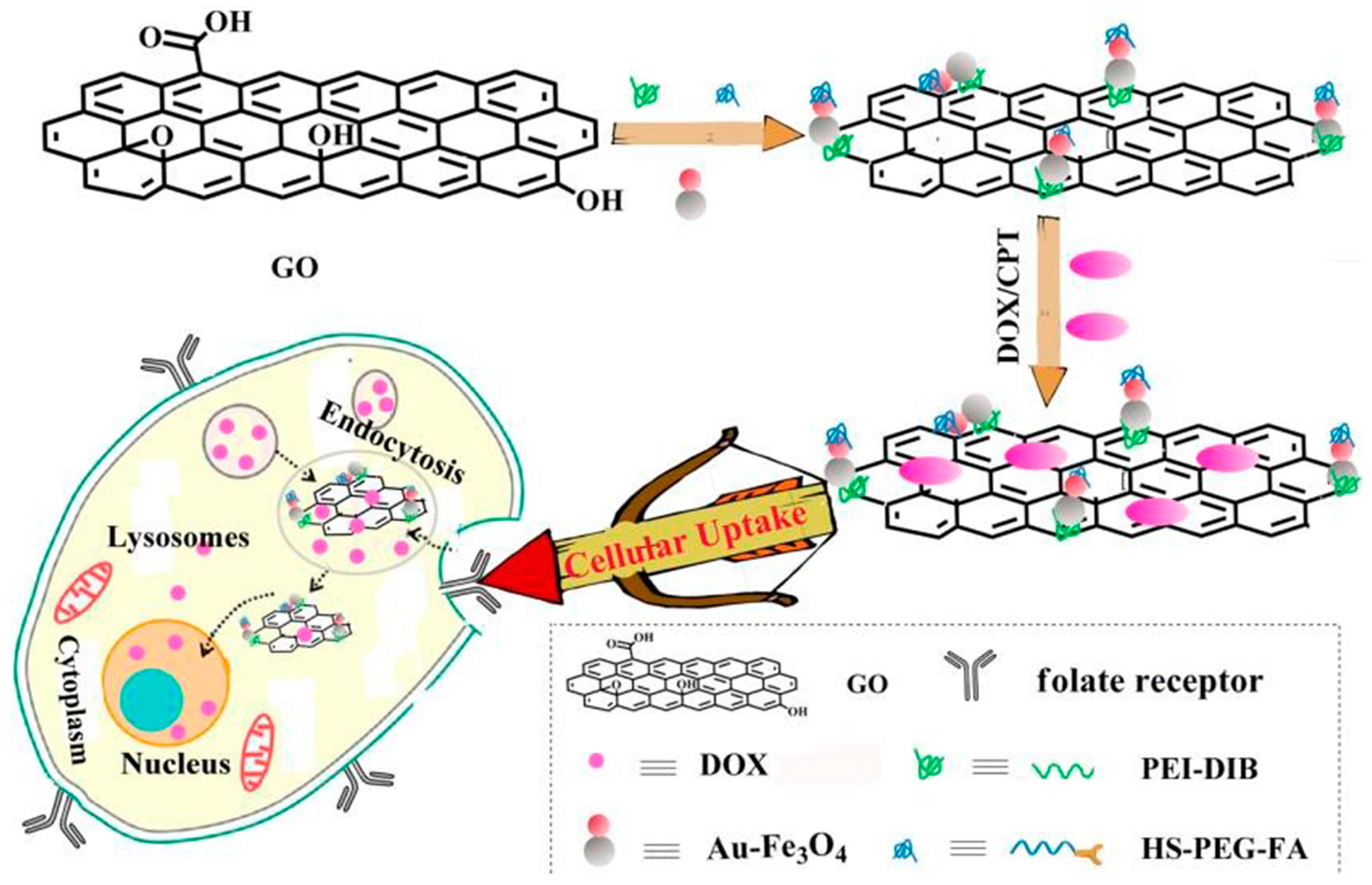
4. Results and Discussion
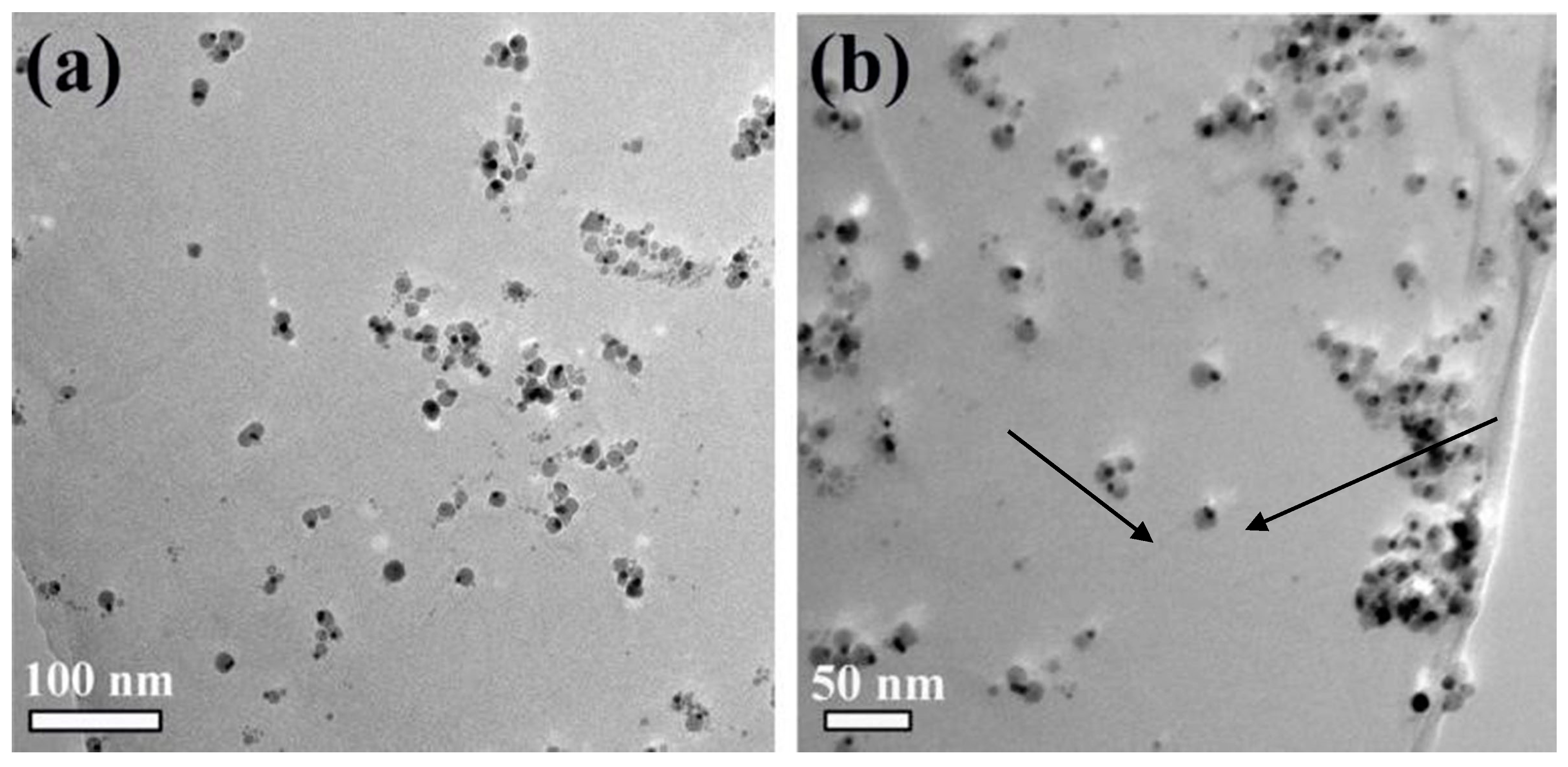
4.1. Morphological Changes of Au-Fe3O4 Nanoparticles on GO Surface before and after Modification
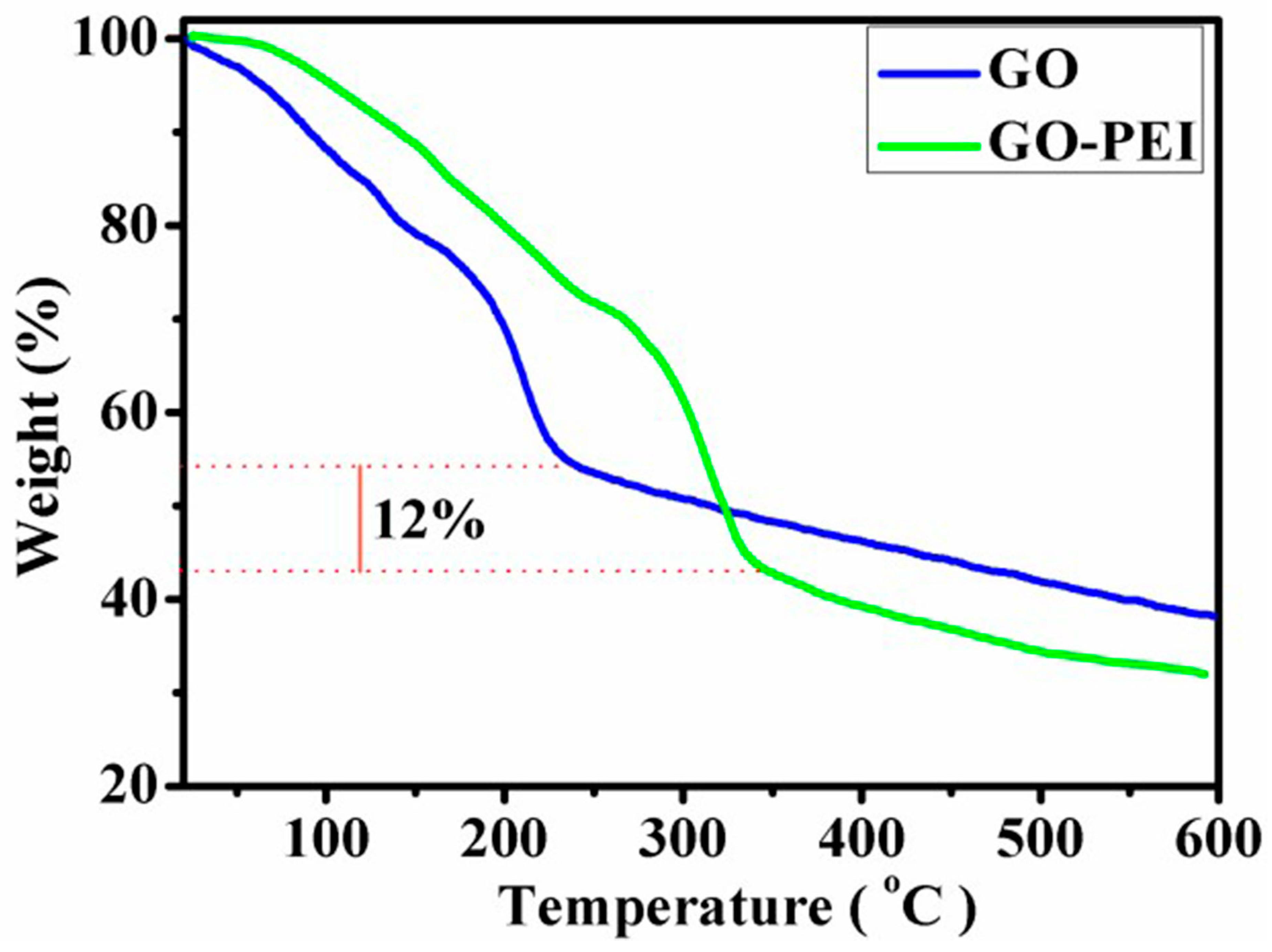
4.2. Thermo Gravimetric Analysis of GO and GO-PEI
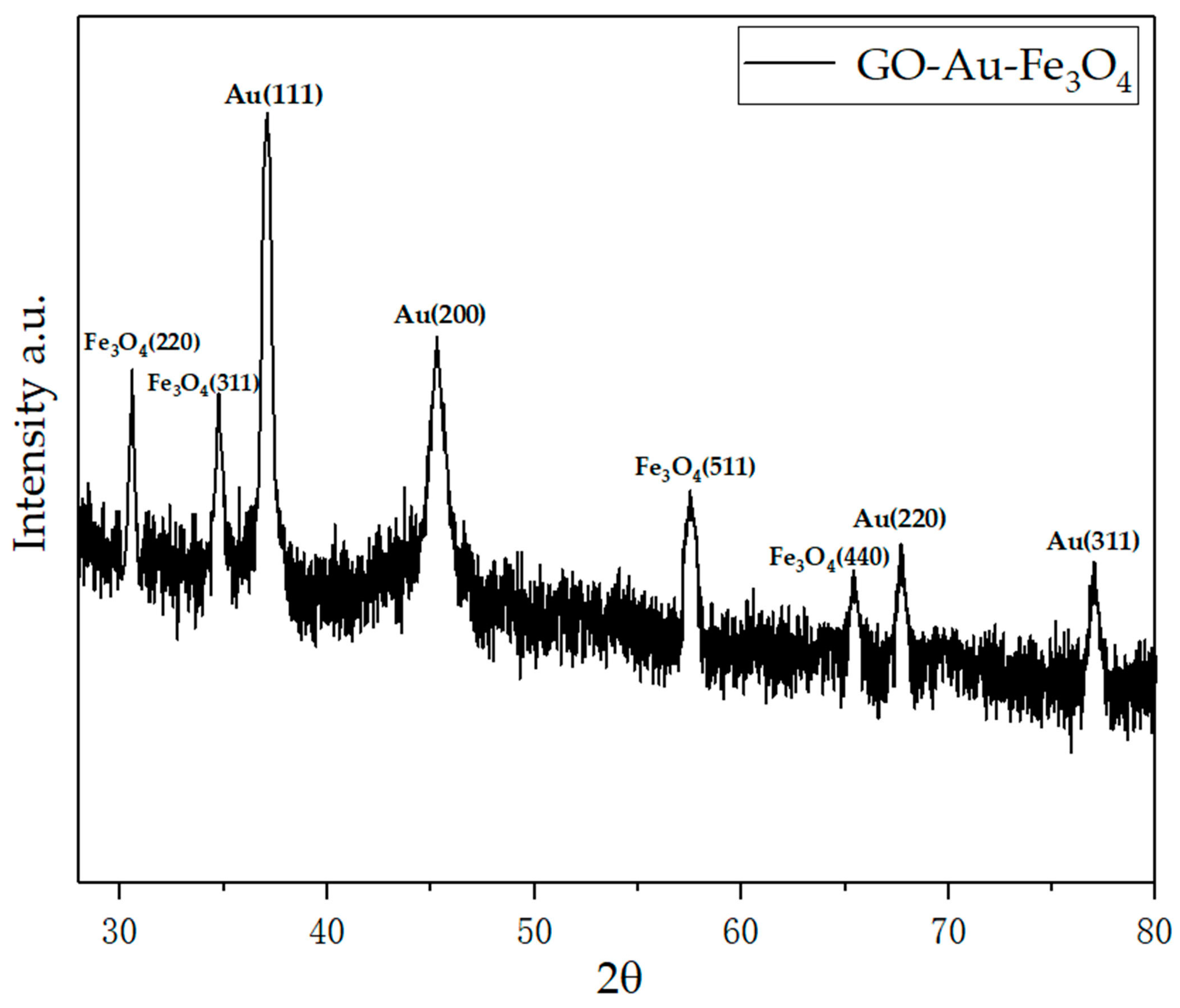
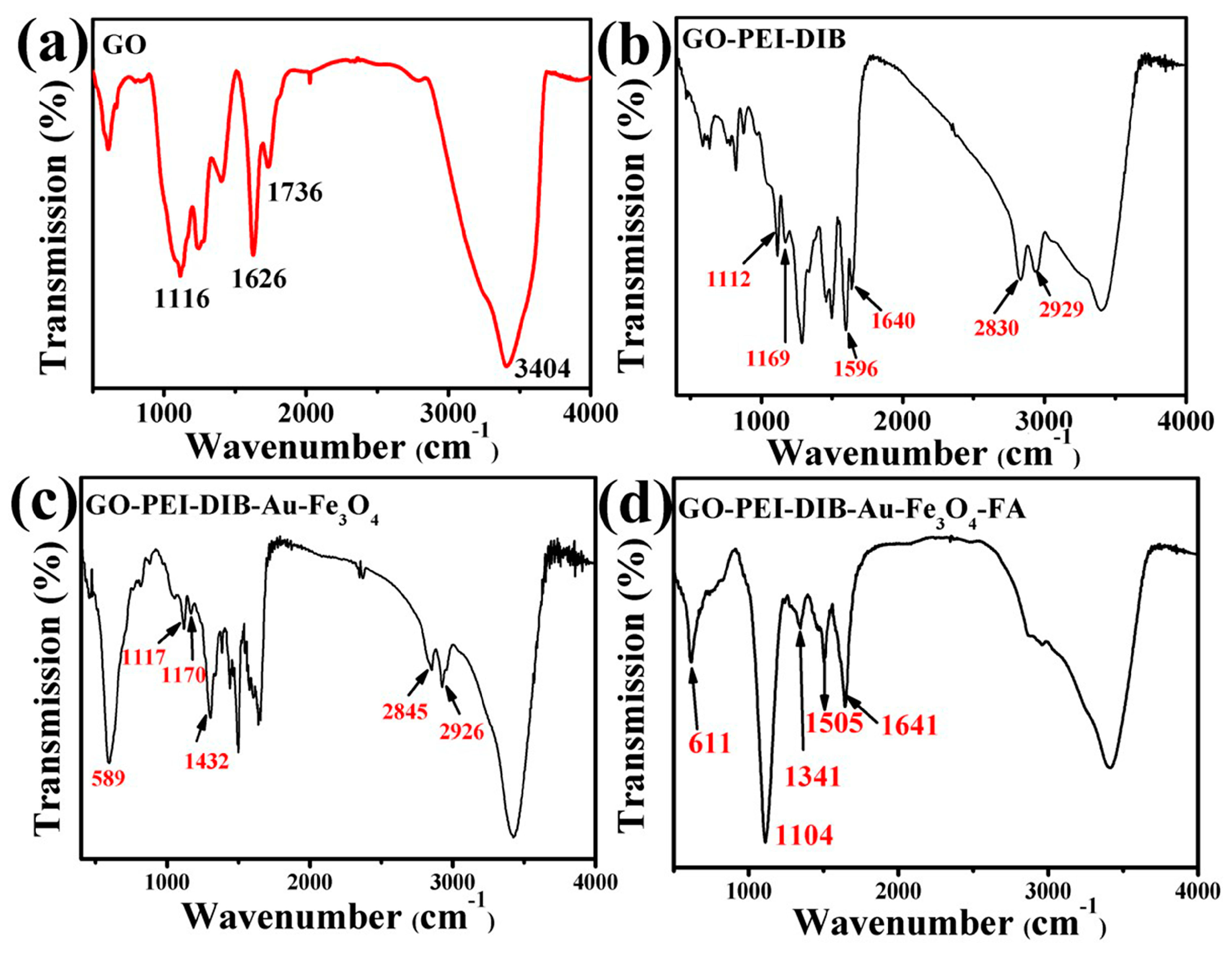
4.3. Infrared Analysis of Graphene-Based Au-Fe3O4 Magnetic Nanocomposites
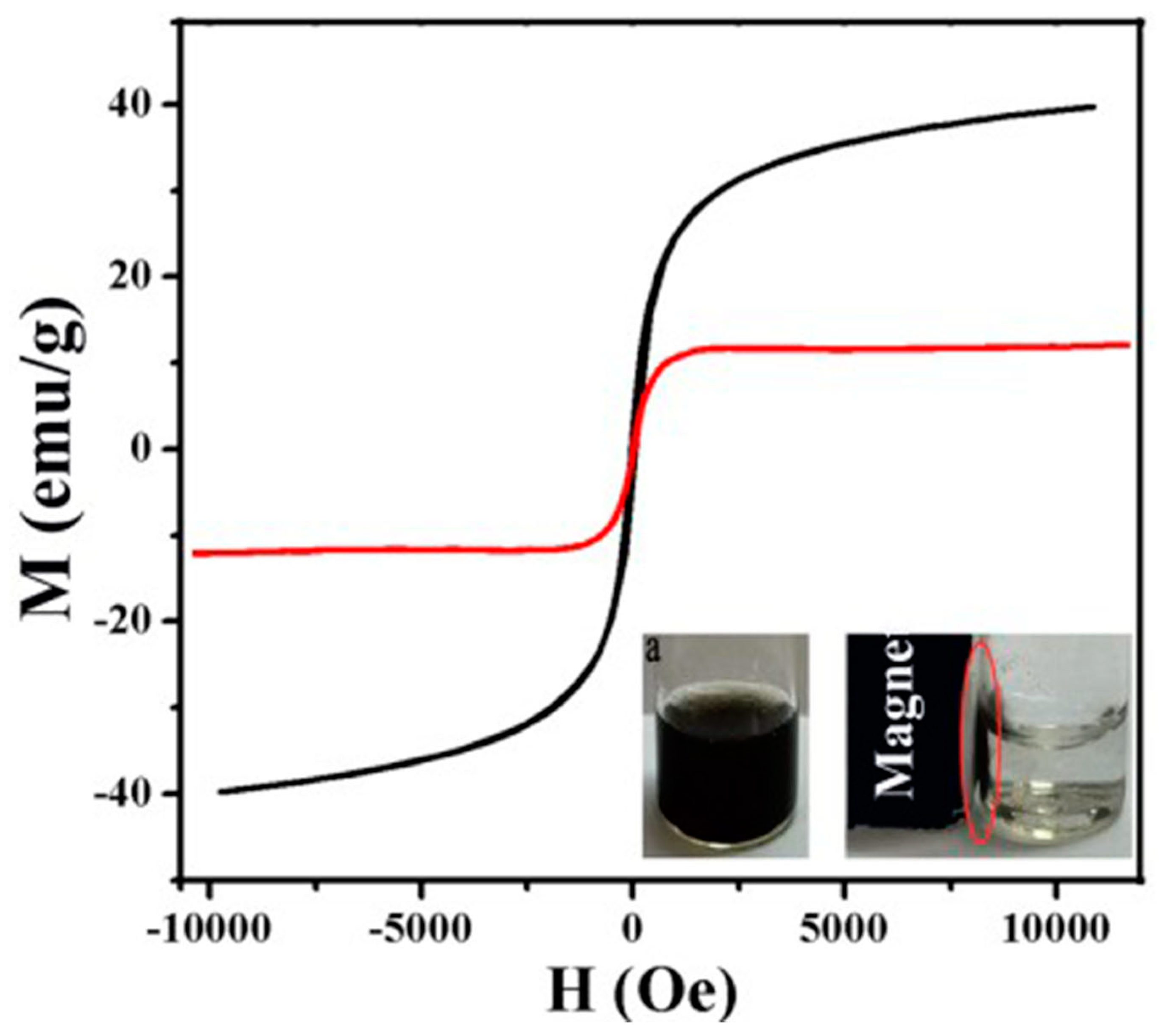
4.4. Magnetic Change of Au-Fe3O4 before and after Modification
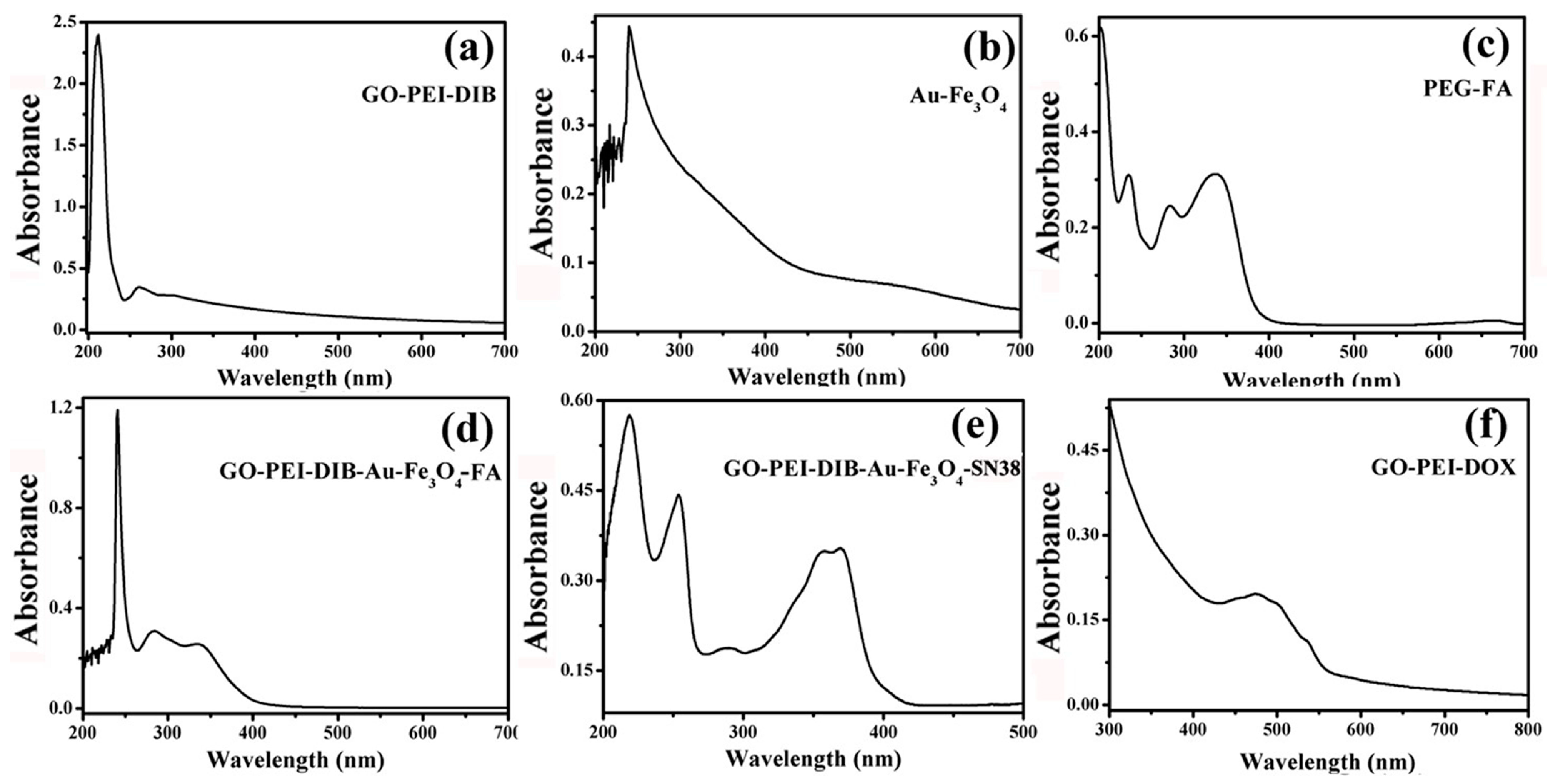
4.5. Graphene-Based Au-Fe3O4 Magnetic Nanocomposites and UV Analysis during Drug Loading
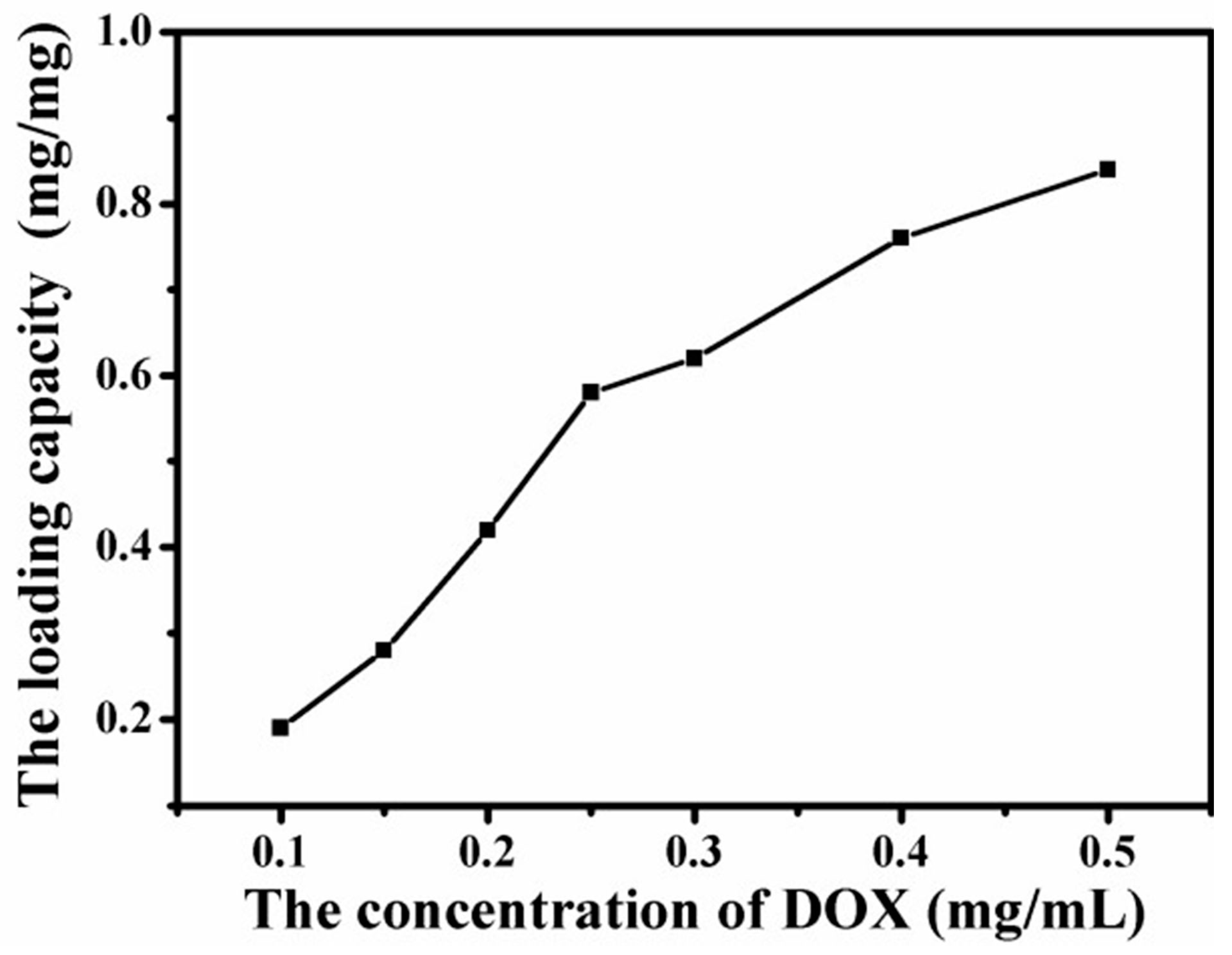
4.6. Loading Capacity of Graphene-Based Au-Fe3O4 Magnetic Nanocomposites for DOX
4.7. Release of DOX Loaded Graphene-Based Au-Fe3O4 Magnetic Nanocomposites under Different pH Conditions
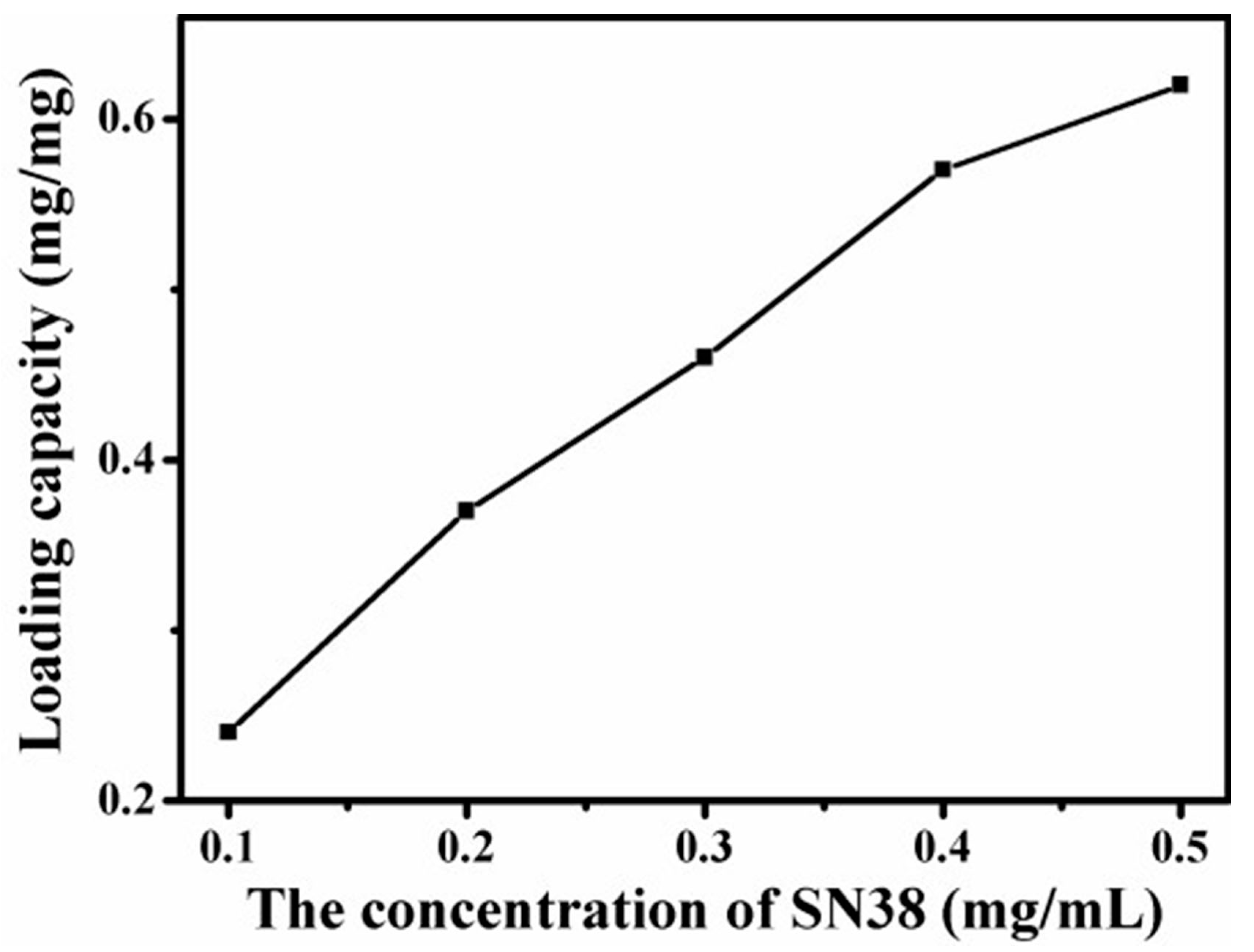
4.8. Loading Capacity of Graphene-Based Au-Fe3O4 Magnetic Nanocomposites for SN38
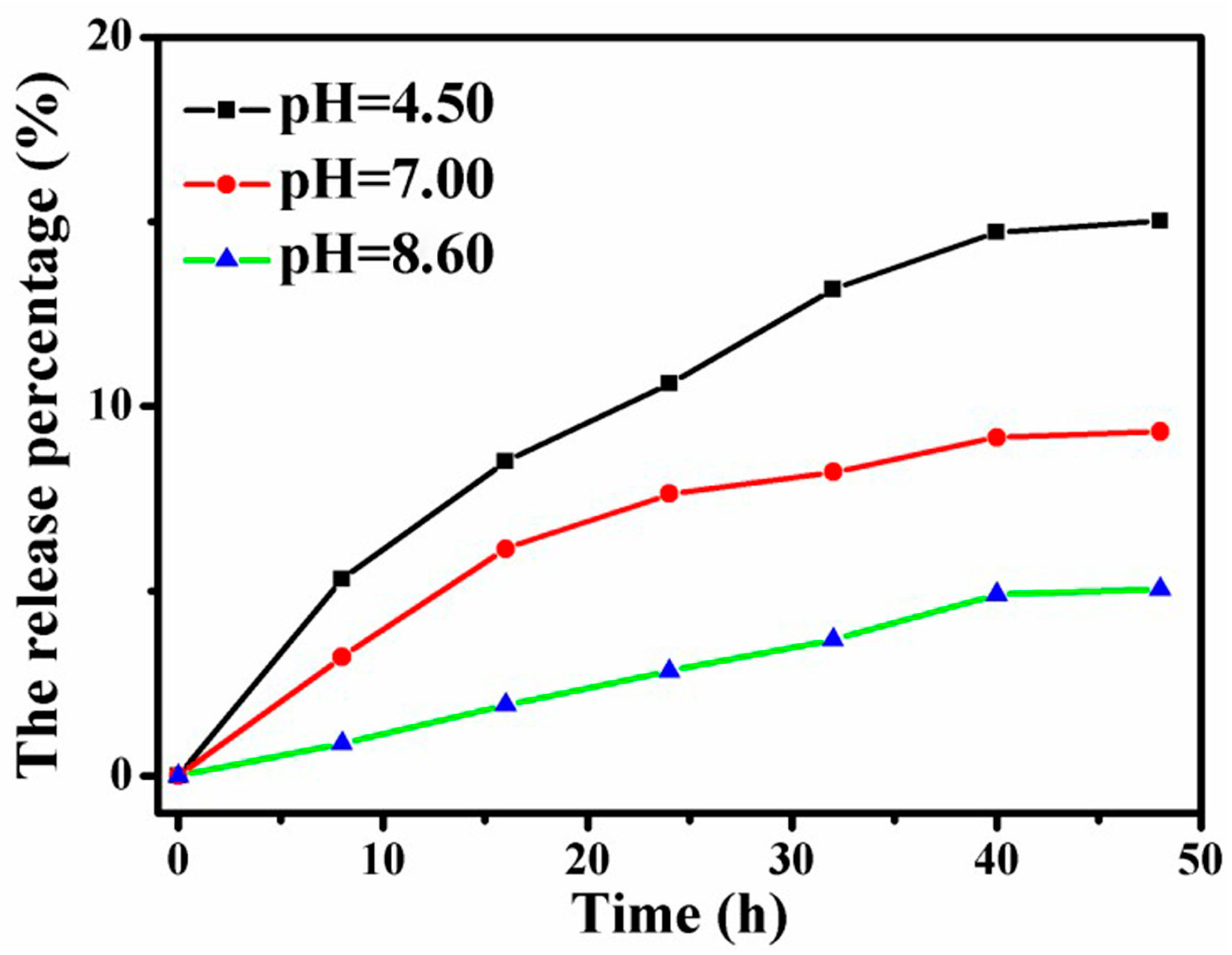
4.9. Release of SN38 Loaded Graphene-Based Au-Fe3O4 Magnetic Nanocomposites under Different pH Conditions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Jiang, J.S.; Du, B.; Gan, Z.F.; Qian, M.; Zhang, P. Preparation and properties of a novel drug delivery system with both magnetic and biomolecular targeting. J. Mater. Sci. Mater. Med. 2009, 20, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, M.H.; Mu, Q.; Stephen, Z.R.; Fang, C.; Zhang, M. Hexanoyl-Chitosan-PEG Copolymer Coated Iron Oxide Nanoparticles for Hydrophobic Drug Delivery. ACS Macro Lett. 2015, 4, 403–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, M.; Han, C.; Wang, X.; Zhang, M.; Zhang, Y.; Shu, J.; Yang, H.; Fang, X.; Yuan, J. Graphene nanohybrids: Excellent electromagnetic properties for the absorbing and shielding of electromagnetic waves. J. Mater. Chem. C 2018, 6, 4586–4602. [Google Scholar] [CrossRef]
- Nooralian, Z.; Parvinzadeh Gashti, M.; Ebrahimi, I. Fabrication of a multifunctional graphene/polyvinylphosphonic acid/cotton nanocomposite via facile spray layer-by-layer assembly. RSC Adv. 2016, 6, 23288–23299. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Liu, L.; Li, X.; Zeng, J.; Jia, X.; Liu, P. Biocompatible graphene oxide nanoparticle-based drug delivery platform for tumor microenvironment-responsive triggered release of doxorubicin. Langmuir 2014, 30, 10419–10429. [Google Scholar] [CrossRef]
- Nie, S.; Emory, S.R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, G.; Marques, P.A.; Granadeiro, C.M.; Nogueira, H.I.; Singh, M.K.; Gracio, J. Surface Modification of Graphene Nanosheets with Gold Nanoparticles: The Role of Oxygen Moieties at Graphene Surface on Gold Nucleation and Growth. Chem. Mater. 2009, 21, 4796–4802. [Google Scholar] [CrossRef]
- Fan, Z.; Kanchanapally, R.; Ray, P.C. Hybrid Graphene Oxide Based Ultrasensitive SERS Probe for Label-Free Biosensing. J. Phys. Chem. Lett. 2013, 4, 3813–3818. [Google Scholar] [CrossRef]
- Gashti, M.P.; Eslami, S. A robust method for producing electromagnetic shielding cellulose via iron oxide pillared clay coating under ultraviolet irradiation. Funct. Mater. Lett. 2015, 8, 1550073. [Google Scholar] [CrossRef]
- Xu, C.; Xie, J.; Ho, D.; Wang, C.; Kohler, N.; Walsh, E.G.; Morgan, J.R.; Chin, Y.E.; Sun, S. Au–Fe3O4 Dumbbell Nanoparticles as Dual-Functional Probes. Angew. Chem. 2008, 47, 173–176. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Wang, Z.; Liu, J.; Zhang, H.; Wang, B.; Yang, Z. A strongly coupled Au/Fe3O4/GO hybrid material with enhanced nanozyme activity for highly sensitive colorimetric detection, and rapid and efficient removal of Hg2+ in aqueous solutions. Nanoscale 2015, 7, 8495–8502. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, G.; Liu, J.; Liu, W.; Zhang, H.; Wang, B. Coordinated assembly of a new 3D mesoporous Fe3O4@Cu2O-graphene oxide framework as a highly efficient and reusable catalyst for the synthesis of quinoxalines. Chem. Commun. 2015, 51, 5069–5072. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Jiang, X.; Chen, X.; Shao, Z.; Yang, W. Doxorubicin-Loaded Magnetic Silk Fibroin Nanoparticles for Targeted Therapy of Multidrug-Resistant Cancer. Adv. Mater. 2015, 26, 7393–7398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, Z.; Zhao, Q.; Huang, J.; Shen, H.; Zhang, Z. Enhanced Chemotherapy Efficacy by Sequential Delivery of siRNA and Anticancer Drugs Using PEI-Grafted Graphene Oxide. Small 2011, 7, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Nandi, S.; Das, P.; Nandi, A.K. Fluorescent graphene oxide via polymer grafting: An efficient nanocarrier for both hydrophilic and hydrophobic drugs. ACS Appl. Mater. Interfaces 2015, 7, 3512–3523. [Google Scholar] [CrossRef] [PubMed]
- Mei, B.C.; Susumu, K.; Medintz, I.L.; Delehanty, J.B.; Mountziaris, T.J.; Mattoussi, H. Modular poly (ethylene glycol) ligands for biocompatible semiconductor and gold nanocrystals with extended pH and ionic stability. J. Mater. Chem. 2008, 18, 4949–4958. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Liu, Z.; Ma, Y.; Huang, Y.; Chen, Y. High-Efficiency Loading and Controlled Release of Doxorubicin Hydrochloride on Graphene Oxide. J. Phys. Chem. C 2008, 112, 17554–17558. [Google Scholar] [CrossRef]
- Zhang, M. Fabrieation of Multifunctional Polymer-Inorganic Nanomaterials for Cancer Theranostics. Ph.D. Thesis, Northeast Normal University in China, Changchun, China, 2018. [Google Scholar]
- Wang, Y.; Liu, X.; Zhang, Q.; Meng, Q. Synthesis of a novel reactive compatibilizer with large surface area and the application in monomer casting nylon/polyethylene–octene elastomer blends. J. Mater. Sci. 2016, 51, 9589–9601. [Google Scholar] [CrossRef]
- Cao, J.; Niu, H.; Du, J.; Yang, L.; Wei, M.; Liu, X.; Liu, Q.; Yang, J. Fabrication of P(NIPAAm-co-AAm) coated optical-magnetic quantum dots/silica core-shell nanocomposites for temperature triggered drug release, bioimaging and in vivo tumor inhibition. J. Mater. Sci. Mater. Med. 2018, 29, 6179–6195. [Google Scholar] [CrossRef]
- Prabha, G.; Raj, V. Synthesis and characterization of chitosan–polyvinylpyrrolidone–bovine serum albumin-coated magnetic iron oxide nanoparticles as potential carrier for delivery of tamoxifen. J. Iran. Chem. Soc. 2018, 15, 871–884. [Google Scholar] [CrossRef]
- Dejam, L.; Elahi, S.M.; Nazari, H.H.; Elahi, H.; Solaymani, S.; Ghaderi, A. Structural and optical characterization of ZnO and AZO thin films: The influence of post-annealing. J. Mater. Sci. Mater. Electron. 2015, 27, 685–696. [Google Scholar] [CrossRef]
- Huang, J.; Zong, C.; Shen, H.; Cao, Y.; Ren, B.; Zhang, Z. Tracking the intracellular drug release from graphene oxide using surface-enhanced Raman spectroscopy. Nanoscale 2013, 5, 10591–10598. [Google Scholar] [CrossRef] [PubMed]
- Gautier, J.; Munnier, E.; Douziech-Eyrolles, L.; Paillard, A.; Dubois, P.; Chourpa, I. SERS spectroscopic approach to study doxorubicin complexes with Fe2+ ions and drug release from SPION-based nanocarriers. Analyst 2013, 138, 7354–7361. [Google Scholar] [CrossRef] [PubMed]


| Reagents | Specifications | Manufacturer |
|---|---|---|
| Ferric acetylacetonate | A.R. | Shanghai Aladdin Biochemical Technology Co., Ltd, Shanghai, China |
| N-(4-Pyridyl)dimethylamine | A.R. | Shanghai Aladdin Biochemical Technology Co., Ltd, Shanghai, China |
| 3,4- protocatechualdehyde oleamine | A.R. | Sigma–Aldrich, St. Louis, MO, USA |
| NHydroxybenzotrizole | A.R. | Sigma–Aldrich, St. Louis, MO, USA |
| Dopamine | A.R. | Sigma–Aldrich, St. Louis, MO, USA |
| Thioglycolic acid; mercaptoacetic acid | A.R. | Sigma–Aldrich, St. Louis, MO, USA |
| Anthranilic acid | A.R. | Sigma–Aldrich, St. Louis, MO, USA |
| Folic acid | A.R. | Sigma–Aldrich, St. Louis, MO, USA |
| Isatoic anhydride | A.R. | Sigma–Aldrich, St. Louis, MO, USA |
| Instrument | Model | Manufacturer |
|---|---|---|
| Fluorescence spectrophotometer | F4500 | Hitachi, Ltd, Tokyo, Japan |
| Transmission electron microscope | HT7800 | Hitachi, Ltd, Tokyo, Japan |
| X-ray Photoelectron Spectrometer | ESCALAB 250 XI | Thermo Fisher Scientific, Hillsboro, OR, USA |
| Fourier transformation infrared spectrometer | IR-960 | Tianjin Ruian Technology Co., Ltd, Tianjin, China |
| Ultraviolet visible spectrophotometer | UV 1750 | Shimazduo, Kyoto, Japan |
| X-ray diffractometer | D8 Advance | BRUKER AXS GMBH, Karlsruhe, Germany |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Huang, S.; Zhang, S.; Luo, Q. Preparation, Characterization and Application of Multi-Mode Imaging Functional Graphene Au-Fe3O4 Magnetic Nanocomposites. Materials 2019, 12, 1978. https://doi.org/10.3390/ma12121978
Sun W, Huang S, Zhang S, Luo Q. Preparation, Characterization and Application of Multi-Mode Imaging Functional Graphene Au-Fe3O4 Magnetic Nanocomposites. Materials. 2019; 12(12):1978. https://doi.org/10.3390/ma12121978
Chicago/Turabian StyleSun, Wei, Shaowen Huang, Siyu Zhang, and Qi Luo. 2019. "Preparation, Characterization and Application of Multi-Mode Imaging Functional Graphene Au-Fe3O4 Magnetic Nanocomposites" Materials 12, no. 12: 1978. https://doi.org/10.3390/ma12121978





