Effect of Inhibitor on Adsorption Behavior and Mechanism of Micro-Zone Corrosion on Carbon Steel
Abstract
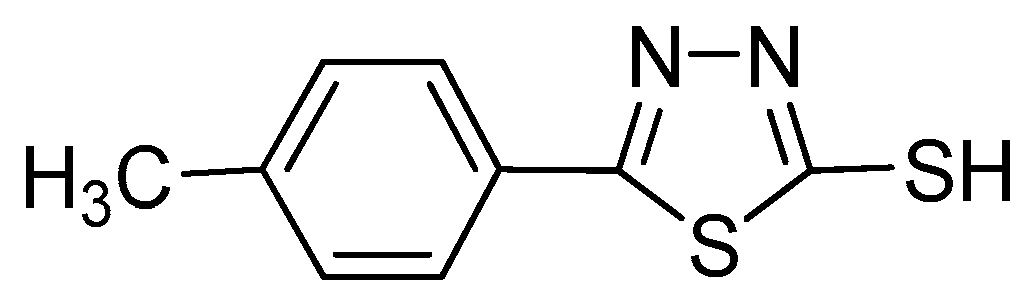
:1. Introduction
2. Experimental Methods
2.1. Materials and Sample Preparation
2.2. EIS Measurements
2.3. Surface Chemical Composition Measurements
2.4. SVET Measurements
3. Results and Discussion
3.1. EIS Measurement Results
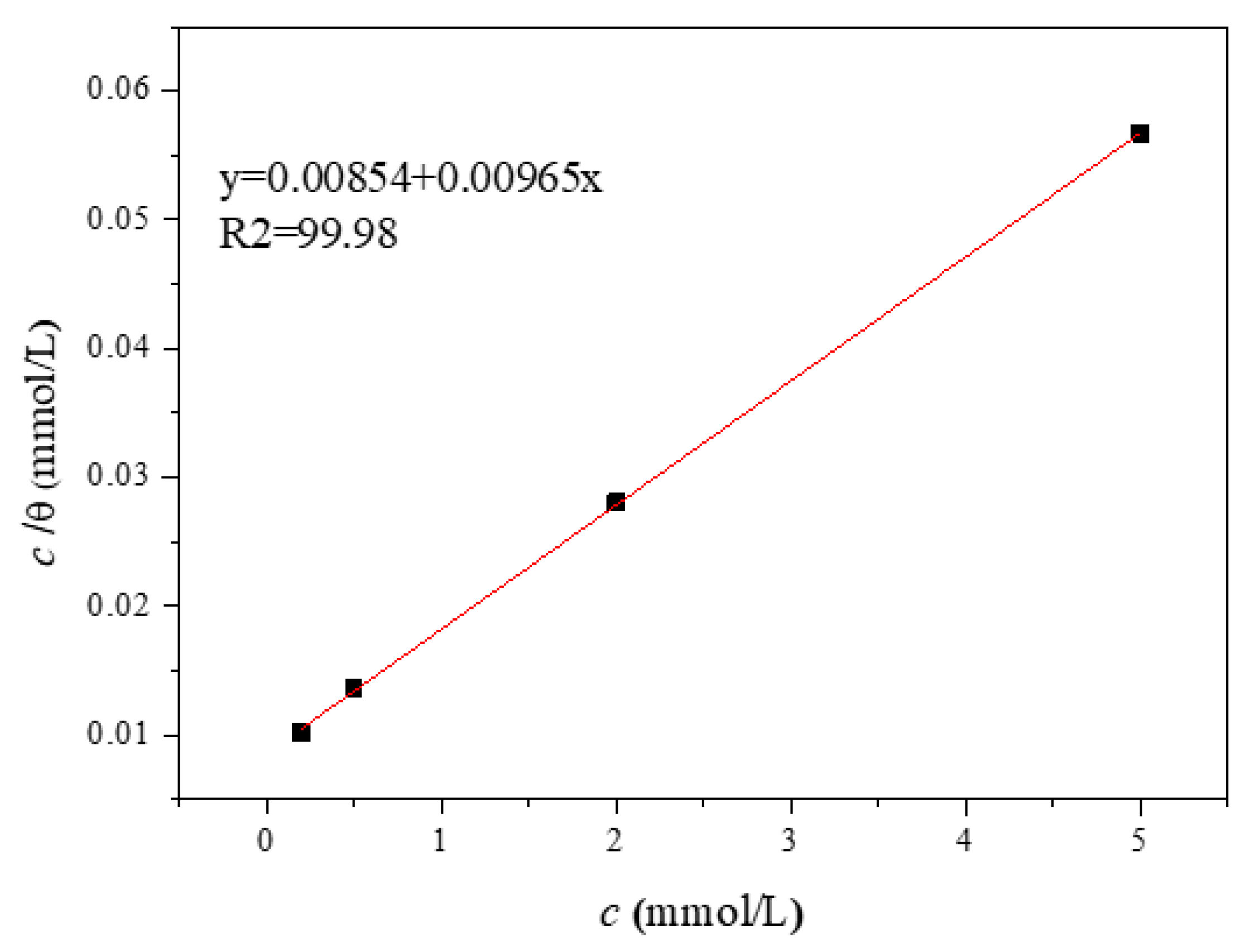
3.2. Adsorption Isotherm Analysis
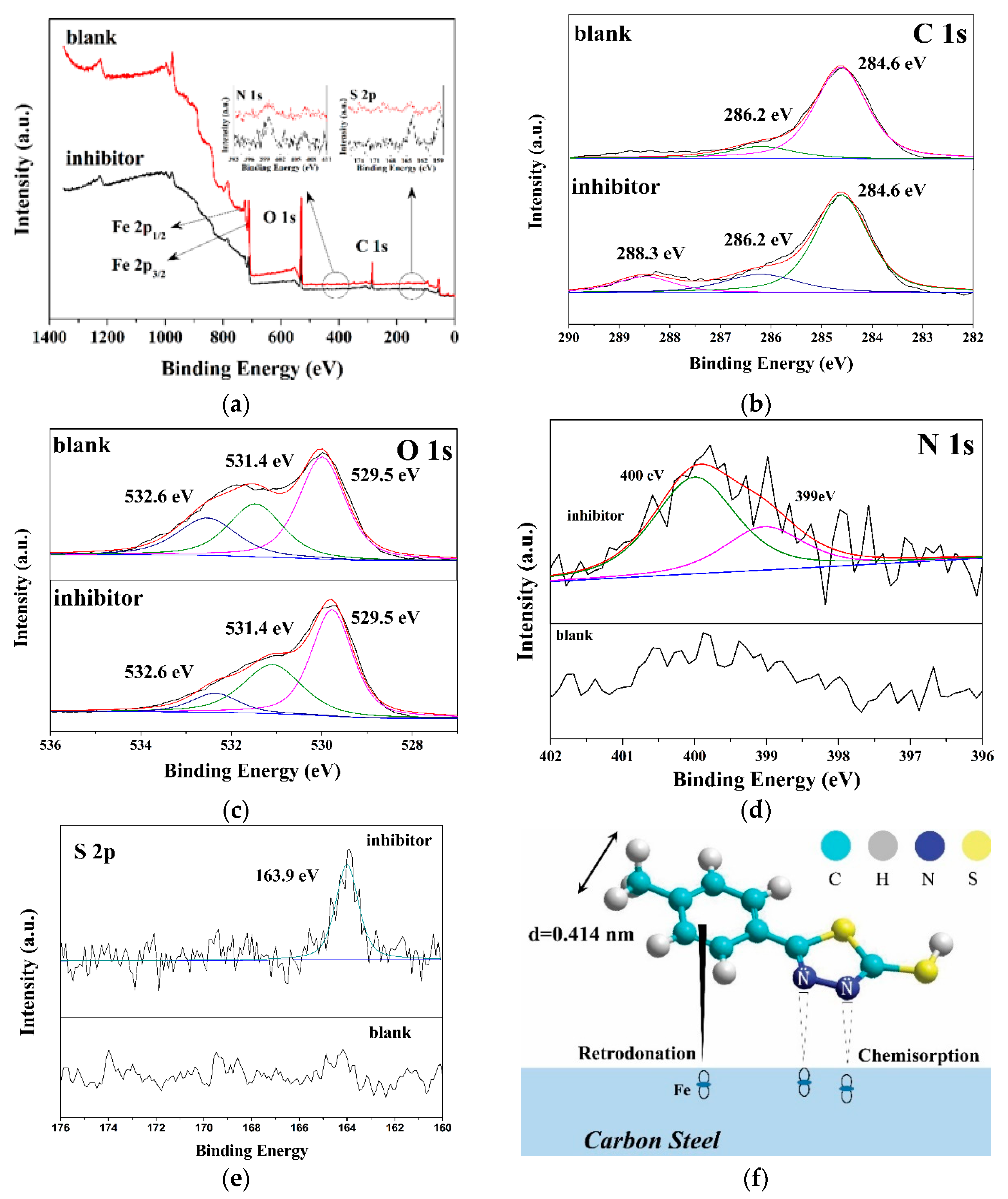
3.3. XPS Analysis
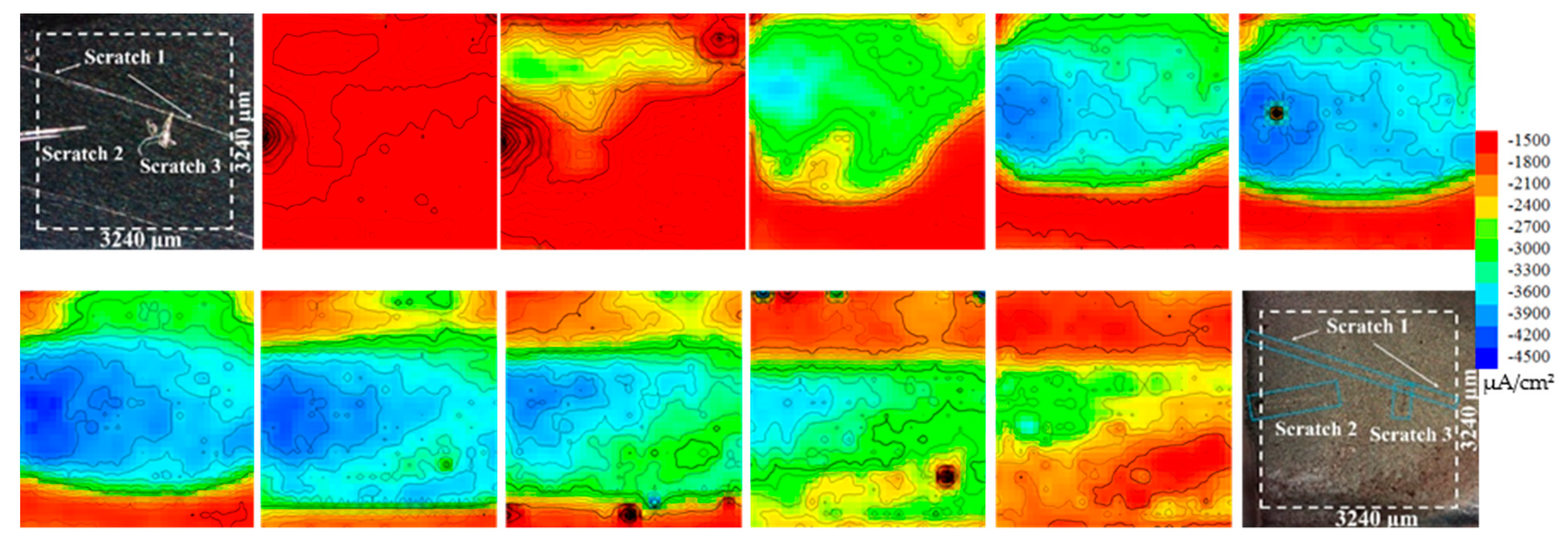
3.4. SVET Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pei, X.; Noel, M.; Green, M.; Fam, A.; Shier, G. Cementitious coatings for improved corrosion resistance of steel reinforcement. Surf. Coat. Technol. 2017, 315, 188–195. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, D.; Chu, H.; Ma, H.; Liu, Y.; Gao, Y.; Shi, J.; Sun, W. The Passive Film Growth Mechanism of New Corrosion-Resistant Steel Rebar in Simulated Concrete Pore Solution: Nanometer Structure and Electrochemical Study. Materials 2017, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Revert, A.-B.; De Weerdt, K.; Hornbostel, K.; Geiker, M.-R. Carbonation-induced corrosion: Investigation of the corrosion onset. Constr. Build. Mater. 2018, 162, 847–856. [Google Scholar] [CrossRef]
- Luo, H.; Su, H.; Dong, C.; Li, X. Passivation and electrochemical behavior of 316L stainless steel in chlorinated simulated concrete pore solution. Appl. Surf. Sci. 2017, 400, 38–48. [Google Scholar] [CrossRef]
- Soriano, C.; Alfantazi, A. Corrosion behavior of galvanized steel due to typical soil organics. Constr. Build. Mater. 2016, 102, 904–912. [Google Scholar] [CrossRef]
- Hsu, C.; Cho, Y.; Liu, T.; Chang, H.; Lien, S. Optimization of residual stress of SiO2/organic silicon stacked layer prepared using inductively coupled plasma deposition. Surf. Coat. Technol. 2017, 320, 293–297. [Google Scholar] [CrossRef]
- Yabuki, A.; Tanabe, S.; Fathona, I.-W. Self-healing polymer coating with the microfibers of superabsorbent polymers provides corrosion inhibition in carbon steel. Surf. Coat. Technol. 2018, 341, 71–77. [Google Scholar] [CrossRef]
- Cole, I.-S. Recent Progress and Required Developments in Atmospheric Corrosion of Galvanised Steel and Zinc. Materials 2017, 10, 1288. [Google Scholar] [CrossRef]
- Park, J.-H.; Park, J.-M. Photo-generated cathodic protection performance of electrophoretically Co-deposited layers of TiO2 nanoparticles and graphene nanoplatelets on steel substrate. Surf. Coat. Technol. 2014, 258, 62–71. [Google Scholar] [CrossRef]
- Chen, C.L.; Sutrisna. Study of W-Co ODS coating on stainless steels by mechanical alloying. Surf. Coat. Technol. 2018, 350, 954–961. [Google Scholar] [CrossRef]
- Zarrouk, A.; Zarrok, H.; Ramli, Y.; Bouachrine, M.; Hammouti, B.; Sahibed-dine, A.; Bentiss, F. Inhibitive properties, adsorption and theoretical study of 3,7-dimethyl-1-(prop-2-yn-1-yl)quinoxalin-2(1H)-one as efficient corrosion inhibitor for carbon steel in hydrochloric acid solution. J. Mol. Liq. 2016, 222, 239–252. [Google Scholar] [CrossRef]
- Tao, Z.; Zhang, S.; Li, W.; Hou, B. Corrosion inhibition of mild steel in acidic solution by some oxo-triazole derivatives. Corros. Sci. 2009, 51, 2588–2595. [Google Scholar] [CrossRef]
- Liu, Y.; Song, Z.; Wang, W.; Jiang, L.; Zhang, Y.; Guo, M.; Song, F.; Xu, N. Effect of ginger extract as green inhibitor on chloride-induced corrosion of carbon steel in simulated concrete pore solutions. J. Clean. Prod. 2019, 214, 298–307. [Google Scholar] [CrossRef]
- Verbruggen, H.; Terryn, H.; De Graeve, I. Inhibitor evaluation in different simulated concrete pore solution for the protection of steel rebars. Constr. Build. Mater. 2016, 124, 887–896. [Google Scholar] [CrossRef]
- Tian, H.; Cheng, Y.-F.; Li, W.; Hou, B. Triazolyl-acylhydrazone derivatives as novel inhibitors for copper corrosion in chloride solutions. Corros. Sci. 2015, 100, 341–352. [Google Scholar] [CrossRef]
- Rivera-Corral, J.-O.; Fajardo, G.; Arliguie, G.; Orozco-Cruz, R.; Deby, F.; Valdez, P. Corrosion behavior of steel reinforcement bars embedded in concrete exposed to chlorides: Effect of surface finish. Constr. Build. Mater. 2017, 147, 815–826. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, C.; Lu, H.; Gao, F.; Ma, H. Influence of phytic acid on the corrosion behavior of iron under acidic and neutral conditions. Electrochim. Acta 2014, 150, 188–196. [Google Scholar] [CrossRef]
- Coelho, L.-B.; Cossement, D.; Olivier, M.-G. Benzotriazole and cerium chloride as corrosion inhibitors for AA2024-T3: An EIS investigation supported by SVET and ToF-SIMS analysis. Corros. Sci. 2018, 130, 177–189. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, H.; Zhang, Z.; Yao, Y. Electrochemical impedance spectroscopy (EIS) of hydration process and drying shrinkage for cement paste with W/C of 0.25 affected by high range water reducer. Constr. Build. Mater. 2017, 131, 536–541. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, S.; Qiang, Y.; Xu, Y.; Guo, L.; Madkour, L.-H.; Chen, S. Experimental and Theoretical Investigation of Thiazolyl Blue as a Corrosion Inhibitor for Copper in Neutral Sodium Chloride Solution. Materials 2018, 11, 1042. [Google Scholar] [CrossRef]
- Unnisa, C.-B.-N.; Devi, G.-N.; Hemapriya, V.; Chitra, S.; Chung, I.; Kim, S.; Prabakaran, M. Linear polyesters as effective corrosion inhibitors for steel rebars in chloride induced alkaline medium—An electrochemical approach. Constr. Build. Mater. 2018, 165, 866–876. [Google Scholar] [CrossRef]
- He, X.; Jiang, Y.; Li, C.; Wang, W.; Hou, B.; Wu, L. Inhibition properties and adsorption behavior of imidazole and 2-phenyl-2-imidazoline on AA5052 in 1.0 M HCl solution. Corros. Sci. 2014, 83, 124–136. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, K.; Yan, L.; Pang, X. Inhibition of the corrosion of X70 and Q235 steel in CO2-saturated brine by imidazoline-based inhibitor. J. Electroanal. Chem. 2017, 791, 83–94. [Google Scholar] [CrossRef]
- Yurt, A.; Ulutas, S.; Dal, H. Electrochemical and theoretical investigation on the corrosion of aluminium in acidic solution containing some Schiff bases. Appl. Surf. Sci. 2006, 253, 919–925. [Google Scholar] [CrossRef]
- Arman, S.-Y.; Omidvar, H.; Tabaian, S.-H.; Sajjadnejad, M.; Fouladvand, S.; Afshar, S. Evaluation of nanostructured S-doped TiO2 thin films and their photoelectrochemical application as photoanode for corrosion protection of 304 stainless steel. Surf. Coat. Technol. 2014, 251, 162–169. [Google Scholar] [CrossRef]
- Wang, X.; Yang, W.; Li, F.; Xue, Y.; Liu, R.; Hao, Y. In Situ Microwave-Assisted Synthesis of Porous N-TiO2/g-C3N4 Heterojunctions with Enhanced Visible-Light Photocatalytic Properties. Ind. Eng. Chem. Res. 2013, 52, 17140–17150. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Mueller, J.; Schloegl, R.; Carlsson, J.-M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef]
- Li, S.; Fu, J. Improvement in corrosion protection properties of TiO2 coatings by chromium doping. Corros. Sci. 2013, 68, 101–110. [Google Scholar] [CrossRef]
- Grosvenor, A.-P.; Kobe, B.-A.; Biesinger, M.-C.; McIntyre, N.-S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, P.; Yu, K.; Zhao, X.; Ding, G. ITO film prepared by ion beam sputtering and its application in high-temperature thermocouple. Vacuum 2017, 146, 31–34. [Google Scholar] [CrossRef]
- Li, C.; Fan, W.; Lu, H.; Ge, Y.; Bai, H.; Shi, W. Fabrication of Au@CdS/RGO/TiO2 heterostructure for photoelectrochemical hydrogen production. New J. Chem. 2016, 40, 2287–2295. [Google Scholar] [CrossRef]
- Hassan, H.-H.; Amin, M.-A.; Gubbala, S.; Sunkara, M.-K. Participation of the dissolved O-2 in the passive layer formation on Zn surface in neutral media. Electrochim. Acta 2007, 52, 6929–6937. [Google Scholar] [CrossRef]
- Tourabi, M.; Nohair, K.; Traisnel, M.; Jama, C.; Bentiss, F. Electrochemical and XPS studies of the corrosion inhibition of carbon steel in hydrochloric acid pickling solutions by 3, 5-bis(2-thienylmethyl)-4-amino-1, 2, 4-triazole. Corros. Sci. 2013, 75, 123–133. [Google Scholar] [CrossRef]
- Kozak, A.-O.; Porada, O.-K.; Ivashchenko, L.-A.; Scrynskyy, P.-L.; Tomila, T.-V.; Manzhara, V.-S. Comparative investigation of Si-C-N Films prepared by plasma enhanced chemical vapour deposition and magnetron sputtering. Appl. Surf. Sci. 2017, 425, 646–653. [Google Scholar] [CrossRef]
- Prabhawalkar, P.-D.; Raole, P.-M.; Kothari, D.-C.; Nair, M.-R. XPS studies at various depths of low-energy n-2+ ions implanted on 304 stainless-steel. Vacuum 1986, 36, 817–820. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, R.; Liu, H.; Liu, Y.; Li, S.; Niu, L. Electrochemical and surface analysis studies of 2-(quinolin-2-yl) quinazoiin-4 (3H) -one as corrosion inhibitor for Q235 steel in hydrochloric acid. J. Mol. Liq. 2016, 222, 671–679. [Google Scholar] [CrossRef]
- Rendon-Nava, D.; Mendoza-Espinosa, D.; Negron-Silva, G.-E.; Tellez-Arreola, J.-L.; Martinez-Torres, A.; Valdez-Calderon, A.; Gonzalez-Montiel, S. Chrysin functionalized NHC-Au (I) complexes: Synthesis, characterization and effects on the nematode Caenorhabditis elegans. New J. Chem. 2017, 41, 2013–2019. [Google Scholar] [CrossRef]
- Lamaka, S.-V.; Taryba, M.; Montemor, M.-F.; Isaacs, H.-S.; Ferreira, M.-G.-S. Quasi-simultaneous measurements of ionic currents by vibrating probe and pH distribution by ion-selective microelectrode. Electrochem. Commun. 2011, 13, 20–23. [Google Scholar] [CrossRef]
- Wang, M.; Liu, M.; Fu, J. An intelligent anticorrosion coating based on pH-responsive smart nanocontainers fabricated via a facile method for protection of carbon steel. J. Mater. Chem. A 2015, 3, 6423–6431. [Google Scholar] [CrossRef]
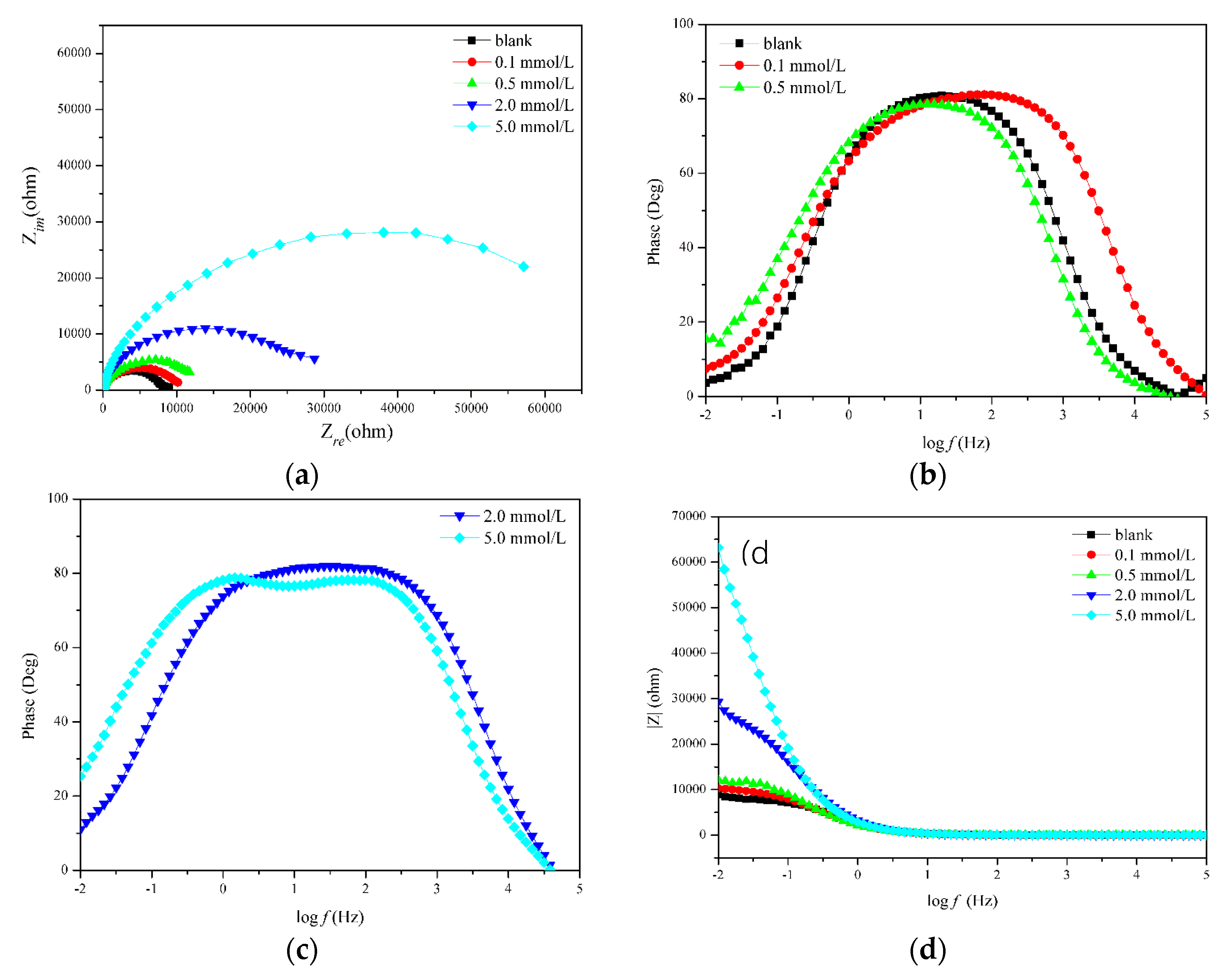
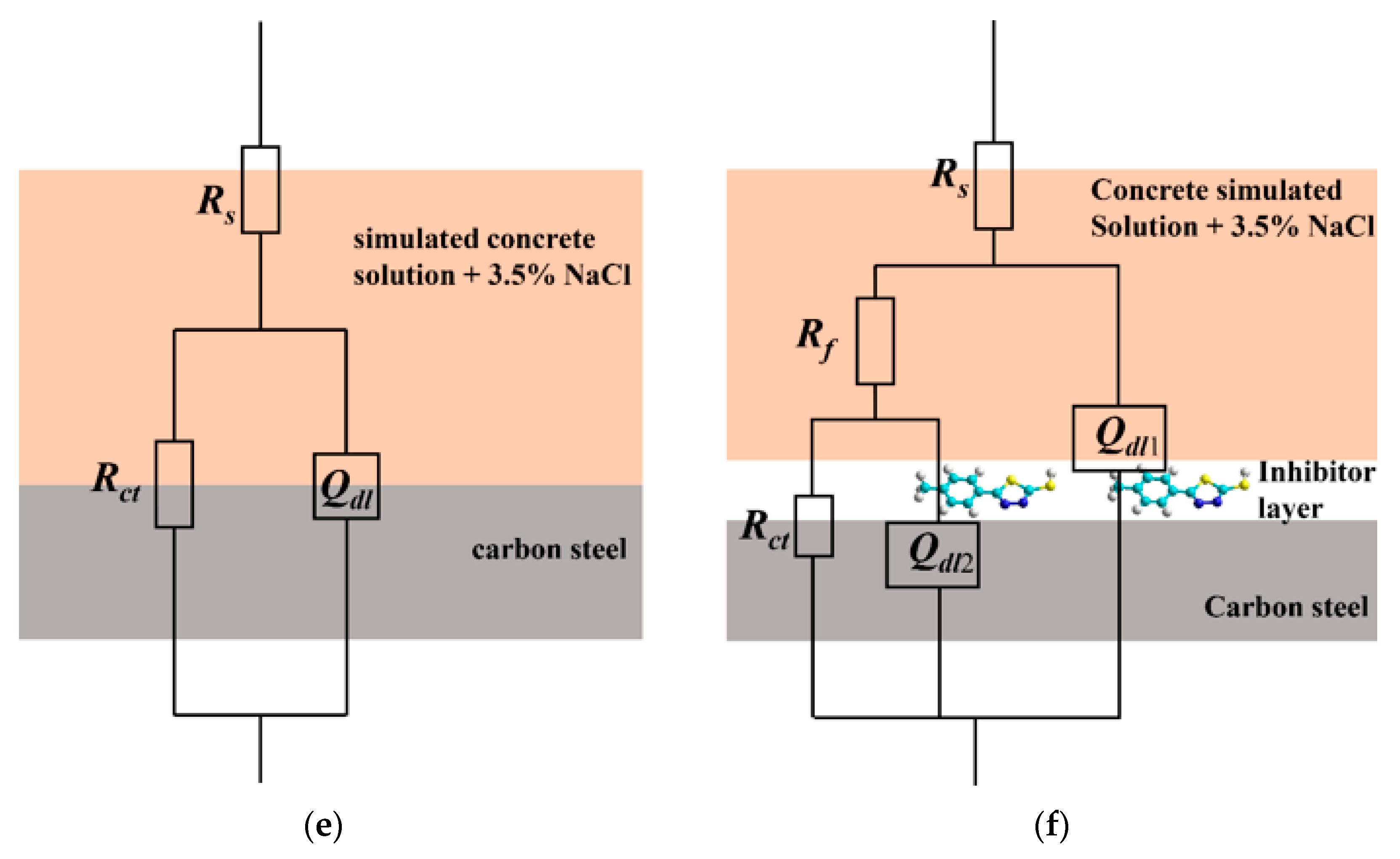
| Sample (mmol/L) | Rs (Ω cm2) | Qdl1 (F cm−2) | n1 | Rf (Ω cm2) | Qdl2 (F cm−2) | n2 | Rct (Ω cm2) | η (%) |
|---|---|---|---|---|---|---|---|---|
| Blank | 4.382 | 6.89 × 10−5 | 0.9245 | – | – | – | 7783 | – |
| 0.1 | 1.191 | 7.316 × 10−5 | 0.9004 | – | – | – | 9691 | 19.7 |
| 0.5 | 6.545 | 8.83 × 10−5 | 0.8892 | – | – | – | 12,320 | 36.8 |
| 2.0 | 1.543 | 4.831 × 10−5 | 0.9374 | 274 | 1.890 × 10−5 | 0.4388 | 32,060 | 75.7 |
| 5.0 | 2.679 | 4.539 × 10−5 | 0.9375 | 678.4 | 2.665 × 10−5 | 0.7467 | 70,650 | 89.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Zhang, Z.; Wu, S.; Jiang, J.; Chu, H. Effect of Inhibitor on Adsorption Behavior and Mechanism of Micro-Zone Corrosion on Carbon Steel. Materials 2019, 12, 1901. https://doi.org/10.3390/ma12121901
Wang F, Zhang Z, Wu S, Jiang J, Chu H. Effect of Inhibitor on Adsorption Behavior and Mechanism of Micro-Zone Corrosion on Carbon Steel. Materials. 2019; 12(12):1901. https://doi.org/10.3390/ma12121901
Chicago/Turabian StyleWang, Fengjuan, Zhifeng Zhang, Shengping Wu, Jinyang Jiang, and Hongyan Chu. 2019. "Effect of Inhibitor on Adsorption Behavior and Mechanism of Micro-Zone Corrosion on Carbon Steel" Materials 12, no. 12: 1901. https://doi.org/10.3390/ma12121901
APA StyleWang, F., Zhang, Z., Wu, S., Jiang, J., & Chu, H. (2019). Effect of Inhibitor on Adsorption Behavior and Mechanism of Micro-Zone Corrosion on Carbon Steel. Materials, 12(12), 1901. https://doi.org/10.3390/ma12121901






