Preparation of Sol-Gel Derived Anticorrosive Coating on Q235 Carbon Steel Substrate with Long-Term Corrosion Prevention Durability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
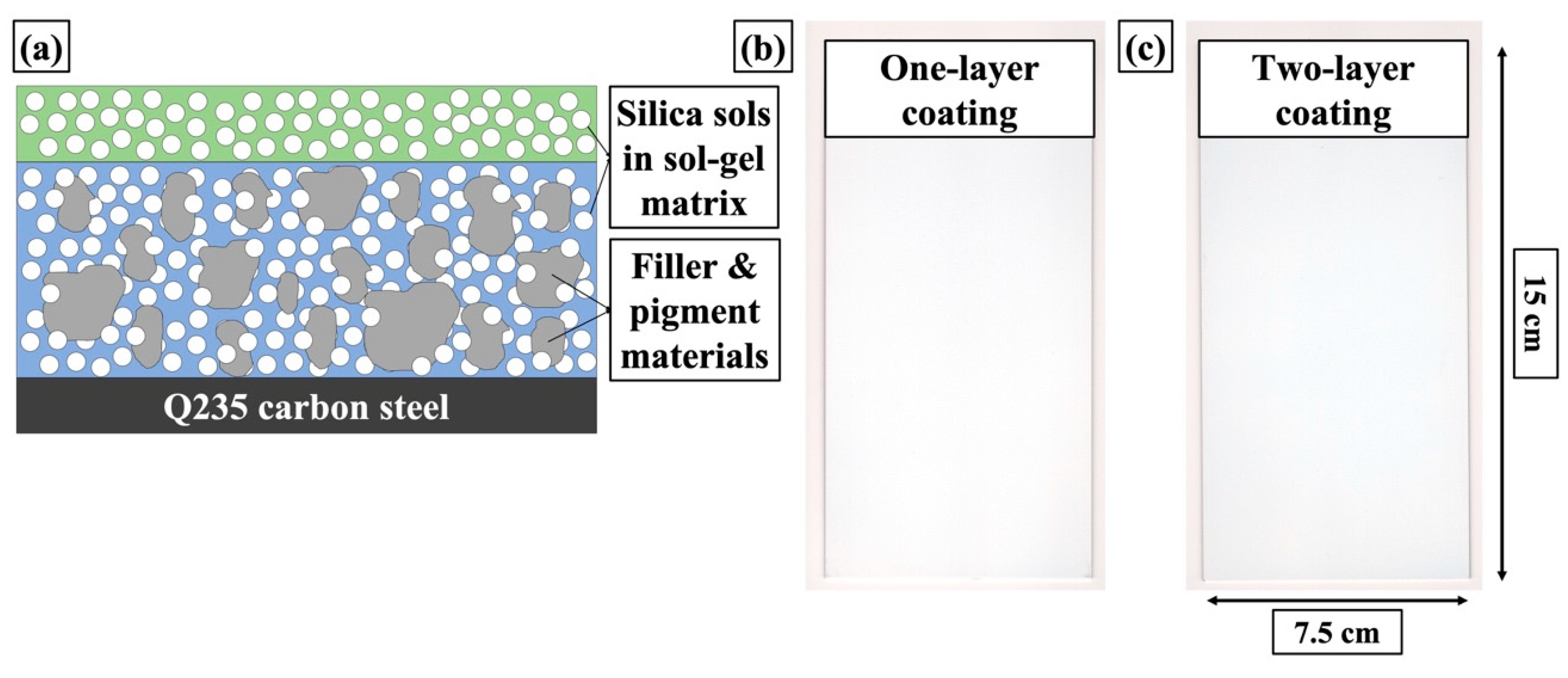
2.2. Preparation of Sol-gel Coatings
2.2.1. Preparation of Liquid Dispersion for Undercoat
2.2.2. Preparation of Liquid Dispersion for Topcoat
2.2.3. Coating Deposition
2.3. Characterizations
2.4. Coating Performance Testing
2.5. Salt Spray Testing
2.6. Outdoor Exposure Testing
3. Results and Discussion
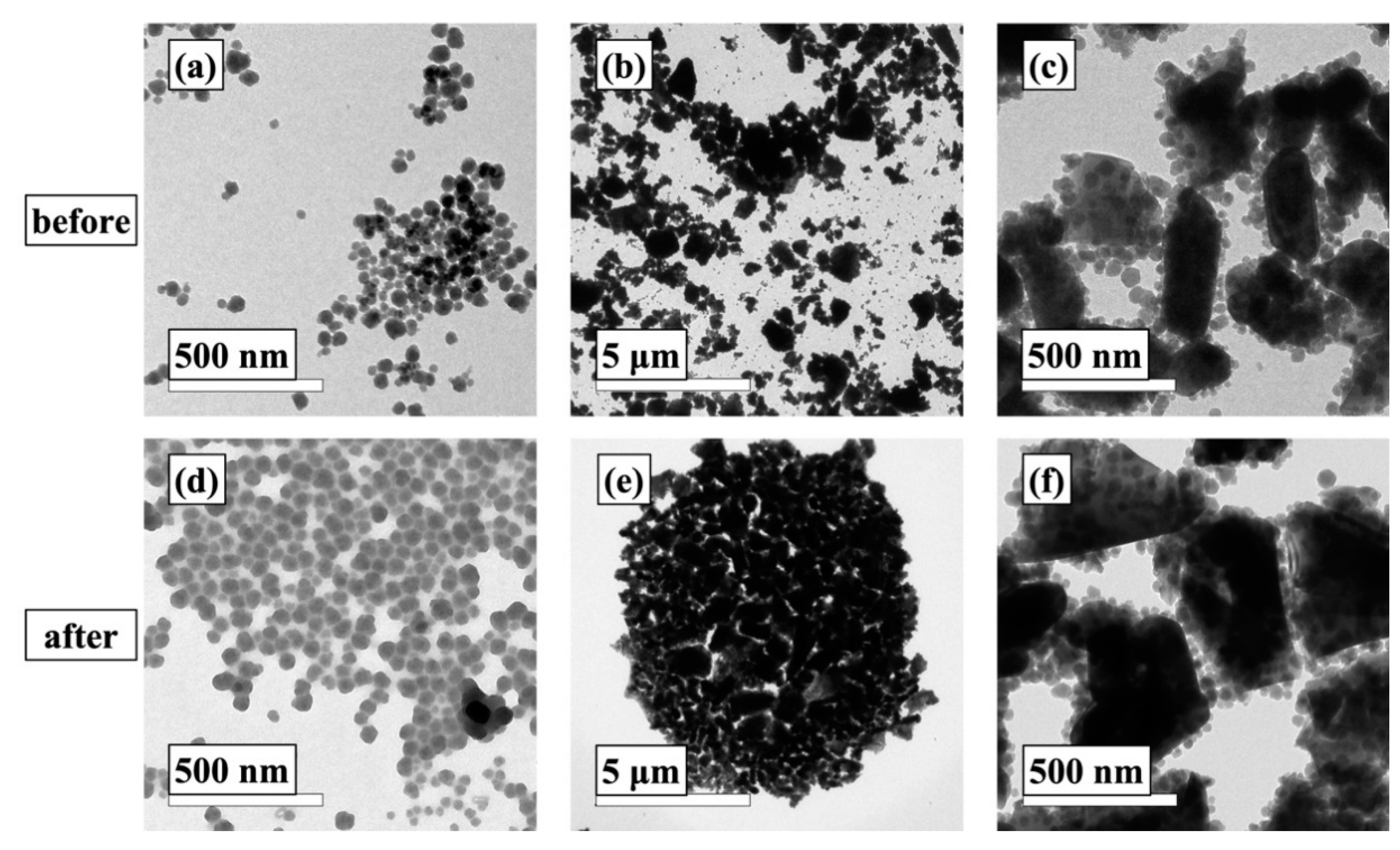
3.1. Preparation of the Sol-gel Coatings
3.2. Coating Morphological Characterization
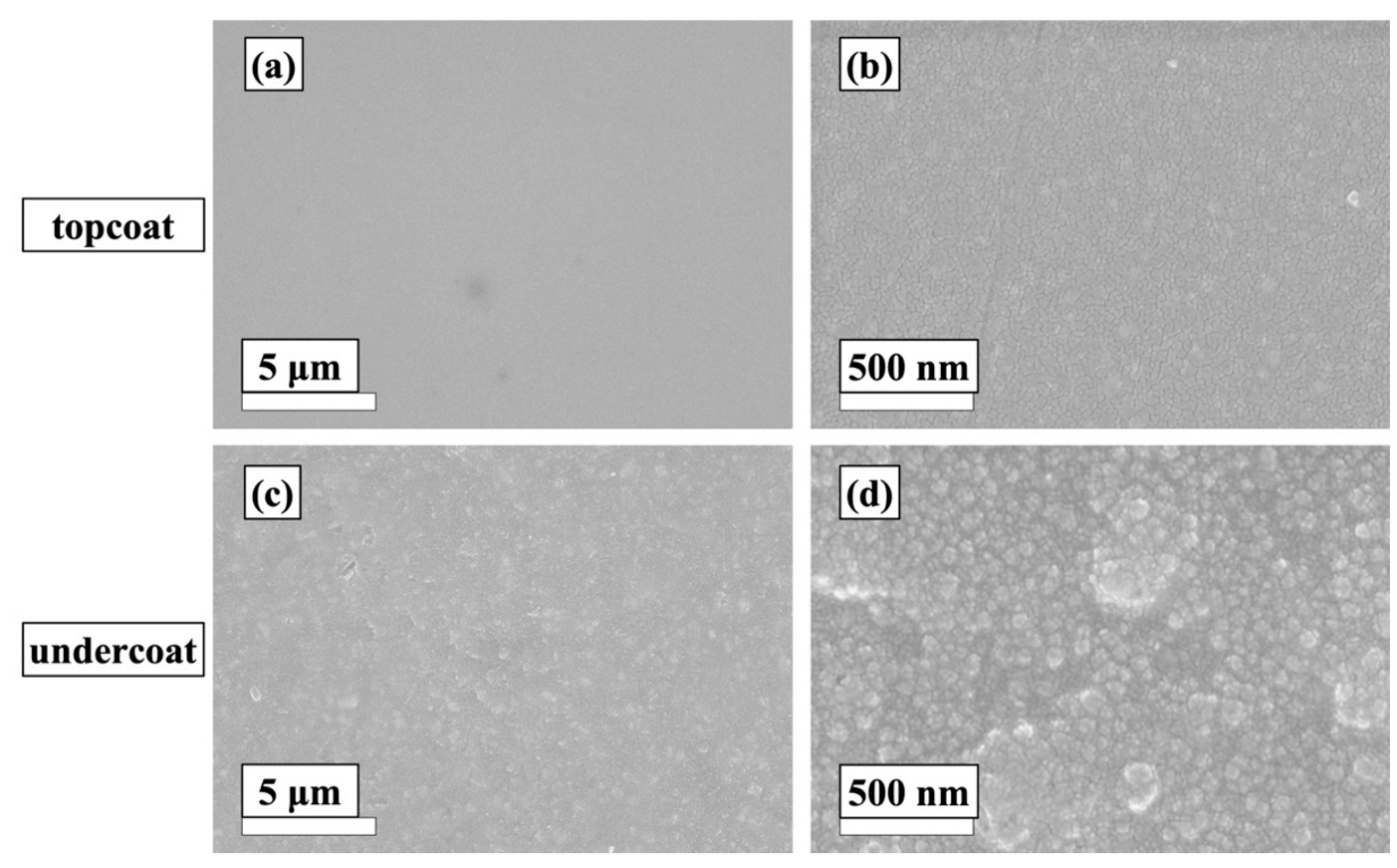
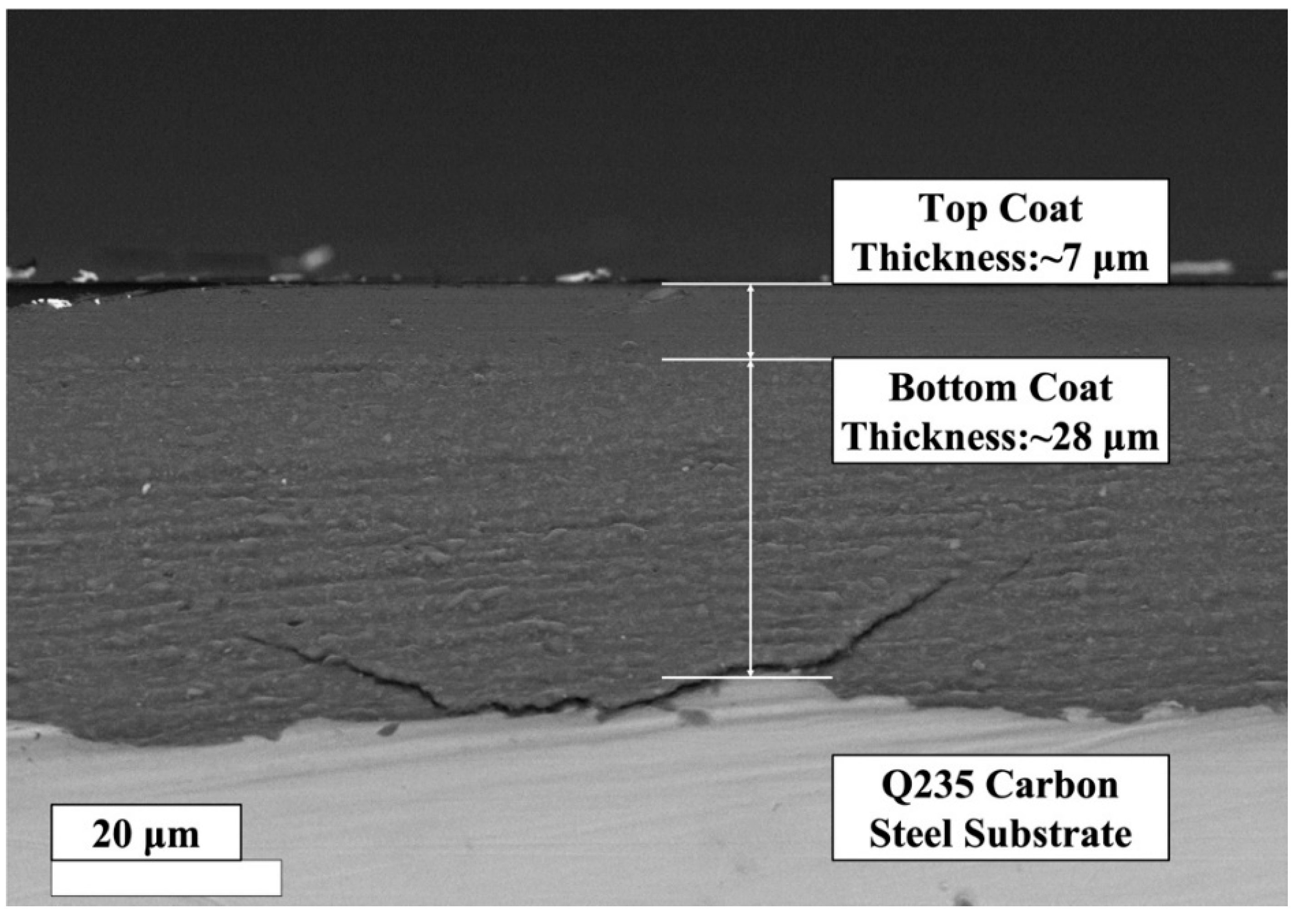
3.2.1. SEM Analysis
3.2.2. EDS Analysis
3.2.3. AFM Analysis
3.3. Coating Anticorrosion Performances
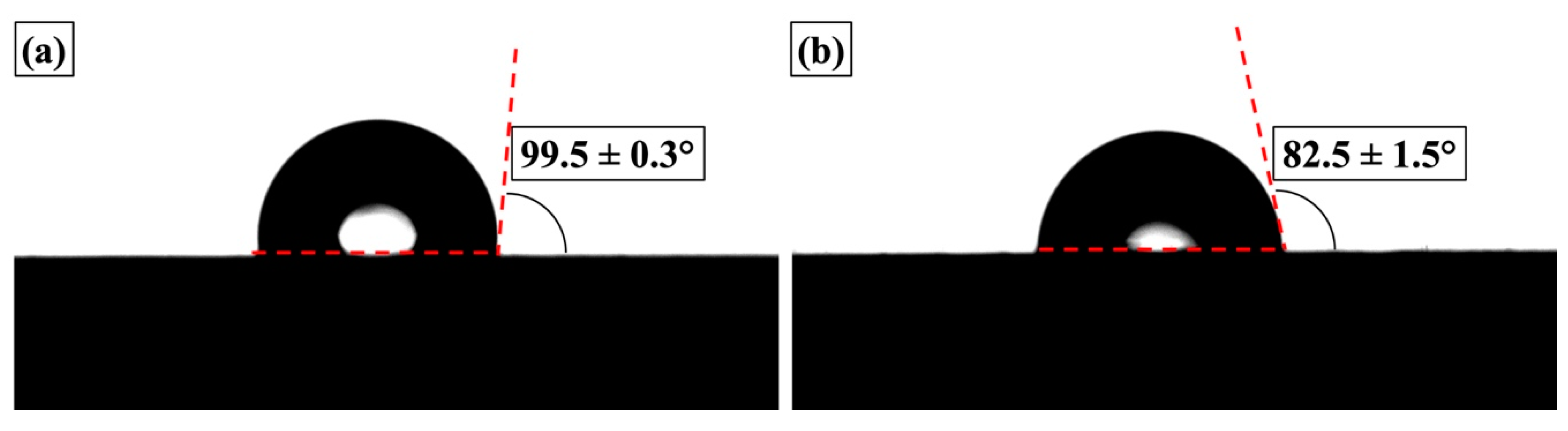
3.3.1. Physical Properties
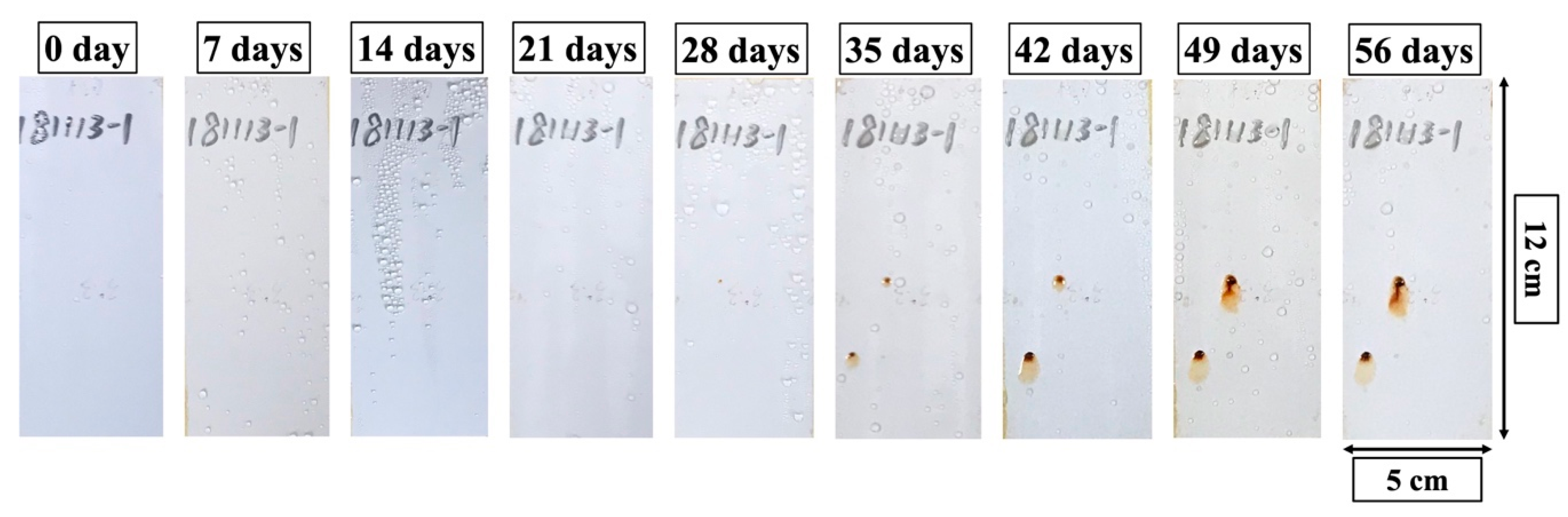
3.3.2. Salt Spray Test
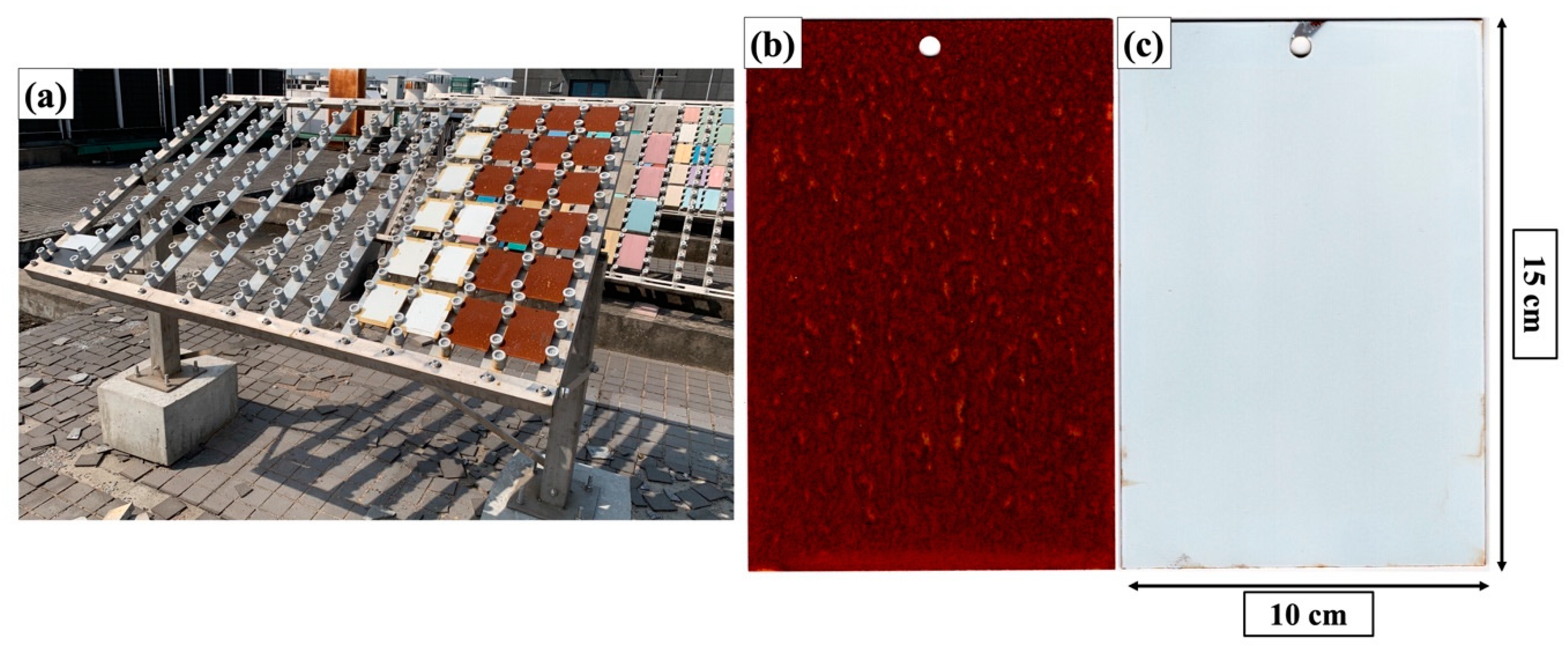
3.3.3. Outdoor Exposure Tests
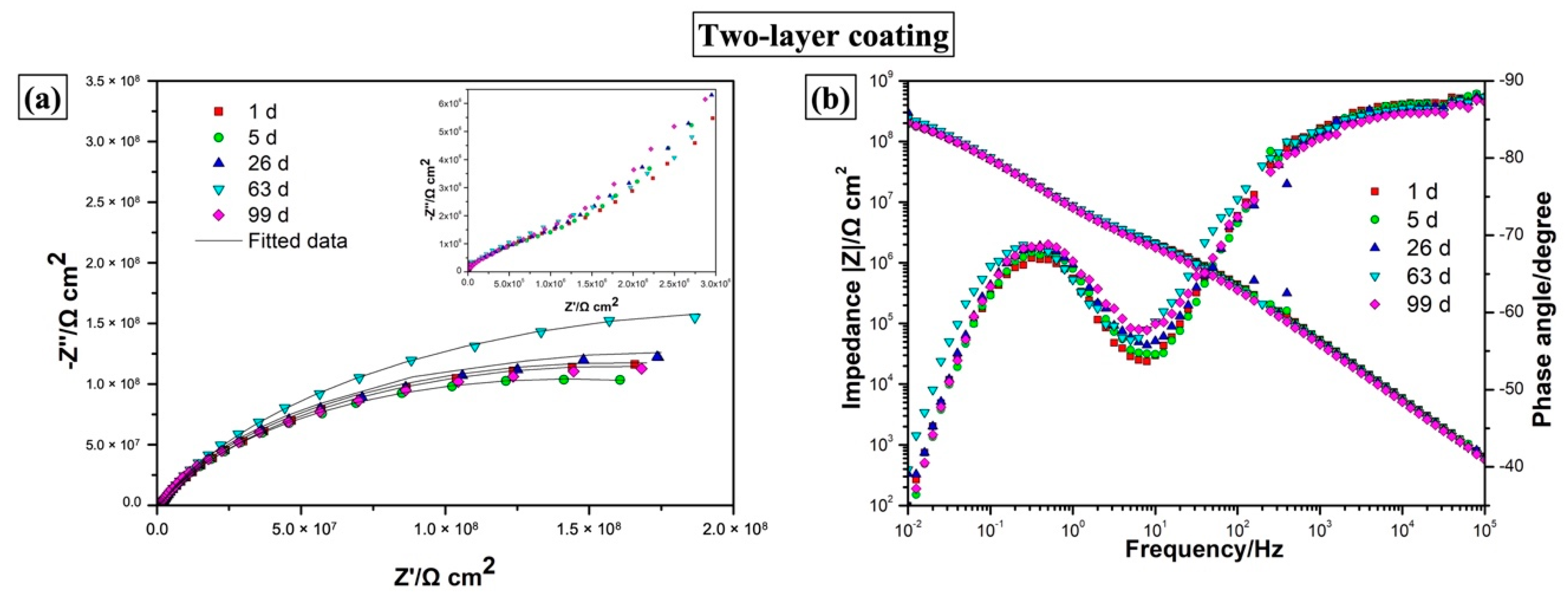
3.3.4. EIS Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hughes, A.E.; Cole, I.S.; Muster, T.H.; Varley, R.J. Designing green, self-healing coatings for metal protection. NPG Asia Mater. 2010, 2, 143. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Organic–inorganic hybrid sol–gel coatings for metal corrosion protection: A review of recent progress. J. Coat. Technol. Res. 2015, 12, 1–35. [Google Scholar] [CrossRef]
- Osborne, J.H. Observations on chromate conversion coatings from a sol–gel perspective. Prog. Org. Coat. 2001, 41, 280–286. [Google Scholar] [CrossRef]
- Borisova, D.; Möhwald, H.; Shchukin, D.G. Influence of embedded nanocontainers on the efficiency of active anticorrosive coatings for aluminum alloys part ii: Influence of nanocontainer position. ACS Appl. Mater. Interfaces 2013, 5, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Borisova, D.; Möhwald, H.; Shchukin, D.G. Influence of embedded nanocontainers on the efficiency of active anticorrosive coatings for aluminum alloys part i: Influence of nanocontainer concentration. ACS Appl. Mater. Interfaces 2012, 4, 2931–2939. [Google Scholar] [CrossRef] [PubMed]
- Snihirova, D.; Lamaka, S.V.; Taryba, M.; Salak, A.N.; Kallip, S.; Zheludkevich, M.L.; Ferreira, M.G.S.; Montemor, M.F. Hydroxyapatite microparticles as feedback-active reservoirs of corrosion inhibitors. ACS Appl. Mater. Interfaces 2010, 2, 3011–3022. [Google Scholar] [CrossRef] [PubMed]
- Zheludkevich, M.L.; Shchukin, D.G.; Yasakau, K.A.; Möhwald, H.; Ferreira, M.G.S. Anticorrosion coatings with self-healing effect based on nanocontainers impregnated with corrosion inhibitor. Chem. Mater. 2007, 19, 402–411. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Zheludkevich, M.; Yasakau, K.; Lamaka, S.; Ferreira, M.G.S.; Möhwald, H. Layer-by-layer assembled nanocontainers for self-healing corrosion protection. Adv. Mater. 2006, 18, 1672–1678. [Google Scholar] [CrossRef]
- Wang, D.; Bierwagen, G.P. Sol–gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Shen, G.X.; Chen, Y.C.; Lin, C.J. Corrosion protection of 316 l stainless steel by a tio2 nanoparticle coating prepared by sol–gel method. Thin Solid Films 2005, 489, 130–136. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Serra, R.; Montemor, M.F.; Salvado, I.M.M.; Ferreira, M.G.S. Corrosion protective properties of nanostructured sol–gel hybrid coatings to aa2024-t3. Surf. Coat. Technol. 2006, 200, 3084–3094. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Montemor, M.F.; Galio, A.F.; Zheludkevich, M.L.; Trindade, C.; Dick, L.F.; Ferreira, M.G.S. Novel hybrid sol–gel coatings for corrosion protection of az31b magnesium alloy. Electrochim. Acta 2008, 53, 4773–4783. [Google Scholar] [CrossRef]
- Tan, A.L.K.; Soutar, A.M.; Annergren, I.F.; Liu, Y.N. Multilayer sol–gel coatings for corrosion protection of magnesium. Surf. Coat. Technol. 2005, 198, 478–482. [Google Scholar] [CrossRef]
- Chou, T.P.; Chandrasekaran, C.; Cao, G.Z. Sol-gel-derived hybrid coatings for corrosion protection. J. Sol-Gel Sci. Technol. 2003, 26, 321–327. [Google Scholar] [CrossRef]
- Milošev, I.; Kapun, B.; Rodič, P.; Iskra, J. Hybrid sol–gel coating agents based on zirconium (iv) propoxide and epoxysilane. J. Sol-Gel Sci. Technol. 2015, 74, 447–459. [Google Scholar] [CrossRef]
- Rodič, P.; Iskra, J.; Milošev, I. A hybrid organic–inorganic sol–gel coating for protecting aluminium alloy 7075-t6 against corrosion in harrison’s solution. J. Sol-Gel Sci. Technol. 2014, 70, 90–103. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Pandarus, V.; Béland, F.; Ilharco, L.M.; Pagliaro, M. The sol–gel route to advanced silica-based materials and recent applications. Chem. Rev. 2013, 113, 6592–6620. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Salvado, I.M.; Ferreira, M.G.S. Sol-gel coatings for corrosion protection of metals. J. Mater. Chem. 2005, 15, 5099–5111. [Google Scholar] [CrossRef]
- Santana, I.; Pepe, A.; Jimenez-Pique, E.; Pellice, S.; Milošev, I.; Ceré, S. Corrosion protection of carbon steel by silica-based hybrid coatings containing cerium salts: Effect of silica nanoparticle content. Surf. Coat. Technol. 2015, 265, 106–116. [Google Scholar] [CrossRef]
- Ballarre, J.; Manjubala, I.; Schreiner, W.H.; Orellano, J.C.; Fratzl, P.; Ceré, S. Improving the osteointegration and bone–implant interface by incorporation of bioactive particles in sol–gel coatings of stainless steel implants. Acta Biomater. 2010, 6, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Rosero-Navarro, N.C.; Pellice, S.A.; Castro, Y.; Aparicio, M.; Duran, A. Improved corrosion resistance of aa2024 alloys through hybrid organic-inorganic sol-gel coatings produced from sols with controlled polymerisation. Surf. Coat. Technol. 2009, 203, 1897–1903. [Google Scholar] [CrossRef]
- Tiringer, U.; Durán, A.; Castro, Y.; Milošev, I. Self-healing effect of hybrid sol-gel coatings based on gptms, teos, sio2 nanoparticles and ce(no3)3 applied on aluminum alloy 7075-t6. J. Electrochem. Soc. 2018, 165, C213–C225. [Google Scholar] [CrossRef]
- Phanasgaonkar, A.; Raja, V.S. Influence of curing temperature, silica nanoparticles- and cerium on surface morphology and corrosion behaviour of hybrid silane coatings on mild steel. Surf. Coat. Technol. 2009, 203, 2260–2271. [Google Scholar] [CrossRef]
- Parhizkar, N.; Ramezanzadeh, B.; Shahrabi, T. Corrosion protection and adhesion properties of the epoxy coating applied on the steel substrate pre-treated by a sol-gel based silane coating filled with amino and isocyanate silane functionalized graphene oxide nanosheets. Appl. Surf. Sci. 2018, 439, 45–59. [Google Scholar] [CrossRef]
- Nezamdoust, S.; Seifzadeh, D.; Rajabalizadeh, Z. Ptms/oh-mwcnt sol-gel nanocomposite for corrosion protection of magnesium alloy. Surf. Coat. Technol. 2018, 335, 228–240. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Zheludkevich, M.; Mohwald, H. Feedback active coatings based on incorporated nanocontainers. J. Mater. Chem. 2006, 16, 4561–4566. [Google Scholar] [CrossRef]
- Zhang, F.; Ju, P.; Pan, M.; Zhang, D.; Huang, Y.; Li, G.; Li, X. Self-healing mechanisms in smart protective coatings: A review. Corros. Sci. 2018, 144, 74–88. [Google Scholar] [CrossRef]
- Zahidah, K.A.; Kakooei, S.; Ismail, M.C.; Raja, P.B. Halloysite nanotubes as nanocontainer for smart coating application: A review. Prog. Org. Coat. 2017, 111, 175–185. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Lamaka, S.V.; Yasakau, K.A.; Zheludkevich, M.L.; Ferreira, M.G.S.; Möhwald, H. Active anticorrosion coatings with halloysite nanocontainers. J. Phys. Chem. C 2008, 112, 958–964. [Google Scholar] [CrossRef]
- Wang, H.; Akid, R.; Gobara, M. Scratch-resistant anticorrosion sol–gel coating for the protection of az31 magnesium alloy via a low temperature sol–gel route. Corros. Sci. 2010, 52, 2565–2570. [Google Scholar] [CrossRef]
- Guglielmi, M. Sol-gel coatings on metals. J. Sol-Gel Sci. Technol. 1997, 8, 443–449. [Google Scholar] [CrossRef]
- AbdolahZadeh, M.; van der Zwaag, S.; Garcia, S.J. Self-healing corrosion-protective sol–gel coatings based on extrinsic and intrinsic healing approaches. In Self-Healing Materials; Hager, M.D., van der Zwaag, S., Schubert, U.S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 185–218. [Google Scholar]
- Barrow, D.A.; Petroff, T.E.; Sayer, M. Thick ceramic coatings using a sol gel based ceramic-ceramic 0–3 composite. Surf. Coat. Technol. 1995, 76, 113–118. [Google Scholar] [CrossRef]
- Gąsiorek, J.; Szczurek, A.; Babiarczuk, B.; Kaleta, J.; Jones, W.; Krzak, J. Functionalizable sol-gel silica coatings for corrosion mitigation. Materials 2018, 11, 197. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.P.; Chandrasekaran, C.; Limmer, S.J.; Seraji, S.; Wu, Y.; Forbess, M.J.; Nguyen, C.; Cao, G.Z. Organic–inorganic hybrid coatings for corrosion protection. J. Non-Cryst. Solids 2001, 290, 153–162. [Google Scholar] [CrossRef]
- Yuan, W.; van Ooij, W.J. Characterization of organofunctional silane films on zinc substrates. J. Colloid Interface Sci. 1997, 185, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Tiringer, U.; Milošev, I.; Durán, A.; Castro, Y. Hybrid sol–gel coatings based on gptms/teos containing colloidal sio2 and cerium nitrate for increasing corrosion protection of aluminium alloy 7075-t6. J. Sol-Gel Sci. Technol. 2018, 85, 546–557. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Akbarian, M.; Ramezanzadeh, M.; Ramezanzadeh, B.; Mahdavian, M. Evaluation of the corrosion protection performance of mild steel coated with hybrid sol-gel silane coating in 3.5 wt.% nacl solution. Prog. Org. Coat. 2018, 123, 190–200. [Google Scholar] [CrossRef]
- Maia, F.; Yasakau, K.A.; Carneiro, J.; Kallip, S.; Tedim, J.; Henriques, T.; Cabral, A.; Venancio, J.; Zheludkevich, M.L.; Ferreira, M.G.S. Corrosion protection of aa2024 by sol-gel coatings modified with mbt-loaded polyurea microcapsules. Chem. Eng. J. 2016, 283, 1108–1117. [Google Scholar] [CrossRef]
- Akhtar, S.; Matin, A.; Kumar, A.M.; Ibrahim, A.; Laoui, T. Enhancement of anticorrosion property of 304 stainless steel using silane coatings. Appl. Surf. Sci. 2018, 440, 1286–1297. [Google Scholar] [CrossRef]
- Liu, X.H.; Cheng, Y.C.; Wang, W.; Liu, F.H.; Hou, B.R. Application of 1d attapulgite as reservoir with benzotriazole for corrosion protection of carbon steel. Mater. Chem. Phys. 2018, 205, 292–302. [Google Scholar] [CrossRef]
- Bonora, P.L.; Deflorian, F.; Fedrizzi, L. Electrochemical impedance spectroscopy as a tool for investigating underpaint corrosion. Electrochim. Acta 1996, 41, 1073–1082. [Google Scholar] [CrossRef]
- Mrad, M.; Dhouibi, L.; Montemor, M.F. Elaboration of γ-glycidoxypropyltrimethoxysilane coating on aa2024-t3 aluminum alloy: Influence of synthesis route on physicochemical and anticorrosion properties. Prog. Org. Coat. 2018, 121, 1–12. [Google Scholar] [CrossRef]
- Liu, X.; Gu, C.; Ma, Z.; Ma, X.; Hou, B. Ph-responsive containers based on modified hollow tio2 for active and passive protection of carbon steel. J. Electrochem. Soc. 2018, 165, C145–C154. [Google Scholar] [CrossRef]

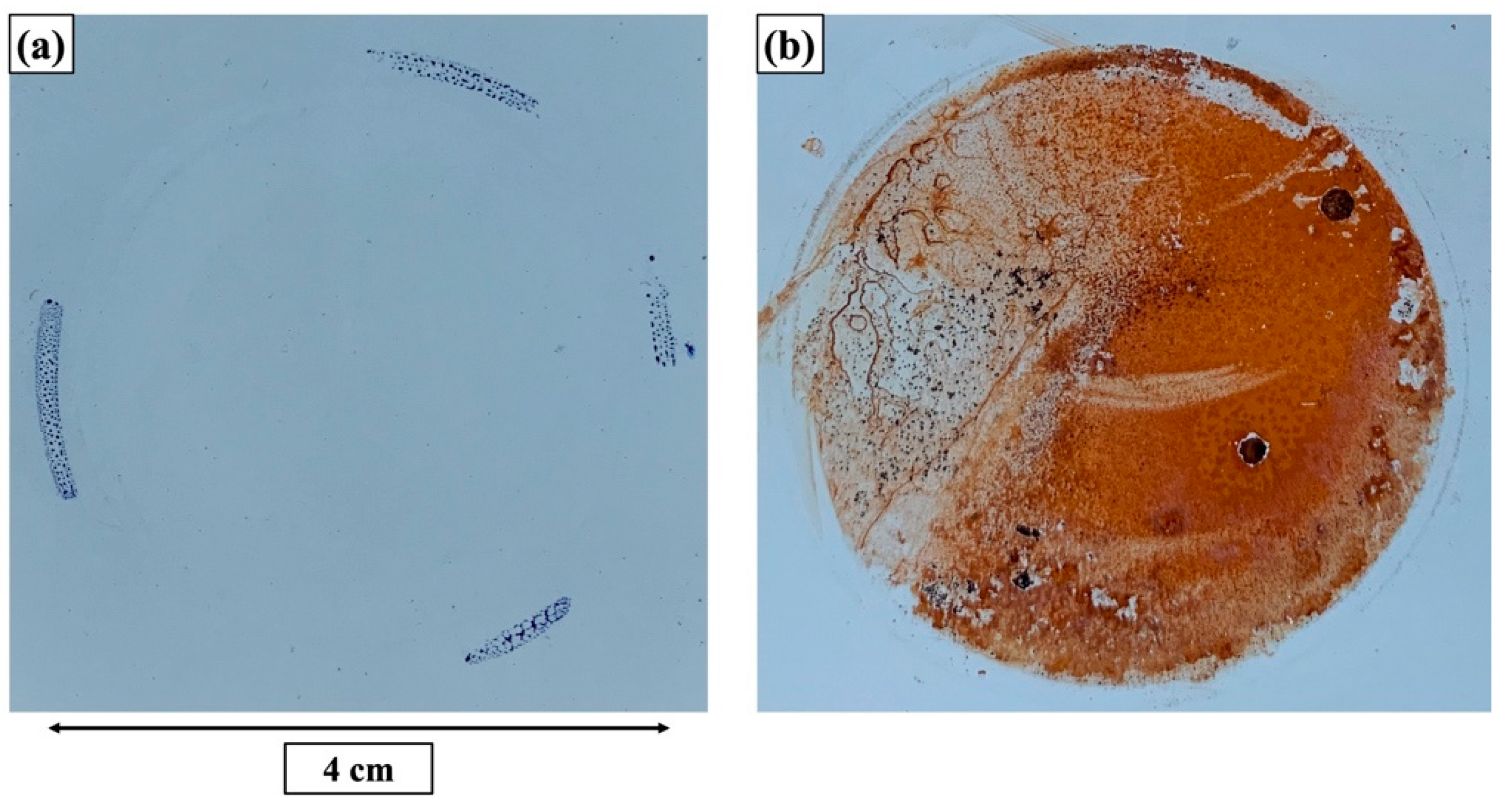
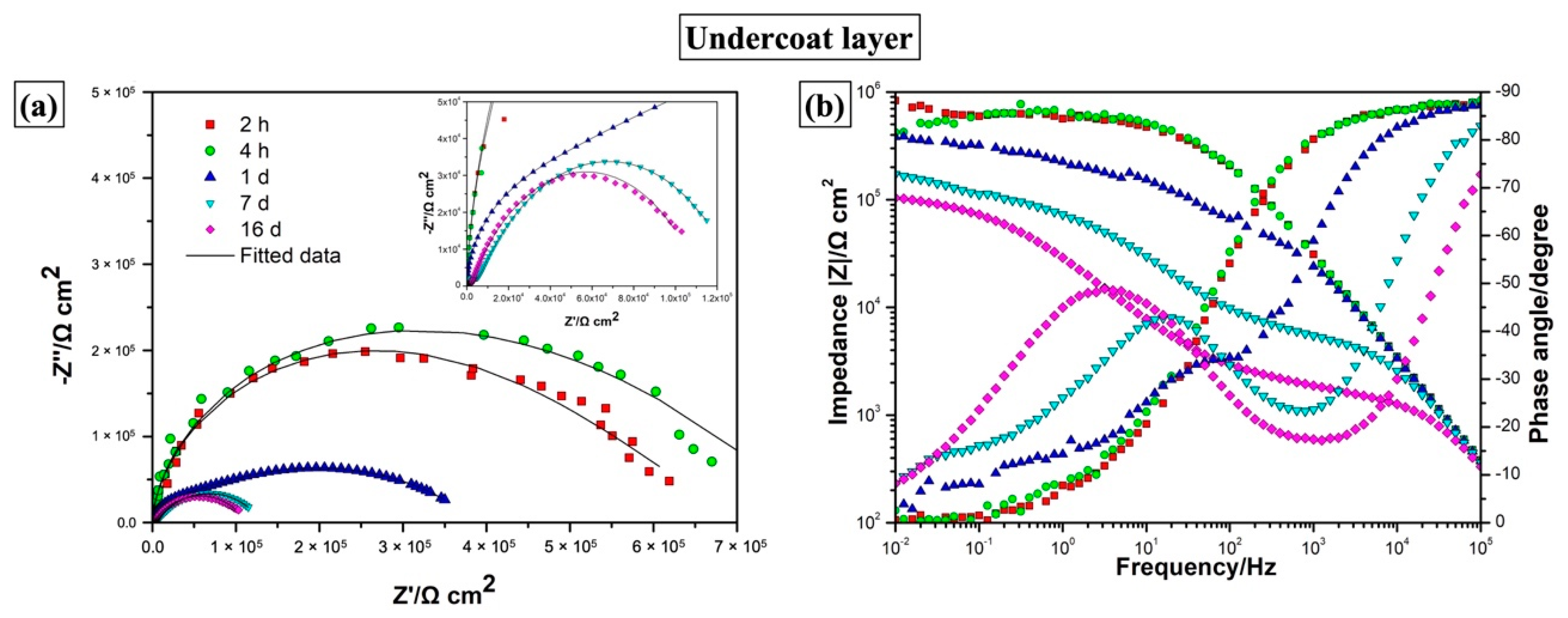
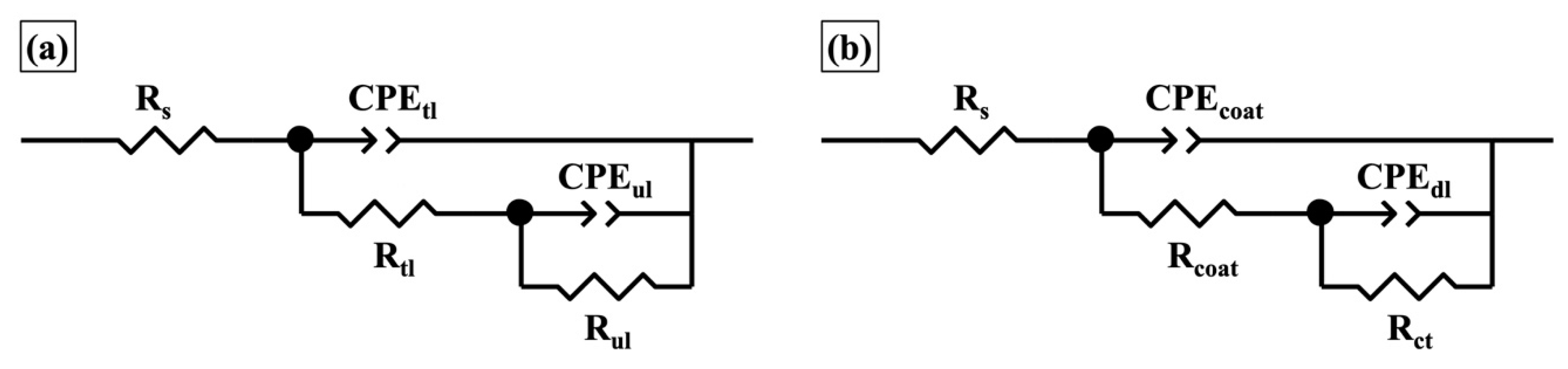
| Elements | Top Layer (Top View) (at.%) | Top Layer (Cross-Section) (at.%) | Underlying Layer (Top View) (at.%) | Underlying Layer (Cross-Section) (at.%) |
|---|---|---|---|---|
| O | 61.8 ± 4.1 | 58.7 ± 4.9 | 62.3 ± 4.4 | 65.6 ± 6.2 |
| Si | 38.1 ± 1.4 | 41.3 ± 1.8 | 31.8 ± 1.3 | 28.8 ± 1.5 |
| Ti | 0.1 ± 0.0 | 0.1 ± 0.0 | 5.9 ± 0.3 | 5.6 ± 0.3 |
| Coating | Immersion Time | Rtl (Ω·cm2) | CPEtl | Rul (Ω·cm2) | CPEul | χ2 10−3 | ||
|---|---|---|---|---|---|---|---|---|
| Y0 (Ω−1·sn·cm−2) | n | Y0 (Ω−1·sn·cm−2) | n | |||||
| Two-layer | 1 d | 2.11 ± 0.02 × 106 | 4.38 ± 0.06 × 10−9 | 0.956 ± 0.001 | 3.17 ± 0.06 × 108 | 2.32 ± 0.02 × 10−8 | 0.778 ± 0.003 | 1.9881 |
| 5 d | 1.90 ± 0.04 × 106 | 4.66 ± 0.07 × 10−9 | 0.952 ± 0.002 | 2.84 ± 0.05 × 108 | 2.35 ± 0.02 × 10−8 | 0.786 ± 0.003 | 1.9358 | |
| 26 d | 1.94 ± 0.06 × 106 | 5.21 ± 0.09 × 10−9 | 0.947 ± 0.002 | 3.18 ± 0.07 × 108 | 2.27 ± 0.02 × 10−8 | 0.789 ± 0.003 | 2.0837 | |
| 63 d | 2.64 ± 0.06 × 106 | 5.49 ± 0.08 × 10−9 | 0.946 ± 0.001 | 4.33 ± 0.11 × 108 | 2.11 ± 0.02 × 10−8 | 0.787 ± 0.004 | 2.2237 | |
| 99 d | 1.82 ± 0.04 × 106 | 6.00 ± 0.09 × 10−9 | 0.941 ± 0.002 | 2.97 ± 0.06 × 108 | 2.23 ± 0.02 × 10−8 | 0.786 ± 0.003 | 1.9318 | |
| One-layer | Immersion Time | Rcoat (Ω·cm2) | CPEcoat | Rct (Ω·cm2) | CPEdl | χ2 10−3 | ||
| 2 h | 1.14 ± 0.43 × 105 | 6.27 ± 0.23 × 10−9 | 0.971 ± 0.003 | 5.88 ± 0.64 × 105 | 1.90 ± 0.20 × 10−7 | 0.409 ± 0.030 | 1.1674 | |
| 4 h | 2.00 ± 0.44 × 105 | 6.46 ± 0.25 × 10−9 | 0.969 ± 0.003 | 6.09 ± 0.75 × 105 | 1.82 ± 0.29 × 10−7 | 0.476 ± 0.050 | 2.9885 | |
| 1 d | 2.21 ± 0.38 × 104 | 5.40 ± 0.49 × 10−9 | 0.984 ± 0.007 | 3.70 ± 0.12 × 105 | 1.05 ± 0.05 × 10−6 | 0.409 ± 0.014 | 5.7427 | |
| 7 d | 4.66 ± 0.08 × 103 | 7.33 ± 0.53 × 10−9 | 0.962 ± 0.006 | 1.27 ± 0.02 × 105 | 2.64 ± 0.06 × 10−6 | 0.620 ± 0.005 | 2.2603 | |
| 16 d | 1.71 ± 0.02 × 103 | 2.49 ± 0.24 × 10−8 | 0.872 ± 0.008 | 1.11 ± 0.01 × 105 | 9.50 ± 0.12 × 10−6 | 0.647 ± 0.003 | 2.2467 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wu, C.; Xue, M.; Cai, J.; Huang, Y.; Yang, H. Preparation of Sol-Gel Derived Anticorrosive Coating on Q235 Carbon Steel Substrate with Long-Term Corrosion Prevention Durability. Materials 2019, 12, 1960. https://doi.org/10.3390/ma12121960
Li Y, Wu C, Xue M, Cai J, Huang Y, Yang H. Preparation of Sol-Gel Derived Anticorrosive Coating on Q235 Carbon Steel Substrate with Long-Term Corrosion Prevention Durability. Materials. 2019; 12(12):1960. https://doi.org/10.3390/ma12121960
Chicago/Turabian StyleLi, Yue, Chunchun Wu, Ming Xue, Jiawen Cai, Yi Huang, and Hui Yang. 2019. "Preparation of Sol-Gel Derived Anticorrosive Coating on Q235 Carbon Steel Substrate with Long-Term Corrosion Prevention Durability" Materials 12, no. 12: 1960. https://doi.org/10.3390/ma12121960
APA StyleLi, Y., Wu, C., Xue, M., Cai, J., Huang, Y., & Yang, H. (2019). Preparation of Sol-Gel Derived Anticorrosive Coating on Q235 Carbon Steel Substrate with Long-Term Corrosion Prevention Durability. Materials, 12(12), 1960. https://doi.org/10.3390/ma12121960




