Alendronate Release from UHMWPE-Based Biomaterials in Relation to Particle Size of the GUR Powder for Manufacturing
Abstract
:1. Introduction
2. Materials and Methods
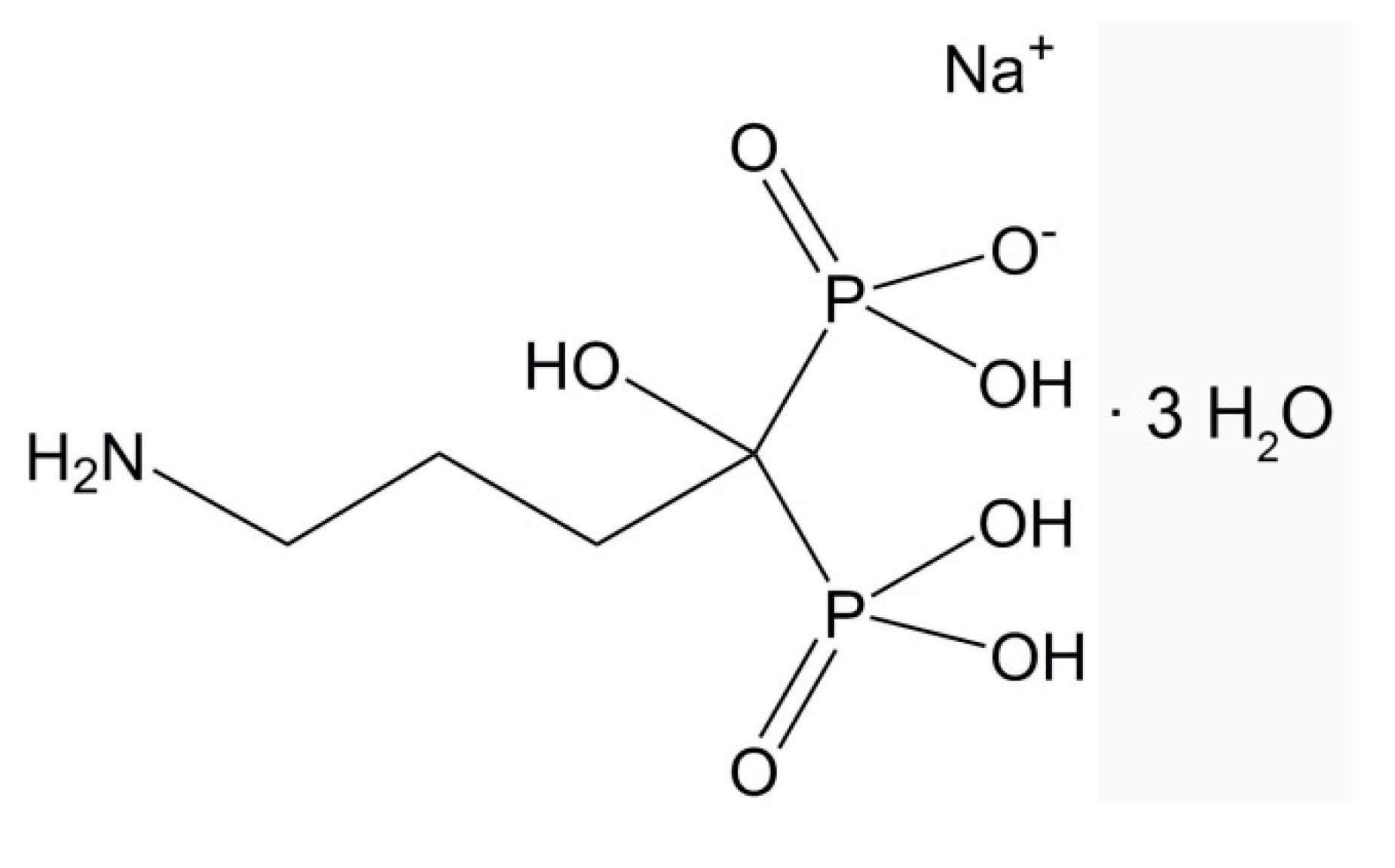
2.1. Reagents and Materials
2.2. Sample Preparation
2.3. Characterization of the GUR and the Molds
2.3.1. Particle Size
2.3.2. Weight and Dimensions
2.3.3. Surface Roughness
2.3.4. Tensile Testing
2.3.5. Crystallinity
2.4. Release Experiments
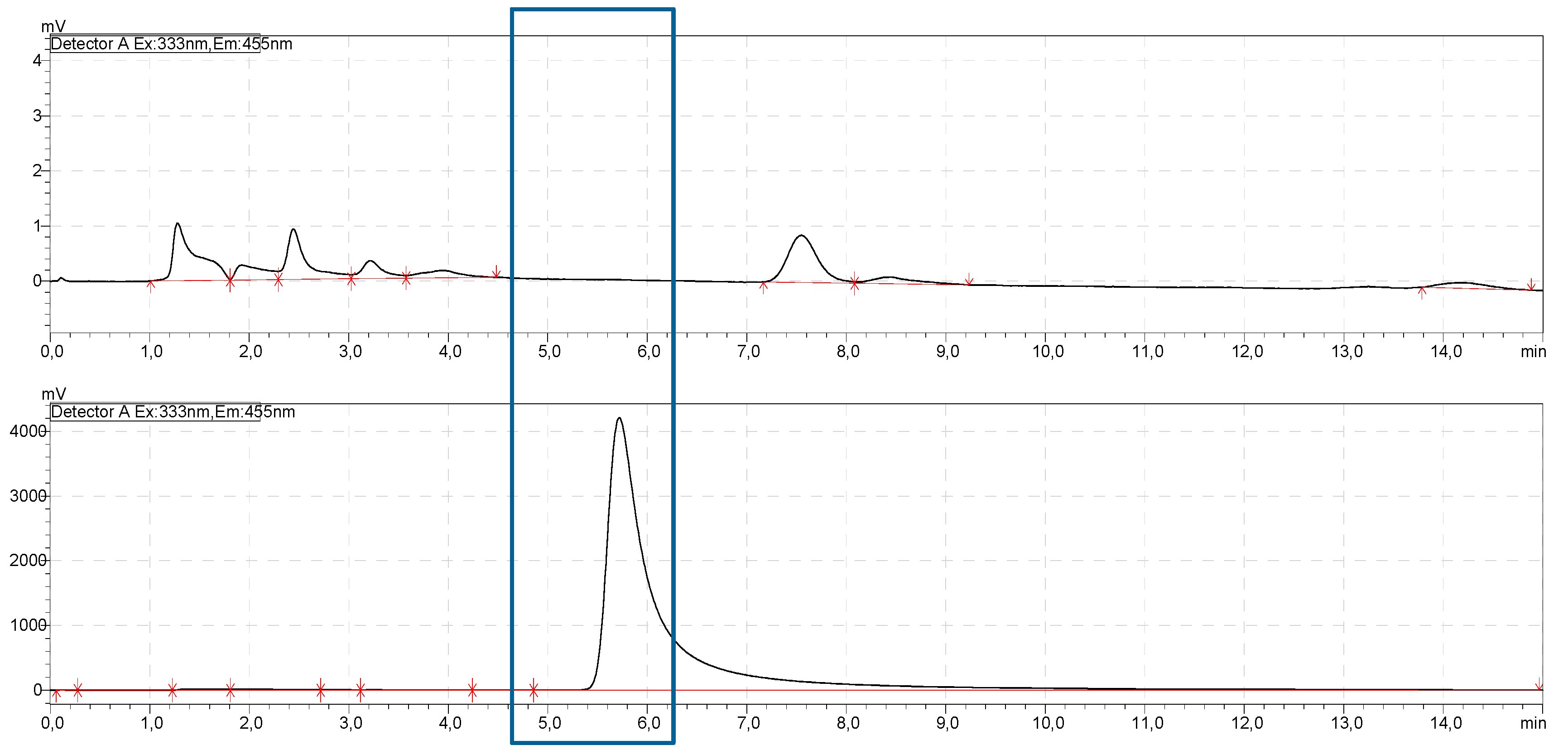
2.5. HPLC
2.5.1. Equipment
2.5.2. Buffer Preparation
2.5.3. Derivatization Solution
2.5.4. Chromatographic Settings
2.5.5. Calibration Curve
2.5.6. Preparing the Specimens
2.6. Biocompatibility Investigations
2.6.1. Live/Dead Assay
2.6.2. Cell Proliferation Test (WST)
2.6.3. LDH Test
2.7. Statistical Analysis
3. Results
3.1. GUR and Specimen Characterization
3.1.1. Particle Size
3.1.2. Weights and Dimensions
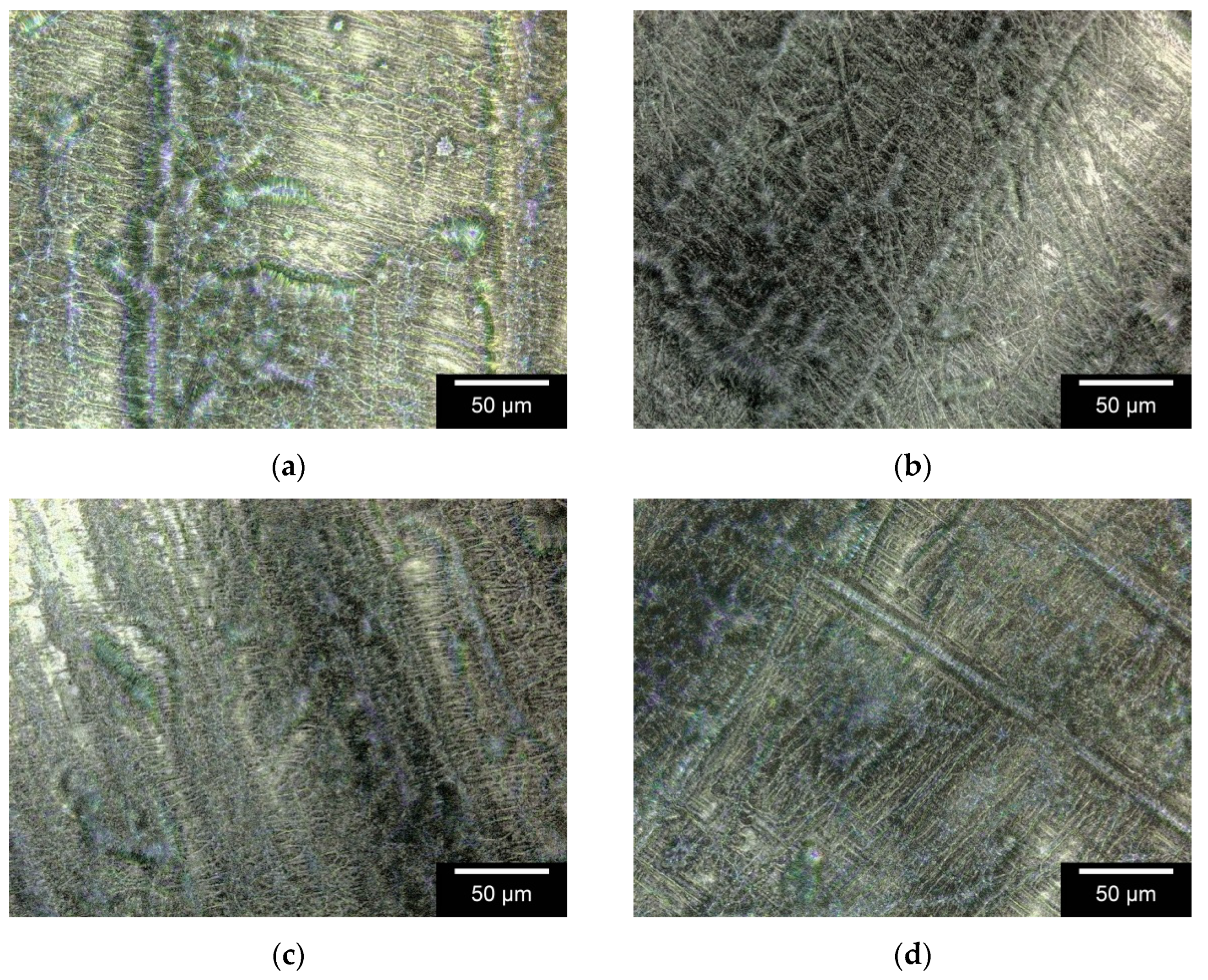
3.1.3. Surface Roughness
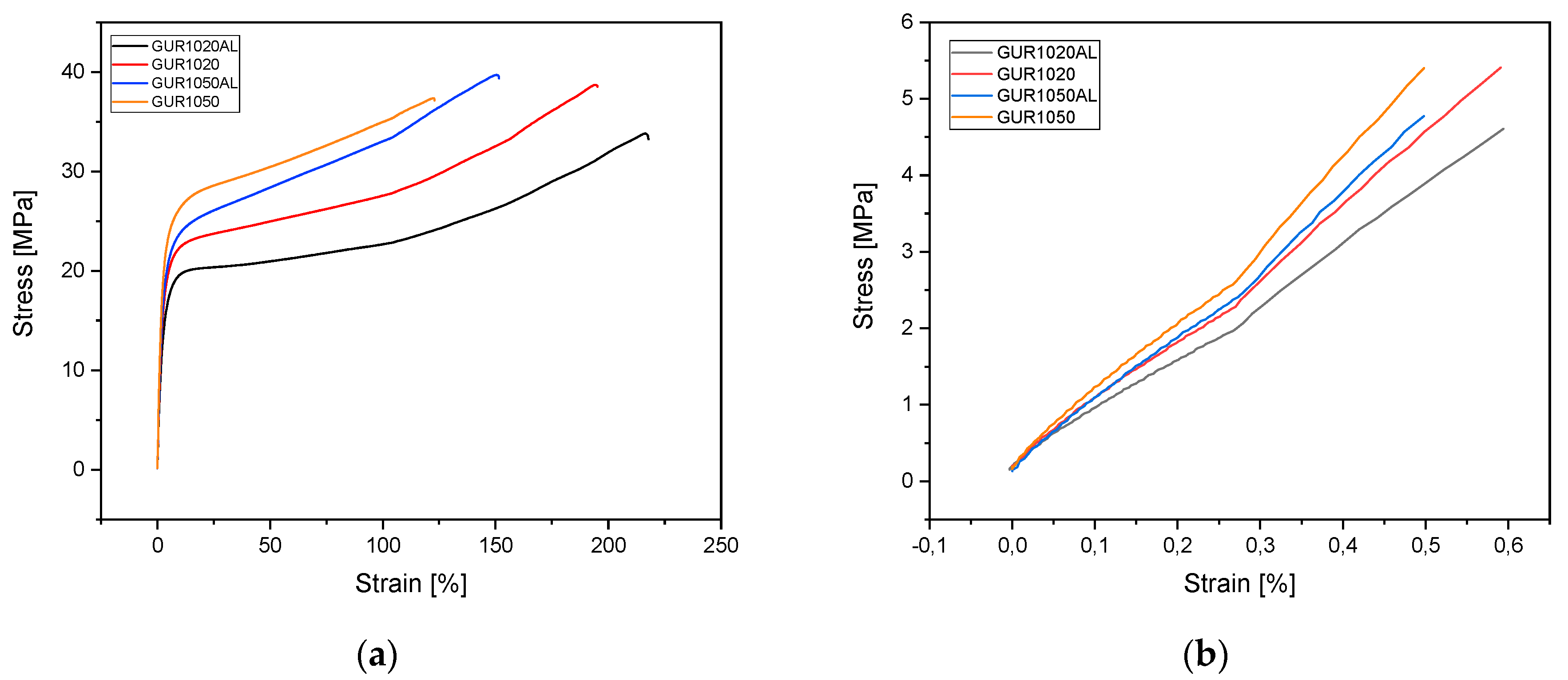
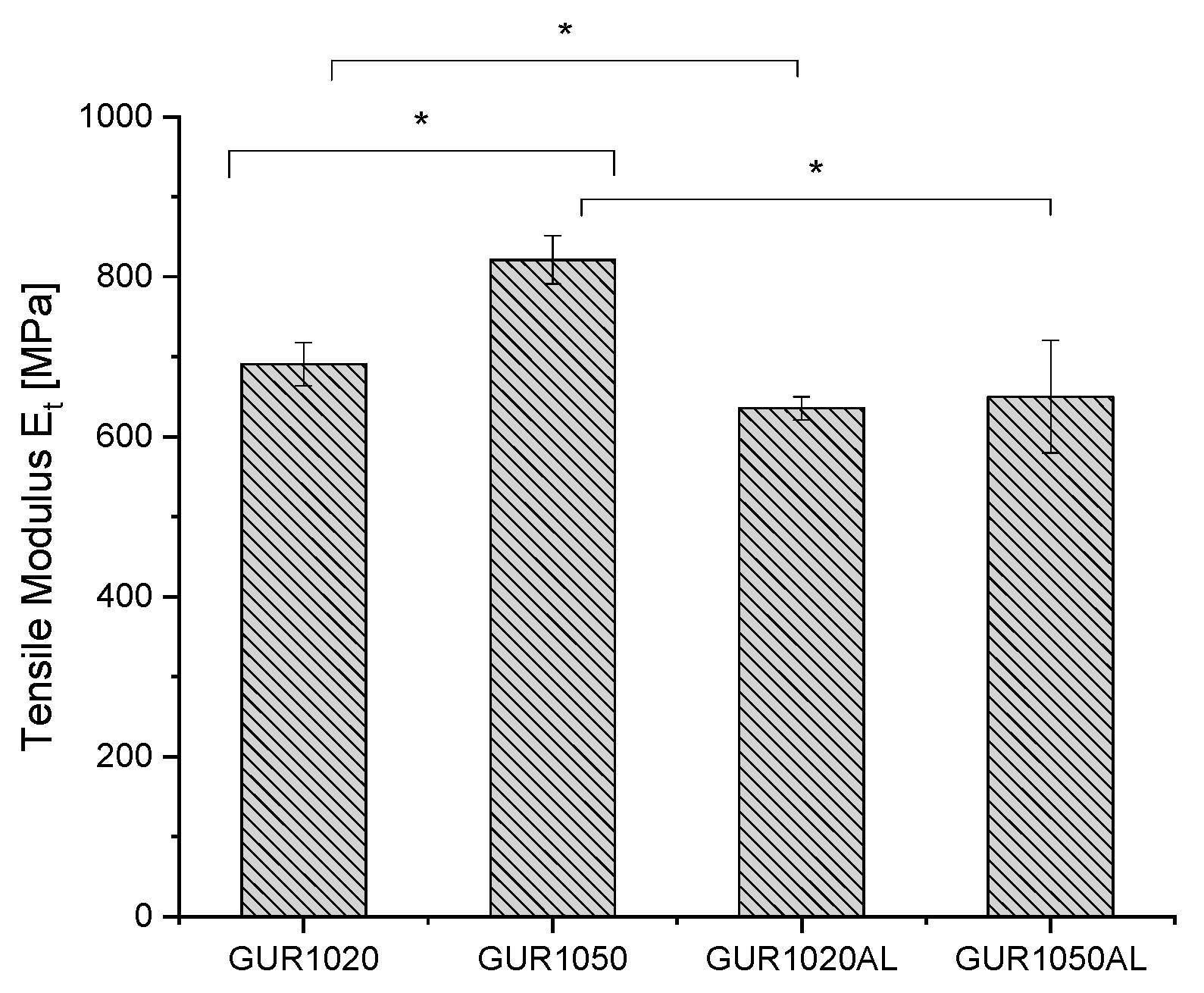
3.1.4. Tensile Tests
3.1.5. DSC
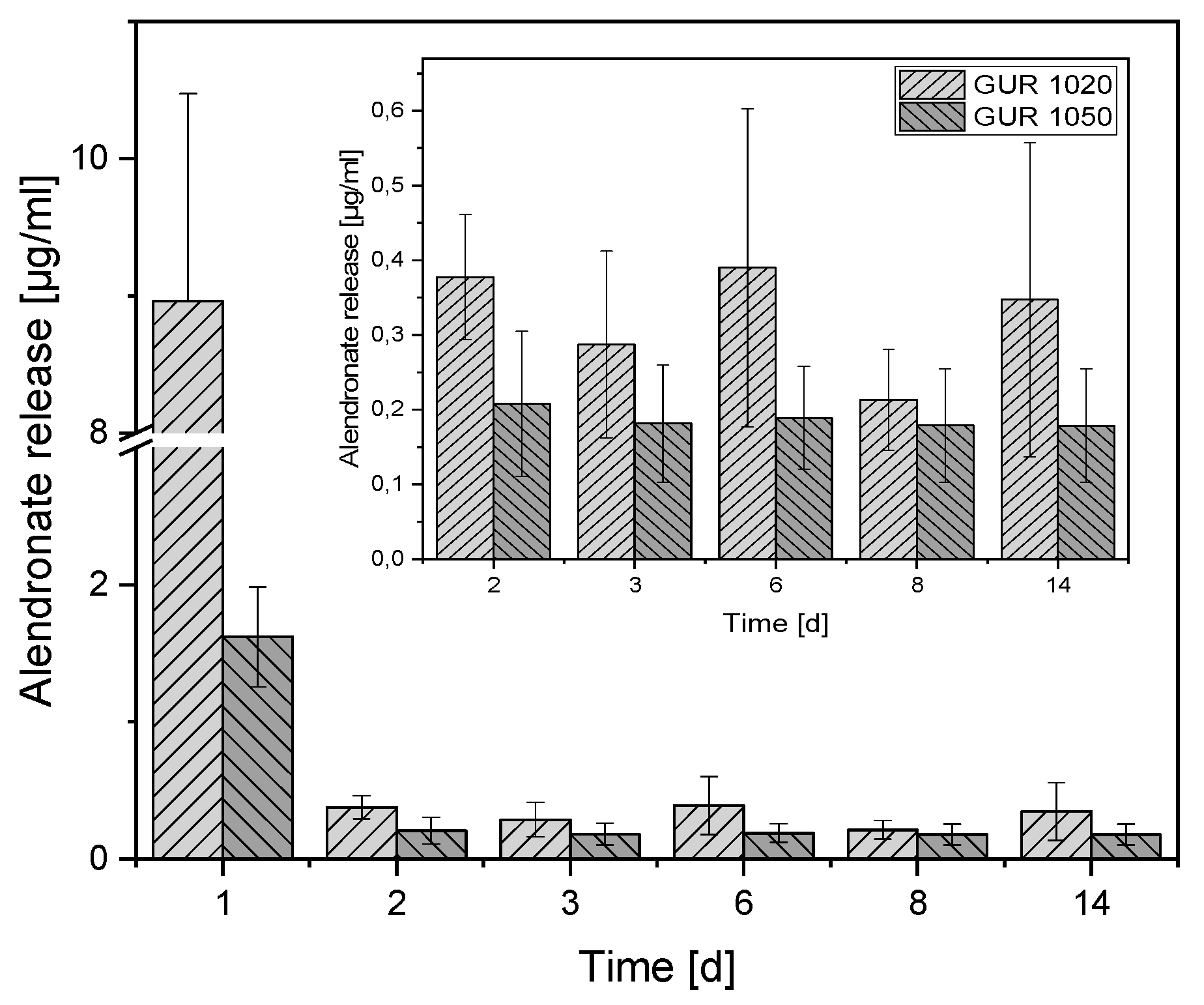
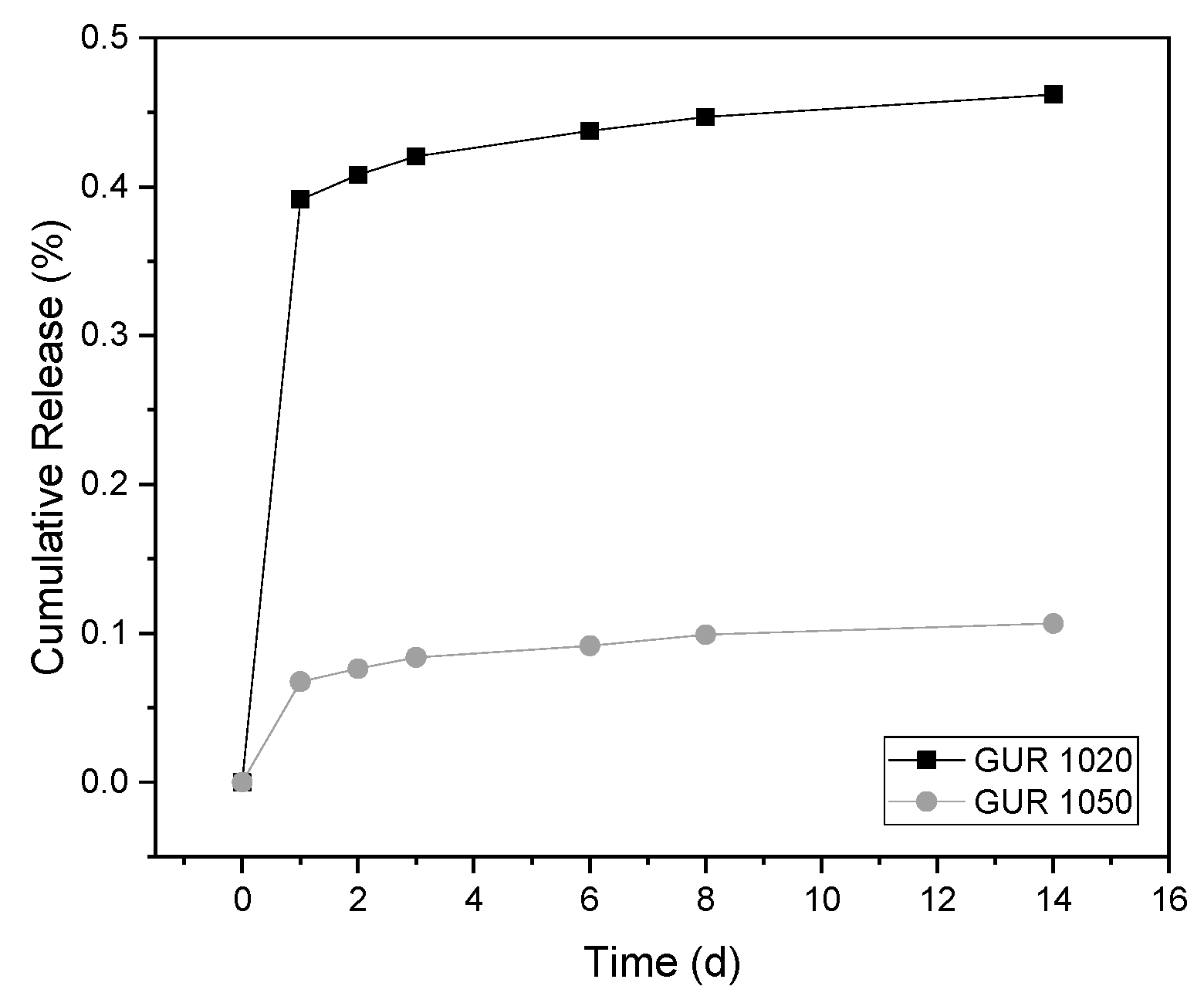
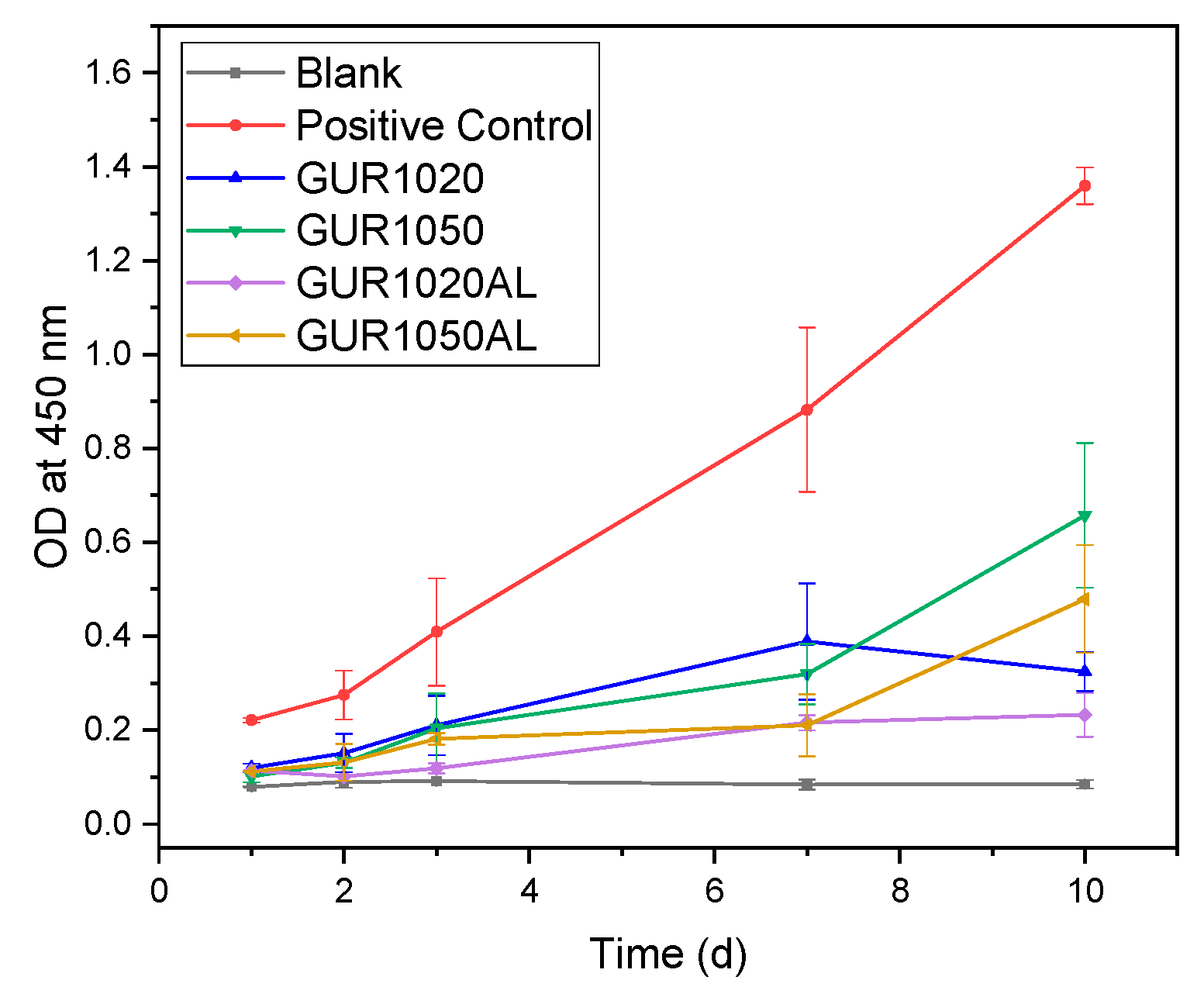
3.2. Release Experiments
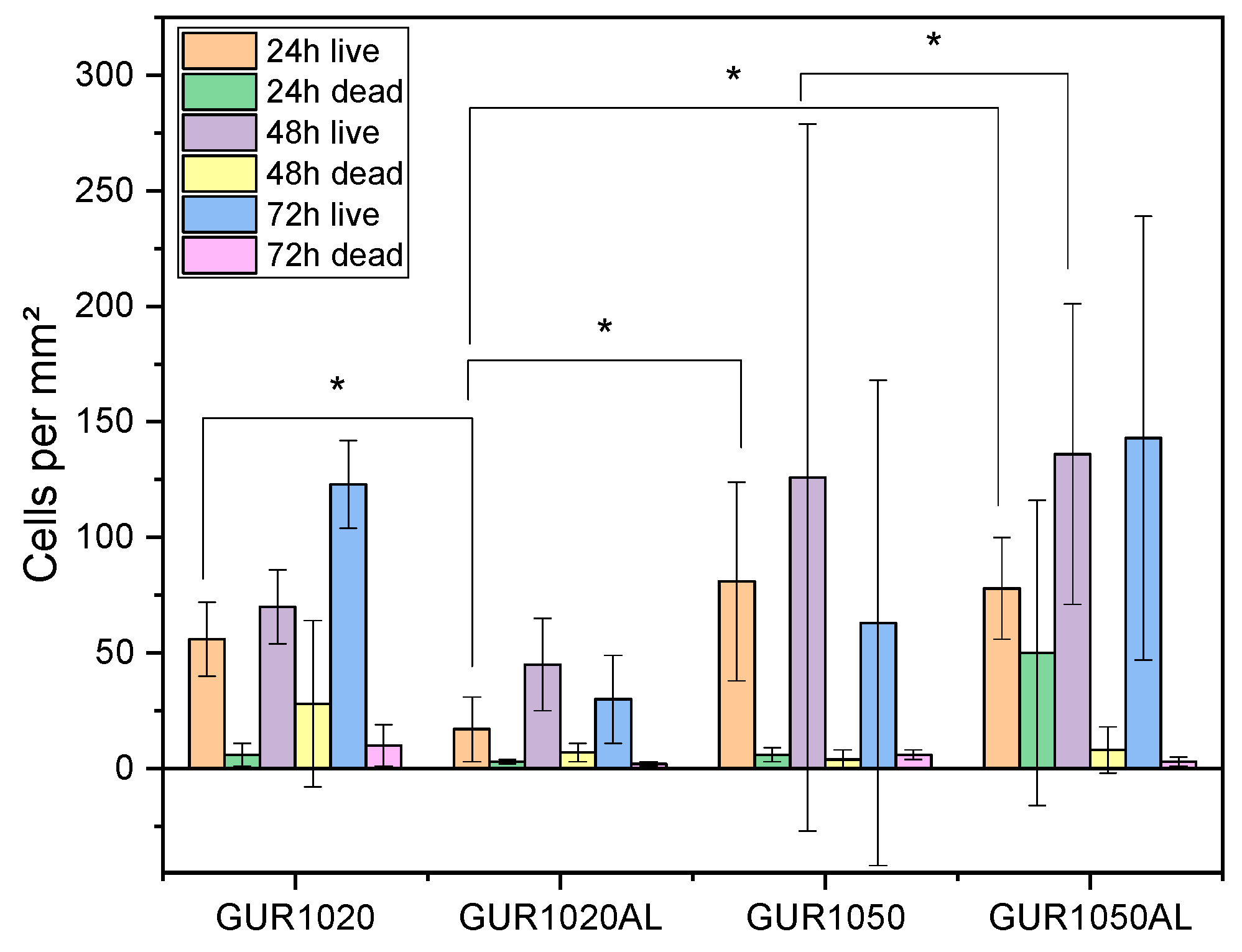
3.3. Biocompatibility
3.3.1. Live/Dead Assay
3.3.2. Cell Proliferation (WST)
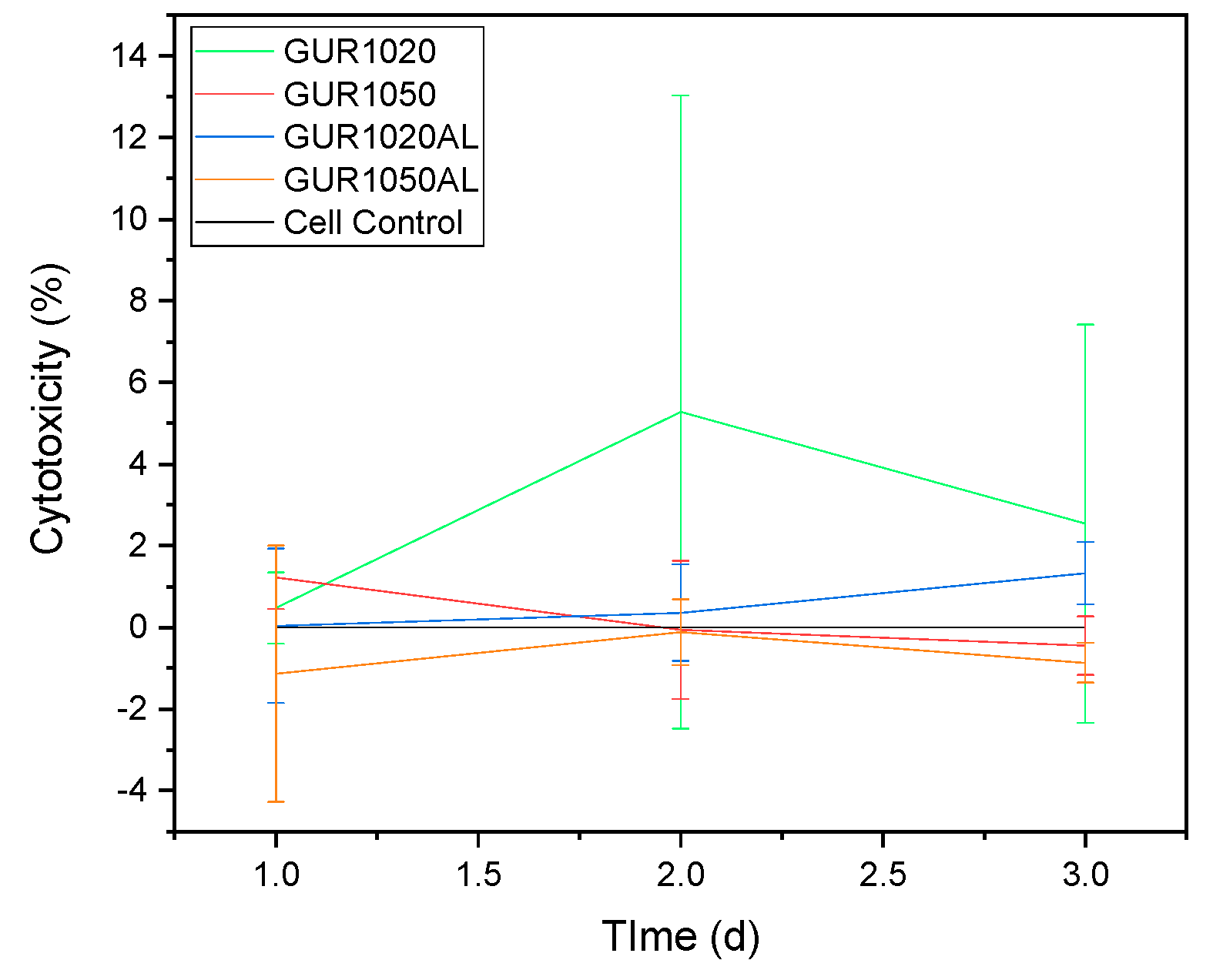
3.3.3. LDH
4. Discussion
4.1. Characterization of the Different GUR Types and the Molds
4.1.1. Particle Size Distribution
4.1.2. Surface Roughness
4.1.3. Tensile Testing
4.1.4. DSC
4.2. Release Experiments
4.3. Biocompatibility
4.3.1. Live/Dead Assay
4.3.2. WST
4.3.3. LDH
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Destatis. Gesundheit-Fallpauschalenbezogene krankenhausstatistik (Drg-Statistik) Operationen UND prozeduren Der Vollstationären Patientinnen UND Patienten in Krankenhäusern (4-Steller); Statistisches Bundesamt (Destatis): Wiesbadem, Germany, 2016; p. 72. [Google Scholar]
- Bleß, H.H.; Kip, M. Weißbuch Gelenkersatz-Versorgungssituation Endoprothetischer Hüft-UND Knieoperationen in Deutschland; Springer: Berlin/Heidelberg, Germany, 2017; p. XII, 142. [Google Scholar]
- Krause, A.; Kirschning, A.; Drager, G. Bioorthogonal metal-free click-ligation of crgd-pentapeptide to alginate. Org. Biomol. Chem. 2012, 10, 5547–5553. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.H. Conquest of a worldwide human disease: Particle-induced periprosthetic osteolysis. Clin. Orthop. Relat. Res. 2004, 429, 39–42. [Google Scholar] [CrossRef]
- Krause, A.; Schonau, E.; Gollhofer, A.; Duran, I.; Ferrari-Malik, A.; Freyler, K.; Ritzmann, R. Alleviation of motor impairments in patients with cerebral palsy: Acute effects of whole-body vibration on stretch reflex response, voluntary muscle activation and mobility. Front. Neurol. 2017, 8, 416. [Google Scholar] [CrossRef] [PubMed]
- Childs, L.M.; Goater, J.J.; O’Keefe, R.J.; Schwarz, E.M. Efficacy of etanercept for wear debris-induced osteolysis. J. Bone Miner. Res. 2001, 16, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Millett, P.J.; Allen, M.J.; Bostrom, M.P.G. Effects of alendronate on particle-induced osteolysis in a rat model. J. Bone Jt. Surg. 2002, 84, 236–249. [Google Scholar] [CrossRef]
- Zhu, F.-B.; Cai, X.Z.; Yan, S.-G.; Zhu, H.-X.; Li, R. The effects of local and systemic alendronate delivery on wear debris-induced osteolysis in vivo. J. Orth. Res. 2010, 28, 893–899. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, O.; Du, J.; Yan, Y.; Liu, C. A new drug delivery system-ciprofloxacine/tricalcium phosphate delivery capsule (ctdc) and its in vitro drug release pattern. J. Tongji Med. Univ. 1997, 17, 160–164. [Google Scholar]
- Olszynski, W.P.; Davison, K.S. Alendronate for the treatment of osteoporosis in men. Expert Opin. Pharmacother. 2008, 9, 491–498. [Google Scholar] [CrossRef]
- Sharpe, M.; Noble, S.; Spencer, C.M. Alendronate: An update of its use in osteoporosis. Drugs 2001, 61, 999–1039. [Google Scholar] [CrossRef]
- Kurtz, S.M. Chapter 4—The Origins of uhmwpe in total hip arthroplasty. In The Uhmwpe Handbook; Kurtz, S.M., Ed.; Academic Press: San Diego, CA, USA, 2004; pp. 53–70. [Google Scholar]
- Kurtz, S.M. Chapter 2—From ethylene gas to uhmwpe component: The process of producing orthopedic implants. In Uhmwpe Biomaterials Handbook, 2nd ed.; Kurtz, S.M., Ed.; Academic Press: Boston, MA, USA, 2009; pp. 7–19. [Google Scholar]
- Liu, Y.; Shi, F.; Gong, K.; Liu, Y.; Zhi, W.; Weng, J.; Qu, S. Study on critical-sized ultra-high molecular weight polyethylene wear particles loaded with alendronate sodium: In vitro release and cell response. J. Mater. Sci. Mater. Med. 2017, 28, 56. [Google Scholar] [CrossRef]
- Gong, K.; Qu, S.; Liu, Y.; Wang, J.; Zhang, Y.; Jiang, C.; Shen, R. The mechanical and tribological properties of uhmwpe loaded aln after mechanical activation for joint replacements. J. Mech. Behav. Biomed. Mater. 2016, 61, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Bai, Y.; Liu, X.; Fu, R.; Duan, K.; Weng, J. Study on in vitro release and cell response to alendronate sodium-loaded ultrahigh molecular weight polyethylene loaded with alendronate sodium wear particles to treat the particles-induced osteolysis. J. Biomed. Mater. Res. A 2013, 101, 394–403. [Google Scholar] [CrossRef] [PubMed]
- James, S.P.; Oldinski, R.; Zhang, M.; Schwartz, H. Chapter 18—uhmwpe/hyaluronan microcomposite biomaterials. In Uhmwpe Biomaterials Handbook, 2nd ed.; Kurtz, S.M., Ed.; Academic Press: Boston, MA, USA, 2009; pp. 259–276. [Google Scholar]
- Rauscher, K.; Vogt, J.; Wilke, I.; Friebe, R. Chemische Tabellen UND Rechentafeln Für Die Analytische Praxis; Verlag Harri Deutsch: Frankfurt/Main, Germany, 2000. [Google Scholar]
- Al Deeb, S.K.; Hamdan, I.I.; Al Najjar, S.M. Spectroscopic and hplc methods for the determination of alendronate in tablets and urine. Talanta 2004, 64, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Validation of analytical procedures: Text and methodology q2(r1). In Proceedings of the International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use I, Geneva, Switzerland, 6 November 1996.
- Manoj Kumar, R.; Gupta, P.; Sharma, S.K.; Mittal, A.; Shekhar, M.; Kumar, V.; Manoj Kumar, B.V.; Roy, P.; Lahiri, D. Sustained drug release from surface modified uhmwpe for acetabular cup lining in total hip implant. Mater. Sci. Eng. C 2017, 77, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Greer, K.; King, R.; Chan, F. The effects of raw material, irradiation dose, and irradiation source on crosslinking of uhmwpe. J. ASTM Int. 2004, 1, 1–11. [Google Scholar] [CrossRef]
- Martin, J.Y.; Schwartz, Z.; Hummert, T.W.; Schraub, D.M.; Simpson, J.; Lankford, J.; Dean, D.D.; Cochran, D.L.; Boyan, B.D. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (mg63). J. Biomed. Mater. Res. 1995, 29, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, D.; Schulz, E.; Fanter, D.; Fuhrmann, H.; Bilda, D. Crystallinity and lateral crystallite size of different uhmw pe materials. J. Appl. Polym. Sci. 1991, 42, 863–866. [Google Scholar] [CrossRef]
- Oral, E.; Wannomae, K.K.; Rowell, S.L.; Muratoglu, O.K. Diffusion of vitamin e in ultra-high molecular weight polyethylene. Biomaterials 2007, 28, 5225–5237. [Google Scholar] [CrossRef]
- Im, G.I.; Qureshi, S.A.; Kenney, J.; Rubash, H.E.; Shanbhag, A.S. Osteoblast proliferation and maturation by bisphosphonates. Biomaterials 2004, 25, 4105–4115. [Google Scholar] [CrossRef]
- Giuliani, N.; Pedrazzoni, M.; Negri, G.; Passeri, G.; Impicciatore, M.; Girasole, G. Bisphosphonates stimulate formation of osteoblast precursors and mineralized nodules in murine and human bone marrow cultures in vitro and promote early osteoblastogenesis in young and aged mice in vivo. Bone 1998, 22, 455–461. [Google Scholar] [CrossRef]
- Xiong, Y.; Yang, H.; Feng, J.; Shi, Z.; Wu, L. Effects of alendronate on the proliferation and osteogenic differentiation of mg-63 cells. J. Int. Med. Res. 2009, 37, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Reinholz, G.G.; Getz, B.; Pederson, L.; Sanders, E.S.; Subramaniam, M.; Ingle, J.N.; Spelsberg, T.C. Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res. 2000, 60, 6001–6007. [Google Scholar] [PubMed]

| Particle Size Classes [µm] | Percentage [%] | |
|---|---|---|
| GUR1020 | GUR1050 | |
| 0.1–10 | 36.52 | 56.76 |
| 11–30 | 32.61 | 26.45 |
| 31–50 | 6.78 | 4.66 |
| 51–70 | 8.06 | 3.25 |
| 71–90 | 5.65 | 1.13 |
| 91–110 | 2.41 | 1.55 |
| 111–130 | 2.33 | 1.45 |
| 131–150 | 1.81 | 1.37 |
| 151–170 | 1.95 | 1.55 |
| 171–190 | 0.82 | 1.06 |
| 191–210 | 0.68 | 0.37 |
| 211–230 | 0.15 | 0.25 |
| 231–250 | 0.15 | 0.06 |
| 251–270 | 0.08 | 0 |
| 271–290 | 0 | 0.06 |
| Specimen | Width [mm] | Length [mm] | Height [mm] | Weight [g] |
|---|---|---|---|---|
| GUR1020 | 9.57 ± 0.28 | 12.72 ± 0.03 | 2.12 ± 0.02 | 0.25 ± 0.01 |
| GUR1050 | 9.49 ± 0.31 | 12.71 ± 0.02 | 2.11 ± 0.01 | 0.24 ± 0.01 |
| GUR1020AL | 9.52 ± 0.20 | 13.02 ± 0.02 | 2.01 ± 0.02 | 0.24 ± 0.01 |
| GUR1050AL | 9.44 ± 0.42 | 12.75 ± 0.04 | 2.13 ± 0.01 | 0.24 ± 0.01 |
| Specimens | GUR1020 | GUR1050 | GUR1020AL | GUR1050AL |
|---|---|---|---|---|
| Sa [µm] | 2.02 ± 0.43 | 2.26 ± 0.38 | 2.57 ± 0.48 | 2.18 ± 0.27 |
| Specimen | Et [MPa] | εtB [%] | σB [MPa] |
|---|---|---|---|
| GUR1050 | 821 ± 30 | 71 ± 5 | 36 ± 10 |
| GUR1050AL | 650 ± 70 | 122 ± 116 | 38 ± 2 |
| GUR1020 | 691 ± 27 | 151 ± 3 | 37 ± 1 |
| GUR1020AL | 635 ± 15 | 174 ± 7 | 34 ± 1 |
| Sample | % Crystallinity |
|---|---|
| GUR1020 | 52.5 ± 2.4 |
| GUR1020AL | 56.2 ± 2.1 |
| GUR1050 | 57.6 ± 2.5 |
| GUR1050AL | 55.2 ± 3.5 |
| Time | cells per mm² | ||||||
|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | |||||
| Specimen | live | dead | live | dead | live | dead | |
| GUR1020 | 56 ± 16 | 6 ± 5 | 70 ± 16 | 28 ± 36 | 123 ± 19 | 10 ± 9 | |
| GUR1020AL | 17 ± 14 | 3 ± 1 | 45 ± 20 | 7 ± 4 | 30 ± 19 | 2 ± 1 | |
| GUR1050 | 81 ± 43 | 6 ± 3 | 126 ± 153 | 4 ± 4 | 63 ± 105 | 6 ± 2 | |
| GUT1050AL | 78 ± 22 | 50 ± 66 | 136 ± 65 | 8 ± 10 | 143 ± 96 | 3 ± 2 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seidenstuecker, M.; Weber, J.; Latorre, S.H.; Straub, B.; Matthes, U.; Friedrich, C.; Mayr, H.O.; Bernstein, A. Alendronate Release from UHMWPE-Based Biomaterials in Relation to Particle Size of the GUR Powder for Manufacturing. Materials 2019, 12, 1832. https://doi.org/10.3390/ma12111832
Seidenstuecker M, Weber J, Latorre SH, Straub B, Matthes U, Friedrich C, Mayr HO, Bernstein A. Alendronate Release from UHMWPE-Based Biomaterials in Relation to Particle Size of the GUR Powder for Manufacturing. Materials. 2019; 12(11):1832. https://doi.org/10.3390/ma12111832
Chicago/Turabian StyleSeidenstuecker, Michael, Julia Weber, Sergio H. Latorre, Brigitte Straub, Ulrich Matthes, Christian Friedrich, Hermann O. Mayr, and Anke Bernstein. 2019. "Alendronate Release from UHMWPE-Based Biomaterials in Relation to Particle Size of the GUR Powder for Manufacturing" Materials 12, no. 11: 1832. https://doi.org/10.3390/ma12111832
APA StyleSeidenstuecker, M., Weber, J., Latorre, S. H., Straub, B., Matthes, U., Friedrich, C., Mayr, H. O., & Bernstein, A. (2019). Alendronate Release from UHMWPE-Based Biomaterials in Relation to Particle Size of the GUR Powder for Manufacturing. Materials, 12(11), 1832. https://doi.org/10.3390/ma12111832






