Molybdenum Dopped Copper Ferrites as Active Catalysts for Alcohols Oxidative Coupling
Abstract
:1. Introduction
2. Results and Discussion
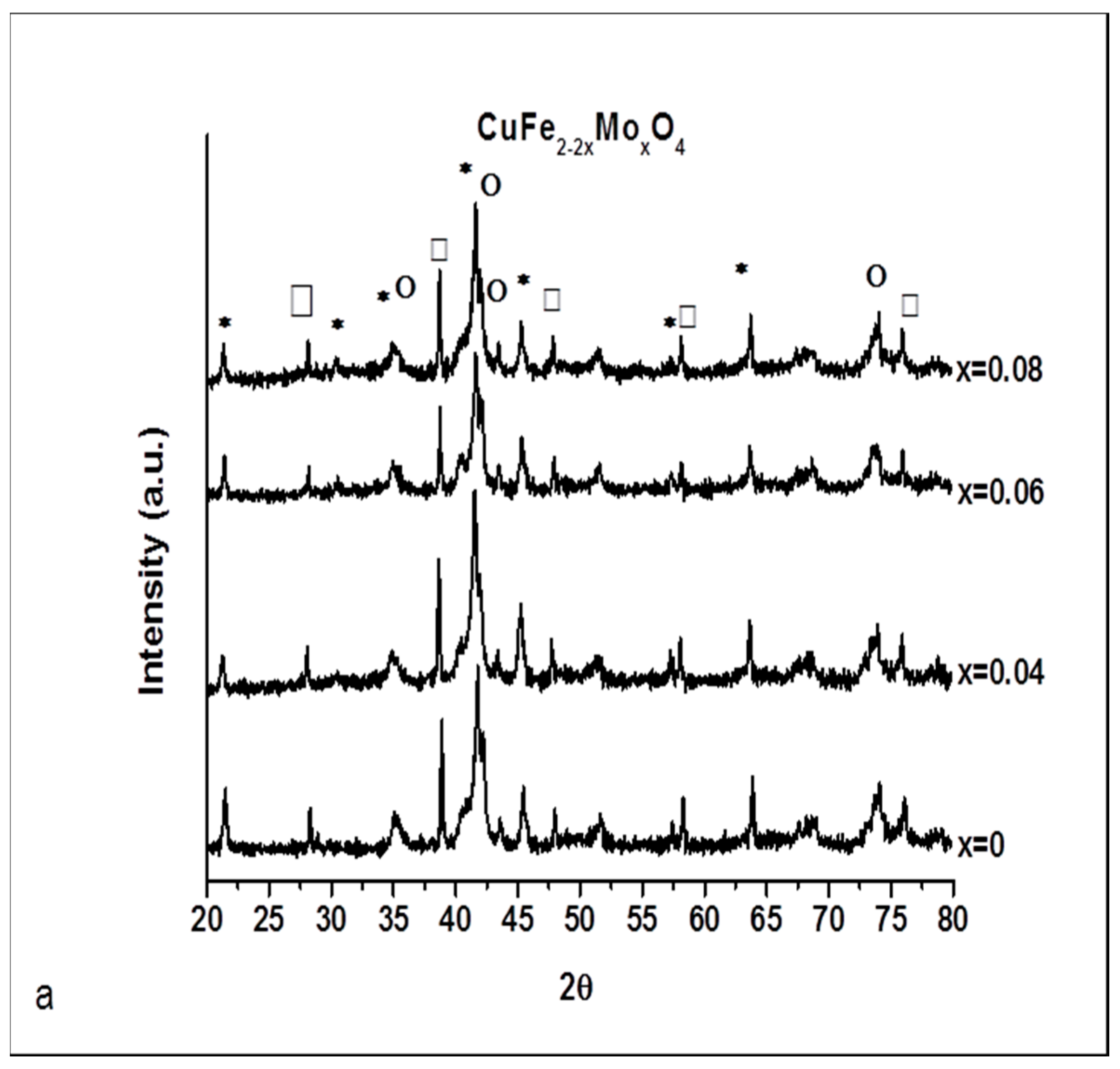
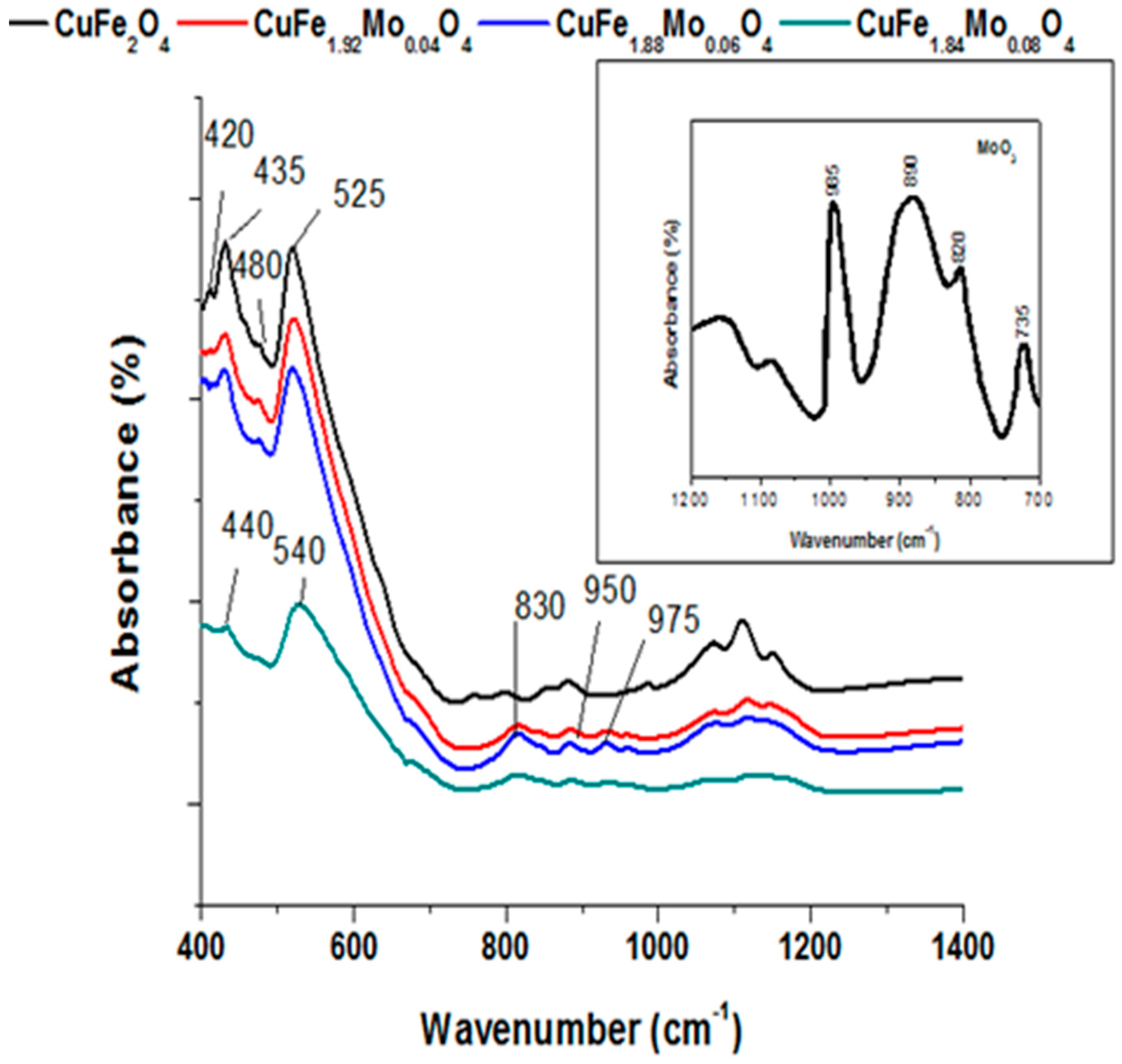
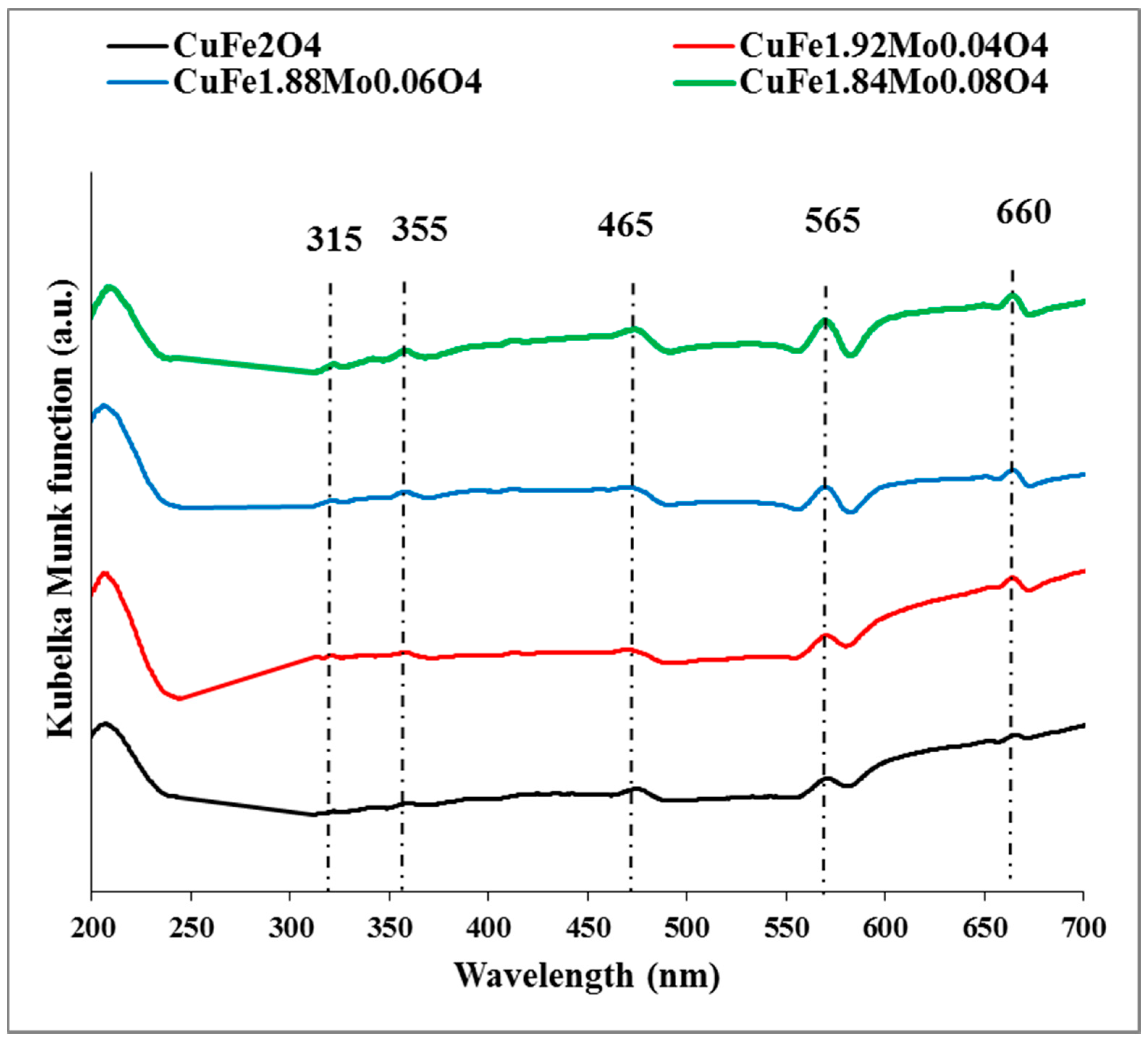
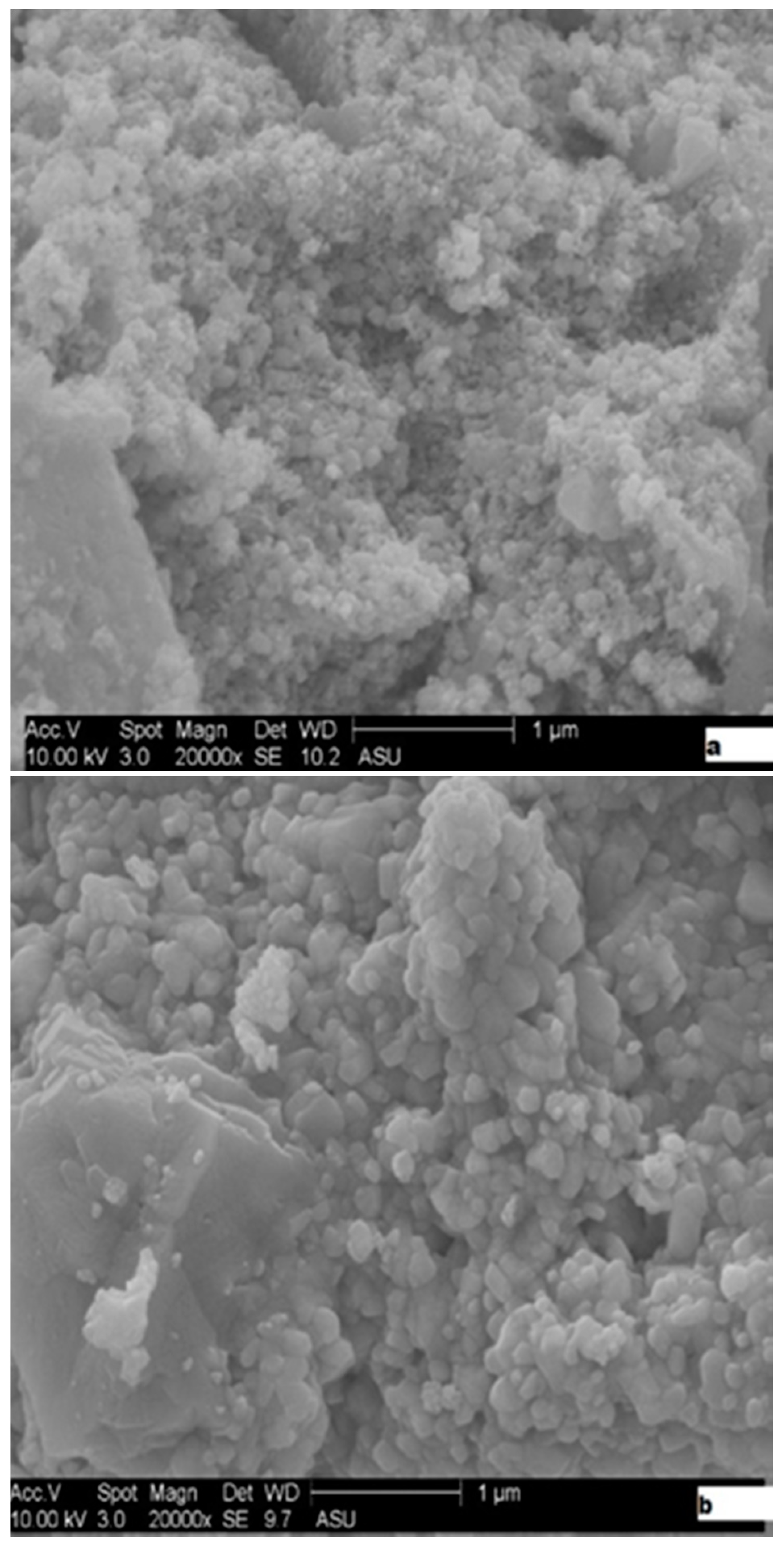
2.1. Catalysts Characterization
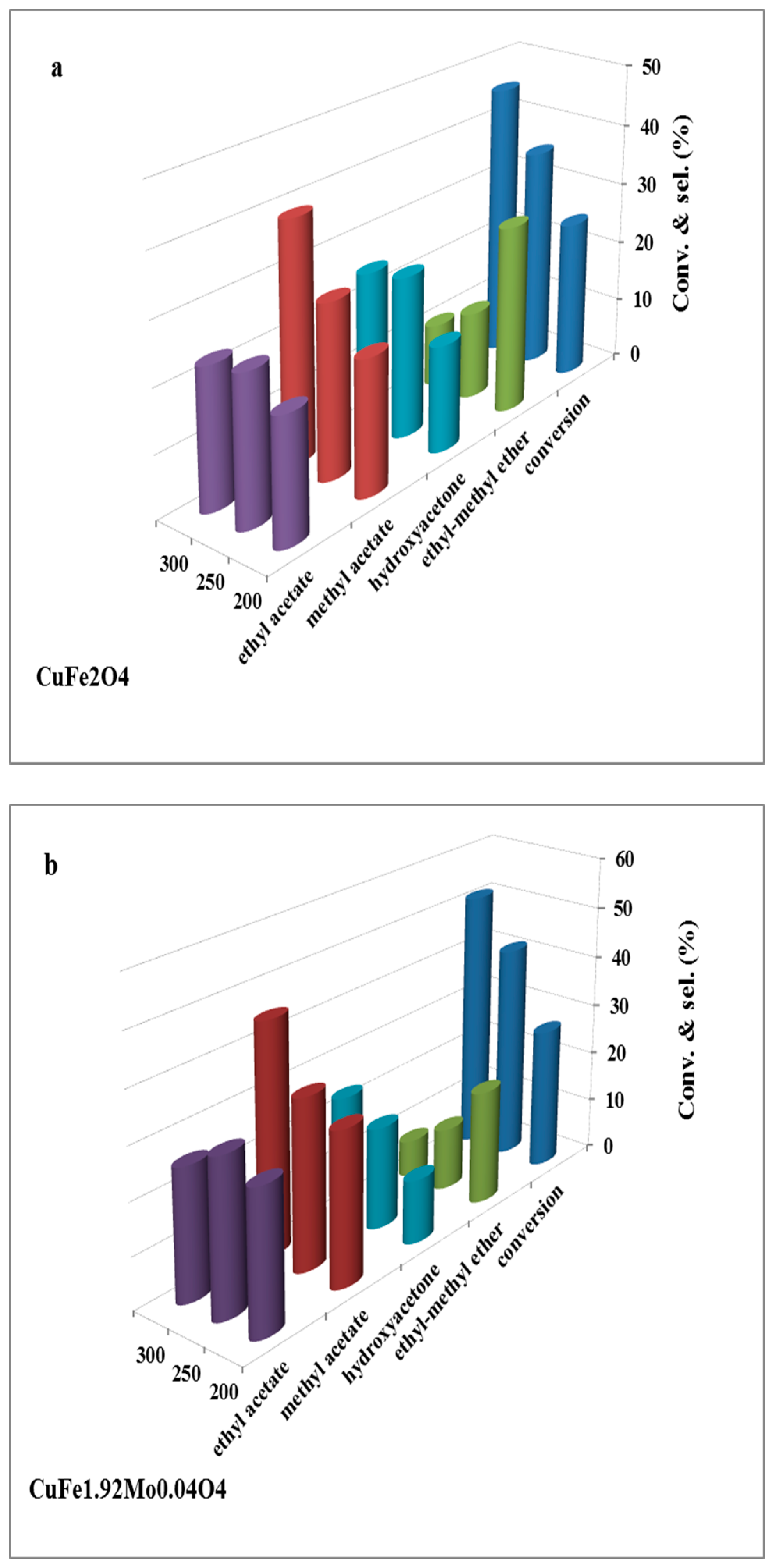
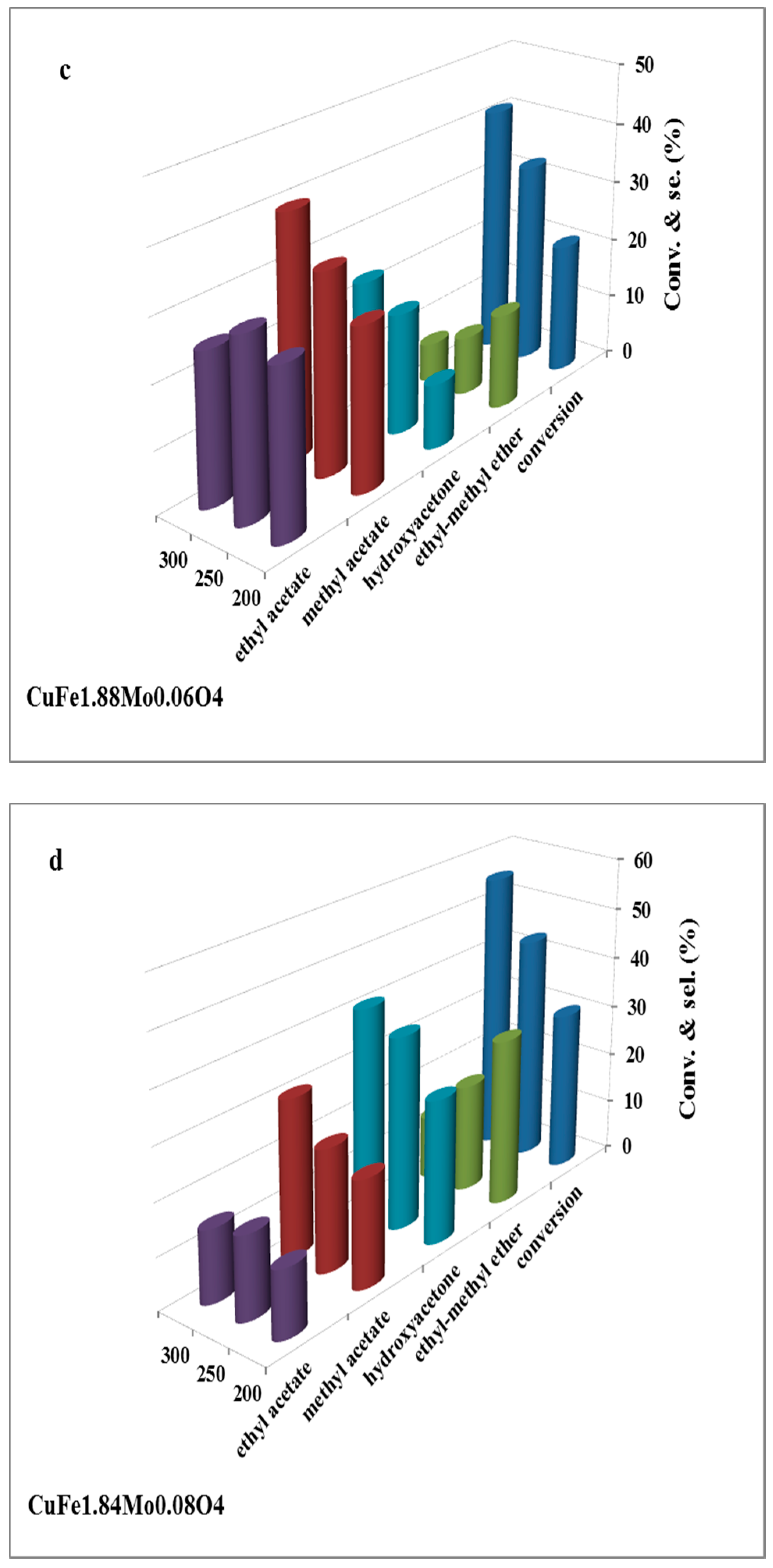
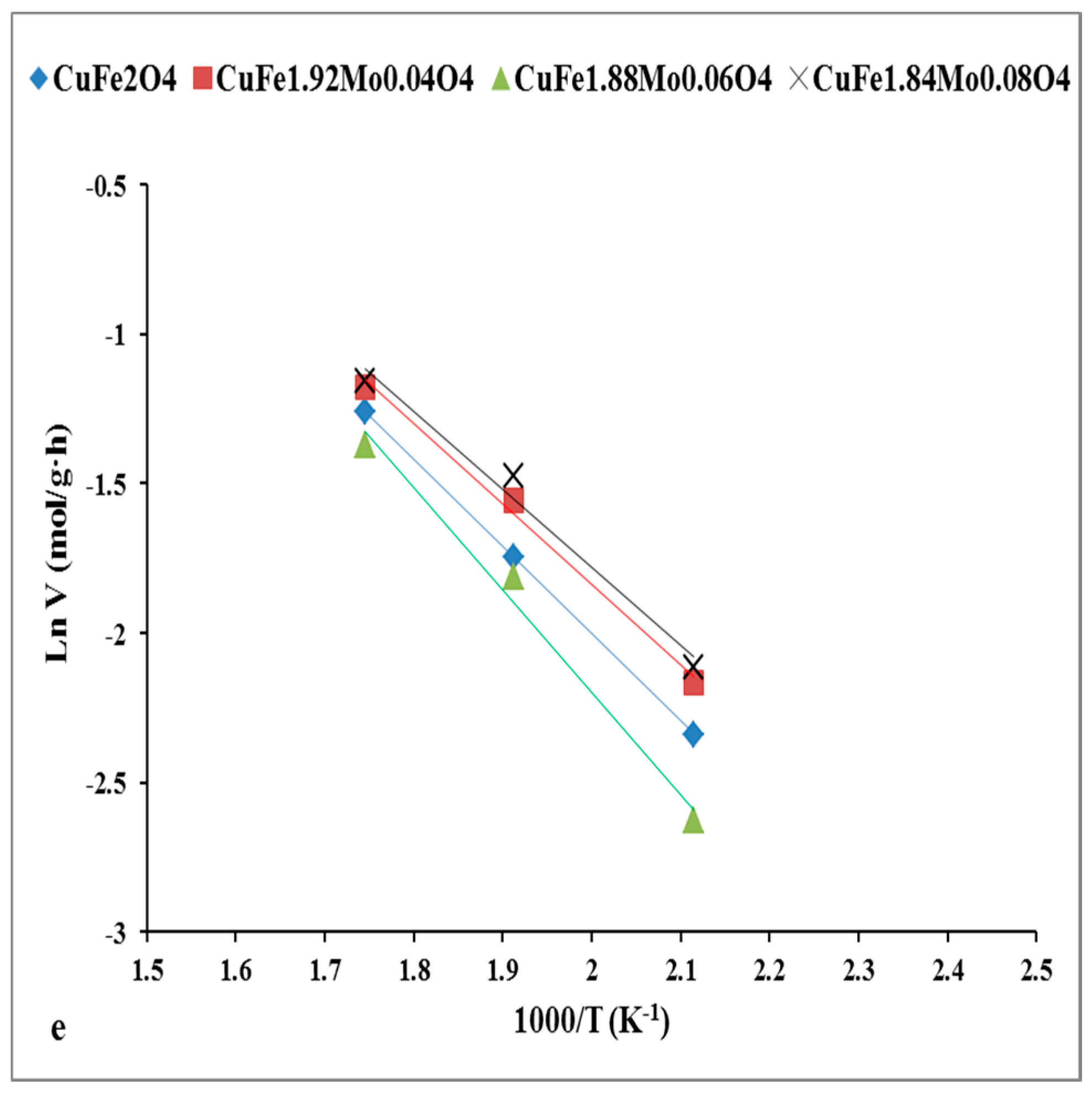
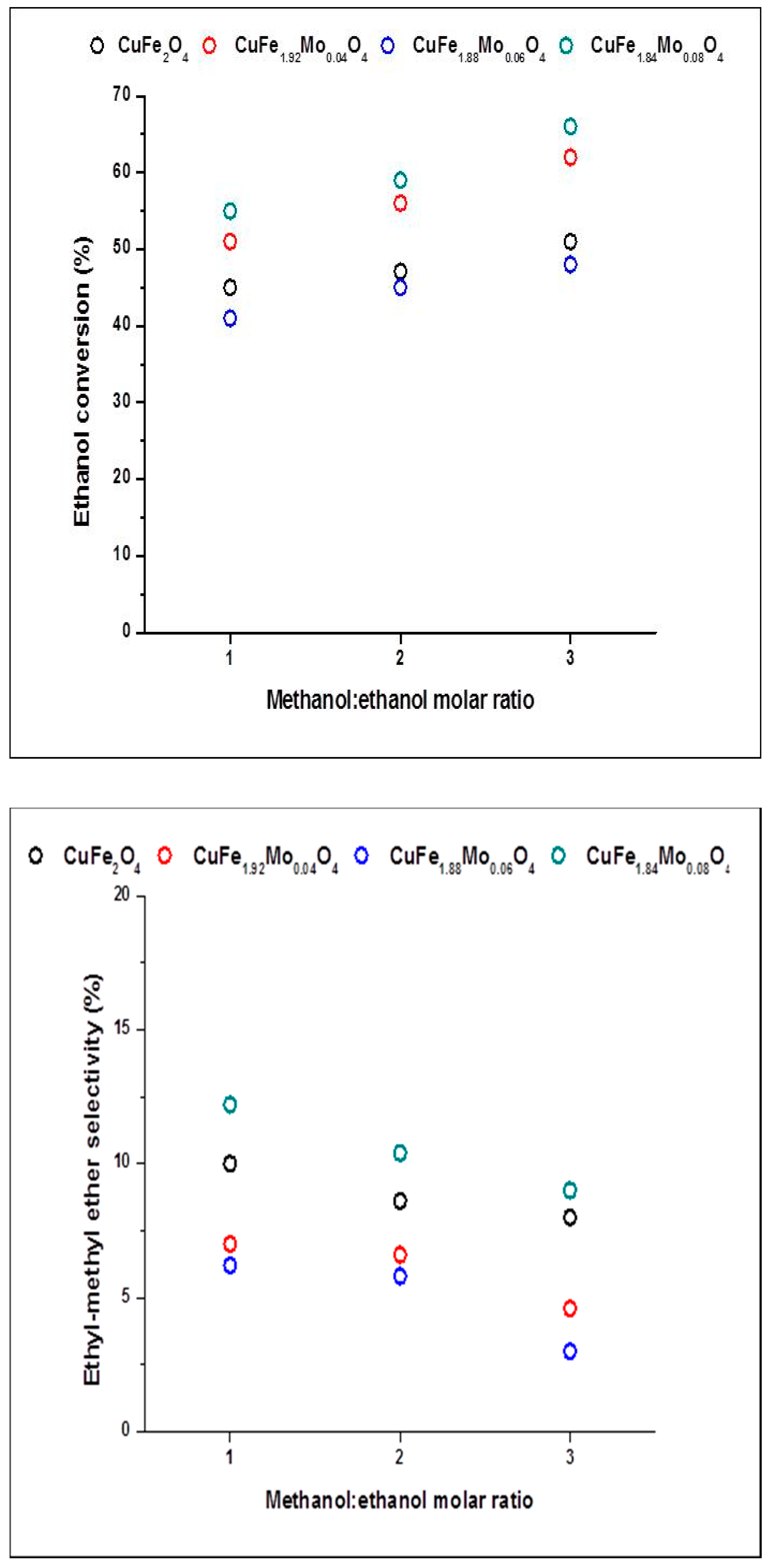
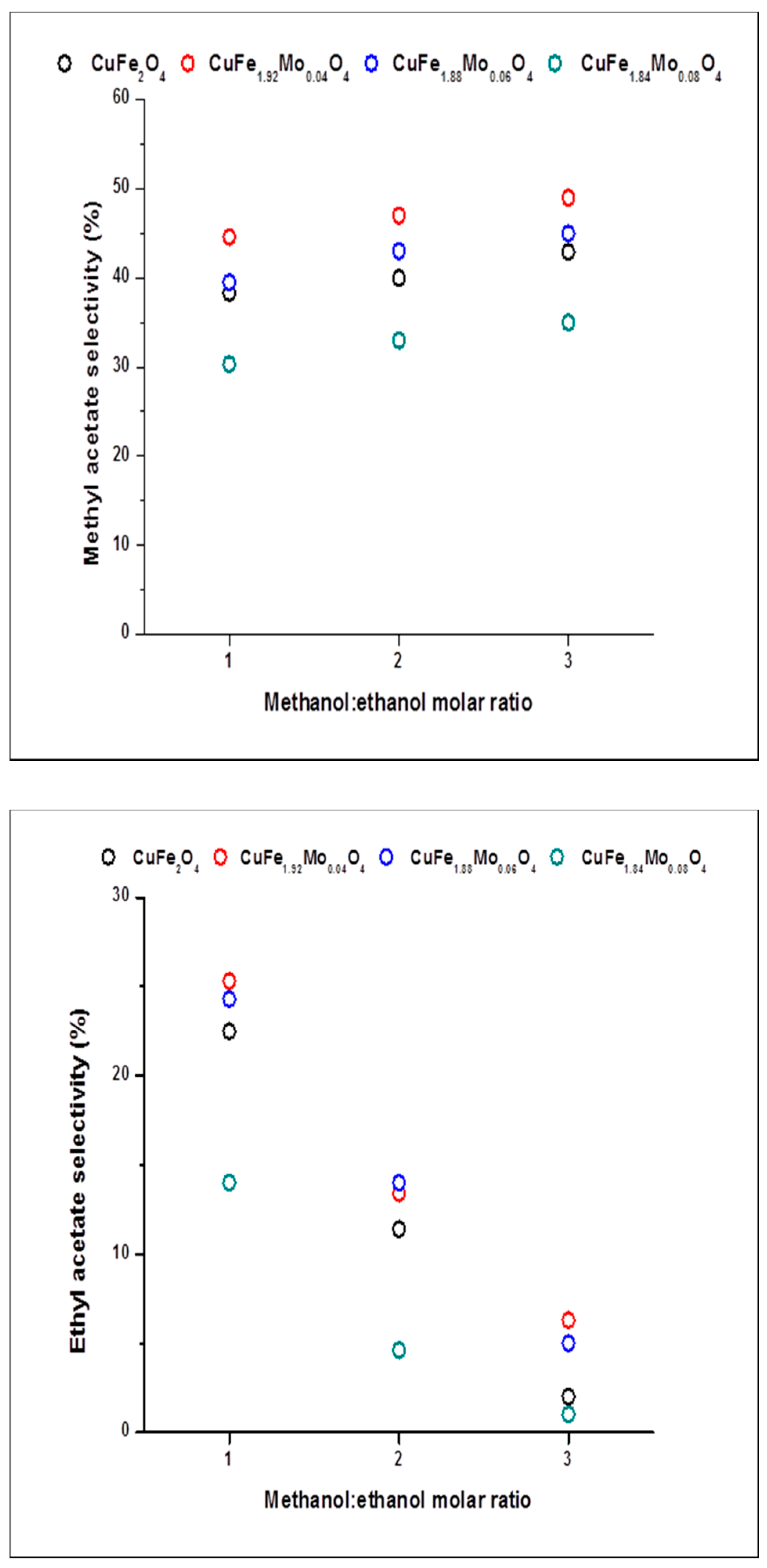
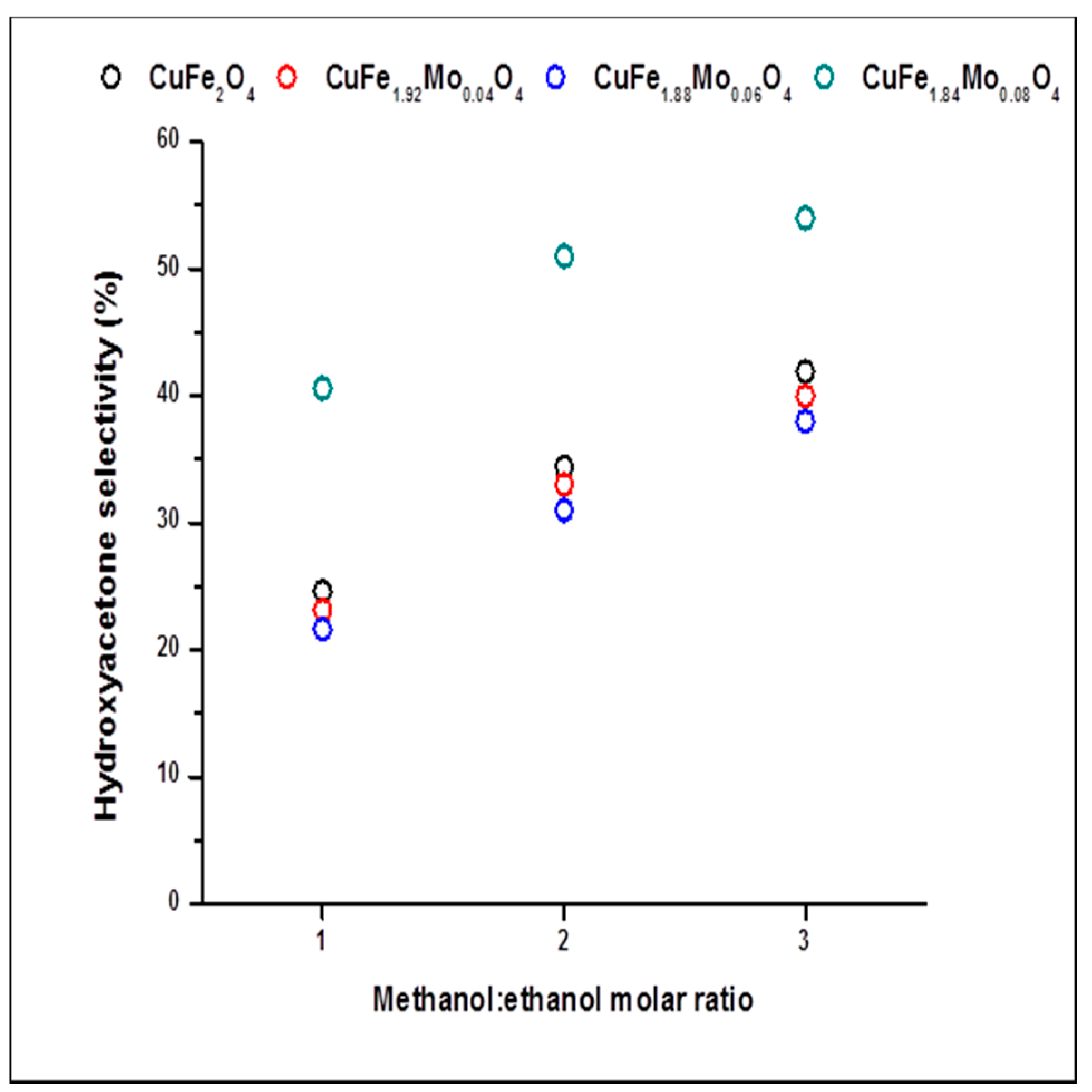
2.2. Catalysts Activity
2.3. Discussion
3. Experimental Part
3.1. Materials and Catalysts’ Synthesis
3.2. Catalysts’ Characterization
3.3. Catalytic Experiments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- León, M.; Díaz, E.; Ordónez, S. Ethanol catalytic condensation over Mg–Al mixed oxides derived from hydrotalcites. Catal. Today 2011, 164, 436–442. [Google Scholar] [CrossRef]
- Stošić, D.F.; Hosoglu, S.; Bennici, A.; Travert, M.; Capron, F.; Dumeignil, J.-L.; Couturier, J.-L.; Dubois, A.; Auroux, A. Methanol and ethanol reactivity in the presence of hydrotalcites with Mg/Al ratios varying from 2 to 7. Catal. Commun. 2017, 89, 14–18. [Google Scholar] [CrossRef]
- Matsumoto, T.; Ohishi, M.; Inoue, S. Selective cross-acyloin condensation catalyzed by thiazolium salt. Formation of 1-hydroxy 2-ones from formaldehyde and other aldehydes. J. Org. Chem. 1985, 50, 603–606. [Google Scholar] [CrossRef]
- Mohamad, M.H.; Awang, R.; Yunus, W.W. A Review of Acetol: Application and Production. Am. J. Appl. Sci. 2011, 8, 1135–1139. [Google Scholar] [CrossRef]
- Sato, S.; Akiyama, M.; Takahashi, R.; Hara, T.; Inui, K.; Yokota, M. Vapor-phase reaction of polyols over copper catalysts. Appl. Catal. A Gen. 2008, 347, 186–191. [Google Scholar] [CrossRef]
- Kinage, A.K.; Upare, P.P.; Kasinathan, P.; Hwang, Y.K.; Chang, J.S. Selective conversion of glycerol to acetol over sodium-doped metal oxide catalysts. Catal. Commun. 2010, 11, 620–623. [Google Scholar] [CrossRef]
- Manova, E.; Tsoncheva, T.; Paneva, D.; Mitov, I.; Tenchev, K.; Petrov, L. Mechanochemically synthesized nano-dimensional iron–cobalt spinel oxides as catalysts for methanol decomposition. Appl. Catal. A Gen. 2004, 277, 119–127. [Google Scholar] [CrossRef]
- Vozniuk, O.; Bazzo, C.; Albonetti, S.; Tanchoux, N.; Bosselet, F.; Millet, J.M.M.; Di Renzo, F.; Cavani, F. Structural Changes of Binary/Ternary Spinel Oxides During Ethanol Anaerobic Decomposition. ChemCatChem 2017, 9, 2219–2230. [Google Scholar] [CrossRef]
- Pardeshi, S.K.; Pawar, R.Y. Optimization of reaction conditions in selective oxidation of styrene over fine crystallite spinel-type CaFe2O4 complex oxide catalyst. Mater. Res. Bull. 2010, 45, 609–615. [Google Scholar] [CrossRef]
- Florea, M.; Alifanti, M.; Parvulescu, V.I.; Mihaila-Tarabasanu, D.; Diamandescu, L.; Feder, M.; Negrila, C.; Frunza, L. Total oxidation of toluene on ferrite-type catalysts. Catal. Today 2009, 141, 361–366. [Google Scholar] [CrossRef]
- Velinov, N.; Petrova, T.; Genova, I.; Ivanov, I.; Tsoncheva, T.; Idakiev, V.; Kunev, B.; Mitov, I. Synthesis and Mössbauer spectroscopic investigation of copper-manganese ferrite catalysts for water-gas shift reaction and methanol decomposition. Mater. Res. Bull. 2017, 95, 556–562. [Google Scholar] [CrossRef]
- Han, H.Y.; Wang, S.F.; Tsai, A.P.; Kameoka, S. Catalysts prepared from copperenickel ferrites for the steam reforming of methanol. J. Power Sources 2015, 281, 138–145. [Google Scholar] [CrossRef]
- Kubacka, A.; Fernández-García, M.; Martínez-Arias, A. Catalytic hydrogen production through WGS or steam reforming of alcohols over Cu, Ni and Co catalysts. Appl. Catal. A Gen. 2016, 518, 2–17. [Google Scholar] [CrossRef]
- Hu, W.; Li, D.; Yang, Y.; Li, T.; Chen, H.; Liu, P. Copper ferrite supported gold nanoparticles as efficient and recyclable catalyst for liquid-phase ethanol oxidation. J. Catal. 2018, 357, 108–117. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, M.; Khajuria, H.; Sharma, S.; Nawaz Sheikh, H. Studies on hydrothermal synthesis of photolumniscent rare earth (Eu3+ & Tb3+) doped NG@FeMoO4 for enhanced visible light photodegradation of methylene blue dye. Solid State Sci. 2018, 76, 38–47. [Google Scholar] [CrossRef]
- Fell, C.R.; Qian, D.; Carroll, K.J.; Chi, M.; Jones, J.L.; Meng, Y.S. Correlation Between Oxygen Vacancy, Microstrain, and Cation Distribution in Lithium-Excess Layered Oxides During the First Electrochemical Cycle. Chem. Mater. 2013, 25, 1621–1629. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Y.; Yin, L.; Chen, C.; Zhang, Y.; Li, D. Y type zeolites/PI membranes for sulfur-free hydrogen source and for fuel cell applications. Int. J. Hydrog. Energy 2014, 39, 642–643. [Google Scholar] [CrossRef]
- Dodi, G.; Hritcu, D.; Draganescu, D.; Radu, D.A.; Popa, M.I. Hexagonal-shaped aminosilane magnetite nanoparticles: Preparation, characterization and hybrid film deposition. Colloids Surf. 2018, 542, 21–30. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, B.; Su, K.; Gu, Y.; Xi, P.; Guo, M. The synthesis of diphenyl carbonate from dimethyl carbonate and phenol over mesoporous MoO3/SiMCM-41. J. Mol. Catal. A Chem. 2008, 289, 100–105. [Google Scholar] [CrossRef]
- Rahimi-Nasrabadi, M.; Mahdi Pourmortazavi, S.; Khalilian-Shalamzar, M. Facile chemical synthesis and structure characterization of copper molybdate nanoparticles. J. Mol. Struct. 2015, 1083, 229–235. [Google Scholar] [CrossRef]
- Thielemann, J.P.; Ressler, T.; Walter, A.; Tzolova-Müller, G.; Hess, C. Structure of molybdenum oxide supported on silica SBA-15 studied by Raman, UV–Vis and X-ray absorption spectroscopy. Appl. Catal. A Gen. 2011, 399, 28–34. [Google Scholar] [CrossRef]
- Bravo-Suarez, J.J.; Subramaniam, B.; Chaudhari, R.V. Vapor-phase methanol and ethanol coupling reactions on CuMgAl mixed metal oxides. Appl. Catal. A Gen. 2013, 455, 234–246. [Google Scholar] [CrossRef]
- Tahir, D.; Tougaard, S. Electronic and optical properties of Cu, CuO and Cu2O studied by electron spectroscopy. J. Phys. Condens. Matter 2012, 24, 175002–175010. [Google Scholar] [CrossRef] [PubMed]
- Grissa, R.; Martinez, H.; Pelé, V.; Cotte, S.; Pecquenard, B.; Le Cras, F. An X-ray photoelectron spectroscopy study of the electrochemical behaviour of iron molybdate thin films in lithium and sodium cells. J. Power Sources 2017, 342, 796–807. [Google Scholar] [CrossRef]
- Lee, Y.J.; Barrera, D.; Luo, K.; Hsu, J.W.P. Hindawi Publishing Corporation, In Situ Chemical Oxidation of Ultrasmall MoOx Nanoparticles in Suspensions. J. Nanotechnol. 2012, 195761. [Google Scholar] [CrossRef]
- Wang, X.; Kang, Q.; Li, D. Catalytic combustion of chlorobenzene over MnOx–CeO2 mixed oxide catalysts. Appl. Catal. B Environ. 2009, 86, 166–175. [Google Scholar] [CrossRef]
- Li, H.; Lu, G.; Dai, Q.; Wang, Y.; Guo, Y.; Guo, Y. Efficient low-temperature catalytic combustion of trichloroethylene over flower-like mesoporous Mn-doped CeO2 microspheres. Appl. Catal. B Environ. 2011, 102, 475–483. [Google Scholar] [CrossRef]
- Fan, J.; Dai, Y.; Li, Y.; Zheng, N.; Guo, J.; Yan, X.; Stucky, D.G. Low-Temperature, Highly Selective, Gas-Phase Oxidation of Benzyl Alcohol over Mesoporous K-Cu-TiO2 with Stable Copper(I) Oxidation State. J. Am. Chem. Soc. 2009, 131, 15568–15569. [Google Scholar] [CrossRef]
- Di Cosimo, J.I.; Apesteguía, C.R.; Ginés, M.J.L.; Iglesia, E. Structural Requirements and Reaction Pathways in Condensation Reactions of Alcohols on MgyAlOx Catalysts. J. Catal. 2000, 190, 261–275. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Madix, R.J.; Friend, C.M. Achieving Optimum Selectivity in Oxygen Assisted Alcohol Cross-Coupling on Gold. J. Am. Chem. Soc. 2010, 132, 16571–16580. [Google Scholar] [CrossRef]
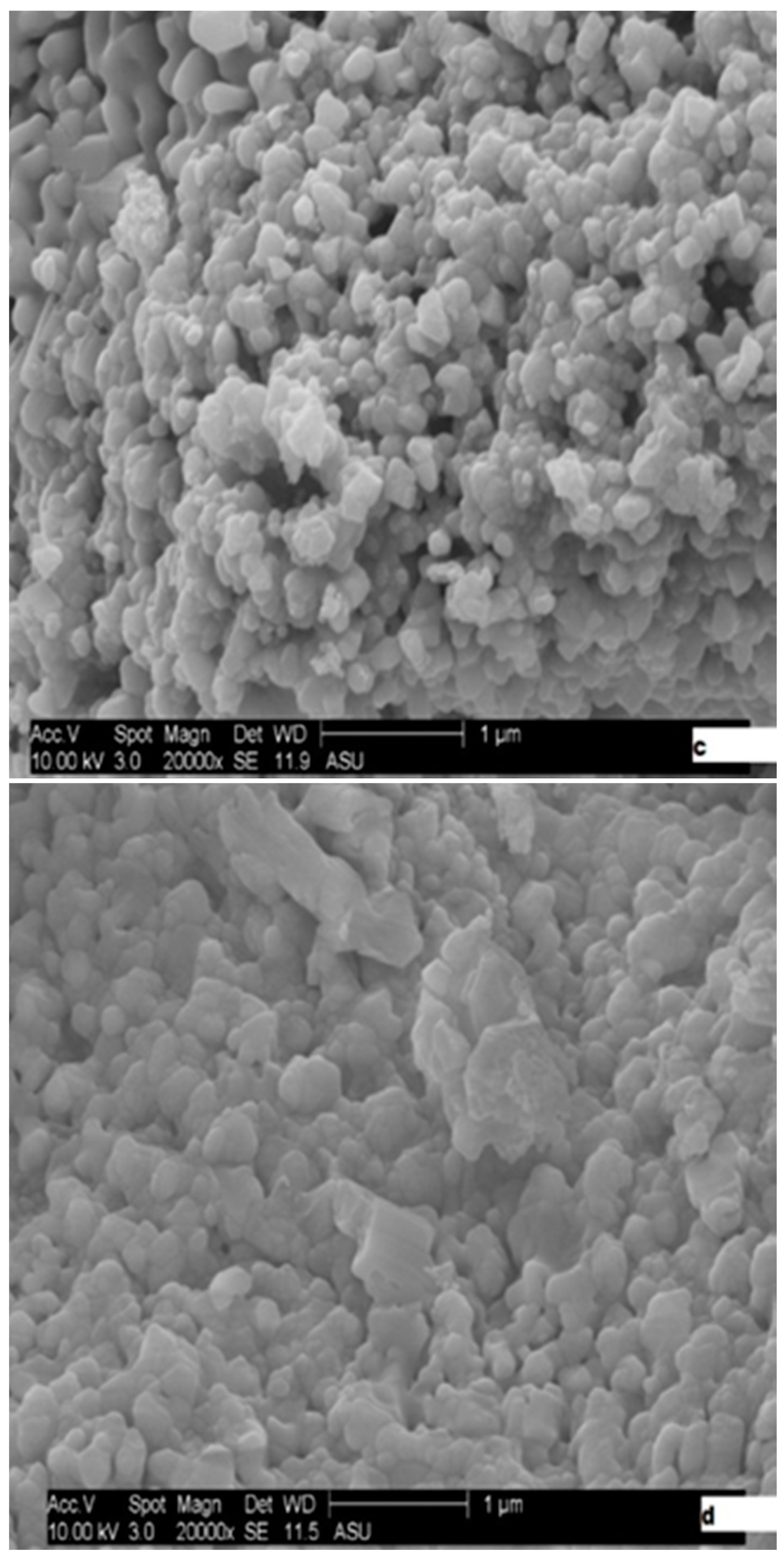
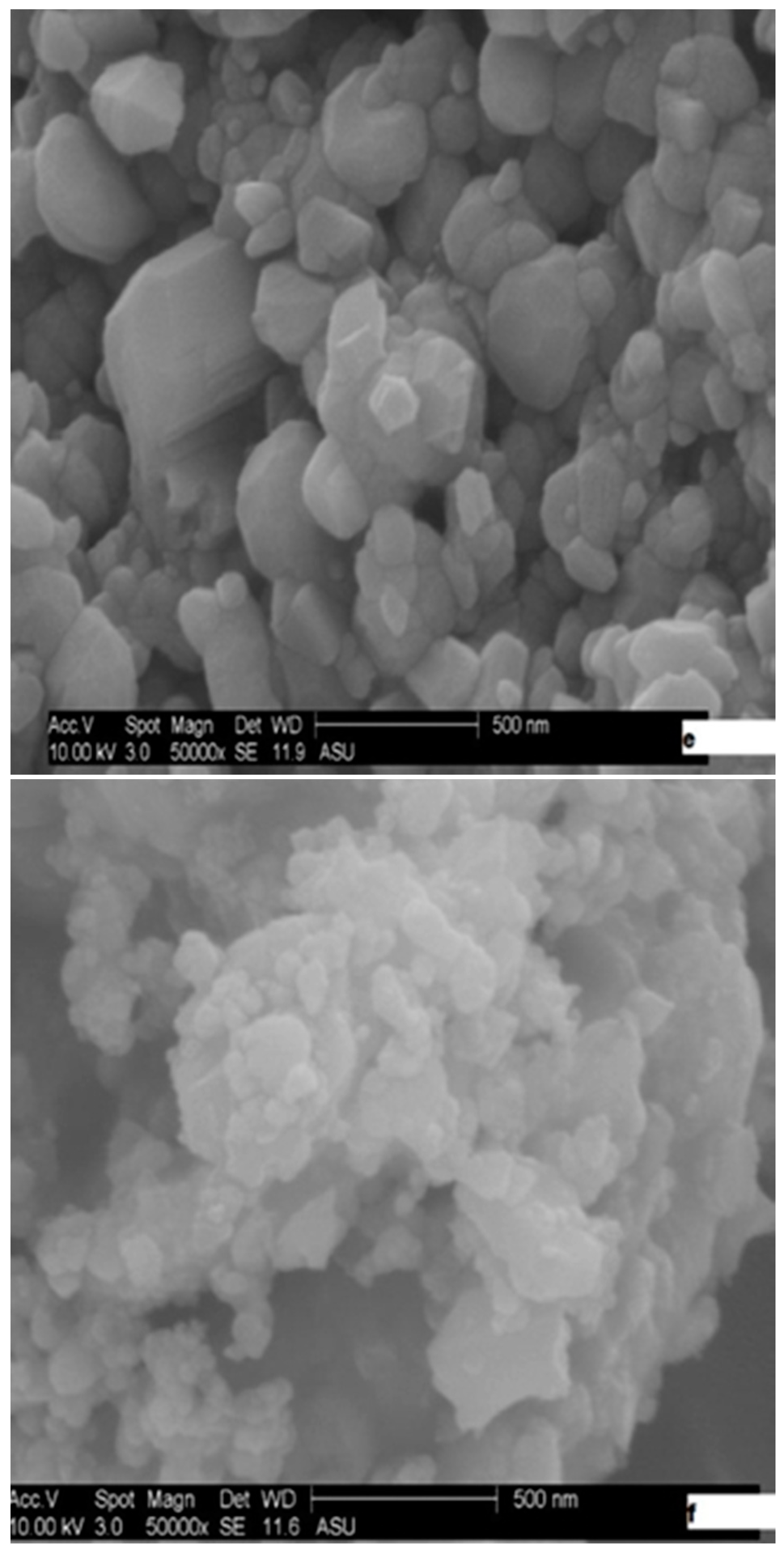
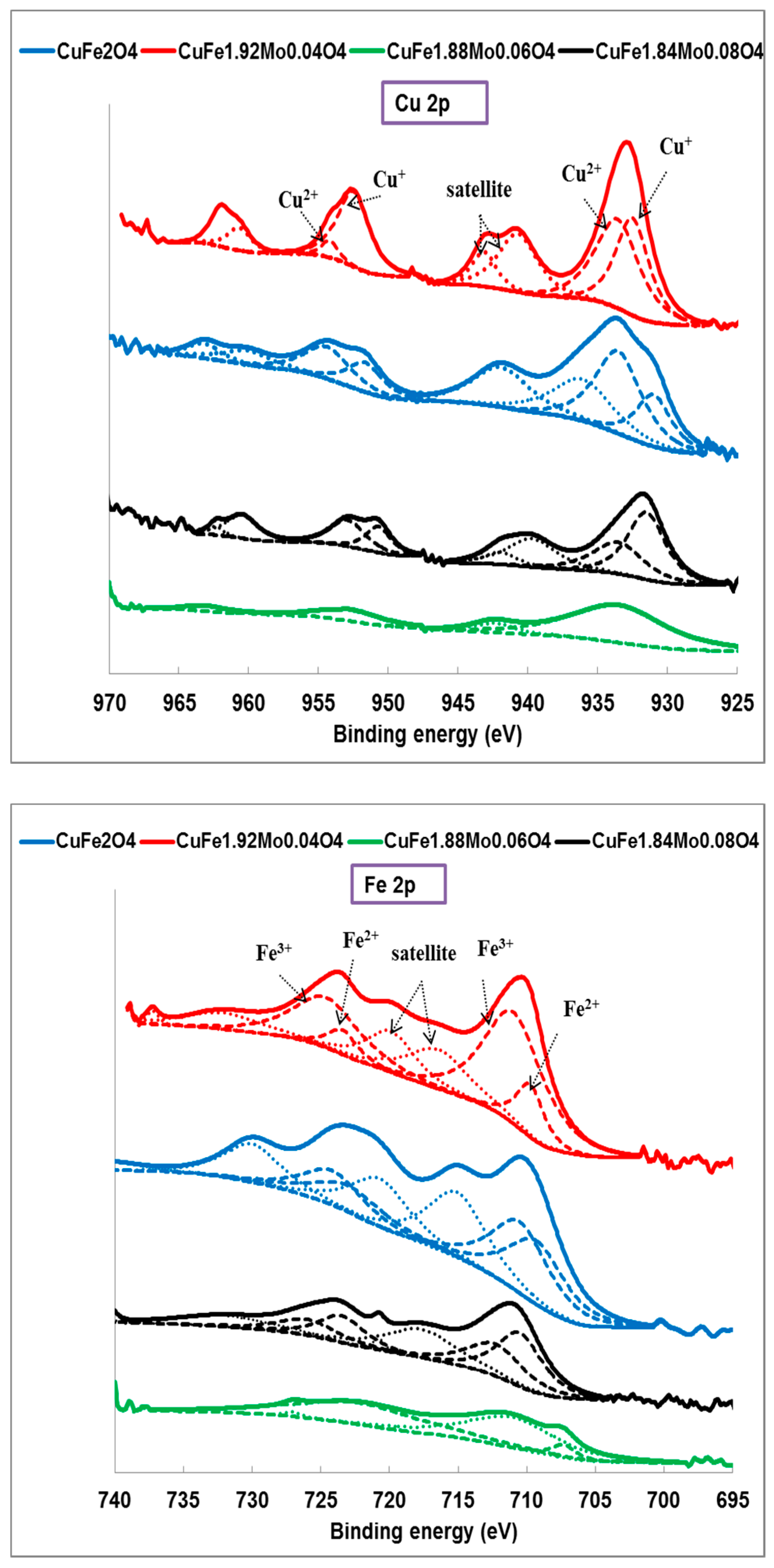
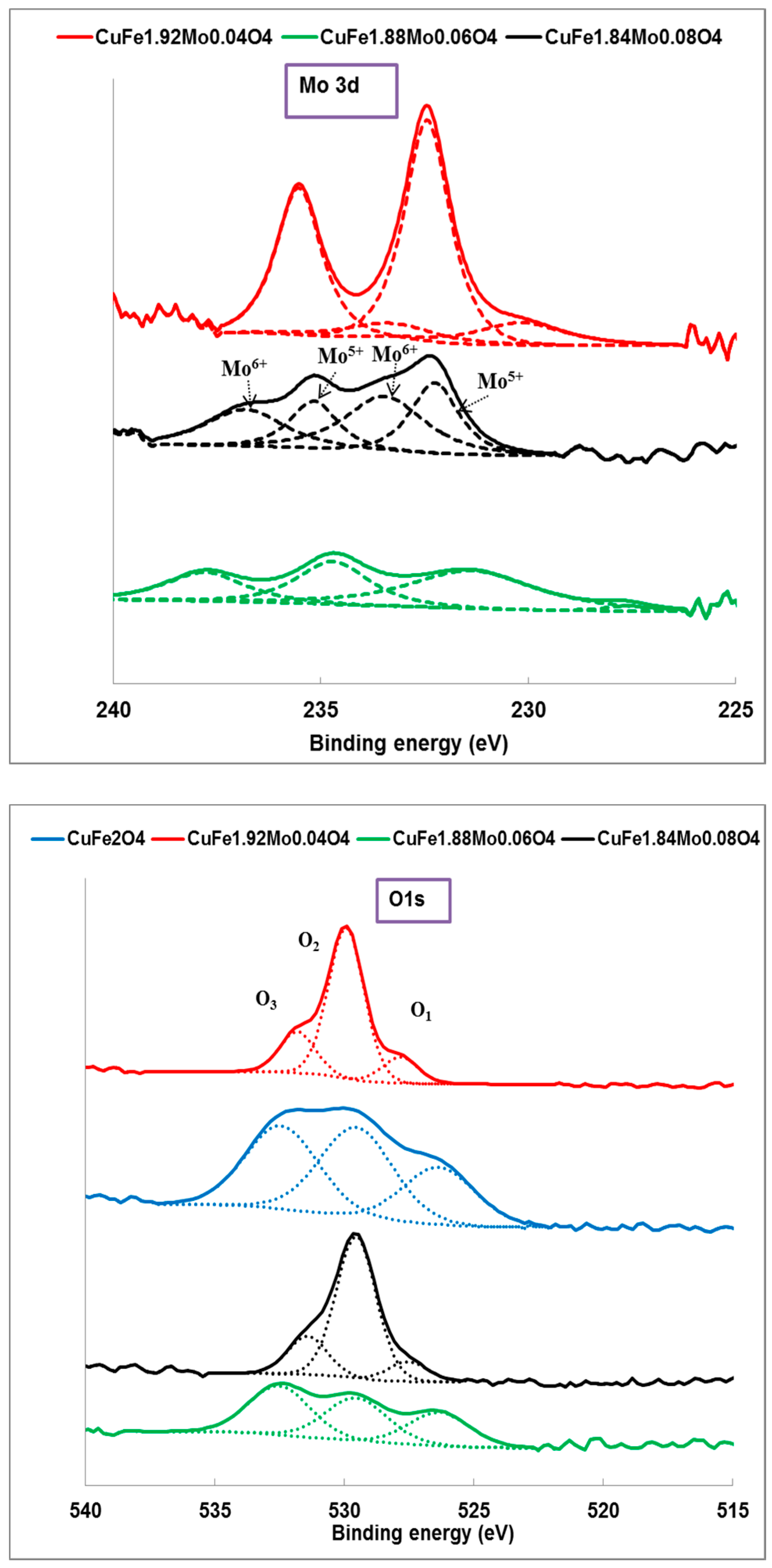

| Catalyst | Wt (%) | At (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Cu | Fe | Mo | O | Cu | Fe | Mo | O | |
| CuFe2O4 | 27.1 | 45.3 | – | 26.9 | 14.6 | 27.7 | – | 57.6 |
| CuFe1.92Mo0.04O4 | 38.7 | 40.5 | 1.7 | 19.1 | 24.0 | 28.5 | 0.7 | 46.8 |
| CuFe1.88Mo0.06O4 | 37.2 | 43.7 | 3.6 | 15.4 | 24.7 | 33.0 | 1.6 | 40.7 |
| CuFe1.84Mo0.08O4 | 24.0 | 45.0 | 5.2 | 25.9 | 13.3 | 28.2 | 1.9 | 56.7 |
| Catalyst | Cu+/Cu2+ | Fe2+/Fe3+ | Mo5+(Mo4+)/Mo6+ | Surface Composition At (%) | ||
|---|---|---|---|---|---|---|
| Cu | Fe | Mo | ||||
| CuFe2O4 | 0.5 | 0.9 | – | 9.1 | 18.2 | 0 |
| CuFe1.92Mo0.04O4 | 0.8 | 0.2 | 0.2 | 9.7 | 12.2 | 4.6 |
| CuFe1.88Mo0.06O4 | 0 | 0 | (0.05) | 8.7 | 13.2 | 4.3 |
| CuFe1.84Mo0.08O4 | 1.6 | 1.3 | 0.8 | 8.4 | 14.5 | 4.8 |
| CuFe1.84Mo0.08O4 (used) | 1.3 | 1.2 | 0.7 | 8.5 | 14.5 | 4.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitran, G.; Chen, S.; Seo, D.-K. Molybdenum Dopped Copper Ferrites as Active Catalysts for Alcohols Oxidative Coupling. Materials 2019, 12, 1871. https://doi.org/10.3390/ma12111871
Mitran G, Chen S, Seo D-K. Molybdenum Dopped Copper Ferrites as Active Catalysts for Alcohols Oxidative Coupling. Materials. 2019; 12(11):1871. https://doi.org/10.3390/ma12111871
Chicago/Turabian StyleMitran, Gheorghiţa, Shaojiang Chen, and Dong-Kyun Seo. 2019. "Molybdenum Dopped Copper Ferrites as Active Catalysts for Alcohols Oxidative Coupling" Materials 12, no. 11: 1871. https://doi.org/10.3390/ma12111871
APA StyleMitran, G., Chen, S., & Seo, D.-K. (2019). Molybdenum Dopped Copper Ferrites as Active Catalysts for Alcohols Oxidative Coupling. Materials, 12(11), 1871. https://doi.org/10.3390/ma12111871






