Studies on Pitting Corrosion of Al–Cu–Li Alloys Part III: Passivation Kinetics of AA2098–T851 Based on the Point Defect Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrode and Solution
2.2. Electrochemical Set-up
3. Results
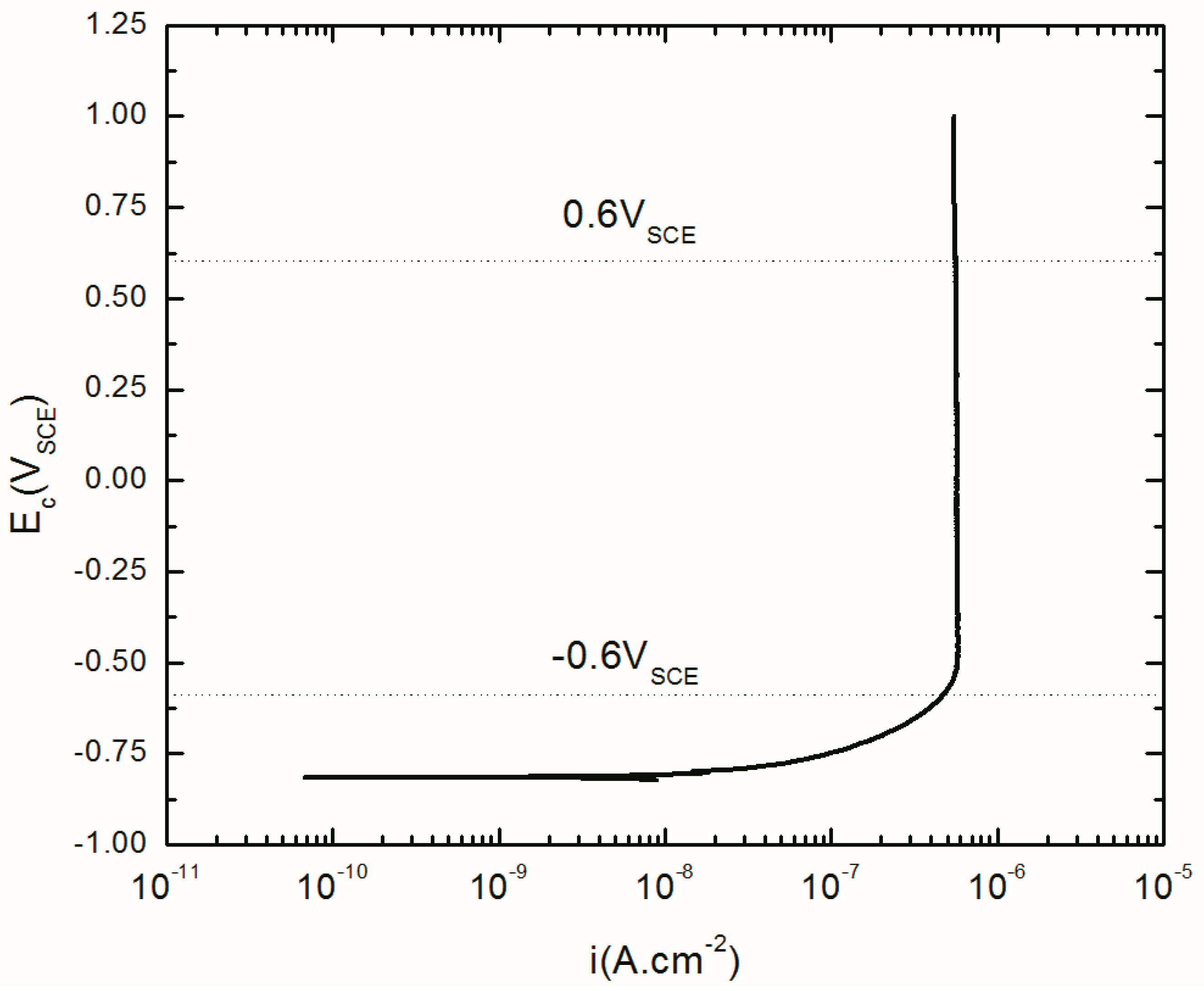
3.1. Potentistatic Polarization
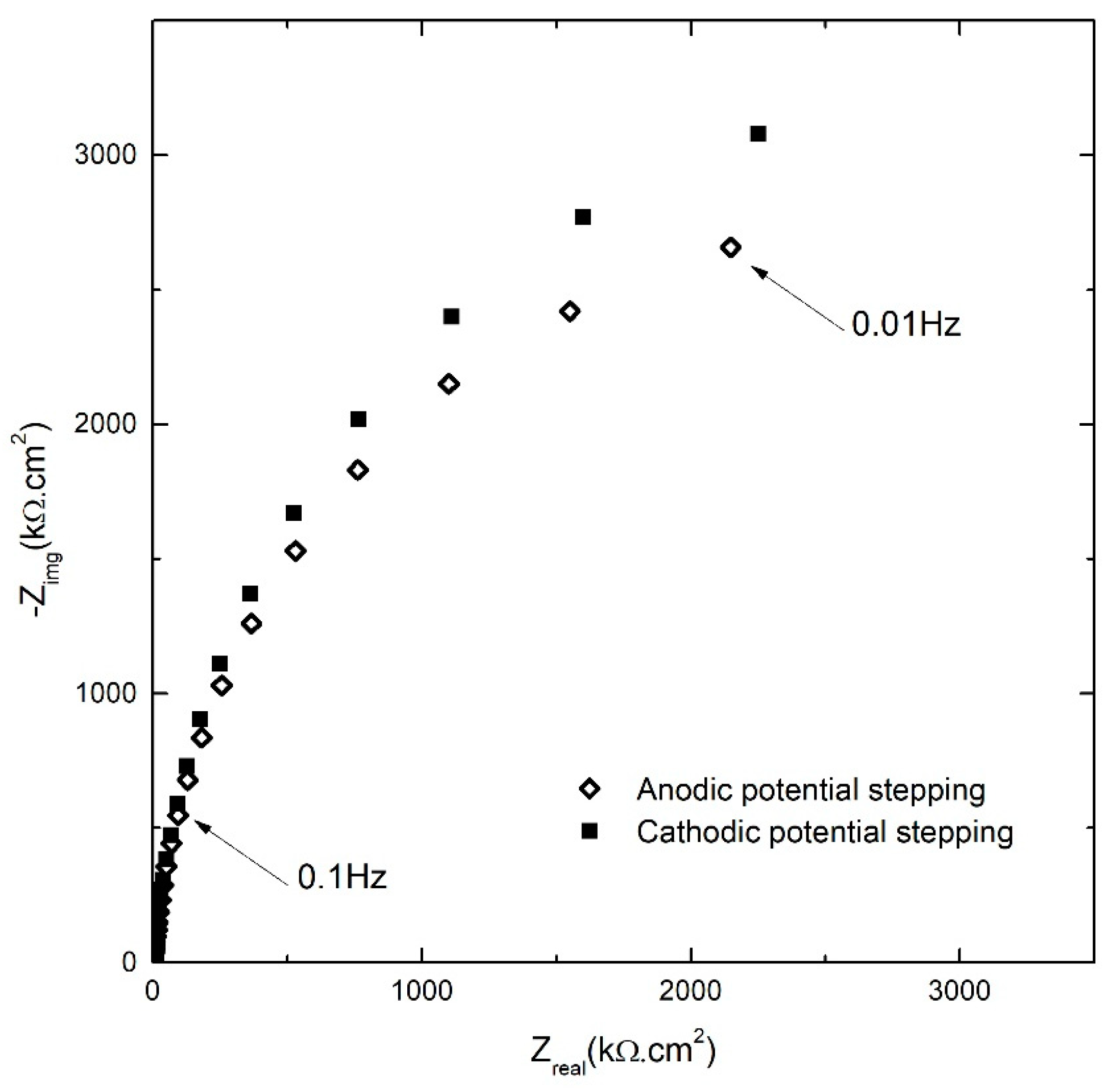
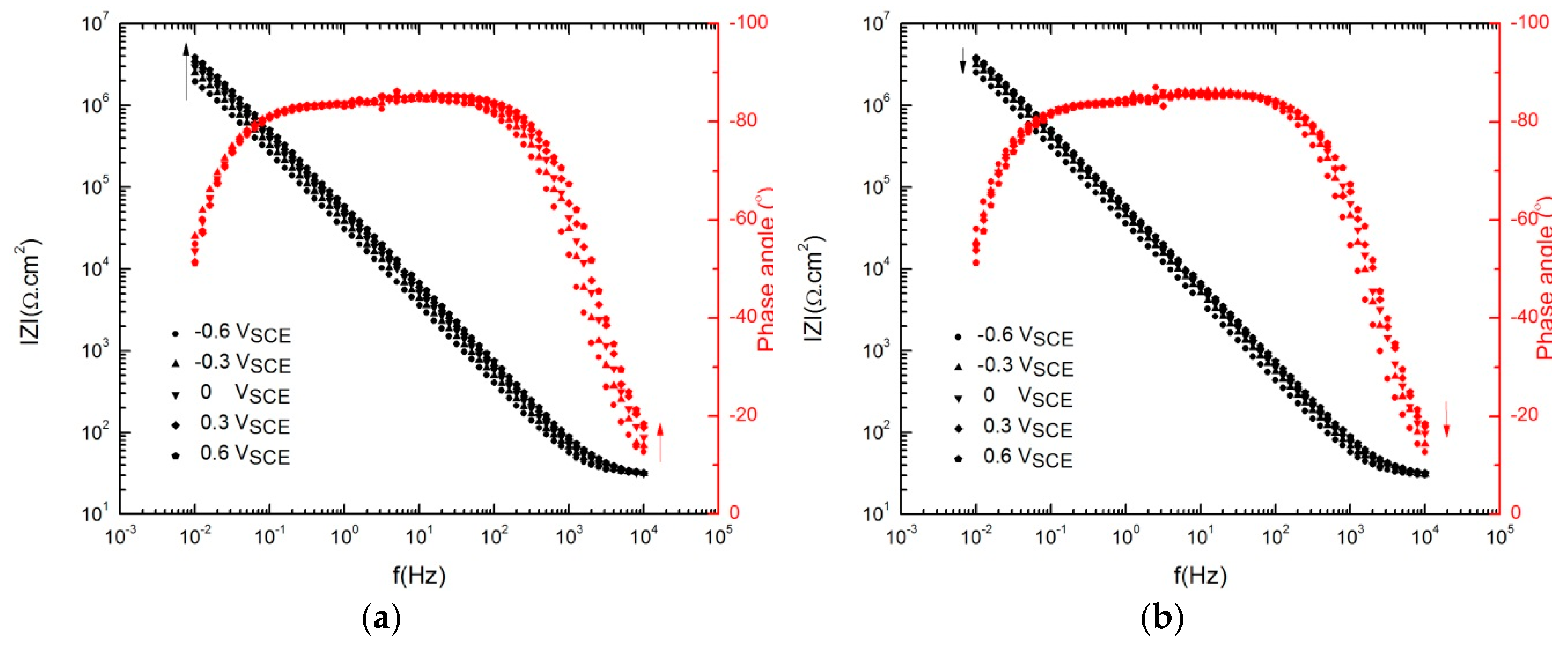
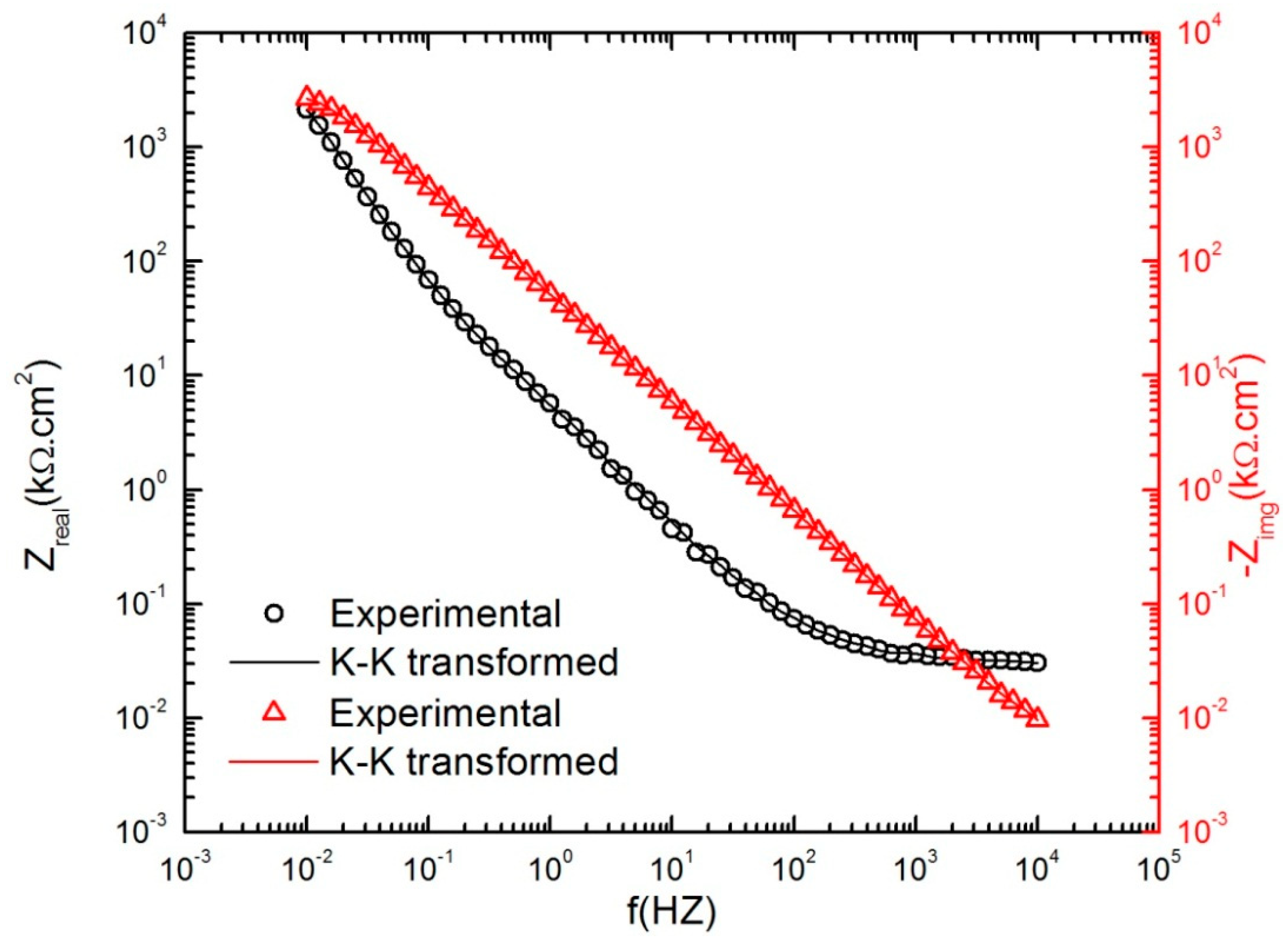
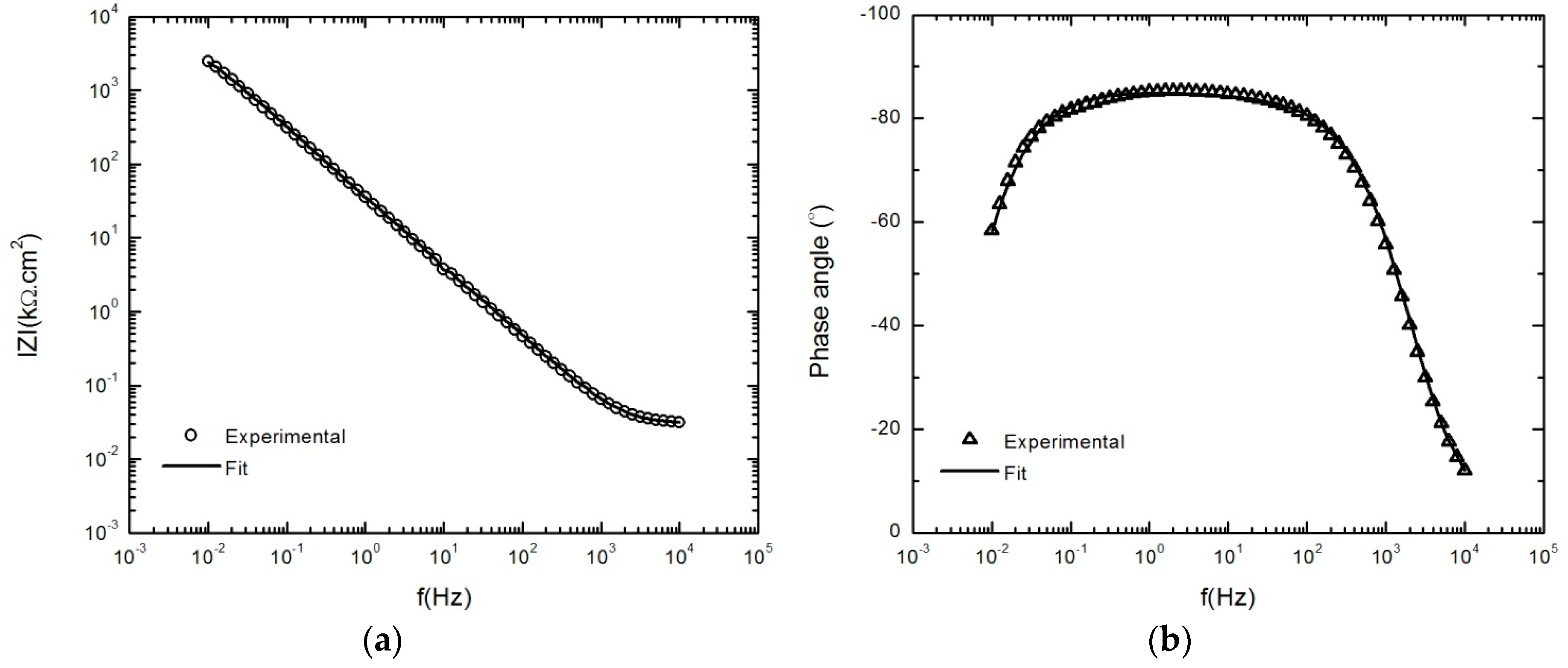
3.2. Impedance Analysis of the Passive Layer
4. Discussions
4.1. The Mixed Potential Model and Optimization of EIS Data
4.2. Quantum Tunneling Definition of Current Density
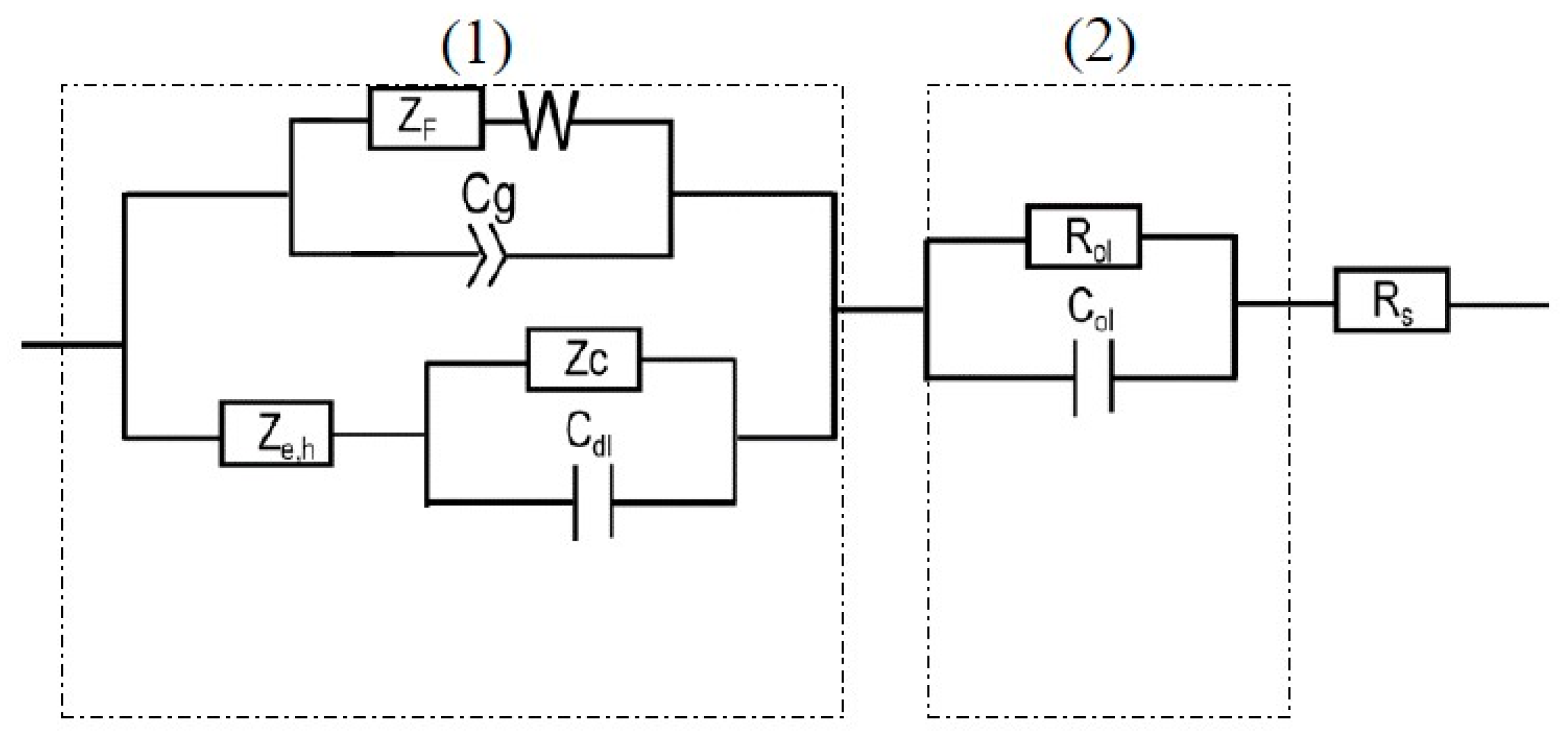
4.3. Equivalent Circuit for EIS Data Fitting
4.4. Optimization Parameters
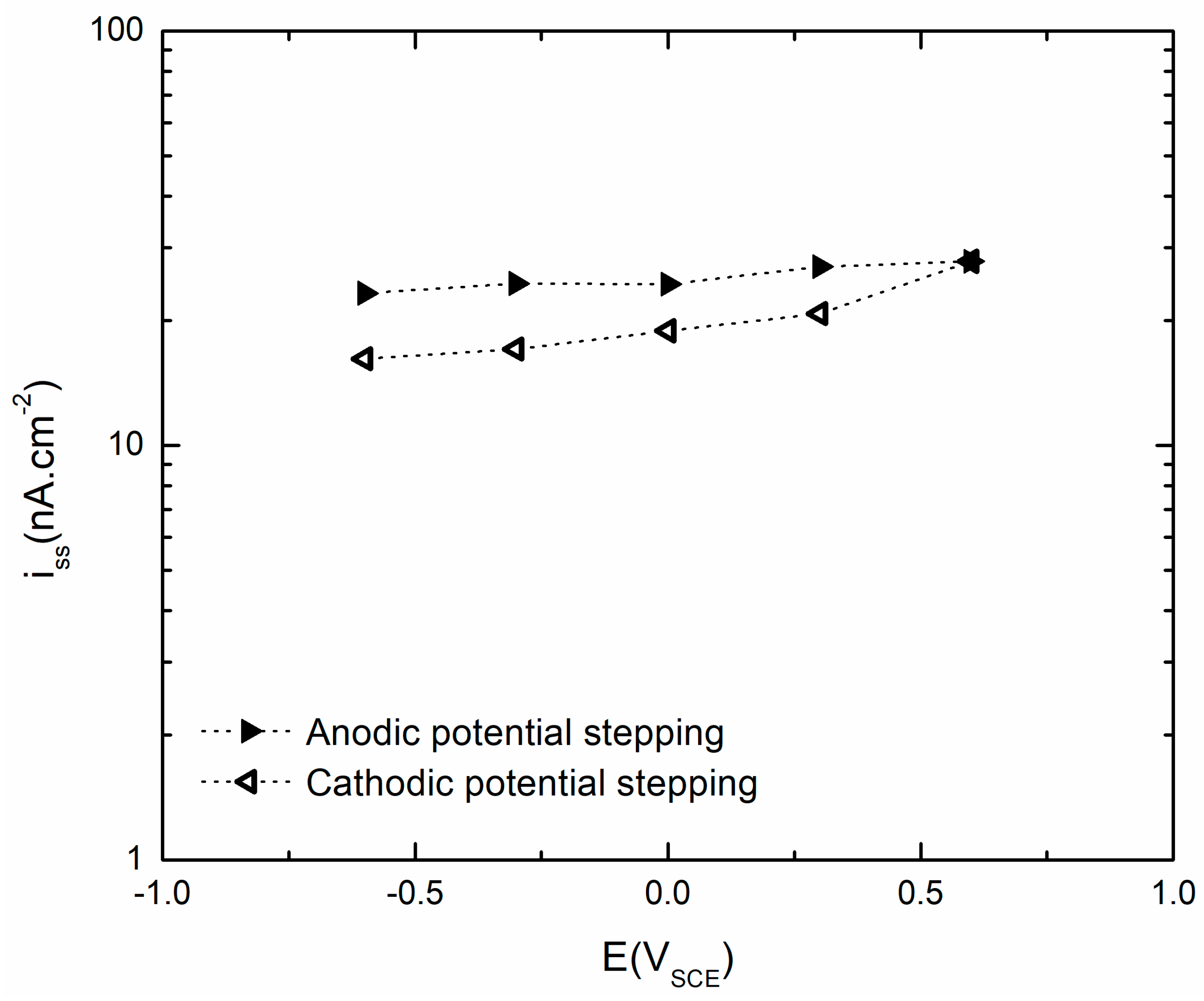
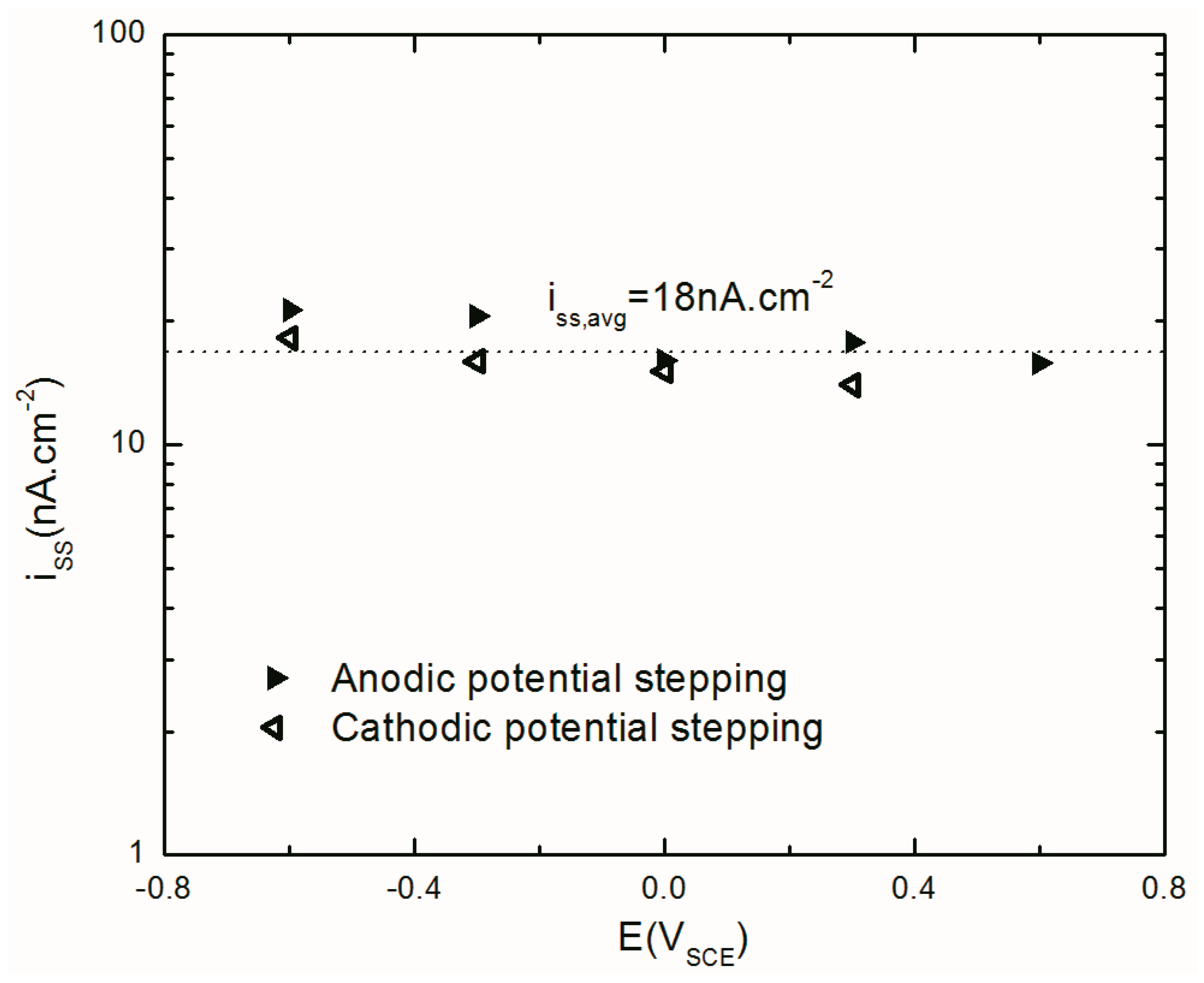
4.4.1. Steady-State Anodic Current Density
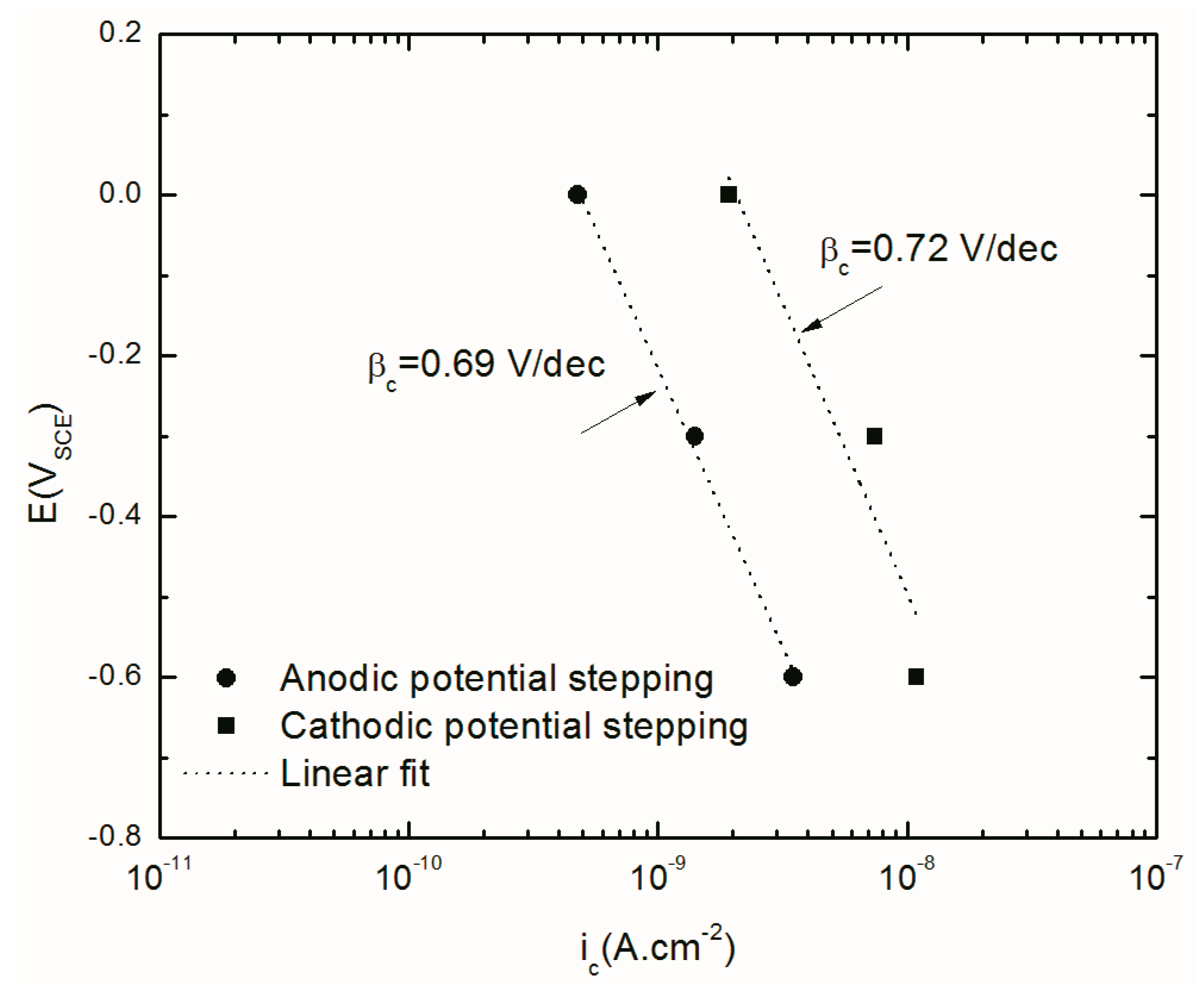
4.4.2. Cathodic Current Density
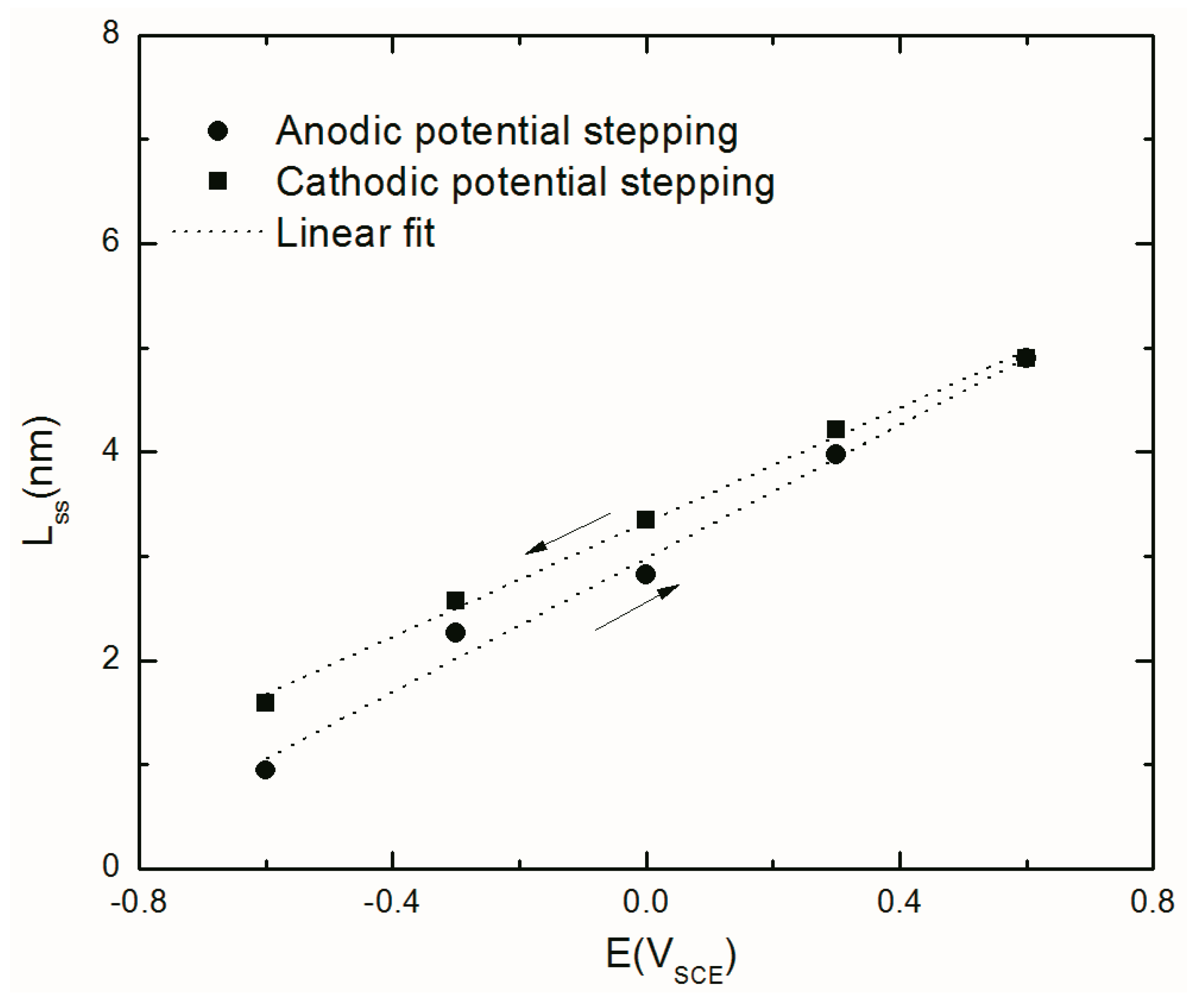
4.4.3. Steady-State Thickness of the Barrier Layer
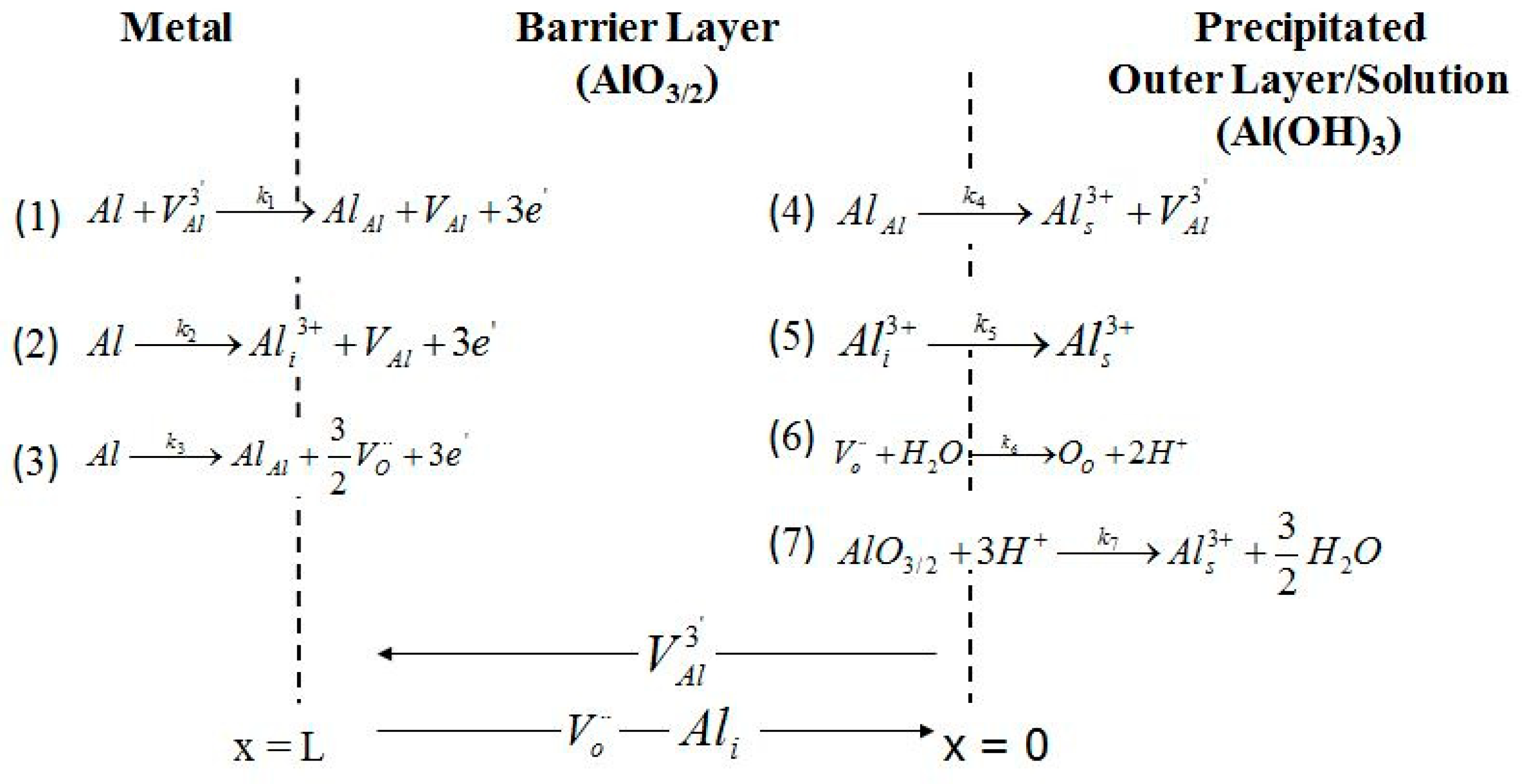
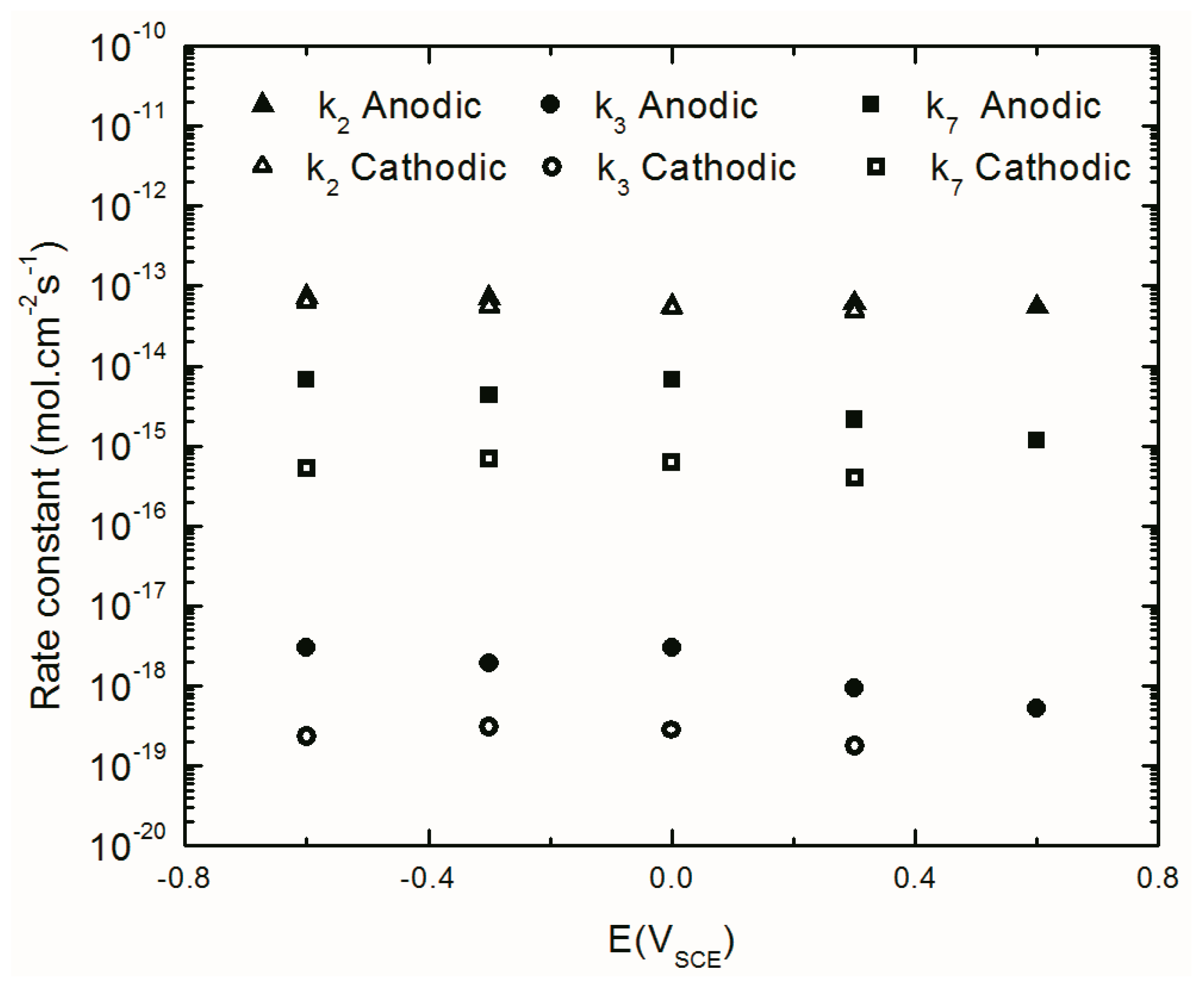
4.4.4. The Kinetics of the Point Defect Reactions
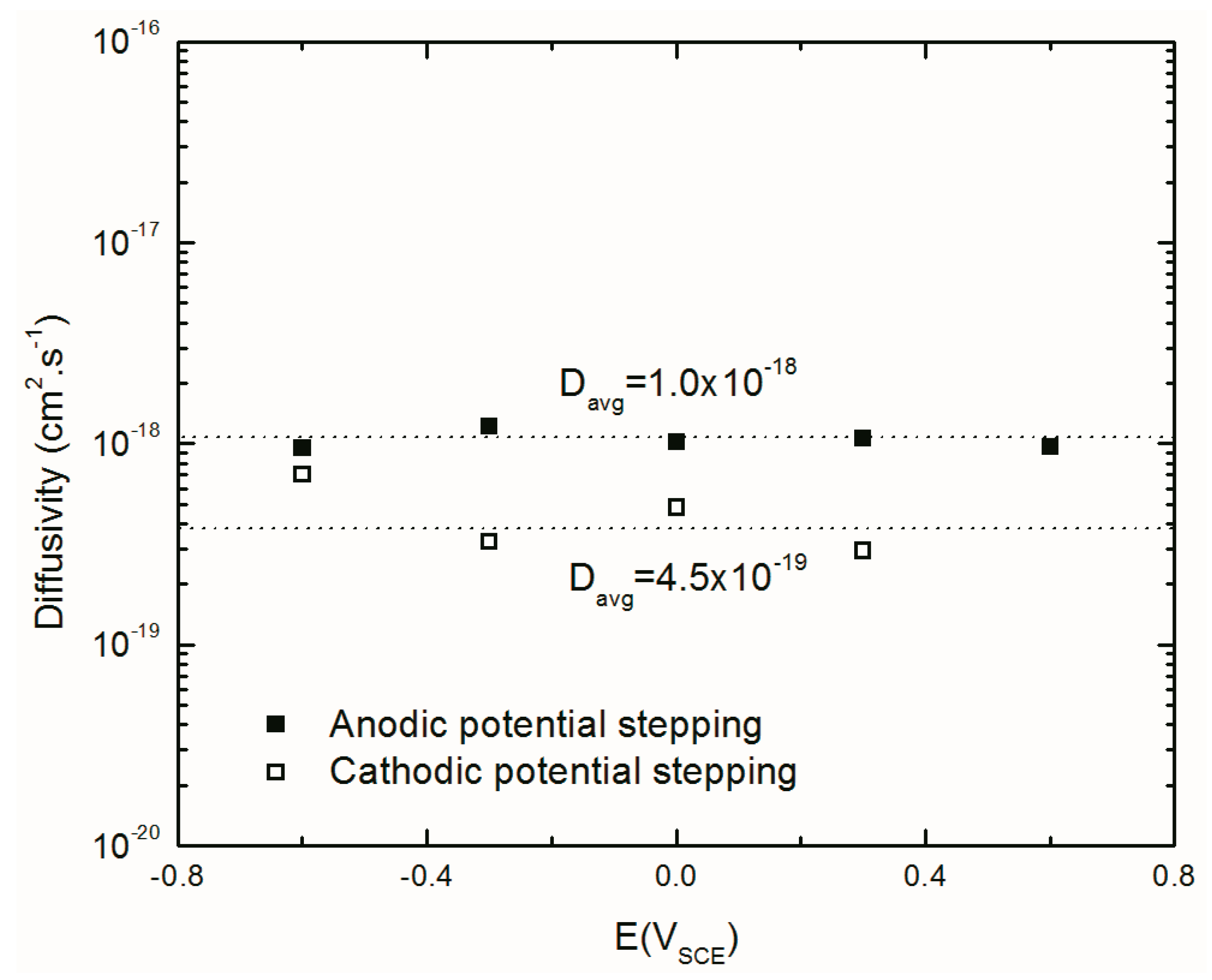
4.4.5. Diffusivity of Aluminum Interstitials in the Barrier Layer
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Starke, E.A.; Staley, J.T. Application of modern aluminum alloys to aircraft. Prog. Aerosp. Sci. 1996, 32, 131–172. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, R.K.; Dwarakadasa, E.S. Anisotropic tensile and fracture behaviour of aluminum lithium alloy 8090. Met. Mater. Process. 1991, 4, 279–292. [Google Scholar]
- Criner, C.B. Aluminum Base Alloy. U.S. Patent No. 2,784,126, 5 March 1957. [Google Scholar]
- Criner, C.B. Aluminum Base Alloy. U.S. Patent No. 2,915,391, 1 December 1959. [Google Scholar]
- Palit, G.C.; Elayaperumal, K. Passivity and pitting of corrosion resistant pure metals Ta, Nb, Ti, Zr, Cr and A1 in chloride solutions. Corros. Sci. 1978, 18, 169–179. [Google Scholar] [CrossRef]
- Ilevbare, G.O.; Scully, J.R.; Yuan, J.; Kelly, R.G. Inhibition of pitting corrosion on aluminum alloy 2024-T3: Effect of soluble chromate additions vs chromate conversion coating. Corrosion 2000, 56, 227–242. [Google Scholar] [CrossRef]
- Buchheit, R.G.; Grant, R.P.; Hlava, P.F.; McKenzie, B.; Zender, G.L. Local dissolution phenomena associated with S phase (Al2CuMg) particles in aluminum alloy 2024-T3. J. Electrochem. Soc. 1997, 144, 2621–2628. [Google Scholar] [CrossRef]
- Chen, G.S.; Gao, M.; Wei, R.P. Microconstituent-induced pitting corrosion in aluminum alloy 2024-T3. Corrosion 1996, 52, 8–15. [Google Scholar] [CrossRef]
- Muller, I.L.; Galvele, J.R. Pitting potential of high purity binary aluminium alloys—I. Al Cu alloys. Pitting and intergranular corrosion. Corros. Sci. 1977, 17, 179191–189193. [Google Scholar] [CrossRef]
- Scully, J.R.; Peebles, D.E.; Romig, A.D.; Frear, D.R.; Hills, C.R. Metallurgical factors influencing the corrosion of aluminum, Al-Cu, and Al-Si alloy thin films in dilute hydrofluoric solution. Metall. Trans. A 1992, 23, 2641–2655. [Google Scholar] [CrossRef]
- Mears, R.B.; Brown, R.H. Causes of corrosion currents. Ind. Eng. Chem. 1941, 33, 1001–1010. [Google Scholar] [CrossRef]
- Frankel, G.S. Pitting corrosion of metals a review of the critical factors. J. Electrochem. Soc. 1998, 145, 2186–2198. [Google Scholar] [CrossRef]
- Lei, X.; Saatchi, A.; Ghanbari, E.; Dang, R.; Li, W.; Wang, N.; Macdonald, D.D. Studies on Pitting Corrosion of Al-Cu-Li Alloys Part I: Effect of Li Addition by Microstructural, Electrochemical, In-situ, and Pit Depth Analysis. Materials 2019, 12, 1600. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, E.; Saatchi, A.; Lei, X.; Macdonald, D.D. Studies on Pitting Corrosion of Al-Cu-Li Alloys Part II: Breakdown Potential and Pitting Initiation. Materials 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Verwey, E. Electrolytic conduction of a solid insulator at high fields The formation of the anodic oxide film on aluminium. Physica 1935, 2, 1059–1063. [Google Scholar] [CrossRef]
- Vetter, K.J.; Gorn, F. Kinetics of layer formation and corrosion processes of passive iron in acid solutions. Electrochim. Acta 1973, 18, 321–326. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Wang, F. Electrochemical corrosion behaviour of microcrystalline aluminium in acidic solutions. Corros. Sci. 2007, 49, 2071–2082. [Google Scholar] [CrossRef]
- Zhao, X. Exfoliation Corrosion Kinetics of High Strength Aluminum Alloys. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2006. [Google Scholar]
- Chao, C.Y.; Lin, L.F.; Macdonald, D.D. A point defect model for anodic passive films I. Film growth kinetics. J. Electrochem. Soc. 1981, 128, 1187–1194. [Google Scholar] [CrossRef]
- Lin, L.F.; Chao, C.Y.; Macdonald, D.D. A point defect model for anodic passive films II. Chemical breakdown and pit initiation. J. Electrochem. Soc. 1981, 128, 1194–1198. [Google Scholar] [CrossRef]
- Lu, P.; Sharifi-Asl, S.; Kursten, B.; Macdonald, D.D. The Irreversibility of the Passive State of Carbon Steel in the Alkaline Concrete Pore Solution under Simulated Anoxic Conditions. J. Electrochem. Soc. 2015, 162, 572–581. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Biaggio, S.R.; Song, H. Steady State Passive Films Interfacial Kinetic Effects and Diagnostic Criteria. J. Electrochem. Soc. 1992, 139, 170–177. [Google Scholar] [CrossRef]
- Martin, F.J.; Cheek, G.T.; O’Grady, W.E.; Natishan, M.P. Impedance studies of the passive film on aluminium. Corros. Sci. 2005, 47, 3187–3201. [Google Scholar] [CrossRef]
- Fernandes, J.C.S.; Picciochi, R.; Belo, M.D.C.; Silva, T.M.E.; Ferreira, M.G.S.E.; Fonseca, I.T. Capacitance and photoelectrochemical studies for the assessment of anodic oxide films on aluminium. Electrochim. Acta 2004, 49, 4701–4707. [Google Scholar] [CrossRef]
- McCafferty, E. Semiconductor aspects of the passive oxide film on aluminum as modified by surface alloying. Corros. Sci. 2003, 45, 301–308. [Google Scholar] [CrossRef]
- Bockris, J.O.; Kang, Y. The protectivity of aluminum and its alloys with transition metals. J. Solid State Electrochem. 1997, 1, 17–35. [Google Scholar] [CrossRef]
- Chang, C.L.; Sankaranarayanan, S.K.; Engelhard, M.H.; Shutthanandan, V.; Ramanathan, S. On the Relationship between Nonstoichiometry and Passivity Breakdown in Ultrathin Oxides: Combined Depth-Dependent Spectroscopy, Mott− Schottky Analysis, and Molecular Dynamics Simulation Studies. J. Phys. Chem. C 2009, 113, 3502–3511. [Google Scholar] [CrossRef]
- Lu, P.; Kursten, B.; Macdonald, D.D. Deconvolution of the Partial Anodic and Cathodic Processes during the Corrosion of Carbon Steel in Concrete Pore Solution under Simulated Anoxic Conditions. Electrochim. Acta 2014, 143, 312–323. [Google Scholar] [CrossRef]
- Gurney, R.W.; Fowler, R.H. The quantum mechanics of electrochemistry. II. Proc. R. Soc. A. Math. Phys. Eng. Sci. 1932, 378–396. [Google Scholar] [CrossRef]
- Gerischer, H. Kinetics of oxidation-reduction reactions on metals and semiconductors. I. General remarks on the electron transition between a solid body and a reduction-oxidation electrolyte. Z. Phys. Chem. Neue Folge 1960, 26, 223–247. [Google Scholar] [CrossRef]
- Ghanbari, E.; Saatchi, A.; Lei, X.; Kovalov, D.; Macdonald, D.D. Passivity Breakdown and Pitting Corrosion of Al- Li Aerospace Alloys. In Proceedings of the DoD Allied Nations Technical Corrosion Conference, Birmingham, AL, USA, 6–10 August 2017. [Google Scholar]
- JSun, B.; Zhang, G.A.; Liu, W.; Lu, M.X. The formation mechanism of corrosion scale and electrochemical characteristic of low alloy steel in carbon dioxide-saturated solution. Corros. Sci. 2012, 57, 131–138. [Google Scholar]
- Macdonald, D.D. Reflections on the history of electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1376–1388. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Urquidi-Macdonald, M. Application of Kramers-Kronig Transforms in the Analysis of Electrochemical Systems I. Polarization Resistance. J. Electrochem. Soc. 1985, 132, 2316–2319. [Google Scholar] [CrossRef]
- Urquidi-Macdonald; Mirna, S.R.; Macdonald, D.D. Applications of Kramers—Kronig transforms in the analysis of electrochemical impedance data—III. Stability and linearity. Electrochim. Acta 1990, 35, 1559–1566. [Google Scholar] [CrossRef]
- Ellis 2: Complex curve fitting for one independent variable IgorExchange. Available online: http://www.igorexchange.com/project/Ellis2 (accessed on 7 August 2012).
- GenCurvefit | IgorExchange. Available online: http://www.igorexchange.com/project/gencurvefit (accessed on 7 August 2012).
- Macdonald, D.D. The point defect model for the passive state. J. Electrochem. Soc. 1992, 139, 3434–3449. [Google Scholar] [CrossRef]
- Macdonald, D.D. On the existence of our metals-based civilization I. Phase-space analysis. J. Electrochem. Soc. 2006, 153, 213–224. [Google Scholar] [CrossRef]
- Macdonald, D.D. Passivity–the key to our metals-based civilization. Pure Appl. Chem. 1999, 71, 951–978. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Urquidi-Macdonald, M. Theory of Steady-State Passive Films. J. Electrochem. Soc. 1990, 137, 2395–2402. [Google Scholar] [CrossRef]
- Sharifi-Asl, S.; Taylor, M.L.; Lu, Z.; Engelhardt, G.R.; Kursten, B.; Macdonald, D.D. Modeling of the electrochemical impedance spectroscopic behavior of passive iron using a genetic algorithm approach. Electrochim. Acta 2013, 102, 161–173. [Google Scholar] [CrossRef]
- Vetter, K.J.; Schultze, J.W. Tunnelübergang der Elektronen und thermische Aktivierung bei der anodischen Sauerstoffentwicklung., Berichte Der Bunsengesellschaft Für Phys. Chemie 1973, 77, 945–953. [Google Scholar]
- Moffat, T.P.; Yang, H.; Fan, F.R.F.; Bard, A.J. Electron-Transfer Reactions on Passive Chromium. J. Electrochem. Soc. 1992, 139, 1992. [Google Scholar] [CrossRef]
- Hamann, C.H. Die Oxidation von Kohlenmonoxid an Platin im sauren Elektrolyten., Berichte Der Bunsengesellschaft Für Phys. Chemie 1971, 75, 542–547. [Google Scholar]
- Schultze, J.W.; Vetter, K.J. The influence of the tunnel probability on the anodic oxygen evolution and other redox reactions at oxide covered platinum electrodes. Electrochim. Acta 1973, 18, 889–896. [Google Scholar] [CrossRef]
- Schuldiner, S. Oxidation of hydrogen on a passive platinum electrode. J. Electrochem. Soc. 1968, 115, 362–365. [Google Scholar] [CrossRef]
- Bao, J.; Macdonald, D.D. Oxidation of hydrogen on oxidized platinum: Part I: The tunneling current. J. Electroanal. Chem. 2007, 600, 205–216. [Google Scholar] [CrossRef]
- Macdonald, D.D. Some personal adventures in passivity—A review of the point defect model for film growth. Elektrokhimiya 2012, 48, 259–284. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Engelhardt, G.R. The point defect model for bilayer passive films. ECS Trans. 2010, 28, 123–144. [Google Scholar]
- Macdonald, D.D. The history of the point defect model for the passive state: A brief review of film growth aspects. Electrochim. Acta 2011, 56, 1761–1772. [Google Scholar] [CrossRef]
- Vijh, A.K. Electrolytic hydrogen evolution reaction on aluminum, oxide-covered electrodes. J. Phys. Chem. 1969, 73, 506–513. [Google Scholar] [CrossRef]
- Torresi, R.M.; Camara, O.R.; de Pauli, C.P.; Giordano, M.C. Hydrogen evolution reaction on anodic titanium oxide films. Electrochim. Acta 1987, 32, 1291–1301. [Google Scholar] [CrossRef]
- Boodts, J.C.F.; Trasatti, S. Hydrogen evolution on iridium oxide cathodes. J. Appl. Electrochem. 1989, 19, 255–262. [Google Scholar] [CrossRef]
- Dickinson, T.; Greef, R.; Wynne-Jones, L. The kinetics of the chlorine electrode reaction at a platinum electrode. Electrochim. Acta 1969, 14, 467–489. [Google Scholar] [CrossRef]
- Schmickler, W. Resonance tunnelling through oxide layers on passive iron electrodes. J. Electroanal. Chem. Interfacial. Electrochem 1977, 83, 387–391. [Google Scholar] [CrossRef]
- Schmickler, W.; Ulstrup, J. A theory of electron transfer reactions at film-covered metal electrodes. Chem. Phys. 1977, 19, 217–232. [Google Scholar] [CrossRef]
- Gerischer, H. The impact of semiconductors on the concepts of electrochemistry. Electrochim. Acta 1990, 35, 1677–1699. [Google Scholar] [CrossRef]
- Bockris, J.; Potter, E.C. The mechanism of the cathodic hydrogen evolution reaction. J. Electrochem. Soc. 1952, 99, 169–186. [Google Scholar] [CrossRef]
- Pentland, N.; Bockris, J.; Sheldon, E. Hydrogen evolution reaction on copper, gold, molybdenum, palladium, rhodium, and iron mechanism and measurement technique under high purity conditions. J. Electrochem. Soc. 1957, 104, 182–194. [Google Scholar] [CrossRef]
- Heyrovský, J. A theory of overpotential. Recl. Des Trav. Chim. Des Pays-Bas. 1927, 46, 582–585. [Google Scholar] [CrossRef]
- Chao, C.Y.; Lin, L.F.; Macdonald, D.D. A point defect model for anodic passive films III. Impedance response. J. Electrochem. Soc. 1982, 129, 1874–1879. [Google Scholar] [CrossRef]

| Reaction | ai (V−1) | bi (cm−1) | ci | Units of |
|---|---|---|---|---|
| (1) | α1(1 − α)χγ | −α1βχK | −α1βχγ | |
| (2) | α2(1 − α)χγ | −α2βχK | −α2βχγ | |
| (3) | α3(1 − α)χγ | −α3βχK | −α3βχγ | |
| (4) | α4αδγ | - | α4βδγ | |
| (5) | α5αδγ | - | α5βδγ | |
| (6) | 2α6αγ | - | 2α6βδγ | |
| (7) | α7α(δ − χ) γ | - | α7(δ − χ)βγ |
| Parameter | Value | Unit | Source |
|---|---|---|---|
| Dependence of the potential drop across bl/ol upon pH (β) | −0.014 | V | Calculated from experiment |
| Electric Field Strength (ε) | 3 × 106 | V cm−1 | Assumed |
| Oxidation state (χ) | 3 | Assigned | |
| Molar volume of oxide per cation(Ω) | 12.91 | cm3 mol−1 | From density |
| Kinetic order of Reaction (7) (n) | 0.5 | - | Assumed |
| Oxidation state (δ) | +3 | - | Assigned |
| Solution resistance (Rs) | 30 | Ω | From EIS |
| Anodic Potential Stepping Direction | Cathodic Potential Stepping Direction | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Eapp (VSCE) | −0.6 | −0.3 | 0 | 0.3 | 0.6 | 0.3 | 0 | −0.3 | −0.6 |
| α | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 |
| α2 | 0.15 | 0.11 | 0.12 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 |
| α3 | 0.29 | 0.25 | 0.27 | 0.25 | 0.23 | 0.25 | 0.25 | 0.24 | 0.26 |
| αc | 0.18 | 0.179 | 0.179 | - | - | - | 0.16 | 0.18 | 0.18 |
| k02 (mol cm−2.s) | 5.3 × 10−10 | 4.3 × 10−10 | 2.8 × 10−10 | 3.0 × 10−10 | 5.8 × 10−10 | 8.9 × 10−10 | 6.3 × 10−10 | 7.9 × 10−10 | 8.8 × 10−10 |
| k03 (mol.cm−2.s) | 1.1 × 10−10 | 8.5 × 10−10 | 4.9 × 10−10 | 5.8 × 10−10 | 1.0 × 10−10 | 7.6 × 10−10 | 8.2 × 10−10 | 9.3 × 10−10 | 5.2 × 10−10 |
| k07 (mol cm−2.s) | 6.8 × 10−15 | 4.3 × 10−15 | 6.8 × 10−15 | 2.1 × 10−15 | 1.2 × 10−15 | 4.0 × 10−16 | 6.3 × 10−16 | 6.9 × 10−16 | 5.3 × 10−16 |
| k2 (mol cm−2.s) | 7.4 × 10−14 | 7.1 × 10−14 | 5.6 × 10−14 | 6.2 × 10−14 | 5.5 × 10−14 | 4.8 × 10−14 | 5.2 × 10−14 | 5.5 × 10−14 | 6.3 × 10−14 |
| k3 (mol cm−2.s) | 3.0 × 10−18 | 1.9 × 10−18 | 3.0 × 10−18 | 9.5 × 10−19 | 5.3 × 10−19 | 1.8 × 10−19 | 2.8 × 10−19 | 3.1 × 10−19 | 2.4 × 10−19 |
| k7 (mol cm−2 s) | 6.8 × 10−15 | 4.3 × 10−15 | 6.8 × 10−15 | 2.1 × 10−15 | 1.2 × 10−15 | 4.0 × 10−16 | 6.3 × 10−16 | 6.9 × 10−16 | 5.3 × 10−16 |
| kC (mol cm−2.s) | 2.8 × 10−14 | 9.4 × 10−14 | 2.7 × 10−13 | - | - | - | 7.2 × 10−13 | 5.2 × 10−13 | 8.7 × 10−14 |
| D (cm2 s−1) | 9.5 × 10−19 | 1.2 × 10−18 | 1.0 × 10−18 | 1.1 × 10−18 | 9.7 × 10−19 | 2.9 × 10−19 | 4.8 × 10−19 | 3.3 × 10−19 | 7.0 × 10−19 |
| Iss (nA cm−2) | 21.3 | 20.6 | 16.1 | 17.8 | 15.8 | 14.0 | 15.1 | 16.0 | 18.2 |
| Ic (nA cm−2) | −3.5 | −1.4 | −0.47 | - | - | - | −1.9 | −7.4 | −10.9 |
| Lss (nm) | 0.95 | 2.26 | 2.82 | 3.97 | 4.90 | 4.21 | 3.35 | 2.57 | 1.59 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghanbari, E.; Saatchi, A.; Lei, X.; Macdonald, D.D. Studies on Pitting Corrosion of Al–Cu–Li Alloys Part III: Passivation Kinetics of AA2098–T851 Based on the Point Defect Model. Materials 2019, 12, 1912. https://doi.org/10.3390/ma12121912
Ghanbari E, Saatchi A, Lei X, Macdonald DD. Studies on Pitting Corrosion of Al–Cu–Li Alloys Part III: Passivation Kinetics of AA2098–T851 Based on the Point Defect Model. Materials. 2019; 12(12):1912. https://doi.org/10.3390/ma12121912
Chicago/Turabian StyleGhanbari, Elmira, Alireza Saatchi, Xiaowei Lei, and Digby D. Macdonald. 2019. "Studies on Pitting Corrosion of Al–Cu–Li Alloys Part III: Passivation Kinetics of AA2098–T851 Based on the Point Defect Model" Materials 12, no. 12: 1912. https://doi.org/10.3390/ma12121912




