Evolution of Zeolite Crystals in Self-Supporting Faujasite Blocks: Effects of Hydrothermal Conditions
Abstract
:1. Introduction
2. Experimental
2.1 Materials
2.2. Sample Preparation
2.3. Materials Characterization
3. Results and Discussion
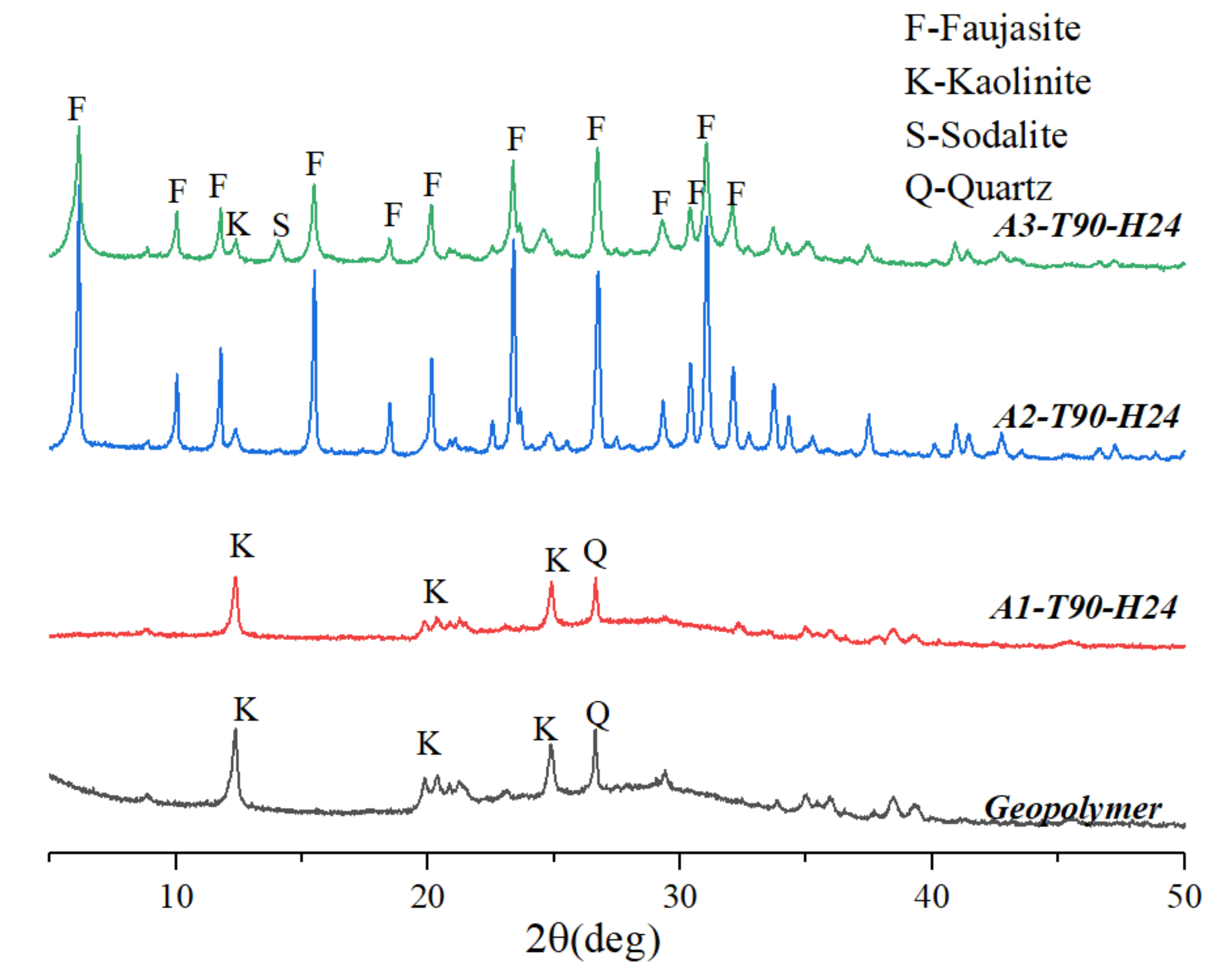
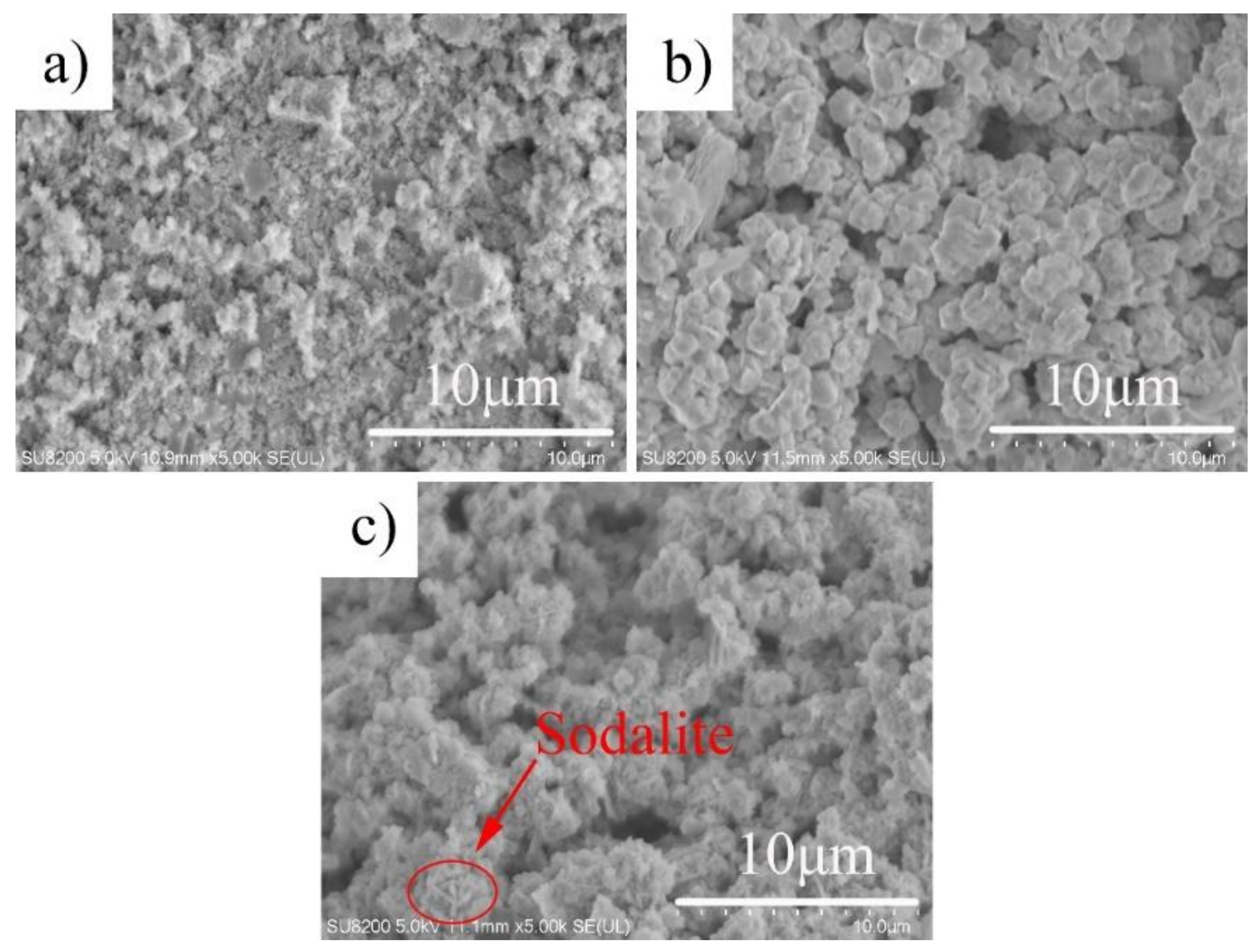
3.1. Effect of Alkalinity
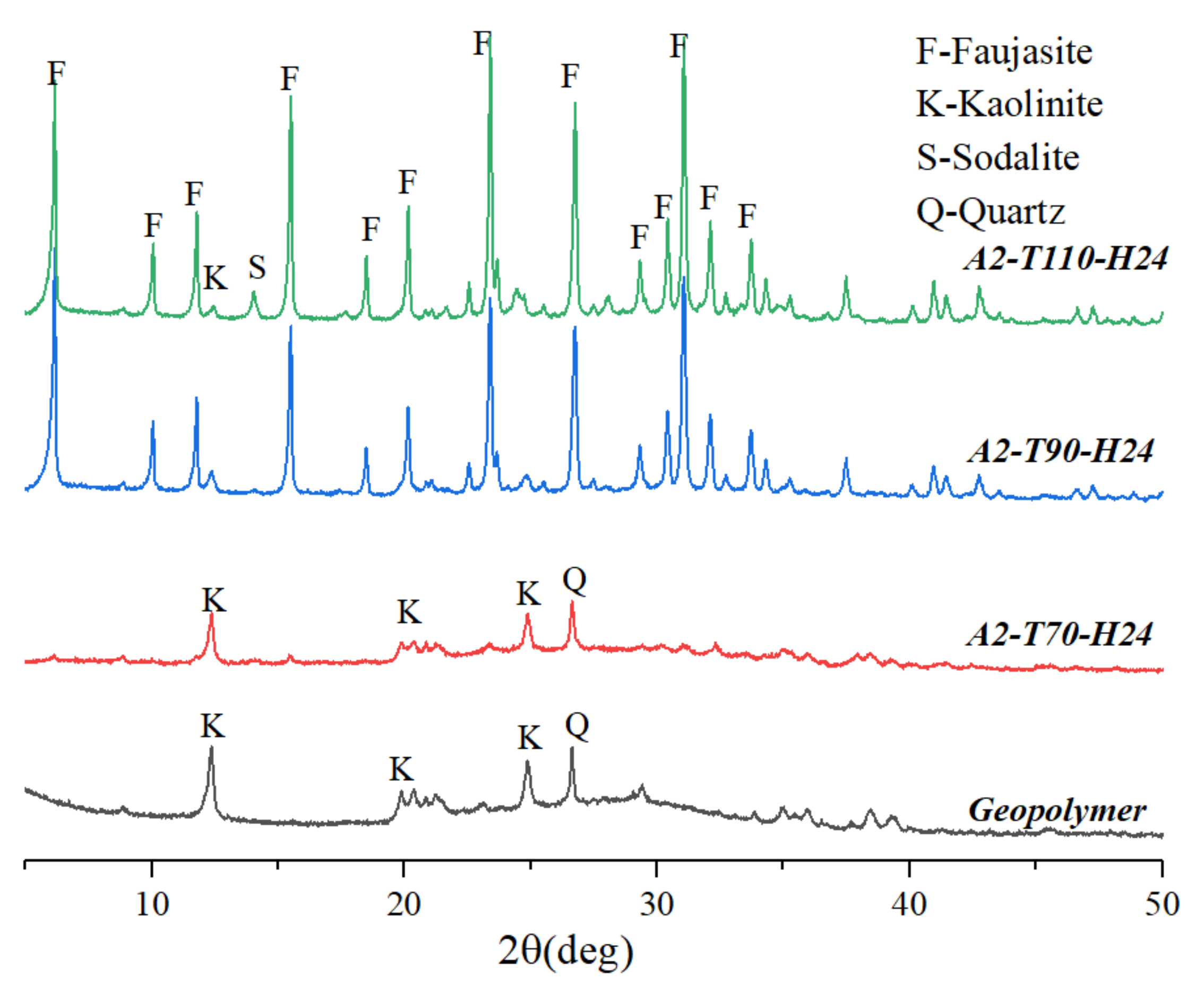
3.2. Effect of Hydrothermal Temperature
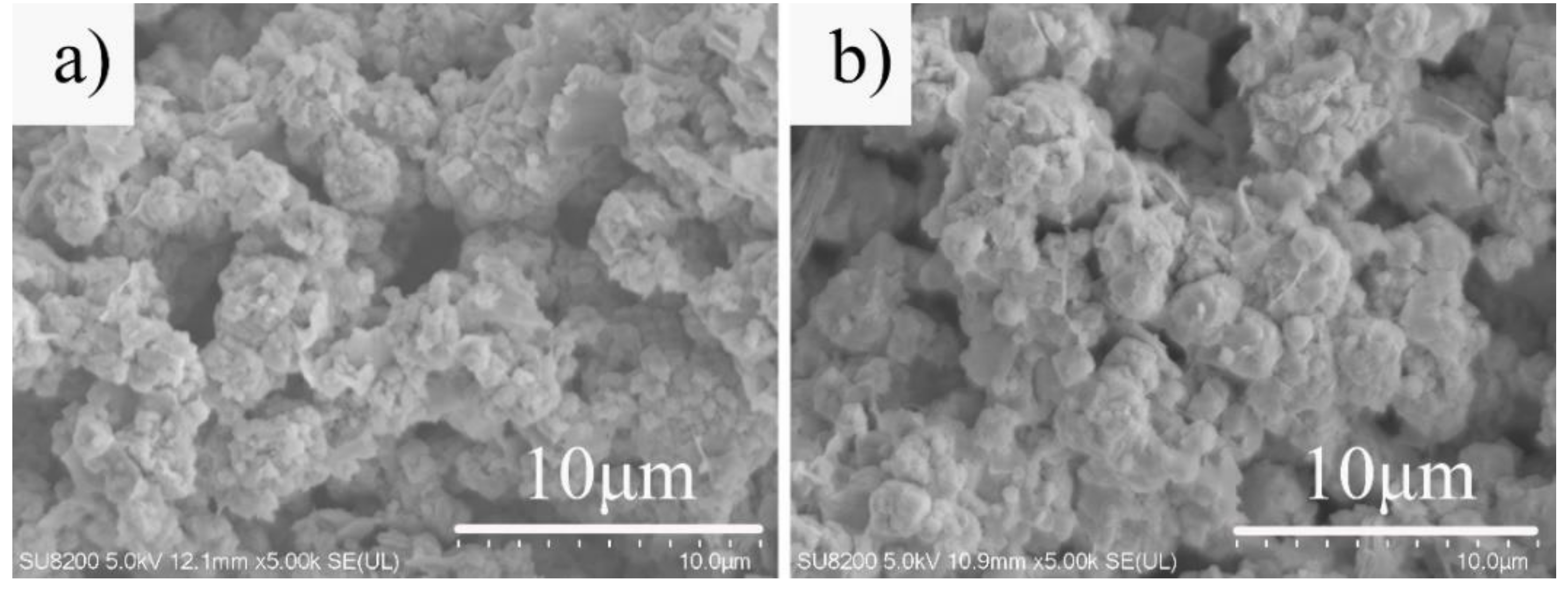
3.3. Effect of Hydrothermal Time
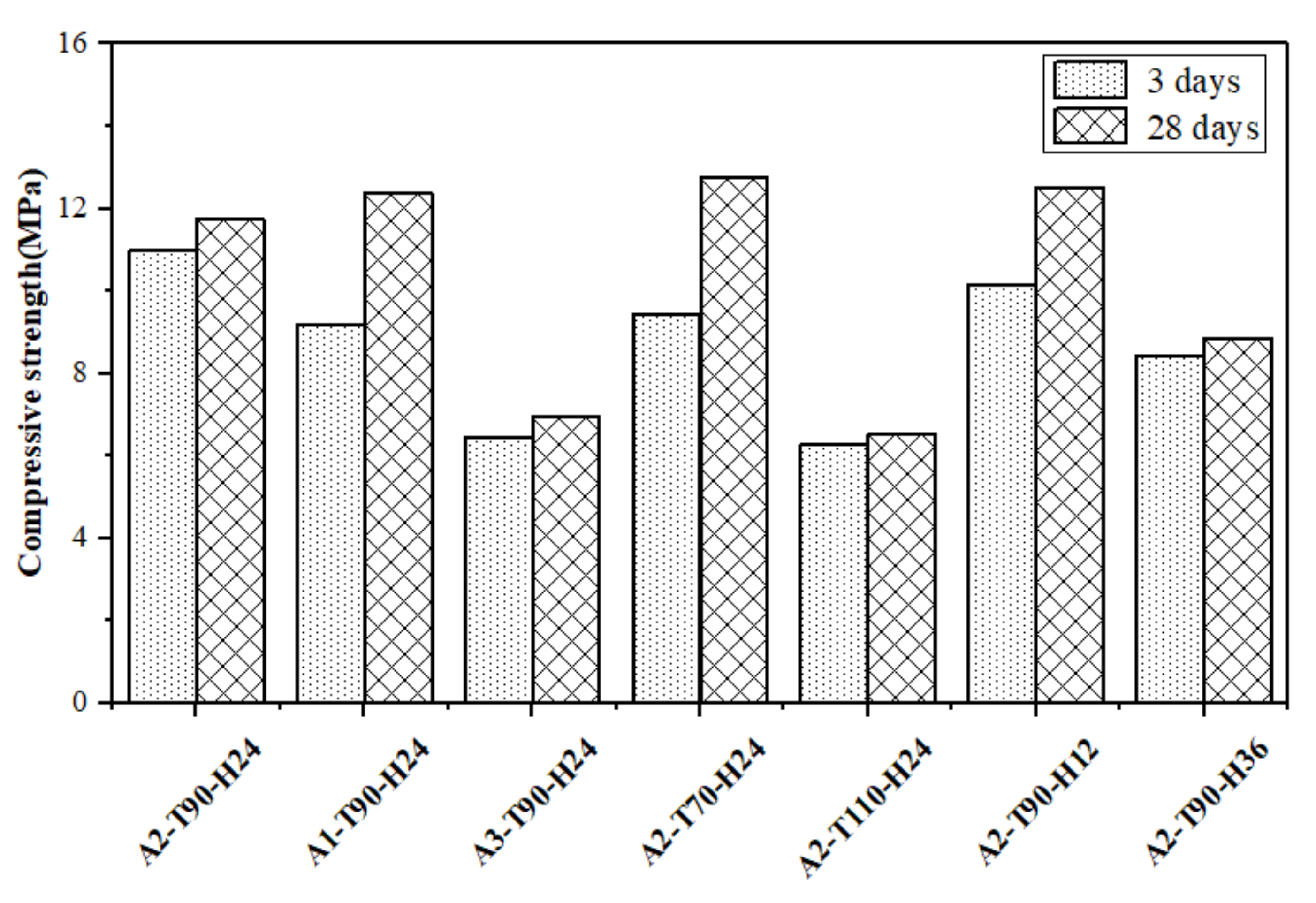
3.4. Compressive Strength Analysis
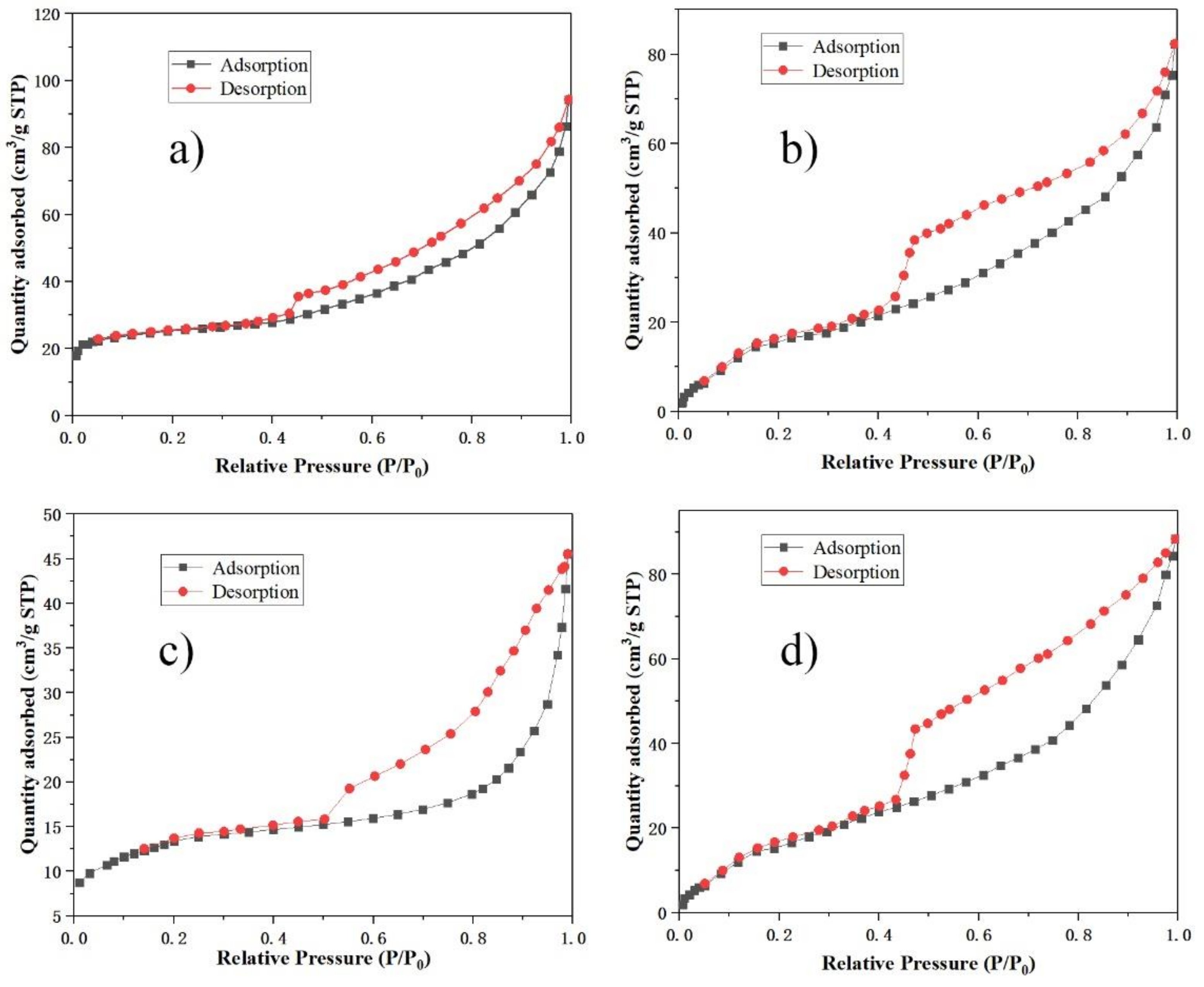
3.5. Pore Structure Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Chai, L.; Yang, Z.; Wang, Y.; Wang, H. Identifying sources and assessing potential risk of heavy metals in soils from direct exposure to children in a mine-impacted city, Changsha, China. J. Environ. Qual. 2010, 39, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.W.; Lee, M.L.; Ko, M.S.; Ueng, T.H.; Yang, S.F. The heavy metal adsorption characteristics on metakaolin-based geopolymer. Appl. Clay Sci. 2012, 56, 90–96. [Google Scholar] [CrossRef]
- Jamil, T.S.; Ibrahim, H.S.; Elmaksoud, I.H.A.; Wakeel, S.T.E. Application of zeolite prepared from Egyptian kaolin for removal of heavy metals: I. Optimum conditions. Desalination 2010, 258, 34–40. [Google Scholar] [CrossRef]
- Khalid, H.R.; Lee, N.K.; Park, S.M.; Abbas, N.; Lee, H.K. Synthesis of geopolymer-supported zeolites via robust one-step method and their adsorption potential. J. Hazard Mater. 2018, 353, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Catizzone, E.; Daele, S.V.; Bianco, M.; Michele, A.D.; Aloise, A.; Migliori, M.; Valtchev, V.; Giordano, G. Catalytic application of ferrierite nanocrystals in vapour-phase dehydration of methanol to dimethyl ether. Appl. Catal. B 2019, 243, 273–282. [Google Scholar] [CrossRef]
- Valtchev, V.; Mintova, S. Hierarchical zeolites. MRS Bull. 2016, 41, 689–693. [Google Scholar] [CrossRef]
- Lee, N.K.; Khalid, H.R.; Lee, H.K. Synthesis of mesoporous geopolymers containing zeolite phases by a hydrothermal treatment. Micropor. Mesopor. Mat. 2016, 229, 22–30. [Google Scholar] [CrossRef]
- Davidovits, J.; Quentin, S. Geopolymers: Inorganic polymerie new materials. J. Therm. Anal. Calorim. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Man-made rock deosynthesis and the resulting development of very early high strength cement. J. Mater. Educ. 1994, 16, 91–139. [Google Scholar]
- Davidovits, J. Geopolymer Chemistry and Application; Institute Geopolymer: Saint-Quentin, France, 2008. [Google Scholar]
- Wang, H.Q.; Yan, C.J.; Li, D.; Zhou, F.; Liu, Y.; Zhou, C.Y.; Komarnenci, S. In situ transformation of geopolymer gels to self-supporting NaX zeolite monoliths with excellent compressive strength. Microp. Mesop. Mater. 2019, 286, 125–132. [Google Scholar] [CrossRef]
- He, Y.; Cui, X.; Liu, K.; Liu, X.; Wang, Y.; Zhang, J. Preparation of self-supporting NaA zeolite membranes using geopolymers. J. Membrane Sci. 2013, 447, 66–72. [Google Scholar] [CrossRef]
- Cui, X.; He, Y.; Liu, L.; Chen, J. NaA zeolite synthesis from geopolymer precursor. Mrs Commun. 2011, 1, 49–51. [Google Scholar]
- Khalid, H.R.; Lee, N.K.; Choudhry, I.; Wang, Z.; Lee, H.K. Evolution of zeolite crystals in geopolymer-supported zeolites: Effects of composition of starting materials. Mater. Lett. 2019, 239, 33–36. [Google Scholar] [CrossRef]
- Liguori, B.; Aprea, P.; Roviello, G.; Ferone, C. Self-supporting zeolites by Geopolymer Gel Conversion (GGC). Microp. Mesop. Mater. 2019, 286, 125–132. [Google Scholar] [CrossRef]
- Chen, S.J.; Zhang, W.W.; Sorge, L.P.; Seo, D.K. Exploratory synthesis of low-silica nanozeolites through geopolymer chemistry. Cryst. Growth Des. 2019, 19, 1167–1171. [Google Scholar] [CrossRef]
- He, Y.; Cui, X.; Liu, L.; Zhang, S.; Zhang, W. Preparation of the NaA zeolite with a high Ca2+ cation exchange capacity by sol-gel method. Rare Metal Mat. Eng. 2012, 41, 157–162. [Google Scholar]
- Qiu, X.; Liu, Y.; Li, D.; Yan, C. Preparation of NaP zeolite block from fly ash-based geopolymer via in situ hydrothermal method. J. Porous Mater. 2015, 22, 291–299. [Google Scholar] [CrossRef]
- Maiti, M.; Sarkar, M.; Xu, S.; Das, S.; Adak, D.; Maiti, S. Application of silica nanoparticles to develop faujasite nanocomposite for heavy metal and carcinogenic dye degradation. Environ. Prog. Sustain. 2019, 38, 15–18. [Google Scholar] [CrossRef]
- Ltaief, O.O.; Benzina, M.; Siffert, S.; Fourmentin, S. Synthesis of faujasite type zeolite from low grade tunisian clay for the removal of heavy metals from aqueous waste by batch process: Kinetic and equilibrium study. CR. Chim. 2015, 18, 1123–1133. [Google Scholar] [CrossRef]
- Sutarno, S.; Yateman, A. Phase transformation in the formation of faujasite from fly ash. Indo. J. Chem. 2005, 5, 278–282. [Google Scholar] [CrossRef]
- Zhang, J.; He, Y.; Wang, Y.P.; Mao, J.; Cui, X.M. Synthesis of a self-supporting faujasite zeolite membrane using geopolymer gel for separation of alcohol/water mixture. Mater. Lett. 2014, 116, 167–170. [Google Scholar] [CrossRef]
- Rossi, A.D.; Simão, L.; Ribeiro, M.J.; Novais, R.M.; Labrincha, J.A.; Hotza, D.; Moreira, R.F.P.M. In-situ synthesis of zeolites by geopolymerization of biomass fly ash and metakaolin. Mater. Lett. 2019, 236, 644–648. [Google Scholar] [CrossRef]



| Sample | SiO2 | Al2O3 | CaO | Fe2O3 | K2O | TiO2 | MgO | SO3 | MnO | P2O5 | Na2O | LOI |
| MK | 48.43 | 38.68 | 1.95 | 0.972 | 0.503 | 0.178 | 0.169 | 0.156 | 0.0654 | 0.0316 | 0.0316 | 8.76 |
| Sample | Hydrothermal Alkalinity/mol·L−1 | Hydrothermal Temperature/°C | Hydrothermal Time/h |
|---|---|---|---|
| Geopolymer | 0 | 0 | 0 |
| A1-T90-H24 | 1 | 90 | 24 |
| A2-T90-H24 | 2 | 90 | 24 |
| A3-T90-H24 | 3 | 90 | 24 |
| A2-T70-H24 | 2 | 70 | 24 |
| A2-T110-H24 | 2 | 110 | 24 |
| A2-T90-H12 | 2 | 90 | 12 |
| A2-T90-H36 | 2 | 90 | 36 |
| Sample | BET Surface Area (m2/g) | Micropore Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|---|
| A2-T90-H24 | 80.36 | 19.7 | 0.24 | 7.86 |
| A3-T90-H24 | 53.16 | 11.8 | 0.19 | 9.27 |
| A2-T110-H24 | 44.42 | 9.6 | 0.16 | 11.36 |
| A2-T90-H36 | 60.42 | 14.3 | 0.21 | 8.94 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, L.; Wang, Z.; Lu, D. Evolution of Zeolite Crystals in Self-Supporting Faujasite Blocks: Effects of Hydrothermal Conditions. Materials 2019, 12, 1965. https://doi.org/10.3390/ma12121965
Guan L, Wang Z, Lu D. Evolution of Zeolite Crystals in Self-Supporting Faujasite Blocks: Effects of Hydrothermal Conditions. Materials. 2019; 12(12):1965. https://doi.org/10.3390/ma12121965
Chicago/Turabian StyleGuan, Liuliu, Zhuangzhuang Wang, and Duyou Lu. 2019. "Evolution of Zeolite Crystals in Self-Supporting Faujasite Blocks: Effects of Hydrothermal Conditions" Materials 12, no. 12: 1965. https://doi.org/10.3390/ma12121965
APA StyleGuan, L., Wang, Z., & Lu, D. (2019). Evolution of Zeolite Crystals in Self-Supporting Faujasite Blocks: Effects of Hydrothermal Conditions. Materials, 12(12), 1965. https://doi.org/10.3390/ma12121965




