Electrode Materials for High-Performance Sodium-Ion Batteries
Abstract
:1. Introduction
1.1. Current Global Energy Scenario
1.2. Current Perspective on LIBs for Energy Storage
1.3. Rationale for SIBs for Energy Storage
2. Anodes
2.1. Carbons
2.2. Alloys
2.2.1. Sn-Based
2.2.2. Sb-Based
2.2.3. Ge-Based
2.2.4. P Based
2.2.5. Other Alloys
2.3. Metal Oxides
2.4. 2-D Materials
2.4.1. Graphene
2.4.2. Phosphorene
2.4.3. Transition Metal Dichalcogenides
2.4.4. MXenes
3. Cathodes
3.1. Layered Transition Metal Oxides (TMOs)
3.2. Polyanionic Compounds
3.2.1. Phosphates
3.2.2. NASICON Compounds and Fluorophosphates
3.3. Prussian Blue
3.4. Organic Materials
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, H.; Kim, H.; Ding, Z.; Lee, M.H.; Lim, K.; Yoon, G.; Kang, K. Recent Progress in Electrode Materials for Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1600943. [Google Scholar] [CrossRef] [Green Version]
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef]
- Haines, A.; Kovats, R.S.; Campbell-Lendrum, D.; Corvalan, C. Climate change and human health: Impacts, vulnerability and public health. Public Health 2006, 120, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, S.; Topal, E. When will fossil fuel reserves be diminished? Energy Policy 2009, 37, 181–189. [Google Scholar] [CrossRef]
- Kruyt, B.; van Vuuren, D.P.; de Vriesac, H.J.M.; Groenenberg, H. Indicators for energy security. Energy Policy 2009, 37, 2166–2181. [Google Scholar] [CrossRef]
- Erdinc, O.; Uzunoglu, M. Optimum design of hybrid renewable energy systems: Overview of different approaches. Renew. Sustain. Energy Rev. 2012, 16, 1412–1425. [Google Scholar] [CrossRef]
- Cavallo, A.J. Energy Storage Technologies for Utility Scale Intermittent Renewable Energy Systems. J. Sol. Energy Eng. 2001, 123, 387–389. [Google Scholar] [CrossRef]
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Hall, P.J.; Bain, E.J. Energy-storage technologies and electricity generation. Energy Policy 2008, 36, 4352–4355. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Nishi, Y. The development of lithium ion secondary batteries. Chem. Rec. 2001, 1, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Wakihara, M. Recent developments in lithium ion batteries. Mater. Sci. Eng. R Rep. 2001, 33, 109–134. [Google Scholar] [CrossRef]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.-M. Nanomaterials for Rechargeable Lithium Batteries. Angew. Chem. 2008, 47, 2930–2946. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Vikstrom, H.; Davidsson, S.; Hook, M. Lithium availability and future production outlooks. Appl. Energy 2013, 110, 252–266. [Google Scholar] [CrossRef] [Green Version]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Kundu, D.; Talaie, E.; Duffort, V.; Nazar, L.F. The Emerging Chemistry of Sodium Ion Batteries for Electrochemical Energy Storage. Angew. Chem. Int. Ed. 2015, 54, 3431–3448. [Google Scholar] [CrossRef]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-González, J.; Rojo, T. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 2012, 5, 5884–5901. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.; Park, K.-Y.; Kim, H.; Kim, S.-W.; Kang, K. Aqueous Rechargeable Li and Na Ion Batteries. Chem. Rev. 2014, 114, 11788–11827. [Google Scholar] [CrossRef]
- Kim, S.W.; Seo, D.H.; Ma, X.; Ceder, G.; Kang, K. Electrode materials for rechargeable sodium-ion batteries: Potential alternatives to current lithium-ion batteries. Adv. Energy Mater. 2012, 2, 710–721. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, L.; Sushko, M.L.; Wang, W.; Schwenzer, B.; Xiao, J.; Nie, Z.; Saraf, L.V.; Yang, Z.; Liu, J. Sodium Ion Insertion in Hollow Carbon Nanowires for Battery Applications. Nano Lett. 2012, 12, 3783–3787. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xiao, L.; Wang, W.; Choi, D.; Nie, Z.; Yu, J.; Saraf, L.V.; Yang, Z.; Liu, J. Reversible Sodium Ion Insertion in Single Crystalline Manganese Oxide Nanowires with Long Cycle Life. Adv. Mater. 2011, 23, 3155–3160. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Ren, Z.; Singh, G. Beyond Graphene Anode Materials for Emerging Metal Ion Batteries and Supercapacitors. Nano-Micro Lett. 2018, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, L.; Zhu, Z.; Hu, Z.; Zhao, Q.; Chen, J. All Organic Sodium-Ion Batteries with Na4C8H2O6. Angew. Chem. 2014, 53, 5892–5896. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Ren, Z.; Singh, G. Molecular polymer-derived ceramics for applications in electrochemical energy storage devices. J. Phys. D Appl. Phys. 2018, 51, 463001. [Google Scholar] [CrossRef]
- Bhandavat, R.; Pei, Z.; Singh, G. Polymer-derived ceramics as anode material for rechargeable Li-ion batteries: A review. Nanomater. Energy 2012, 1, 324–337. [Google Scholar] [CrossRef]
- Doeff, M.M.; Ma, Y.; Visco, S.J.; De Jonghe, L.C. Electrochemical insertion of sodium into carbon. J. Electrochem. Soc. 1993, 140, L169–L170. [Google Scholar] [CrossRef]
- Alcántara, R.; Jiménez-Mateos, J.M.; Lavela, P.; Tirado, J.L. Carbon black: A promising electrode material for sodium-ion batteries. Electrochem. Commun. 2001, 3, 639–642. [Google Scholar] [CrossRef]
- Alcántara, R.; Madrigal, F.F.; Lavela, P.; Tirado, J.; Mateos, J.J.; De Salazar, C.G.; Stoyanova, R.; Zhecheva, E. Characterisation of mesocarbon microbeads (MCMB) as active electrode material in lithium and sodium cells. Carbon 2000, 38, 1031–1041. [Google Scholar] [CrossRef]
- Komaba, S.; Murata, W.; Ishikawa, T.; Yabuuchi, N.; Ozeki, T.; Nakayama, T.; Ogata, A.; Gotoh, K.; Fujiwara, K. Electrochemical Na insertion and solid electrolyte interphase for hard-carbon electrodes and application to Na-Ion batteries. Adv. Funct. Mater. 2011, 21, 3859–3867. [Google Scholar] [CrossRef]
- Luo, W.; Jian, Z.; Xing, Z.; Wang, W.; Bommier, C.; Lerner, M.M.; Ji, X. Electrochemically Expandable Soft Carbon as Anodes for Na-Ion Batteries. ACS Cent. Sci. 2015, 1, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Song, H.; Li, Y.; Chen, Y.; Chen, X.; Zhou, J.; Ma, Z.; Wan, X.; Tian, P.; Wu, J. Rod-like ordered mesoporous carbons with various lengths as anode materials for sodium ion battery. Electrochim. Acta 2016, 218, 285–293. [Google Scholar] [CrossRef]
- Cao, B.; Liu, H.; Xu, B.; Lei, Y.F.; Chen, X.H.; Song, H.H. Mesoporous soft carbon as an anode material for sodium ion batteries with superior rate and cycling performance. J. Mater. Chem. A 2016, 4, 6472–6478. [Google Scholar] [CrossRef]
- Zhu, H.; Shen, F.; Luo, W.; Zhu, S.; Zhao, M.; Natarajan, B.; Dai, J.; Zhou, L.; Ji, X.; Yassar, R.S. Low temperature carbonization of cellulose nanocrystals for high performance carbon anode of sodium-ion batteries. Nano Energy 2017, 33, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.; He, K.; Zhu, Y.J.; Han, F.D.; Xu, Y.H.; Matsuda, I.; Ishii, Y.; Cumings, J.; Wang, C.S. Expanded graphite as superior anode for sodium-ion batteries. Nat. Commun. 2014, 5, 10. [Google Scholar] [CrossRef]
- Luo, W.; Wan, J.; Ozdemir, B.; Bao, W.; Chen, Y.; Dai, J.; Lin, H.; Xu, Y.; Gu, F.; Barone, V.; et al. Potassium Ion Batteries with Graphitic Materials. Nano Lett. 2015, 15, 7671–7677. [Google Scholar] [CrossRef]
- Moriwake, H.; Kuwabara, A.; Fisher, C.A.J.; Ikuhara, Y. Why is sodium-intercalated graphite unstable? RSC Adv. 2017, 7, 36550–36554. [Google Scholar] [CrossRef] [Green Version]
- Jache, B.; Adelhelm, P. Use of Graphite as a Highly Reversible Electrode with Superior Cycle Life for Sodium-Ion Batteries by Making Use of Co-Intercalation Phenomena. Angew. Chem. Int. Ed. 2014, 53, 10169–10173. [Google Scholar] [CrossRef]
- Yao, X.H.; Ke, Y.J.; Ren, W.H.; Wang, X.P.; Xiong, F.Y.; Yang, W.; Qin, M.S.; Li, Q.; Mai, L.Q. Defect-Rich Soft Carbon Porous Nanosheets for Fast and High-Capacity Sodium-Ion Storage. Adv. Energy Mater. 2019, 9, 1803260. [Google Scholar] [CrossRef]
- Cheng, D.L.; Yang, L.C.; Zhu, M. High-performance anode materials for Na-ion batteries. Rare Met. 2018, 37, 167–180. [Google Scholar] [CrossRef]
- Yun, Y.S.; Park, K.-Y.; Lee, B.; Cho, S.Y.; Park, Y.-U.; Hong, S.J.; Kim, B.H.; Gwon, H.; Kim, H.; Lee, S.; et al. Sodium-Ion Storage in Pyroprotein-Based Carbon Nanoplates. Adv. Mater. 2015, 27, 6914–6921. [Google Scholar] [CrossRef] [PubMed]
- Saurel, D.; Orayech, B.; Xiao, B.; Carriazo, D.; Li, X.; Rojo, T. From Charge Storage Mechanism to Performance: A Roadmap toward High Specific Energy Sodium-Ion Batteries through Carbon Anode Optimization. Adv. Energy Mater. 2018, 8, 1703268. [Google Scholar] [CrossRef]
- Luo, W.; Schardt, J.; Bommier, C.; Wang, B.; Razink, J.; Simonsen, J.; Ji, X.L. Carbon nanofibers derived from cellulose nanofibers as a long-life anode material for rechargeable sodium-ion batteries. J. Mater. Chem. A 2013, 1, 10662–10666. [Google Scholar] [CrossRef]
- Li, W.H.; Zeng, L.C.; Yang, Z.Z.; Gu, L.; Wang, J.Q.; Liu, X.W.; Cheng, J.X.; Yu, Y. Free-standing and binder-free sodium-ion electrodes with ultralong cycle life and high rate performance based on porous carbon nanofibers. Nanoscale 2014, 6, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Lotfabad, E.M.; Ding, J.; Cui, K.; Kohandehghan, A.; Kalisvaart, W.P.; Hazelton, M.; Mitlin, D. High-Density Sodium and Lithium Ion Battery Anodes from Banana Peels. ACS Nano 2014, 8, 7115–7129. [Google Scholar] [CrossRef]
- Zhang, F.; Yao, Y.G.; Wan, J.Y.; Henderson, D.; Zhang, X.G.; Hu, L.B. High Temperature Carbonized Grass as a High Performance Sodium Ion Battery Anode. ACS Appl. Mater. Interfaces 2017, 9, 391–397. [Google Scholar] [CrossRef]
- Hou, H.S.; Shao, L.D.; Zhang, Y.; Zou, G.Q.; Chen, J.; Ji, X.B. Large-Area Carbon Nanosheets Doped with Phosphorus: A High-Performance Anode Material for Sodium-Ion Batteries. Adv. Sci. 2017, 4, 1600243. [Google Scholar] [CrossRef]
- Hu, X.D.; Sun, X.H.; Yoo, S.J.; Evanko, B.; Fan, F.R.; Cai, S.; Zheng, C.M.; Hu, W.B.; Stucky, G.D. Nitrogen-rich hierarchically porous carbon as a high-rate anode material with ultra-stable cyclability and high capacity for capacitive sodium-ion batteries. Nano Energy 2019, 56, 828–839. [Google Scholar] [CrossRef]
- Shen, Y.L.; Sun, S.J.; Yang, M.; Zhao, X.Y. Typha-derived hard carbon for high-performance sodium ion storage. J. Alloys Compd. 2019, 784, 1290–1296. [Google Scholar] [CrossRef]
- Zhao, X.; Ding, Y.; Xu, Q.; Yu, X.; Liu, Y.; Shen, H. Low-Temperature Growth of Hard Carbon with Graphite Crystal for Sodium-Ion Storage with High Initial Coulombic Efficiency: A General Method. Adv. Energy Mater. 2019, 9, 1803648. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.; Park, Y.U.; Kim, J.; Hwang, I.; Kang, K. Sodium Storage Behavior in Natural Graphite using Ether-based Electrolyte Systems. Adv. Funct. Mater. 2015, 25, 534–541. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.; Li, Z.; Kohandehghan, A.; Cui, K.; Xu, Z.; Zahiri, B.; Tan, X.; Lotfabad, E.M.; Olsen, B.C.; et al. Carbon Nanosheet Frameworks Derived from Peat Moss as High Performance Sodium Ion Battery Anodes. Acs Nano 2013, 7, 11004–11015. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Tang, K.; Song, K.; van Aken, P.A.; Yu, Y.; Maier, J. Nitrogen doped porous carbon fibres as anode materials for sodium ion batteries with excellent rate performance. Nanoscale 2014, 6, 1384–1389. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Fu, L.; White, R.J.; Yu, L.; Titirici, M.-M.; Antonietti, M.; Maier, J. Hollow Carbon Nanospheres with Superior Rate Capability for Sodium-Based Batteries. Adv. Energy Mater. 2012, 2, 873–877. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Wu, X.; Yu, J.; Wang, Y.; Hu, Y.-S.; Li, H.; Chen, L.; Huang, X. Amorphous monodispersed hard carbon micro-spherules derived from biomass as a high performance negative electrode material for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 71–77. [Google Scholar] [CrossRef]
- Shen, F.; Zhu, H.; Luo, W.; Wan, J.; Zhou, L.; Dai, J.; Zhao, B.; Han, X.; Fu, K.; Hu, L. Chemically Crushed Wood Cellulose Fiber towards High-Performance Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 23291–23296. [Google Scholar] [CrossRef]
- Xie, F.; Xu, Z.; Jensen, A.C.S.; Au, H.; Lu, Y.; Araullo-Peters, V.; Drew, A.J.; Hu, Y.-S.; Titirici, M.-M. Hard–Soft Carbon Composite Anodes with Synergistic Sodium Storage Performance. Adv. Funct. Mater. 2019, 1901072. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.-S.; Titirici, M.-M.; Chen, L.; Huang, X. Hard Carbon Microtubes Made from Renewable Cotton as High-Performance Anode Material for Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1600659. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, P.; Liang, X.; Chen, C.; Xiang, H. High-yield microstructure-controlled amorphous carbon anode materials through a pre-oxidation strategy for sodium ion batteries. J. Alloys Compd. 2019, 786, 468–474. [Google Scholar] [CrossRef]
- Li, L.; Zheng, Y.; Zhang, S.L.; Yang, J.P.; Shao, Z.P.; Guo, Z.P. Recent progress on sodium ion batteries: Potential high-performance anodes. Energy Environ. Sci. 2018, 11, 2310–2340. [Google Scholar] [CrossRef]
- Chevrier, V.L.; Ceder, G. Challenges for Na-ion Negative Electrodes. J. Electrochem. Soc. 2011, 158, A1011–A1014. [Google Scholar] [CrossRef]
- Han, X.; Liu, C.; Sun, J.; Sendek, A.D.; Yang, W. Density functional theory calculations for evaluation of phosphorene as a potential anode material for magnesium batteries. RSC Adv. 2018, 8, 7196–7204. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Xu, Y.; Zhu, Y.; Culver, J.N.; Lundgren, C.A.; Xu, K.; Wang, C. Tin-Coated Viral Nanoforests as Sodium-Ion Battery Anodes. ACS Nano 2013, 7, 3627–3634. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Jing, M.; Yang, Y.; Zhang, Y.; Zhu, Y.; Song, W.; Yang, X.; Ji, X. Sb porous hollow microspheres as advanced anode materials for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 2971–2977. [Google Scholar] [CrossRef]
- Yue, C.; Yu, Y.; Sun, S.; He, X.; Chen, B.; Lin, W.; Xu, B.; Zheng, M.; Wu, S.; Li, J.; et al. High Performance 3D Si/Ge Nanorods Array Anode Buffered by TiN/Ti Interlayer for Sodium-Ion Batteries. Adv. Funct. Mater. 2015, 25, 1386–1392. [Google Scholar] [CrossRef]
- Xu, G.-L.; Chen, Z.; Zhong, G.-M.; Liu, Y.; Yang, Y.; Ma, T.; Ren, Y.; Zuo, X.; Wu, X.-H.; Zhang, X. Nanostructured black phosphorus/Ketjenblack–multiwalled carbon nanotubes composite as high performance anode material for sodium-ion batteries. Nano Lett. 2016, 16, 3955–3965. [Google Scholar] [CrossRef]
- Xu, Y.H.; Zhu, Y.J.; Liu, Y.H.; Wang, C.S. Electrochemical Performance of Porous Carbon/Tin Composite Anodes for Sodium-Ion and Lithium-Ion Batteries. Adv. Energy Mater. 2013, 3, 128–133. [Google Scholar] [CrossRef]
- Kim, Y.; Ha, K.-H.; Oh, S.M.; Lee, K.T. High-Capacity Anode Materials for Sodium-Ion Batteries. Chem. Eur. J. 2014, 20, 11980–11992. [Google Scholar] [CrossRef]
- Zhu, H.L.; Jia, Z.; Chen, Y.C.; Weadock, N.; Wan, J.Y.; Vaaland, O.; Han, X.G.; Li, T.; Hu, L.B. Tin Anode for Sodium-Ion Batteries Using Natural Wood Fiber as a Mechanical Buffer and Electrolyte Reservoir. Nano Lett. 2013, 13, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Zhang, N.; Jiao, L.F.; Tao, Z.L.; Chen, J. Ultrasmall Sn Nanoparticles Embedded in Carbon as High-Performance Anode for Sodium-Ion Batteries. Adv. Funct. Mater. 2015, 25, 214–220. [Google Scholar] [CrossRef]
- Mao, M.L.; Yan, F.L.; Cui, C.Y.; Ma, J.M.; Zhang, M.; Wang, T.H.; Wang, C.S. Pipe-Wire TiO2-Sn@Carbon Nanofibers Paper Anodes for Lithium and Sodium Ion Batteries. Nano Lett. 2017, 17, 3830–3836. [Google Scholar] [CrossRef] [PubMed]
- Sha, M.; Zhang, H.; Nie, Y.T.; Nie, K.Q.; Lv, X.X.; Sun, N.; Xie, X.K.; Ma, Y.Y.; Sun, X.H. Sn nanoparticles@ nitrogen-doped carbon nanofiber composites as high-performance anodes for sodium-ion batteries. J. Mater. Chem. A 2017, 5, 6277–6283. [Google Scholar] [CrossRef]
- Pan, L.; Huang, H.J.; Zhong, M.; Niederberger, M. Hydrogel-derived foams of nitrogen-doped carbon loaded with Sn nanodots for high-mass-loading Na-ion storage. Energy Storage Mater. 2019, 16, 519–526. [Google Scholar] [CrossRef]
- Darwiche, A.; Marino, C.; Sougrati, M.T.; Fraisse, B.; Stievano, L.; Monconduit, L. Better Cycling Performances of Bulk Sb in Na-Ion Batteries Compared to Li-Ion Systems: An Unexpected Electrochemical Mechanism. J. Am. Chem. Soc. 2012, 134, 20805–20811. [Google Scholar] [CrossRef] [PubMed]
- Allan, P.K.; Griffin, J.M.; Darwiche, A.; Borkiewicz, O.J.; Wiaderek, K.M.; Chapman, K.W.; Morris, A.J.; Chupas, P.J.; Monconduit, L.; Grey, C.P. Tracking Sodium-Antimonide Phase Transformations in Sodium-Ion Anodes: Insights from Operando Pair Distribution Function Analysis and Solid-State NMR Spectroscopy. J. Am. Chem. Soc. 2016, 138, 2352–2365. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.J.; Han, X.G.; Xu, Y.H.; Liu, Y.H.; Zheng, S.Y.; Xu, K.; Hu, L.B.; Wang, C.S. Electrospun Sb/C Fibers for a Stable and Fast Sodium-Ion Battery Anode. ACS Nano 2013, 7, 6378–6386. [Google Scholar] [CrossRef]
- Wu, L.; Hu, X.H.; Qian, J.F.; Pei, F.; Wu, F.Y.; Mao, R.J.; Ai, X.P.; Yang, H.X.; Cao, Y.L. Sb-C nanofibers with long cycle life as an anode material for high-performance sodium-ion batteries. Energy Environ. Sci. 2014, 7, 323–328. [Google Scholar] [CrossRef]
- Zhao, W.X.; Zou, L.; Ma, X.Q.; Zhang, W.L.; Li, Y.D.; Wang, G.Z.; Zhang, P.; Xia, L.P. Ultrafine Sb nanoparticles embedded in nitrogen-doped carbon nanofibers as ultralong cycle durability and high-rate anode materials for reversible sodium storage. Electrochim. Acta 2019, 300, 396–403. [Google Scholar] [CrossRef]
- Cui, C.Y.; Xu, J.T.; Zhang, Y.Q.; Wei, Z.X.; Mao, M.L.; Lian, X.; Wang, S.Y.; Yang, C.Y.; Fan, X.L.; Ma, J.M.; et al. Antimony Nanorod Encapsulated in Cross-Linked Carbon for High-Performance Sodium Ion Battery Anodes. Nano Lett. 2019, 19, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Chockla, A.M.; Klavetter, K.C.; Mullins, C.B.; Korgel, B.A. Solution-Grown Germanium Nanowire Anodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2012, 4, 4658–4664. [Google Scholar] [CrossRef] [PubMed]
- Li, W.W.; Wang, X.F.; Liu, B.; Xu, J.; Liang, B.; Luo, T.; Luo, S.J.; Chen, D.; Shen, G.Z. Single-crystalline metal germanate nanowire-carbon textiles as binder-free, self-supported anodes for high-performance lithium storage. Nanoscale 2013, 5, 10291–10299. [Google Scholar] [CrossRef] [PubMed]
- Abel, P.R.; Lin, Y.M.; de Souza, T.; Chou, C.Y.; Gupta, A.; Goodenough, J.B.; Hwang, G.S.; Heller, A.; Mullins, C.B. Nanocolumnar Germanium Thin Films as a High-Rate Sodium-Ion Battery Anode Material. J. Phys. Chem. C 2013, 117, 18885–18890. [Google Scholar] [CrossRef]
- Stojic, M.; Kostic, D.; Stosic, B. The behavior of sodium in Ge, Si and GaAs. Physica B+C 1986, 138, 125–128. [Google Scholar] [CrossRef]
- Wang, X.; Fan, L.; Gong, D.; Zhu, J.; Zhang, Q.; Lu, B. Core–Shell Ge@Graphene@TiO2 Nanofibers as a High-Capacity and Cycle-Stable Anode for Lithium and Sodium Ion Battery. Adv. Funct. Mater. 2016, 26, 1104–1111. [Google Scholar] [CrossRef]
- Luo, W.; Shen, F.; Bommier, C.; Zhu, H.L.; Ji, X.L.; Hu, L.B. Na-Ion Battery Anodes: Materials and Electrochemistry. Acc. Chem. Res. 2016, 49, 231–240. [Google Scholar] [CrossRef]
- Qian, J.F.; Wu, X.Y.; Cao, Y.L.; Ai, X.P.; Yang, H.X. High Capacity and Rate Capability of Amorphous Phosphorus for Sodium Ion Batteries. Angew. Chem. Int. Ed. 2013, 52, 4633–4636. [Google Scholar] [CrossRef]
- Kim, Y.; Park, Y.; Choi, A.; Choi, N.-S.; Kim, J.; Lee, J.; Ryu, J.H.; Oh, S.M.; Lee, K.T. An Amorphous Red Phosphorus/Carbon Composite as a Promising Anode Material for Sodium Ion Batteries. Adv. Mater. 2013, 25, 3045–3049. [Google Scholar] [CrossRef]
- Liu, S.; Xu, H.; Bian, X.; Feng, J.; Liu, J.; Yang, Y.; Yuan, C.; An, Y.; Fan, R.; Ci, L. Hollow nanoporous red phosphorus as an advanced anode for sodium-ion batteries. J. Mater. Chem. A 2018, 6, 12992–12998. [Google Scholar] [CrossRef]
- Yao, S.; Cui, J.; Huang, J.; Huang, J.-Q.; Chong, W.G.; Qin, L.; Mai, Y.-W.; Kim, J.-K. Rational Assembly of Hollow Microporous Carbon Spheres as P Hosts for Long-Life Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702267. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, T.; Chuang, C.; Lu, Y.-R.; Chan, T.-S.; Du, Z.; Ji, H.; Wan, L.-J. Synergy of Black Phosphorus–Graphite–Polyaniline-Based Ternary Composites for Stable High Reversible Capacity Na-Ion Battery Anodes. ACS Appl. Mater. Interfaces 2019, 11, 16656–16661. [Google Scholar] [CrossRef] [PubMed]
- Webb, S.A.; Baggetto, L.; Bridges, C.A.; Veith, G.M. The electrochemical reactions of pure indium with Li and Na: Anomalous electrolyte decomposition, benefits of FEC additive, phase transitions and electrode performance. J. Power Sources 2014, 248, 1105–1117. [Google Scholar] [CrossRef]
- Darwiche, A.; Dugas, R.; Fraisse, B.; Monconduit, L. Reinstating lead for high-loaded efficient negative electrode for rechargeable sodium-ion battery. J. Power Sources 2016, 304, 1–8. [Google Scholar] [CrossRef]
- Liu, S.; Feng, J.K.; Bian, X.F.; Liu, J.; Xu, H. Advanced arrayed bismuth nanorod bundle anode for sodium-ion batteries. J. Mater. Chem. A 2016, 4, 10098–10104. [Google Scholar] [CrossRef]
- Wang, C.C.; Wang, L.B.; Li, F.J.; Cheng, F.Y.; Chen, J. Bulk Bismuth as a High-Capacity and Ultralong Cycle-Life Anode for Sodium-Ion Batteries by Coupling with Glyme-Based Electrolytes. Adv. Mater. 2017, 29, 1702212. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xu, R.; Yao, Y.; Ye, S.F.; Zhou, X.F.; Yu, Y. Multicore-Shell Bi@N-doped Carbon Nanospheres for High Power Density and Long Cycle Life Sodium- and Potassium-Ion Anodes. Adv. Funct. Mater. 2019, 29, 1809195. [Google Scholar] [CrossRef]
- Ji, L.; Gu, M.; Shao, Y.; Li, X.; Engelhard, M.H.; Arey, B.W.; Wang, W.; Nie, Z.; Xiao, J.; Wang, C.; et al. Controlling SEI Formation on SnSb-Porous Carbon Nanofibers for Improved Na Ion Storage. Adv. Mater. 2014, 26, 2901–2908. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kopold, P.; Wu, C.; van Aken, P.A.; Maier, J.; Yu, Y. Uniform yolk–shell Sn4P3@C nanospheres as high-capacity and cycle-stable anode materials for sodium-ion batteries. Energy Environ. Sci. 2015, 8, 3531–3538. [Google Scholar] [CrossRef]
- Li, W.; Hu, C.; Zhou, M.; Tao, H.; Wang, K.; Cheng, S.; Jiang, K. Carbon-coated Mo3Sb7 composite as anode material for sodium ion batteries with long cycle life. J. Power Sources 2016, 307, 173–180. [Google Scholar] [CrossRef]
- Edison, E.; Ling, W.C.; Aravindan, V.; Madhavi, S. Highly Stable Intermetallic FeSn2-Graphite Composite Anode for Sodium-Ion Batteries. ChemElectroChem 2017, 4, 1932–1936. [Google Scholar] [CrossRef]
- Gao, H.; Niu, J.; Zhang, C.; Peng, Z.; Zhang, Z. A Dealloying Synthetic Strategy for Nanoporous Bismuth–Antimony Anodes for Sodium Ion Batteries. ACS Nano 2018, 12, 3568–3577. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.-H.; Kim, T.-H.; Hong, K.-S.; Kwon, H.-S. Template-Free Electrochemical Synthesis of Sn Nanofibers as High-Performance Anode Materials for Na-Ion Batteries. ACS Nano 2014, 8, 11824–11835. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Dai, Z.; Bao, J.; Guo, Y.-G. Wet milled synthesis of an Sb/MWCNT nanocomposite for improved sodium storage. J. Mater. Chem. A 2013, 1, 13727–13731. [Google Scholar] [CrossRef]
- Wu, L.; Lu, H.; Xiao, L.; Ai, X.; Yang, H.; Cao, Y. Electrochemical properties and morphological evolution of pitaya-like Sb@C microspheres as high-performance anode for sodium ion batteries. J. Mater. Chem. A 2015, 3, 5708–5713. [Google Scholar] [CrossRef]
- Li, P.; Guo, X.; Wang, S.; Zang, R.; Li, X.; Man, Z.; Li, P.; Liu, S.; Wu, Y.; Wang, G. Two-dimensional Sb@TiO2−x nanoplates as a high-performance anode material for sodium-ion batteries. J. Mater. Chem. A 2019, 7, 2553–2559. [Google Scholar] [CrossRef]
- Nie, A.; Gan, L.-Y.; Cheng, Y.; Tao, X.; Yuan, Y.; Sharifi-Asl, S.; He, K.; Asayesh-Ardakani, H.; Vasiraju, V.; Lu, J.; et al. Ultrafast and Highly Reversible Sodium Storage in Zinc-Antimony Intermetallic Nanomaterials. Adv. Funct. Mater. 2016, 26, 543–552. [Google Scholar] [CrossRef]
- Darwiche, A.; Toiron, M.; Sougrati, M.T.; Fraisse, B.; Stievano, L.; Monconduit, L. Performance and mechanism of FeSb2 as negative electrode for Na-ion batteries. J. Power Sources 2015, 280, 588–592. [Google Scholar] [CrossRef]
- Jian, Z.; Zhao, B.; Liu, P.; Li, F.; Zheng, M.; Chen, M.; Shi, Y.; Zhou, H. Fe2O3 nanocrystals anchored onto graphene nanosheets as the anode material for low-cost sodium-ion batteries. Chem. Commun. 2014, 50, 1215–1217. [Google Scholar] [CrossRef]
- Yang, F.; Yu, F.; Zhang, Z.; Zhang, K.; Lai, Y.; Li, J. Bismuth Nanoparticles Embedded in Carbon Spheres as Anode Materials for Sodium/Lithium-Ion Batteries. Chem. Eur. J. 2016, 22, 2333–2338. [Google Scholar] [CrossRef]
- Xie, H.; Kalisvaart, W.P.; Olsen, B.C.; Luber, E.J.; Mitlin, D.; Buriak, J.M. Sn–Bi–Sb alloys as anode materials for sodium ion batteries. J. Mater. Chem. A 2017, 5, 9661–9670. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, Y.; Choi, A.; Woo, S.; Mok, D.; Choi, N.-S.; Jung, Y.S.; Ryu, J.H.; Oh, S.M.; Lee, K.T. Tin Phosphide as a Promising Anode Material for Na-Ion Batteries. Adv. Mater. 2014, 26, 4139–4144. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, S.; Kravchyk, K.; Ibáñez, M.; Krumeich, F.; Widmer, R.; Nasiou, D.; Meyns, M.; Llorca, J.; Arbiol, J.; et al. SnP nanocrystals as anode materials for Na-ion batteries. J. Mater. Chem. A 2018, 6, 10958–10966. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-O.; Manthiram, A. The facile synthesis and enhanced sodium-storage performance of a chemically bonded CuP2/C hybrid anode. Chem. Commun. 2016, 52, 4337–4340. [Google Scholar] [CrossRef] [PubMed]
- Delmas, C.; Braconnier, J.-J.; Fouassier, C.; Hagenmuller, P. Electrochemical intercalation of sodium in NaxCoO2 bronzes. Solid State Ion. 1981, 3–4, 165–169. [Google Scholar] [CrossRef]
- Tian, L.; Zhuang, Q.; Li, J.; Wu, C.; Shi, Y.; Sun, S. The production of self-assembled Fe2O3–graphene hybrid materials by a hydrothermal process for improved Li-cycling. Electrochim. Acta 2012, 65, 153–158. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, C.; Lu, T.; Guo, Z.; Zhang, D.; Ma, J.; Zhu, S. Simple fabrication of a Fe2O3/carbon composite for use in a high-performance lithium ion battery. Carbon 2013, 52, 565–573. [Google Scholar] [CrossRef]
- Zou, M.; Li, J.; Wen, W.; Chen, L.; Guan, L.; Lai, H.; Huang, Z. Silver-incorporated composites of Fe2O3 carbon nanofibers as anodes for high-performance lithium batteries. J. Power Sources 2014, 270, 468–474. [Google Scholar] [CrossRef]
- Shen, L.; Yu, Y. Greener and cheaper. Nat. Energy 2017, 2, 836–837. [Google Scholar] [CrossRef]
- Klein, F.; Jache, B.; Bhide, A.; Adelhelm, P. Conversion reactions for sodium-ion batteries. Phys. Chem. Chem. Phys. 2013, 15, 15876–15887. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Wang, Y.; Jiao, L. CuO Quantum Dots Embedded in Carbon Nanofibers as Binder-Free Anode for Sodium Ion Batteries with Enhanced Properties. Small 2016, 12, 4865–4872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Han, X.; Liu, Y.; Hu, X.; Zhao, Q.; Chen, J. 3D Porous γ-Fe2O3@C nanocomposite as high-performance anode material of Na-ion batteries. Adv. Energy Mater. 2015, 5, 1401123. [Google Scholar] [CrossRef]
- Wu, L.; Lang, J.; Wang, R.; Guo, R.; Yan, X. Electrospinning Synthesis of Mesoporous MnCoNiOx@Double-Carbon Nanofibers for Sodium-Ion Battery Anodes with Pseudocapacitive Behavior and Long Cycle Life. ACS Appl. Mater. Interfaces 2016, 8, 34342–34352. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, K.; Nithya, C.; Kundoly Purushothaman, B.; Kumar, N.; Gopukumar, S. Sb2O4@rGO nanocomposite anode for high performance sodium-ion batteries. ACS Sustain. Chem. Eng. 2017, 5, 5090–5098. [Google Scholar] [CrossRef]
- Li, K.; Zhang, J.; Lin, D.; Wang, D.-W.; Li, B.; Lv, W.; Sun, S.; He, Y.-B.; Kang, F.; Yang, Q.-H.; et al. Evolution of the electrochemical interface in sodium ion batteries with ether electrolytes. Nat. Commun. 2019, 10, 725. [Google Scholar] [CrossRef]
- Dinh, C.-T.; Nguyen, T.-D.; Kleitz, F.; Do, T.-O. Shape-controlled synthesis of highly crystalline titania nanocrystals. ACS Nano 2009, 3, 3737–3743. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ma, C.; Zhu, Q.-C.; Xu, S.-M.; Wei, X.; Wu, Y.-M.; Tang, W.-P.; Wang, K.-X.; Chen, J.-S. Well-ordered mesoporous Fe2O3/C composites as high performance anode materials for sodium-ion batteries. Dalton Trans. 2017, 46, 5025–5032. [Google Scholar] [CrossRef]
- Zoller, F.; Peters, K.; Zehetmaier, P.M.; Zeller, P.; Döblinger, M.; Bein, T.; Sofer, Z.K.; Fattakhova-Rohlfing, D. Making Ultrafast High-Capacity Anodes for Lithium-Ion Batteries via Antimony Doping of Nanosized Tin Oxide/Graphene Composites. Adv. Funct. Mater. 2018, 28, 1706529. [Google Scholar] [CrossRef]
- Hasa, I.; Verrelli, R.; Hassoun, J. Transition metal oxide-carbon composites as conversion anodes for sodium-ion battery. Electrochim. Acta 2015, 173, 613–618. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Wang, G.; Xia, Y.; Wang, H. One-dimensional hybrid nanocomposite of high-density monodispersed Fe3O4 nanoparticles and carbon nanotubes for high-capacity storage of lithium and sodium. J. Mater. Chem. A 2016, 4, 18532–18542. [Google Scholar] [CrossRef]
- Wang, T.; Qu, J.; Legut, D.; Qin, J.; Li, X.; Zhang, Q. Unique Double-Interstitialcy Mechanism and Interfacial Storage Mechanism in the Graphene/Metal Oxide as the Anode for Sodium-Ion Batteries. Nano Lett. 2019, 19, 3122–3130. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, X.; Bai, Z.; Yan, B.; Li, D.; Sun, X. Morphology-dependent performance of nanostructured Ni 3 S 2 /Ni anode electrodes for high performance sodium ion batteries. Nano Energy 2016, 26, 533–540. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, Z.; Chen, M.; Zou, C.; Jin, H.; Wang, S.; Gu, Q.; Chou, S. P2-type Na2/3Ni1/3Mn2/3O2 as a cathode material with high-rate and long-life for sodium ion storage. J. Mater. Chem. A 2019, 7, 9215–9221. [Google Scholar] [CrossRef]
- Hosono, E.; Saito, T.; Hoshino, J.; Okubo, M.; Saito, Y.; Nishio-Hamane, D.; Kudo, T.; Zhou, H. High power Na-ion rechargeable battery with single-crystalline Na0.44MnO2 nanowire electrode. J. Power Sources 2012, 217, 43–46. [Google Scholar] [CrossRef]
- Qi, L.-Y.; Zhang, Y.-W.; Zuo, Z.-C.; Xin, Y.-L.; Yang, C.-K.; Wu, B.; Zhang, X.-X.; Zhou, H.-H. In situ quantization of ferroferric oxide embedded in 3D microcarbon for ultrahigh performance sodium-ion batteries. J. Mater. Chem. A 2016, 4, 8822–8829. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Wang, Y.; Liu, H.; Huang, Z. Superior sodium-ion storage performance of Co3O4@nitrogen-doped carbon: Derived from a metal–organic framework. J. Mater. Chem. A 2016, 4, 5428–5435. [Google Scholar] [CrossRef]
- Lee, J.-I.; Song, J.; Cha, Y.; Fu, S.; Zhu, C.; Li, X.; Lin, Y.; Song, M.-K. Multifunctional SnO2/3D graphene hybrid materials for sodium-ion and lithium-ion batteries with excellent rate capability and long cycle life. Nano Res. 2017, 10, 4398–4414. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, Y.-E.; Sheng, J.; Li, F.; Tang, B.; Zhang, Y.; Zhou, Z. T-Nb2O5/C Nanofibers Prepared through Electrospinning with Prolonged Cycle Durability for High-Rate Sodium–Ion Batteries Induced by Pseudocapacitance. Small 2017, 13, 1702588. [Google Scholar] [CrossRef]
- Cao, K.; Jiao, L.; Pang, W.K.; Liu, H.; Zhou, T.; Guo, Z.; Wang, Y.; Yuan, H. Na2Ti6O13 Nanorods with Dominant Large Interlayer Spacing Exposed Facet for High-Performance Na-Ion Batteries. Small 2016, 12, 2991–2997. [Google Scholar] [CrossRef]
- Li, N.; Liao, S.; Sun, Y.; Song, H.W.; Wang, C.X. Uniformly dispersed self-assembled growth of Sb2O3/Sb@graphene nanocomposites on a 3D carbon sheet network for high Na-storage capacity and excellent stability. J. Mater. Chem. A 2015, 3, 5820–5828. [Google Scholar] [CrossRef]
- Cai, Y.; Fang, G.; Zhou, J.; Liu, S.; Luo, Z.; Pan, A.; Cao, G.; Liang, S. Metal-organic framework-derived porous shuttle-like vanadium oxides for sodium-ion battery application. Nano Res. 2018, 11, 449–463. [Google Scholar] [CrossRef]
- Wang, X.; Cao, K.; Wang, Y.; Jiao, L. Controllable N-Doped CuCo2O4@C Film as a Self-Supported Anode for Ultrastable Sodium-Ion Batteries. Small 2017, 13, 1700873. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xu, Y.; Wang, C.; Li, Q.; Xiang, J.; Liang, L.; Wu, M.; Zhao, H.; Lei, Y. Amorphous TiO2 inverse opal anode for high-rate sodium ion batteries. Nano Energy 2017, 31, 514–524. [Google Scholar] [CrossRef]
- Kalubarme, R.S.; Inamdar, A.I.; Bhange, D.S.; Im, H.; Gosavi, S.W.; Park, C.-J. Nickel-titanium oxide as a novel anode material for rechargeable sodium-ion batteries. J. Mater. Chem. A 2016, 4, 17419–17430. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, L.; Zhang, Y.; Zhao, H.; Kong, L.; Gao, S. Confined formation of monoclinic Na4Ti5O12 nanoparticles embedded into porous CNTs: Towards enhanced electrochemical performances for sodium ion batteries. New J. Chem. 2018, 42, 19340–19343. [Google Scholar] [CrossRef]
- Wang, S.; Cao, F.; Li, Y.; Zhang, Z.; Zhou, D.; Yang, Y.; Tang, Z. MoS2-Coupled Carbon Nanosheets Encapsulated on Sodium Titanate Nanowires as Super-Durable Anode Material for Sodium-Ion Batteries. Adv. Sci. 2019, 6, 1900028. [Google Scholar] [CrossRef] [PubMed]
- Raccichini, R.; Varzi, A.; Passerini, S.; Scrosati, B. The role of graphene for electrochemical energy storage. Nat. Mater. 2014, 14, 271–279. [Google Scholar] [CrossRef]
- Ying, H.; Han, W.-Q. Metallic Sn-Based Anode Materials: Application in High-Performance Lithium-Ion and Sodium-Ion Batteries. Adv. Sci. 2017, 4, 1700298. [Google Scholar] [CrossRef]
- Li, D.; Kaner, R.B. Graphene-Based Materials. Science 2008, 320, 1170–1171. [Google Scholar] [CrossRef]
- Liu, N.; Chortos, A.; Lei, T.; Jin, L.; Kim, T.R.; Bae, W.-G.; Zhu, C.; Wang, S.; Pfattner, R.; Chen, X.; et al. Ultratransparent and stretchable graphene electrodes. Sci. Adv. 2017, 3, e1700159. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.-H.; Kim, P.; Choi, J.-Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Huang, B.; Pan, Z.; Su, X.; Shao, Z.; An, L. Advances in three-dimensional graphene-based materials: Configurations, preparation and application in secondary metal (Li, Na, K, Mg, Al)-ion batteries. Energy Environ. Sci. 2019. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.W. Two-dimensional nanoarchitectures for lithium storage. Adv. Mater. 2012, 24, 4097–4111. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Mei, L.; Liang, J.; Zhao, Z.; Lee, C.; Fei, H.; Ding, M.; Lau, J.; Li, M.; Wang, C.; et al. Three-dimensional holey-graphene/niobia composite architectures for ultrahigh-rate energy storage. Science 2017, 356, 599–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Zeng, H.; Han, D.; Qiao, K.; Xing, W.; Rood, M.J.; Yan, Z. Nitrogen and Sulfur Co-Doped Graphene Nanosheets to Improve Anode Materials for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 37172–37180. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Chen, S.; Fu, Q.; Sun, Y.; Zhang, Y.; Lin, N.; Gao, P.; Yang, J.; Qian, Y. Layered-Structure SbPO4/Reduced Graphene Oxide: An Advanced Anode Material for Sodium Ion Batteries. ACS Nano 2018, 12, 12869–12878. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Zhang, L.; Xie, F.; Vasileff, A.; Qiao, S.-Z. Graphitic Carbon Nitride (g-C3N4)-Derived N-Rich Graphene with Tuneable Interlayer Distance as a High-Rate Anode for Sodium-Ion Batteries. Adv. Mater. 2019, 1901261. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, G.; Wang, L.; Tang, L.; Zhu, M.; Wu, C.; Dou, S.-X.; Wu, M. Graphene-Encapsulated CuP2: A Promising Anode Material with High Reversible Capacity and Superior Rate-Performance for Sodium-Ion Batteries. Nano Lett. 2019, 19, 2575–2582. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, X.; Mi, L.; Liu, C.; Zhang, J.; Cui, S.; Feng, X.; Cao, Y.; Shen, C. High-Performance Flexible Freestanding Anode with Hierarchical 3D Carbon-Networks/Fe7S8/Graphene for Applicable Sodium-Ion Batteries. Adv. Mater. 2019, 31, 1806664. [Google Scholar] [CrossRef]
- Ling, C.; Mizuno, F. Boron-doped graphene as a promising anode for Na-ion batteries. Phys. Chem. Chem. Phys. 2014, 16, 10419–10424. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, D.-M.; Zhang, C.; Zhang, Y.; Liang, Q.; Chen, S.; Weng, Q.; Zhou, M.; Xue, Y.; Liu, J.; et al. “Protrusions” or “holes” in graphene: Which is the better choice for sodium ion storage? Energy Environ. Sci. 2017, 10, 979–986. [Google Scholar] [CrossRef]
- Guo, X.; Zheng, S.; Zhang, G.; Xiao, X.; Li, X.; Xu, Y.; Xue, H.; Pang, H. Nanostructured graphene-based materials for flexible energy storage. Energy Storage Mater. 2017, 9, 150–169. [Google Scholar] [CrossRef]
- Samuel Reich, E. Phosphorene excites materials scientists. Nature 2014, 506, 19. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Neal, A.T.; Zhu, Z.; Luo, Z.; Xu, X.; Tománek, D.; Ye, P.D. Phosphorene: An Unexplored 2D Semiconductor with a High Hole Mobility. ACS Nano 2014, 8, 4033–4041. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Zheng, G.; Lee, H.-W.; Liu, N.; Wang, H.; Yao, H.; Yang, W.; Cui, Y. Formation of Stable Phosphorus–Carbon Bond for Enhanced Performance in Black Phosphorus Nanoparticle–Graphite Composite Battery Anodes. Nano Lett. 2014, 14, 4573–4580. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Luo, Z.; Tan, H.T.; Li, B.; Sun, S.; Li, Z.; Zong, Y.; Xu, Z.J.; Yang, Y.; et al. An Air-Stable Densely Packed Phosphorene–Graphene Composite Toward Advanced Lithium Storage Properties. Adv. Energy Mater. 2016, 6, 1600453. [Google Scholar] [CrossRef]
- Khandelwal, A.; Mani, K.; Karigerasi, M.H.; Lahiri, I. Phosphorene—The two-dimensional black phosphorous: Properties, synthesis and applications. Mater. Sci. Eng. B 2017, 221, 17–34. [Google Scholar] [CrossRef]
- Padilha, J.E.; Fazzio, A.; da Silva, A.J.R. van der Waals Heterostructure of Phosphorene and Graphene: Tuning the Schottky Barrier and Doping by Electrostatic Gating. Phys. Rev. Lett. 2015, 114, 066803. [Google Scholar] [CrossRef]
- Tao, Y.; Huang, T.; Ding, C.; Yu, F.; Tan, D.; Wang, F.; Xie, Q.; Yao, S. Few-layer phosphorene: An emerging electrode material for electrochemical energy storage. Appl. Mater. Today 2019, 15, 18–33. [Google Scholar] [CrossRef]
- Sresht, V.; Pádua, A.A.H.; Blankschtein, D. Liquid-Phase Exfoliation of Phosphorene: Design Rules from Molecular Dynamics Simulations. ACS Nano 2015, 9, 8255–8268. [Google Scholar] [CrossRef]
- Liu, N.; Becton, M.; Zhang, L.; Chen, H.; Zeng, X.; Pidaparti, R.; Wang, X. A coarse-grained model for mechanical behavior of phosphorene sheets. Phys. Chem. Chem. Phys. 2019, 21, 1884–1894. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, Y.; Yu, H.; Yan, C.; Liu, Y.; Hong, S.; Tao, H.; Robertson, A.W.; Wang, Z.; Pádua, A.A.H. New solvent-stabilized few-layer black phosphorus for antibacterial applications. Nanoscale 2018, 10, 12543–12553. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Hou, H.; Zhang, Y.; Wang, C.; Qiu, X.; Ji, X. Layer-Tunable Phosphorene Modulated by the Cation Insertion Rate as a Sodium-Storage Anode. Adv. Mater. 2017, 29, 1702372. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Shen, X.; Yao, J.; Park, J. Graphene nanosheets for enhanced lithium storage in lithium ion batteries. Carbon 2009, 47, 2049–2053. [Google Scholar] [CrossRef]
- Sun, J.; Lee, H.-W.; Pasta, M.; Yuan, H.; Zheng, G.; Sun, Y.; Li, Y.; Cui, Y. A phosphorene–graphene hybrid material as a high-capacity anode for sodium-ion batteries. Nat. Nanotechnol. 2015, 10, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Batmunkh, M.; Bat-Erdene, M.; Shapter, J.G. Phosphorene and Phosphorene-Based Materials—Prospects for Future Applications. Adv. Mater. 2016, 28, 8586–8617. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hou, H.; Zou, G.; Shi, W.; Shuai, H.; Li, J.; Ji, X. Electrochemical exfoliation of graphene-like two-dimensional nanomaterials. Nanoscale 2019, 11, 16–33. [Google Scholar] [CrossRef]
- Wu, S.; Hui, K.S.; Hui, K.N. 2D Black Phosphorus: From Preparation to Applications for Electrochemical Energy Storage. Adv. Sci. 2018, 5, 1700491. [Google Scholar] [CrossRef]
- Evaristo, M.; Polcar, T.; Cavaleiro, A. Tribological behaviour of C-alloyed transition metal dichalcogenides (TMD) coatings in different environments. Int. J. Mech. Mater. Des. 2008, 4, 137–143. [Google Scholar] [CrossRef]
- Yang, E.; Ji, H.; Jung, Y. Two-Dimensional Transition Metal Dichalcogenide Monolayers as Promising Sodium Ion Battery Anodes. J. Phys. Chem. C 2015, 119, 26374–26380. [Google Scholar] [CrossRef]
- Kang, W.; Wang, Y.; Xu, J. Recent progress in layered metal dichalcogenide nanostructures as electrodes for high-performance sodium-ion batteries. J. Mater. Chem. A 2017, 5, 7667–7690. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically Thin MoS2: A New Direct-Gap Semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [PubMed]
- Splendiani, A.; Sun, L.; Zhang, Y.; Li, T.; Kim, J.; Chim, C.Y.; Galli, G.; Wang, F. Emerging photoluminescence in monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef]
- Joensen, P.; Frindt, R.F.; Morrison, S.R. Single-layer MoS2. Mater. Res. Bull. 1986, 21, 457–461. [Google Scholar] [CrossRef]
- Kolobov, A.V.; Tominaga, J. Two-Dimensional Transition-Metal Dichalcogenides; Springer: Cham, Switzerland, 2016; Volume 239. [Google Scholar]
- Park, J.; Kim, J.-S.; Park, J.-W.; Nam, T.-H.; Kim, K.-W.; Ahn, J.-H.; Wang, G.; Ahn, H.-J. Discharge mechanism of MoS2 for sodium ion battery: Electrochemical measurements and characterization. Electrochim. Acta 2013, 92, 427–432. [Google Scholar] [CrossRef]
- Wang, J.; Luo, C.; Gao, T.; Langrock, A.; Mignerey, A.C.; Wang, C. An Advanced MoS2/Carbon Anode for High-Performance Sodium-Ion Batteries. Small 2015, 11, 473–481. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, D.; Kang, J.; Feng, J.; Bechtel, H.A.; Wang, L.-W.; Cairns, E.J.; Guo, J. Electrochemical Reaction Mechanism of the MoS2 Electrode in a Lithium-Ion Cell Revealed by in Situ and Operando X-ray Absorption Spectroscopy. Nano Lett. 2018, 18, 1466–1475. [Google Scholar] [CrossRef]
- David, L.; Singh, G. Reduced Graphene Oxide Paper Electrode: Opposing Effect of Thermal Annealing on Li and Na Cyclability. J. Phys. Chem. C 2014, 118, 28401–28408. [Google Scholar] [CrossRef]
- Xie, X.; Makaryan, T.; Zhao, M.; Van Aken, K.L.; Gogotsi, Y.; Wang, G. MoS2 Nanosheets Vertically Aligned on Carbon Paper: A Freestanding Electrode for Highly Reversible Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1502161. [Google Scholar] [CrossRef]
- Xie, X.; Ao, Z.; Su, D.; Zhang, J.; Wang, G. MoS2/Graphene Composite Anodes with Enhanced Performance for Sodium-Ion Batteries: The Role of the Two-Dimensional Heterointerface. Adv. Funct. Mater. 2015, 25, 1393–1403. [Google Scholar] [CrossRef]
- Cho, J.S.; Ju, H.S.; Lee, J.-K.; Kang, Y.C. Carbon/two-dimensional MoTe2 core/shell-structured microspheres as an anode material for Na-ion batteries. Nanoscale 2017, 9, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, T.; Chen, W.; Chang, K.; Ma, L.; Huang, G.; Chen, D.; Lee, J.Y. CTAB-assisted synthesis of single-layer MoS2–graphene composites as anode materials of Li-ion batteries. J. Mater. Chem. A 2013, 1, 2202–2210. [Google Scholar] [CrossRef]
- Ai, K.; Ruan, C.; Shen, M.; Lu, L. MoS2 Nanosheets with Widened Interlayer Spacing for High-Efficiency Removal of Mercury in Aquatic Systems. Adv. Funct. Mater. 2016, 26, 5542–5549. [Google Scholar] [CrossRef]
- Niu, F.; Yang, J.; Wang, N.; Zhang, D.; Fan, W.; Yang, J.; Qian, Y. MoSe2-Covered N,P-Doped Carbon Nanosheets as a Long-Life and High-Rate Anode Material for Sodium-Ion Batteries. Adv. Funct. Mater. 2017, 27, 1700522. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Singh, G. Two-Dimensional Anode Materials for Non-lithium Metal-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 932–955. [Google Scholar] [CrossRef]
- Pang, J.; Mendes, R.G.; Bachmatiuk, A.; Zhao, L.; Ta, H.Q.; Gemming, T.; Liu, H.; Liu, Z.; Rummeli, M.H. Applications of 2D MXenes in energy conversion and storage systems. Chem. Soc. Rev. 2019, 48, 72–133. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Transition Metal Carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Lukatskaya, M.; Mashtalir, O.; Ren, C.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.; Naguib, M.; Simon, P.; Barsoum, M.; Gogotsi, Y. Cation Intercalation and High Volumetric Capacitance of Two-Dimensional Titanium Carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Dall’Agnese, Y.; Naguib, M.; Gogotsi, Y.; Barsoum, M.W.; Zhuang, H.L.; Kent, P.R.C. Prediction and Characterization of MXene Nanosheet Anodes for Non-Lithium-Ion Batteries. ACS Nano 2014, 8, 9606–9615. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-X. Prediction of Mobility, Enhanced Storage Capacity, and Volume Change during Sodiation on Interlayer-Expanded Functionalized Ti3C2 MXene Anode Materials for Sodium-Ion Batteries. J. Phys. Chem. C 2016, 120, 5288–5296. [Google Scholar] [CrossRef]
- Zhao, M.-Q.; Xie, X.; Ren, C.E.; Makaryan, T.; Anasori, B.; Wang, G.; Gogotsi, Y. Hollow MXene Spheres and 3D Macroporous MXene Frameworks for Na-Ion Storage. Adv. Mater. 2017, 29, 1702410. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Kretschmer, K.; Anasori, B.; Sun, B.; Wang, G.; Gogotsi, Y. Porous Ti3C2Tx MXene for Ultrahigh-Rate Sodium-Ion Storage with Long Cycle Life. ACS Appl. Nano Mater. 2018, 1, 505–511. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, M.-Q.; Anasori, B.; Maleski, K.; Ren, C.E.; Li, J.; Byles, B.W.; Pomerantseva, E.; Wang, G.; Gogotsi, Y. Porous heterostructured MXene/carbon nanotube composite paper with high volumetric capacity for sodium-based energy storage devices. Nano Energy 2016, 26, 513–523. [Google Scholar] [CrossRef]
- Kim, K.-T.; Ali, G.; Chung, K.Y.; Yoon, C.S.; Yashiro, H.; Sun, Y.-K.; Lu, J.; Amine, K.; Myung, S.-T. Anatase Titania Nanorods as an Intercalation Anode Material for Rechargeable Sodium Batteries. Nano Lett. 2014, 14, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kajiyama, S.; Iinuma, H.; Hosono, E.; Oro, S.; Moriguchi, I.; Okubo, M.; Yamada, A. Pseudocapacitance of MXene nanosheets for high-power sodium-ion hybrid capacitors. Nat. Commun. 2015, 6, 6544. [Google Scholar] [CrossRef]
- Yang, E.; Ji, H.; Kim, J.; Kim, H.; Jung, Y. Exploring the possibilities of two-dimensional transition metal carbides as anode materials for sodium batteries. Phys. Chem. Chem. Phys. 2015, 17, 5000–5005. [Google Scholar] [CrossRef]
- Mashtalir, O.; Cook, K.M.; Mochalin, V.N.; Crowe, M.; Barsoum, M.W.; Gogotsi, Y. Dye adsorption and decomposition on two-dimensional titanium carbide in aqueous media. J. Mater. Chem. A 2014, 2, 14334–14338. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Liang, Q.; Liu, X.; Weng, Q.; Liu, J.; Yang, Y.; Dai, Z.; Ding, K.; Bando, Y.; et al. Amorphous Phosphorus/Nitrogen-Doped Graphene Paper for Ultrastable Sodium-Ion Batteries. Nano Lett. 2016, 16, 2054–2060. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Y.-Z.; Zhang, F.; Liang, H.; Ming, F.; Alshareef, H.N.; Gao, Z. Partially Reduced Holey Graphene Oxide as High Performance Anode for Sodium-Ion Batteries. Adv. Energy Mater. 2019, 9, 1803215. [Google Scholar] [CrossRef]
- Wang, S.; Gong, F.; Yang, S.; Liao, J.; Wu, M.; Xu, Z.; Chen, C.; Yang, X.; Zhao, F.; Wang, B.; et al. Graphene Oxide-Template Controlled Cuboid-Shaped High-Capacity VS4 Nanoparticles as Anode for Sodium-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1801806. [Google Scholar] [CrossRef]
- Liu, S.; Xu, H.; Bian, X.; Feng, J.; Liu, J.; Yang, Y.; Yuan, C.; An, Y.; Fan, R.; Ci, L. Nanoporous Red Phosphorus on Reduced Graphene Oxide as Superior Anode for Sodium-Ion Batteries. ACS Nano 2018, 12, 7380–7387. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Wang, Y.; Lv, H.; Chen, S.; van Aken, P.A.; Wu, X.; Maier, J.; Yu, Y. Ultrathin Ti2Nb2O9 Nanosheets with Pseudocapacitive Properties as Superior Anode for Sodium-Ion Batteries. Adv. Mater. 2018, 30, 1804378. [Google Scholar] [CrossRef]
- Li, G.; Luo, D.; Wang, X.; Seo, M.H.; Hemmati, S.; Yu, A.; Chen, Z. Enhanced Reversible Sodium-Ion Intercalation by Synergistic Coupling of Few-Layered MoS2 and S-Doped Graphene. Adv. Funct. Mater. 2017, 27, 1702562. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, Q.; Zhang, N.; Lei, K.; Li, F.; Chen, J. Facile Spraying Synthesis and High-Performance Sodium Storage of Mesoporous MoS2/C Microspheres. Adv. Funct. Mater. 2016, 26, 911–918. [Google Scholar] [CrossRef]
- Wang, L.P.; Yu, L.; Wang, X.; Srinivasan, M.; Xu, Z.J. Recent developments in electrode materials for sodium ion batteries. J. Mater. Chem. A 2015, 3, 9353–9378. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium Batteries and Cathode Materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef]
- Delmas, C.; Olazcuaga, R.; Le Flem, G.; Hagenmuller, P.; Cherkaoui, F.; Brochu, R. Crystal chemistry of the Na1+xZr2−xLx(PO4)3 (L = Cr, In, Yb) solid solutions. Mater. Res. Bull. 1981, 16, 285–290. [Google Scholar] [CrossRef]
- Shibata, T.; Kobayashi, W.; Moritomo, Y. Sodium Ion Diffusion in Layered NaxCoO2. Appl. Phys. Express 2013, 6, 097101. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Yoshida, H.; Komaba, S. Crystal Structures and Electrode Performance of Alpha-NaFeO2 for Rechargeable Sodium Batteries. Electrochemistry 2012, 80, 716–719. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, H.; Yabuuchi, N.; Komaba, S. NaFe0.5Co0.5O2 as high energy and power positive electrode for Na-ion batteries. Electrochem. Commun. 2013, 34, 60–63. [Google Scholar] [CrossRef] [Green Version]
- Yabuuchi, N.; Kajiyama, M.; Iwatate, J.; Nishikawa, H.; Hitomi, S.; Okuyama, R.; Usui, R.; Yamada, Y.; Komaba, S. P2-type Nax[Fe1/2Mn1/2]O2 made from earth-abundant elements for rechargeable Na batteries. Nat. Mater. 2012, 11, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Zhang, K.; Chen, J. Recent Advances and Prospects of Cathode Materials for Sodium-Ion Batteries. Adv. Mater. 2015, 27, 5343–5364. [Google Scholar] [CrossRef]
- Li, X.; Wu, D.; Zhou, Y.-N.; Liu, L.; Yang, X.-Q.; Ceder, G. O3-type Na(Mn0.25Fe0.25Co0.25Ni0.25)O2: A quaternary layered cathode compound for rechargeable Na ion batteries. Electrochemistry Communications 2014, 49, 51–54. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Yang, L.; Zhou, C.; Lu, B.; Liu, J.; Ouyang, L.; Hu, R.; Liu, J.; Zhu, M. Co-Substitution Enhances the Rate Capability and Stabilizes the Cyclic Performance of O3-Type Cathode NaNi0.45–xMn0.25Ti0.3CoxO2 for Sodium-Ion Storage at High Voltage. ACS Appl. Mater. Interfaces 2019, 11, 7906–7913. [Google Scholar] [CrossRef]
- Hasa, I.; Buchholz, D.; Passerini, S.; Scrosati, B.; Hassoun, J. High Performance Na0.5[Ni0.23Fe0.13Mn0.63]O2 Cathode for Sodium-Ion Batteries. Adv. Energy Mater. 2014, 4, 1400083. [Google Scholar] [CrossRef]
- You, Y.; Kim, S.O.; Manthiram, A. A Honeycomb-Layered Oxide Cathode for Sodium-Ion Batteries with Suppressed P3–O1 Phase Transition. Adv. Energy Mater. 2017, 7, 1601698. [Google Scholar] [CrossRef]
- Kim, D.; Lee, E.; Slater, M.; Lu, W.; Rood, S.; Johnson, C.S. Layered Na[Ni1/3Fe1/3Mn1/3]O2 cathodes for Na-ion battery application. Electrochem. Commun. 2012, 18, 66–69. [Google Scholar] [CrossRef]
- Sathiya, M.; Hemalatha, K.; Ramesha, K.; Tarascon, J.M.; Prakash, A.S. Synthesis, Structure, and Electrochemical Properties of the Layered Sodium Insertion Cathode Material: NaNi1/3Mn1/3Co1/3O2. Chem. Mater. 2012, 24, 1846–1853. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, X.; Xu, S.; Bai, J.; Xiao, R.; Hu, Y.-S.; Li, H.; Yang, X.-Q.; Chen, L.; Huang, X. A zero-strain layered metal oxide as the negative electrode for long-life sodium-ion batteries. Nat. Commun. 2013, 4, 2365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, S.; Schuppert, N.; Bates, A.; Lee, S.C.; Park, S. Novel mesoporous microspheres of Al and Ni doped LMO spinels and their performance as cathodes in secondary lithium ion batteries. Int. J. Green Energy 2017, 14, 656–664. [Google Scholar] [CrossRef]
- Kim, D.; Kang, S.-H.; Slater, M.; Rood, S.; Vaughey, J.T.; Karan, N.; Balasubramanian, M.; Johnson, C.S. Enabling Sodium Batteries Using Lithium-Substituted Sodium Layered Transition Metal Oxide Cathodes. Adv. Energy Mater. 2011, 1, 333–336. [Google Scholar] [CrossRef]
- Billaud, J.; Singh, G.; Armstrong, A.R.; Gonzalo, E.; Roddatis, V.; Armand, M.; Rojo, T.; Bruce, P.G. Na0.67Mn1−xMgxO2 (0 ≤ x ≤ 0.2): A high capacity cathode for sodium-ion batteries. Energy Environ. Sci. 2014, 7, 1387–1391. [Google Scholar] [CrossRef]
- Hamani, D.; Ati, M.; Tarascon, J.M.; Rozier, P. NaxVO2 as possible electrode for Na-ion batteries. Electrochem. Commun. 2011, 13, 938–941. [Google Scholar] [CrossRef]
- Jian, Z.; Hu, Y.S.; Ji, X.; Chen, W. NASICON-Structured Materials for Energy Storage. Adv. Mater. 2017, 29, 1601925. [Google Scholar] [CrossRef]
- Tang, W.; Song, X.; Du, Y.; Peng, C.; Lin, M.; Xi, S.; Tian, B.; Zheng, J.; Wu, Y.; Pan, F.; et al. High-performance NaFePO4 formed by aqueous ion-exchange and its mechanism for advanced sodium ion batteries. J. Mater. Chem. A 2016, 4, 4882–4892. [Google Scholar] [CrossRef]
- Galceran, M.; Saurel, D.; Acebedo, B.; Roddatis, V.V.; Martin, E.; Rojo, T.; Casas-Cabanas, M. The mechanism of NaFePO4 (de)sodiation determined by in situ X-ray diffraction. Phys. Chem. Chem. Phys. 2014, 16, 8837–8842. [Google Scholar] [CrossRef]
- Boucher, F.; Gaubicher, J.; Cuisinier, M.; Guyomard, D.; Moreau, P. Elucidation of the Na2/3FePO4 and Li2/3FePO4 Intermediate Superstructure Revealing a Pseudouniform Ordering in 2D. J. Am. Chem. Soc. 2014, 136, 9144–9157. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Seo, D.-H.; Kim, H.; Park, I.; Yoo, J.-K.; Jung, S.-K.; Park, Y.-U.; Goddard Iii, W.A.; Kang, K. Unexpected discovery of low-cost maricite NaFePO4 as a high-performance electrode for Na-ion batteries. Energy Environ. Sci. 2015, 8, 540–545. [Google Scholar] [CrossRef]
- Oh, S.-M.; Myung, S.-T.; Hassoun, J.; Scrosati, B.; Sun, Y.-K. Reversible NaFePO4 electrode for sodium secondary batteries. Electrochem. Commun. 2012, 22, 149–152. [Google Scholar] [CrossRef]
- Li, C.; Miao, X.; Chu, W.; Wu, P.; Tong, D.G. Hollow amorphous NaFePO4 nanospheres as a high-capacity and high-rate cathode for sodiumion batteries. J. Mater. Chem. A 2015, 3, 8265–8271. [Google Scholar] [CrossRef]
- Ali, G.; Lee, J.-H.; Susanto, D.; Choi, S.-W.; Cho, B.W.; Nam, K.-W.; Chung, K.Y. Polythiophene-Wrapped Olivine NaFePO4 as a Cathode for Na-Ion Batteries. Appl. Mater. Interfaces 2016, 8, 15422–15429. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Liu, Q.; Xiao, L.; Ai, X.; Yang, H.; Cao, Y. High-Performance Olivine NaFePO4 Microsphere Cathode Synthesized by Aqueous Electrochemical Displacement Method for Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 17977–17984. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Hong, H.Y.P.; Kafalas, J.A. Fast Na+-ion transport in skeleton structures. Mater. Res. Bull. 1976, 11, 203–220. [Google Scholar] [CrossRef]
- Alpen, U.V.; Bell, M.F.; Höfer, H.H. Compositional dependence of the electrochemical and structural parameters in the Nasicon system (Na1+xSixZr2P3−xO12). Solid State Ion. 1981, 3–4, 215–218. [Google Scholar]
- Clearfield, A.; Jerus, P.; Cotman, R.N. Hydrothermal and solid state synthesis of sodium zirconium silicophosphates. Solid State Ion. 1981, 5, 301–304. [Google Scholar] [CrossRef]
- Tamura, S.; Imanaka, N.; Adachi, G. Trivalent ion conduction in NASICON type solid electrolyte prepared by ball milling. Solid State Ion. 2002, 154–155, 767–771. [Google Scholar] [CrossRef]
- Engell, J.; Mortensen, S.; Møller, L. Fabrication of Nasicon electrolytes from metal alkoxide derived gels. Solid State Ion. 1983, 9–10, 877–884. [Google Scholar] [CrossRef]
- Delmas, C.; Cherkaoui, F.; Nadiri, A.; Hagenmuller, P. A nasicon-type phase as intercalation electrode: NaTi2(PO4)3. Mater. Res. Bull. 1987, 22, 631–639. [Google Scholar] [CrossRef]
- Kang, J.; Baek, S.; Mathew, V.; Gim, J.; Song, J.; Park, H.; Chae, E.; Rai, A.K.; Kim, J. High rate performance of a Na3V2(PO4)3/C cathode prepared by pyro-synthesis for sodium-ion batteries. J. Mater. Chem. 2012, 22, 20857–20860. [Google Scholar] [CrossRef]
- Liu, J.; Tang, K.; Song, K.; Aken, P.A.V.; Yu, Y.; Maie, J. Electrospun Na3V2(PO4)3/C nanofibers as stable cathode materials for sodium-ion batteries. Nanoscale 2014, 6, 5081–5086. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Xiao, L.; Qian, J.; Cao, Y.; Ai, X.; Huang, Y.; Yang, H. 3D Graphene Decorated NaTi2(PO4)3 Microspheres as a Superior High-Rate and Ultracycle-Stable Anode Material for Sodium Ion Batteries. Adv. Energy Mater. 2016, 6, 1502197. [Google Scholar] [CrossRef]
- Gao, H.; Seymour, I.D.; Xin, S.; Xue, L.; Henkelman, G.; Goodenough, J.B. Na3MnZr(PO4)3: A High-Voltage Cathode for Sodium Batteries. J. Am. Chem. Soc. 2018, 140, 18192–18199. [Google Scholar] [CrossRef]
- Liu, Q.; Meng, X.; Wei, Z.; Wang, D.; Gao, Y.; Wei, Y.; Du, F.; Chen, G. Core/Double-Shell Structured Na3V2(PO4)2F3@C Nanocomposite as the High Power and Long Lifespan Cathode for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 31709–31715. [Google Scholar] [CrossRef]
- Lim, S.Y.; Kim, H.; Shakoor, R.A.; Jung, Y.; Choi, J.W. Electrochemical and Thermal Properties of NASICON Structured Na3V2(PO4)3 as a Sodium Rechargeable Battery Cathode: A Combined Experimental and Theoretical Study. J. Electrochem. Soc. 2012, 159, A1393–A1397. [Google Scholar] [CrossRef]
- Chen, S.; Wu, C.; Shen, L.; Zhu, C.; Huang, Y.; Xi, K.; Maier, J.; Yu, Y. Challenges and Perspectives for NASICON-Type Electrode Materials for Advanced Sodium-Ion Batteries. Adv. Mater. 2017, 29, 1700431. [Google Scholar] [CrossRef]
- Anantharamulu, N.; Rao, K.K.; Rambabu, G.; Kumar, B.V.; Radha, V.; Vithal, M. A wide-ranging review on Nasicon type materials. J. Mater. Sci. 2011, 46, 2821–2837. [Google Scholar] [CrossRef]
- Kim, H.; Lim, H.; Kim, H.-S.; Kim, K.J.; Byun, D.; Choi, W. Polydopamine-derived N-doped carbon-wrapped Na3V2(PO4)3 cathode with superior rate capability and cycling stability for sodium-ion batteries. Nano Res. 2019, 12, 397–404. [Google Scholar] [CrossRef]
- Aragón, M.J.; Lavela, P.; Ortiz, G.F.; Alcántara, R.; Tirado, J.L. Insight into the Electrochemical Sodium Insertion of Vanadium Superstoichiometric NASICON Phosphate. Inorg. Chem. 2017, 56, 11845–11853. [Google Scholar] [CrossRef] [PubMed]
- Pang, G.; Yuan, C.; Nie, P.; Ding, B.; Zhu, J.; Zhang, X. Synthesis of NASICON-type structured NaTi2(PO4)3–graphene nanocomposite as an anode for aqueous rechargeable Na-ion batteries. Nanoscale 2014, 6, 6328–6334. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Q.; Chen, C.; Li, M.; Meng, X.; Bie, X.; Wei, Y.; Huang, Y.; Du, F.; Wang, C.; et al. NASICON-Structured NaTi2(PO4)3@C Nanocomposite as the Low Operation-Voltage Anode Material for High-Performance Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 2238–2246. [Google Scholar] [CrossRef] [PubMed]
- Di Vona, M.L.; Licoccia, S.; Montanaro, L.; Traversa, E. Sol−Gel Synthesis of NASICON: 1D and 2D NMR Investigation. Chem. Mater. 1999, 11, 1336–1341. [Google Scholar] [CrossRef]
- Paolella, A.; Faure, C.; Timoshevskii, V.; Marras, S.; Bertoni, G.; Guerfi, A.; Vijh, A.; Armand, M.; Zaghib, K. A review on hexacyanoferrate-based materials for energy storage and smart windows: Challenges and perspectives. J. Mater. Chem. A 2017, 5, 18919–18932. [Google Scholar] [CrossRef]
- Karyakin, A.A. Prussian Blue and Its Analogues: Electrochemistry and Analytical Applications. Electroanalysis 2001, 13, 813–819. [Google Scholar] [CrossRef]
- Ma, F.; Li, Q.; Wang, T.; Zhang, H.; Wu, G. Energy storage materials derived from Prussian blue analogues. Sci. Bull. 2017, 62, 358–368. [Google Scholar] [CrossRef] [Green Version]
- Pasta, M.; Wessells, C.D.; Huggins, R.A.; Cui, Y. A high-rate and long cycle life aqueous electrolyte battery for grid-scale energy storage. Nat. Commun. 2012, 3, 1149. [Google Scholar] [CrossRef] [Green Version]
- You, Y.; Wu, X.-L.; Yin, Y.-X.; Guo, Y.-G. High-quality Prussian blue crystals as superior cathode materials for room-temperature sodium-ion batteries. Energy Environ. Sci. 2014, 7, 1643–1647. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Cheng, J.; Goodenough, J.B. Prussian blue: A new framework of electrode materials for sodium batteries. Chem. Commun. 2012, 48, 6544–6546. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-J.; Chou, S.-L.; Wang, J.-Z.; Kang, Y.-M.; Wang, J.-L.; Liu, Y.; Gu, Q.-F.; Liu, H.-K.; Dou, S.-X. Facile Method To Synthesize Na-Enriched Na1+xFeFe(CN)6 Frameworks as Cathode with Superior Electrochemical Performance for Sodium-Ion Batteries. Chem. Mater. 2015, 27, 1997–2003. [Google Scholar] [CrossRef]
- Yue, Y.; Binder, A.J.; Guo, B.; Zhang, Z.; Qiao, Z.-A.; Tian, C.; Dai, S. Mesoporous Prussian Blue Analogues: Template-Free Synthesis and Sodium-Ion Battery Applications. Angew. Chem. 2014, 53, 3134–3137. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, C.; Wei, C.; Hu, L.; Qian, J.; Cao, Y.; Ai, X.; Wang, J.; Yang, H. Highly Crystallized Na2CoFe(CN)6 with Suppressed Lattice Defects as Superior Cathode Material for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 5393–5399. [Google Scholar] [CrossRef]
- Yang, D.; Xu, J.; Liao, X.-Z.; He, Y.-S.; Liu, H.; Ma, Z.-F. Structure optimization of Prussian blue analogue cathode materials for advanced sodium ion batteries. Chem. Commun. 2014, 50, 13377–13380. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Liu, C.; Zhang, C.; Ma, W.; Wang, K.; Li, Z.; Lu, X.; Cao, G. Enhanced storage of sodium ions in Prussian blue cathode material through nickel doping. J. Mater. Chem. A 2017, 5, 9604–9610. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bates, A.; Schuppert, N.; Son, B.; Kim, J.G.; Choi, J.S.; Choi, M.J.; Lee, D.-H.; Kwon, O.; Jasinski, J.; et al. A study of a novel Na ion battery and its anodic degradation using sodium rich prussian blue cathode coupled with different titanium based oxide anodes. J. Power Sources 2015, 286, 276–289. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, S.; Wang, B.; Li, Y.; Sun, W.; Lu, Y.; Yan, M.; Song, B.; Dou, S. Prussian Blue@C Composite as an Ultrahigh-Rate and Long-Life Sodium-Ion Battery Cathode. Adv. Funct. Mater. 2016, 26, 5315–5321. [Google Scholar] [CrossRef]
- Itaya, K.; Shoji, N.; Uchida, I. Catalysis of the reduction of molecular oxygen to water at Prussian blue modified electrodes. J. Am. Chem. Soc. 1984, 106, 3423–3429. [Google Scholar] [CrossRef]
- Kaye, S.S.; Long, J.R. Hydrogen Storage in the Dehydrated Prussian Blue Analogues M3[Co(CN)6]2 (M = Mn, Fe, Co, Ni, Cu, Zn). J. Am. Chem. Soc. 2005, 127, 6506–6507. [Google Scholar] [CrossRef]
- Mullaliu, A.; Conti, P.; Aquilanti, G.; Plaisier, J.R.; Stievano, L.; Giorgetti, M. Operando XAFS and XRD Study of a Prussian Blue Analogue Cathode Material: Iron Hexacyanocobaltate. Condens. Matter 2018, 3, 36. [Google Scholar] [CrossRef]
- Mullaliu, A.; Aquilanti, G.; Conti, P.; Plaisier, J.R.; Fehse, M.; Stievano, L.; Giorgetti, M. Copper Electroactivity in Prussian Blue-Based Cathode Disclosed by Operando XAS. J. Phys. Chem. C 2018, 122, 15868–15877. [Google Scholar] [CrossRef]
- Wessells, C.D.; Huggins, R.A.; Cui, Y. Copper hexacyanoferrate battery electrodes with long cycle life and high power. Nat. Commun. 2011, 2, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Sun, M.; Guo, S.; Qian, J.; Liu, Y.; Cao, Y.; Ai, X.; Yang, H. Vacancy-Free Prussian Blue Nanocrystals with High Capacity and Superior Cyclability for Aqueous Sodium-Ion Batteries. ChemNanoMat 2015, 1, 188–193. [Google Scholar] [CrossRef]
- Wang, L.; Song, J.; Qiao, R.; Wray, L.A.; Hossain, M.A.; Chuang, Y.-D.; Yang, W.; Lu, Y.; Evans, D.; Lee, J.-J.; et al. Rhombohedral Prussian White as Cathode for Rechargeable Sodium-Ion Batteries. J. Am. Chem. Soc. 2015, 137, 2548–2554. [Google Scholar] [CrossRef] [PubMed]
- Trócoli, R.; La Mantia, F. An Aqueous Zinc-Ion Battery Based on Copper Hexacyanoferrate. ChemSusChem 2015, 8, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-H.; Myoung, N.; Hong, H.-G. Facile and controllable synthesis of Prussian blue on chitosan-functionalized graphene nanosheets for the electrochemical detection of hydrogen peroxide. Electrochim. Acta 2012, 81, 37–43. [Google Scholar] [CrossRef]
- Wu, X.; Jin, S.; Zhang, Z.; Jiang, L.; Mu, L.; Hu, Y.-S.; Li, H.; Chen, X.; Armand, M.; Chen, L.; et al. Unraveling the storage mechanism in organic carbonyl electrodes for sodium-ion batteries. Sci. Adv. 2015, 1, e1500330. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, P.; Yang, J.; Gong, G.; Guo, L.; Chen, X. Renewable-Juglone-Based High-Performance Sodium-Ion Batteries. Adv. Mater. 2015, 27, 2348–2354. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2014, 7, 19–29. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, R.; Nan, J.; Shu, D.; Qiu, Y.; Chou, S.-L. Quinone Electrode Materials for Rechargeable Lithium/Sodium Ion Batteries. Adv. Energy Mater. 2017, 7, 1700278. [Google Scholar] [CrossRef]
- Song, Z.; Zhan, H.; Zhou, Y. Polyimides: Promising Energy-Storage Materials. Angew. Chem. Int. Ed. 2010, 49, 8444–8448. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhu, L.; Cao, Y.; Ai, X.; Yang, H.X. An aniline-nitroaniline copolymer as a high capacity cathode for Na-ion batteries. Electrochem. Commun. 2012, 21, 36–38. [Google Scholar] [CrossRef]
- Deng, W.; Liang, X.; Wu, X.; Qian, J.; Cao, Y.; Ai, X.; Feng, J.; Yang, H. A low cost, all-organic Na-ion Battery Based on Polymeric Cathode and Anode. Sci. Rep. 2013, 3, 2671. [Google Scholar] [CrossRef]
- Yao, M.; Kuratani, K.; Kojima, T.; Takeichi, N.; Senoh, H.; Kiyobayashi, T. Indigo carmine: An organic crystal as a positive-electrode material for rechargeable sodium batteries. Sci. Rep. 2014, 4, 3650. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-G.; Yuan, S.; Ma, D.-L.; Huang, X.-L.; Meng, F.-L.; Zhang, X.-B. Tailored Aromatic Carbonyl Derivative Polyimides for HighPower and Long-Cycle Sodium-Organic Batteries. Adv. Energy Mater. 2014, 4, 1601651. [Google Scholar] [CrossRef]
- Luo, W.; Allen, M.; Raju, V.; Ji, X. An Organic Pigment as a High-Performance Cathode for Sodium-Ion Batteries. Adv. Energy Mater. 2014, 4, 1400544. [Google Scholar] [CrossRef]
- Manuel, J.; Zhao, X.; Cho, K.-K.; Kim, J.-K.; Ahn, J.-H. Ultralong Life Organic Sodium Ion Batteries Using a Polyimide/Multiwalled Carbon Nanotubes Nanocomposite and Gel Polymer Electrolyte. ACS Sustain. Chem. Eng. 2018, 6, 8159–8166. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Q. Recent progress in rechargeable lithium batteries with organic materials as promising electrodes. J. Mater. Chem. A 2016, 4, 7091–7106. [Google Scholar] [CrossRef]
- Xie, J.; Gu, P.; Zhang, Q. Nanostructured Conjugated Polymers: Toward High-Performance Organic Electrodes for Rechargeable Batteries. ACS Energy Lett. 2017, 2, 1985–1996. [Google Scholar] [CrossRef]
- Liang, Y.; Jing, Y.; Gheytani, S.; Lee, K.-Y.; Liu, P.; Facchetti, A.; Yao, Y. Universal quinone electrodes for long cycle life aqueous rechargeable batteries. Nat. Mater. 2017, 16, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Akutagawa, N.; Satoh, M.; Wada, J.; Masuda, T. Charge/Discharge Properties of Organometallic Batteries Fabricated with Ferrocene–Containing Polymers. Macromol. Rapid Commun. 2008, 29, 1944–1949. [Google Scholar] [CrossRef]
- Nakahara, K.; Iwasa, S.; Satoh, M.; Morioka, Y.; Iriyama, J.; Suguro, M.; Hasegawa, E. Rechargeable batteries with organic radical cathodes. Chem. Phys. Lett. 2002, 359, 351–354. [Google Scholar] [CrossRef]
- Renault, S.; Brandell, D.; Gustafsson, T.; Edström, K. Improving the electrochemical performance of organic Li-ion battery electrodes. Chem. Commun. 2013, 49, 1945–1947. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, Z.; Chen, M.; Gu, Q.; Dou, Y.; Sun, Z.; Chou, S.; Dou, S.X. Multiangular Rod-Shaped Na0.44MnO2 as Cathode Materials with High Rate and Long Life for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 3644–3652. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; He, W.; Pei, F.; Wu, F.; Wu, Y.; Qian, J.; Cao, Y.; Ai, X.; Yang, H. Synthesis and electrochemical behaviors of layered Na0.67[Mn0.65Co0.2Ni0.15]O2 microflakes as a stable cathode material for sodium-ion batteries. J. Mater. Chem. A 2013, 1, 3895–3899. [Google Scholar] [CrossRef]
- Guo, S.; Li, Q.; Liu, P.; Chen, M.; Zhou, H. Environmentally stable interface of layered oxide cathodes for sodium-ion batteries. Nat. Commun. 2017, 8, 135. [Google Scholar] [CrossRef]
- Han, D.-W.; Ku, J.-H.; Kim, R.-H.; Yun, D.-J.; Lee, S.-S.; Doo, S.-G. Aluminum Manganese Oxides with Mixed Crystal Structure: High-Energy-Density Cathodes for Rechargeable Sodium Batteries. ChemSusChem 2014, 7, 1870–1875. [Google Scholar] [CrossRef]
- Oh, S.-M.; Myung, S.-T.; Hwang, J.-Y.; Scrosati, B.; Amine, K.; Sun, Y.-K. High Capacity O3-Type Na[Li0.05(Ni0.25Fe0.25Mn0.5)0.95]O2 Cathode for Sodium Ion Batteries. Chem. Mater. 2014, 26, 6165–6171. [Google Scholar] [CrossRef]
- Fang, Y.; Xiao, L.; Qian, J.; Ai, X.; Yang, H.; Cao, Y. Mesoporous Amorphous FePO4 Nanospheres as High-Performance Cathode Material for Sodium-Ion Batteries. Nano Lett. 2014, 14, 3539–3543. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.; Han, X.; Pellegrinelli, C.; Zhu, Y.; Zhu, H.; Wan, J.; Chung, A.C.; Vaaland, O.; Wang, C.; et al. Porous Amorphous FePO4 Nanoparticles Connected by Single-Wall Carbon Nanotubes for Sodium Ion Battery Cathodes. Nano Lett. 2012, 12, 5664–5668. [Google Scholar] [CrossRef] [PubMed]
- Wongittharom, N.; Lee, T.-C.; Wang, C.-H.; Wang, Y.-C.; Chang, J.-K. Electrochemical performance of Na/NaFePO4 sodium-ion batteries with ionic liquid electrolytes. J. Mater. Chem. A 2014, 2, 5655–5661. [Google Scholar] [CrossRef]
- Barpanda, P.; Liu, G.; Ling, C.D.; Tamaru, M.; Avdeev, M.; Chung, S.-C.; Yamada, Y.; Yamada, A. Na2FeP2O7: A Safe Cathode for Rechargeable Sodium-ion Batteries. Chem. Mater. 2013, 25, 3480–3487. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, W.; Wang, Y.; Feng, P.; Wang, K.; Cheng, S.; Jiang, K. Controllable construction of 3D-skeleton-carbon coated Na3V2(PO4)3 for high-performance sodium ion battery cathode. Nano Energy 2016, 20, 11–19. [Google Scholar] [CrossRef]
- Jung, Y.H.; Lim, C.H.; Kim, D.K. Graphene-supported Na3V2(PO4)3 as a high rate cathode material for sodium-ion batteries. J. Mater. Chem. A 2013, 1, 11350–11354. [Google Scholar] [CrossRef]
- Sun, Q.; Ren, Q.-Q.; Fu, Z.-W. NASICON-type Fe2(MoO4)3 thin film as cathode for rechargeable sodium ion battery. Electrochem. Commun. 2012, 23, 145–148. [Google Scholar] [CrossRef]
- Peng, M.; Li, B.; Yan, H.; Zhang, D.; Wang, X.; Xia, D.; Guo, G. Ruthenium-Oxide-Coated Sodium Vanadium Fluorophosphate Nanowires as High-Power Cathode Materials for Sodium-Ion Batteries. Angew. Chem. 2015, 54, 6452–6456. [Google Scholar] [CrossRef]
- Yu, S.-H.; Shokouhimehr, M.; Hyeon, T.; Sung, Y.-E. Iron Hexacyanoferrate Nanoparticles as Cathode Materials for Lithium and Sodium Rechargeable Batteries. ECS Electrochem. Lett. 2013, 2, A39–A41. [Google Scholar] [CrossRef]
- Xie, M.; Xu, M.; Huang, Y.; Chen, R.; Zhang, X.; Lia, L.; Wu, F. Na2NixCo1−xFe(CN)6: A class of Prussian blue analogs with transition metal elements as cathode materials for sodium ion batteries. Electrochem. Commun. 2015, 59, 91–94. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Chen, S.; Li, G.; Quan, J.; Xu, E.; Song, L.; Jiang, Y. Crystallographic-plane tuned Prussian-blue wrapped with RGO: A high-capacity, long-life cathode for sodium-ion batteries. J. Mater. Chem. A 2017, 5, 3569–3577. [Google Scholar] [CrossRef]
- Banda, H.; Damien, D.; Nagarajan, K.; Hariharan, M.; Shaijumon, M.M. A polyimide based all-organic sodium ion battery. J. Mater. Chem. A 2015, 3, 10453–10458. [Google Scholar] [CrossRef]
- Xu, F.; Xia, J.; Shi, W. Anthraquinone-based polyimide cathodes for sodium secondary batteries. Electrochem. Commun. 2015, 60, 117–120. [Google Scholar] [CrossRef]
- Song, Z.; Qian, Y.; Zhang, T.; Otani, M.; Zhou, H. Poly(benzoquinonyl sulfide) as a High-Energy Organic Cathode for Rechargeable Li and Na Batteries. Adv. Sci. 2015, 2, 1500124. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Hong, J.; Lopez, J.; Sun, Y.; Feng, D.; Lim, K.; Chueh, W.C.; Toney, M.F.; Cui, Y.; Bao, Z. High-performance sodium–organic battery by realizing four-sodium storage in disodium rhodizonate. Nat. Energy 2017, 2, 861–868. [Google Scholar] [CrossRef]
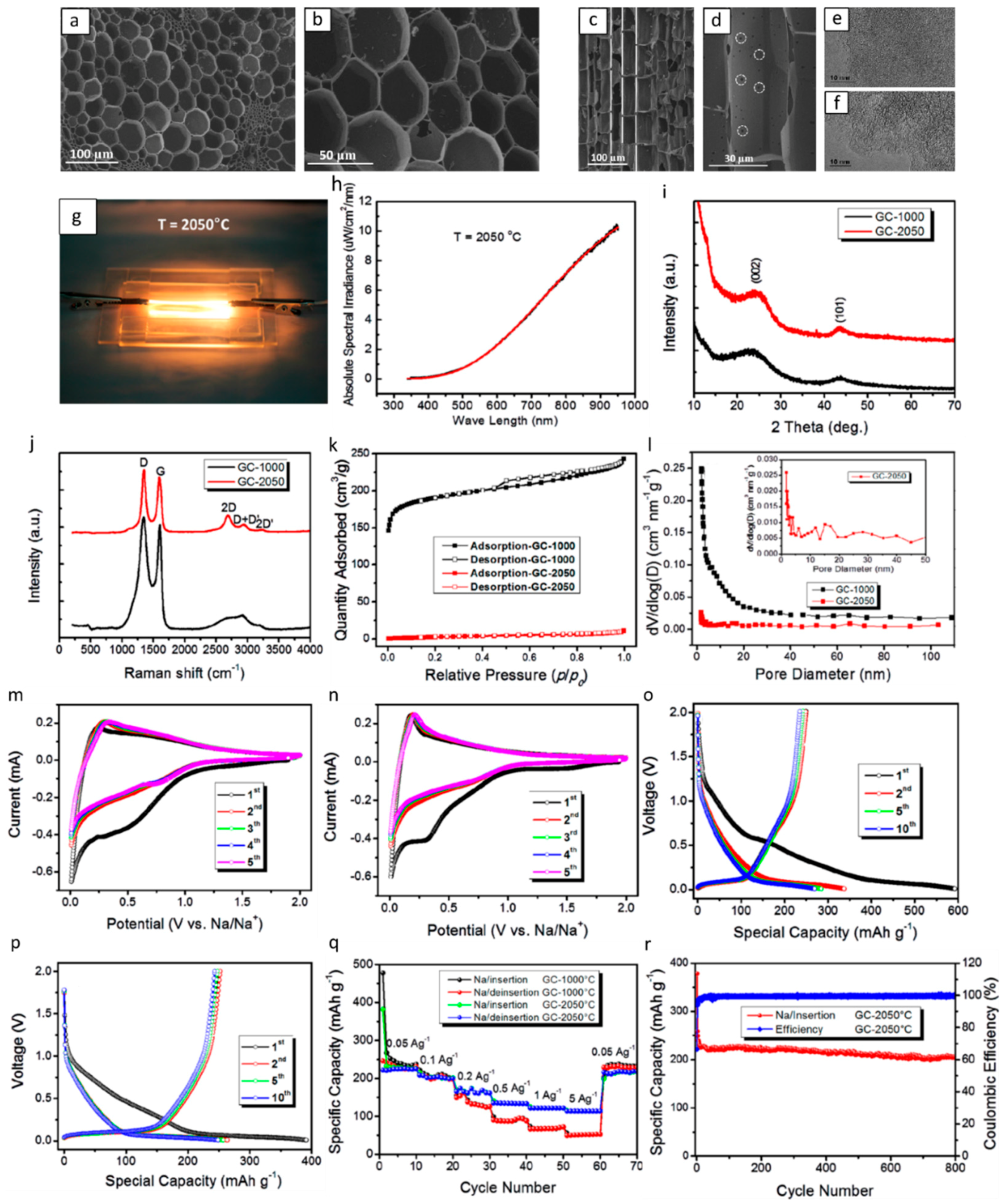





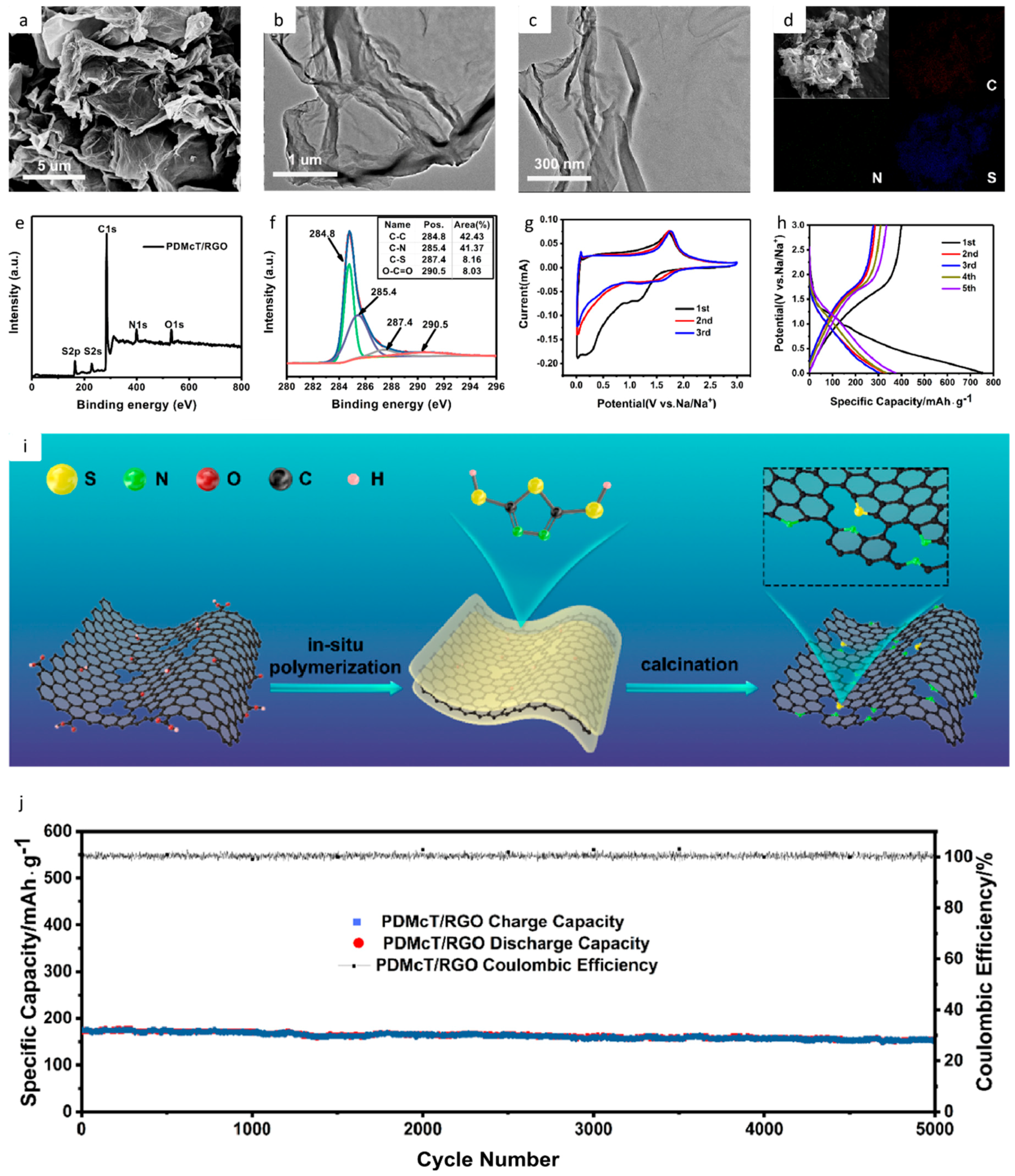





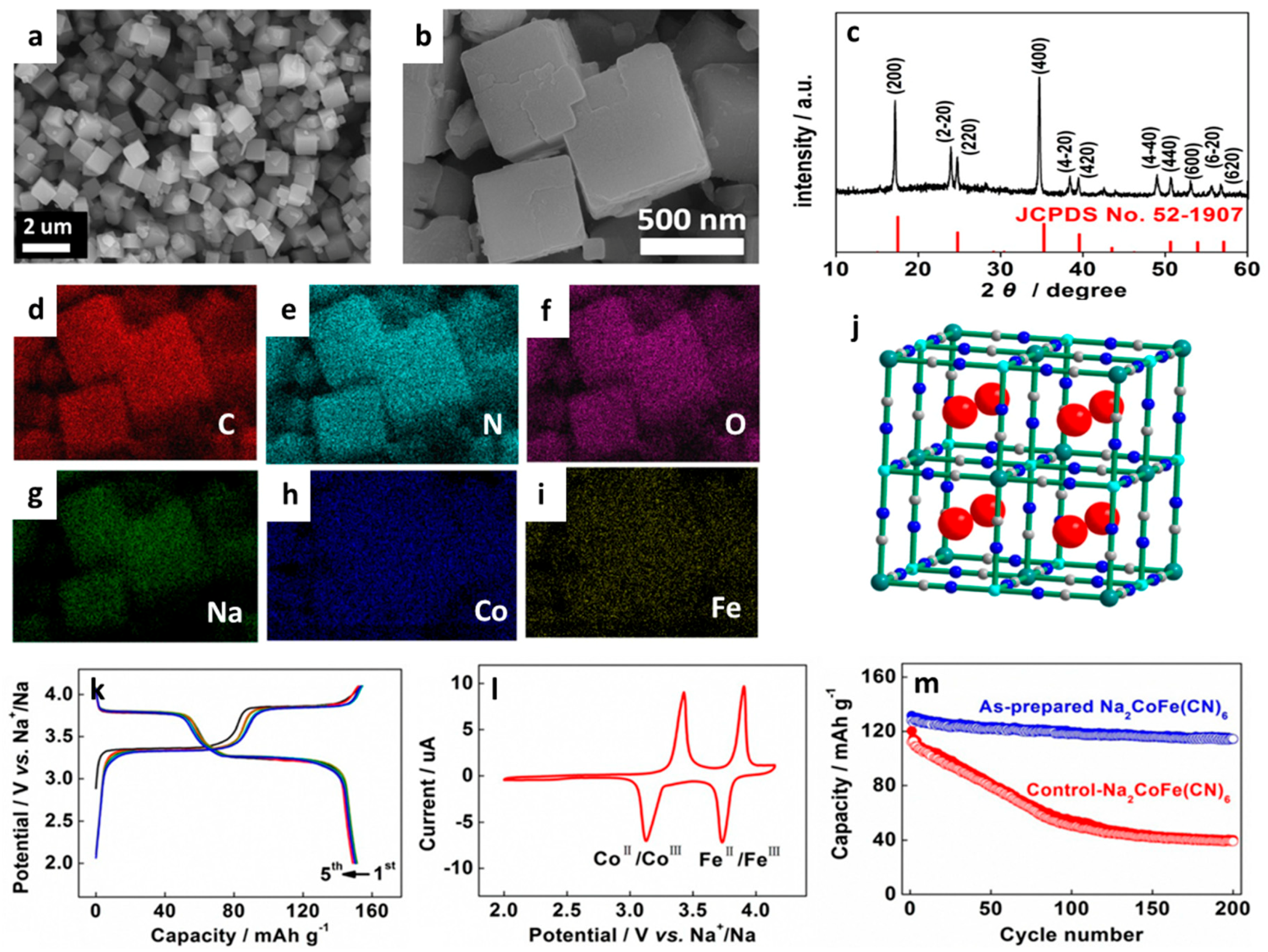

| Type of Carbon Anode | Electrolyte Chemistry | Voltage Range (V) | Performance * | Reference |
|---|---|---|---|---|
| Natural Graphite | 1 M NaPF6 in DEGDME (-) | 0.01–3.00 | 150/2500/100 | [53] |
| Carbon nanosheets derived from peat moss | 1 M NaClO4 in EC/DEC (1:1) | 0.01–3.00 | 255/210/100 | [54] |
| Soft Carbon | 1 M NaPF6 in EC/DEC (1:1) | 0.01–2.00 | 114/300/1000 | [33] |
| Nitrogen doped porous carbon | 1 M NaClO4 in PC (-) | 0.01–3.00 | 243/100/50 | [55] |
| Hollow carbon nanospheres | 1 M NaClO4 in PC (-) | 0.01–3.00 | 160/100/100 | [56] |
| Hard carbon micro-spherules | 1 M NaClO4 in EC/DEC (1:1) | 0.01–3.00 | 290/100/30 | [57] |
| Wood fiber derived hard carbon | 1 M NaClO4 in EC/DEC (1:1) | 0.01–2.5 | 196/200/100 | [58] |
| Hard-soft composite carbon | 1 M NaClO4 in EC/DEC (1:1) | 0.01–2.5 | 191/100/150 | [59] |
| Hard carbon microtubes (HCTs) | 0.8 M NaPF6 in EC/DMC (1:1) | 0.01–2.5 | 305/100/30 | [60] |
| Microstructure-controlled amorphous carbon | 1 M NaPF6 in EC/DMC (1:1) | 0.01–3.00 | 190/200/300 | [61] |
| Type of Alloy-Based Anode | Electrolyte Chemistry | Voltage Range (V) | Performance * | Reference |
|---|---|---|---|---|
| Sn coated viral nanoforests | 1 M NaClO4 in EC/DEC (1:1) | 0.01–1.5 | 405/150/50 | [65] |
| Sn nanofibers | 1 M NaClO4 in PC with 2% FEC | 0.001–0.65 | 776.26/100/84.7 | [103] |
| Sb/MWCNT (Multi-walled carbon nanotube) | 1 M NaClO4 in PC (1:1) with 5/10% FEC | 0.01–2.5 | ~382/120/200 | [104] |
| Sb nanocrystals | 1 M NaPF6 in EC/DMC (-) | 0.02–1.5 | ~550/100/660 | [93] |
| Sb@C microspheres | 1 M NaPF6 in EC/DEC (1:1) | 0.01–3.00 | ~584/100/200 | [105] |
| Sb@TiO2 | 1 M NaClO4 in PC (1:1) with 5% FEC | 0.01–3.00 | 541/100/100 | [106] |
| Zn4Sb3 | 1 M NaClO4 in PC (1:1) with 5% FEC | 0.01–2.0 | 290/200/414 | [107] |
| FeSb2 | 1 M NaClO4 in PC with 5% FEC | 0.01–1.2 | 540/130/36 | [108] |
| SnSb@carbon nanocable | 1 M NaClO4 in PC (1:1) with 5% FEC | 0.005–1.5 | 360/100/100 | [109] |
| Si/Ge nanorod | 1 M NaPF6 in EC/DEC (1:1) | 0.001–1.5 | 20 μAh cm−2/200/10 μA cm−2 | [67] |
| Bi@C microsphere | 1 M NaClO4 in EC/PC (1:1) | 0.01–2.0 | 123.5/100/100 | [110] |
| Sn-Bi-Sb | 1 M NaClO4 in PC (1:1) with 5% FEC | 0.01–2.0 | 621/100/200 | [111] |
| Sn4P3 | 1 M NaClO4 in EC/DEC (1:1) | 0.01–1.5 | ~700/100/100 | [112] |
| SnP nanocrystals | 5 M NaFSI in DME | 0.005–1.5 | 600/200/100 | [113] |
| Cu2P/C | 1 M NaClO4 in EC/DEC (1:1) with 5% FEC | 0.01–1.5 | 430/100/200 | [114] |
| Type of Metal Oxide Anode | Electrolyte Chemistry | Voltage Range | Performance * | Reference |
|---|---|---|---|---|
| P2-Na2/3Co1/3Ti2/3O2 | 1M of NaClO4 in EC/DEC/3 wt.% FEC | 4.0–2.0 | 64.9/400/1C a | [133] |
| Tunnel-Na0.44MnO2 | 1M NaClO4 in PC | 2–3.8 | 82/1000/0.42 C a | [134] |
| Fe3O4 QD@C-GN | 1M NaFP6 in EC/DMC | 0–3.0 | 343/1000/2000 | [135] |
| Co3O4@NC | 1M NaClO4 in PC/PEC | 0–3.0 | 175/1100/1000 | [136] |
| SnO2/3D graphene | 1M NaPF6 in EC/DEC/10 wt.% FEC | 0–2.8 | 223/350/80 | [137] |
| T-Nb2O5/CNF | 1M NaClO4 in PC/EC/5 wt.% PEC | 0–2.8 | 150/5000/1000 | [138] |
| Na2Ti6O13 nanorods | 1M NaClO4 in PC and FEC | 0–2.5 | 109/2800/1000 | [139] |
| Sb2O3/Sb@Gr-CSN | 1M NaClO4 in EC/DMC | 0–2.5 | 487/275/100 | [140] |
| V2O3/C | 1M NaClO4 in DMC/EC | 0–3.0 | 181/1000/2000 | [141] |
| 3-CCO@C | 1M NaClO4 in DMC/EC | 0–3.0 | 314/1000/1000 | [142] |
| Inverse opal am-TiO2 | 1M NaPF6 in DEG-DME | 0–3.0 | 86.7/500/500 | [143] |
| Nickel-titanium oxide | 1M NaPF6 in DMC/EC/5 wt.% FEC | 0–2.5 | 491/200/50 | [144] |
| M-Na4Ti5O12/CNT | 1M NaClO4 in DMC/EC | 0.01–3.0 | 84.4/500/100 | [145] |
| Na2Ti2O5∙H2O/MoS2-C | 1M NaClO4 in DMC/EC/5 wt.% FEC | 0–3.0 | 201.1/16,000/8000 | [81] |
| Type of 2D Materials Anode | Electrolyte Chemistry | Voltage Range (V) | Performance * | Reference |
|---|---|---|---|---|
| P/N-doped graphene | 1M NaPF6 in EC/DEC | - | 809/350/1500 | [212] |
| HRGO300 | 1M NaClO4 in EC/DMC/FEC | 0–3.0 | 163/3000/2000 | [213] |
| VSG2 | ester-based | 0–3.0 | 402/300/500 | [214] |
| Nanoporous RP on rGO | 1M NaClO4 in PC/5 wt.% FEC | 0.01–2.0 | 775.3/1500/5120 | [215] |
| SbPO4/rGO | 1M NaClO4 in PC | 0–1.5 | 100/1000/1000 | [156] |
| Ti2Nb2O9 nanosheets | 1M NaPF6 in diethylene glycol dimethyl ether | ~0.1–3.0 | ~160/500/800 | [216] |
| MoS2/S-doped graphene | 1M NaPF6 in DEC/EC | 0.005–3.0 | 309/500/1000 | [217] |
| Mesoporous MoS2/C | 1M NaClO4 in DMC/EC/5 wt.% FEC | 0.05–3.0 | 390/2500/1000 | [218] |
| MoSe2@N, P-carbon nanosheet | 1M NaPF6 in DEC/EC/5 wt.% FE | 0–3.0 | 168/1000/500 | [196] |
| Type of Cathode | Electrolyte Chemistry | Voltage Range (V) | Performance * | Reference |
|---|---|---|---|---|
| Layered TMOs | ||||
| Na0.44MnO2 | 1M NaClO4 in EC:DEC (1:1) | 2.0–3.8 | 80/2000/0.1 C b | [306] |
| Na0.67[Mn0.65Co0.2Ni0.15]O2 | 1M NaPF6 in EC:DEC (1:1) | 2.0–4.4 | 129/50/20 | [307] |
| NaMnTi0.1Ni0.1O2 | 1M NaClO4 in PEC | 1.5–4.2 | 186/500/0.1 C b | [308] |
| NaxAl0.1Mn0.9O2 | 1M NaPF6 in PEC | 2.0–3.8 | 160/100/0.1 C b | [309] |
| Na[Li0.05(Ni0.25Fe0.25Mn0.5)0.95]O2 | 1M NaClO4 in PEC:FEC (1:1) | 1.75–4.3 | 177/200/0.1 C b | [310] |
| Phosphates | ||||
| Mesoporous amorphous FePO4 | 1M NaPF6 in EC:DEC (1:1) | 1.5–3.8 | 151/160/20 | [311] |
| Amorphous FePO4/CNT | 1M NaClO4 in EC:DMC (1:1) | 2.25–3.75 | 66/300/50 | [312] |
| NaFePO4 | NaTFSI/BMP–TFSI (IL) | 2.0–3.75 | 120/100/0.05 C b | [313] |
| NaFePO4 | 1M NaPF6 in EC:DMC (1:1) | 1.5–4.0 | 150/200/0.1 C b | [240] |
| Na2Fe2P2O7 | 1M NaClO4 in PC | 2.0–4.0 | 90/30/0.1 C b | [314] |
| NASICON and Fluorophosphates | ||||
| Na3V2(PO4)3 | 1M NaClO4 in PC | 2.5–3.8 | 66.5/50/1 C b | [259] |
| C coated Na3V2(PO4)3 | 1M NaPF6 in EC:DMC (1:1) | 2.5–4.0 | 113/8000/2 C b | [315] |
| Graphene supported Na3V2(PO4)3 | 1M NaClO4 in PC | 2.5–3.8 | 90/100/1 C b | [316] |
| Fe2(MoO4)3 | 1M NaClO4 in EC:DMC (1:1) | 1.5–3.5 | 94/100/1 C b | [317] |
| Na3V2O2(PO4)2F | 1M NaClO4 in FEC | 2.5–4.3 | 100/1000/20 C b | [318] |
| PB | ||||
| Na4Fe(CN)6 | 1M NaClO4 in EC/DEC (1:1) | 2.0–4.2 | 100/70/5 | [319] |
| Na2NiCo1−xFe(CN)6 | 1M NaPF6 in EC/DEC (1:1) | 2.0–4.2 | 124/100/50 | [320] |
| NaxCoFe(CN)6 | 1M Na2SO4 (pH = 7) | 0.5–2.0 | 100/200/600 | [285] |
| K0.33FeFe(CN)6/rGO | 1M NaClO4 in EC/DEC (1:1) | 2.0–3.8 | 160/1000/0.5 C b | [321] |
| Na0.647Fe[Fe(CN)6]0.93.2.6H2O/C | 1M NaPF6 in EC/DEC (1:1) | 2.0–4.0 | 130/200/50 | [279] |
| Organic | ||||
| Polyimide (PI) | 1M NaPF6 in PC | 1.5–3.0 | 180/50/50 | [322] |
| Anthraquinone based PI | 1M NaPF6 in DME:DOL (1:1) | 1.5–3.5 | 179/150/50 | [323] |
| Poly(benzoquinonyl sulfide) (PBQS) | 1M NaTFSI/DOL DME | 1.5–4.0 | 268/1000/50 | [324] |
| Disodium rhodizonate | 1M NaClO4 in PC | 0.0–3.1 | 498/50/50 | [325] |
| Juglone/rGO | 1M NaClO4 in EC:DMC (1:1) | 0.0–2.5 | 760/100/100 | [290] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukherjee, S.; Bin Mujib, S.; Soares, D.; Singh, G. Electrode Materials for High-Performance Sodium-Ion Batteries. Materials 2019, 12, 1952. https://doi.org/10.3390/ma12121952
Mukherjee S, Bin Mujib S, Soares D, Singh G. Electrode Materials for High-Performance Sodium-Ion Batteries. Materials. 2019; 12(12):1952. https://doi.org/10.3390/ma12121952
Chicago/Turabian StyleMukherjee, Santanu, Shakir Bin Mujib, Davi Soares, and Gurpreet Singh. 2019. "Electrode Materials for High-Performance Sodium-Ion Batteries" Materials 12, no. 12: 1952. https://doi.org/10.3390/ma12121952
APA StyleMukherjee, S., Bin Mujib, S., Soares, D., & Singh, G. (2019). Electrode Materials for High-Performance Sodium-Ion Batteries. Materials, 12(12), 1952. https://doi.org/10.3390/ma12121952






