Effect of Heat Curing Method on the Mechanical Strength of Alkali-Activated Slag Mortar after High-Temperature Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Characterization
2.2. Mixture Proportion
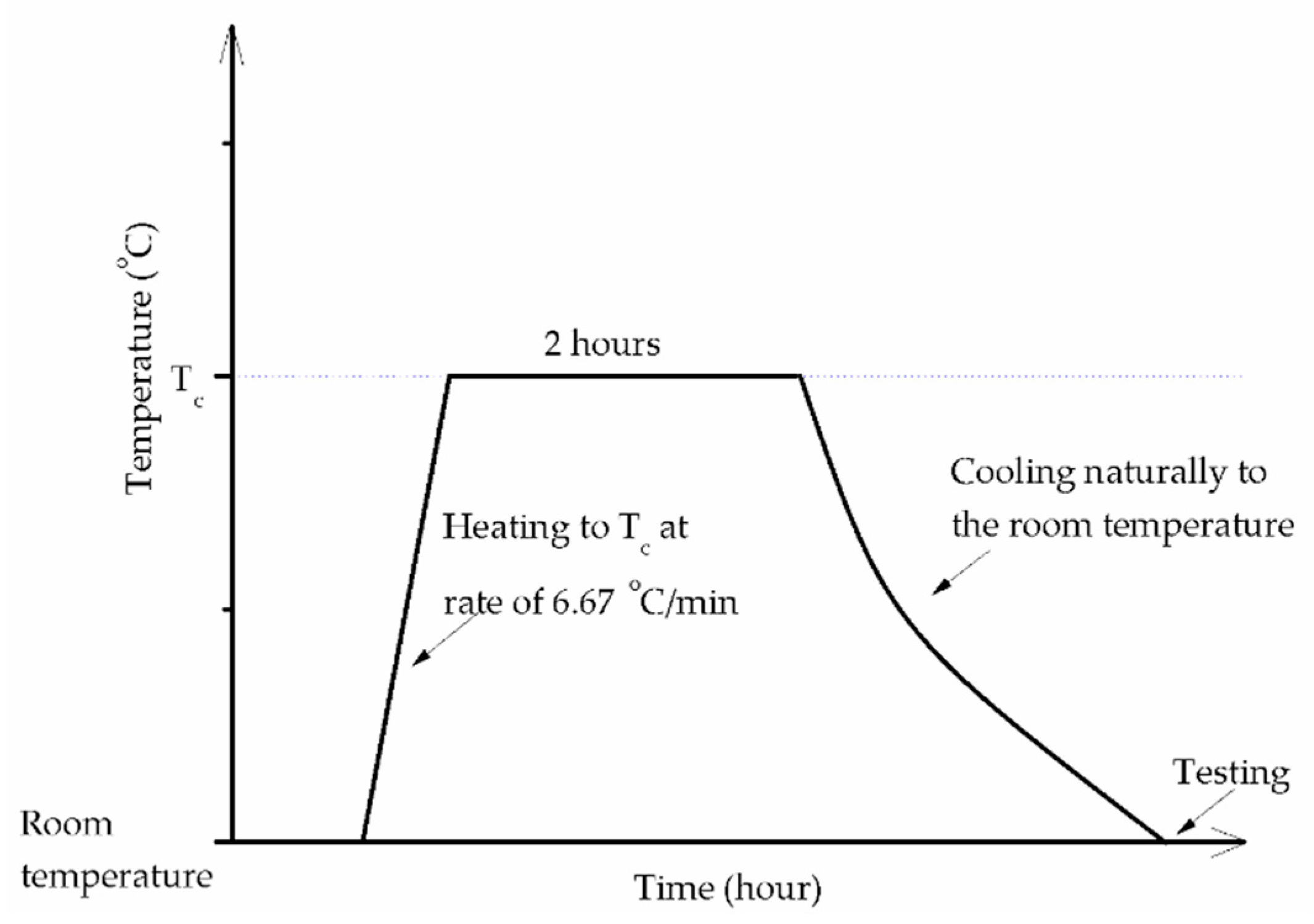
2.3. Experimental Methods
3. Results and Discussion
3.1. Reference Mechanical Strengths
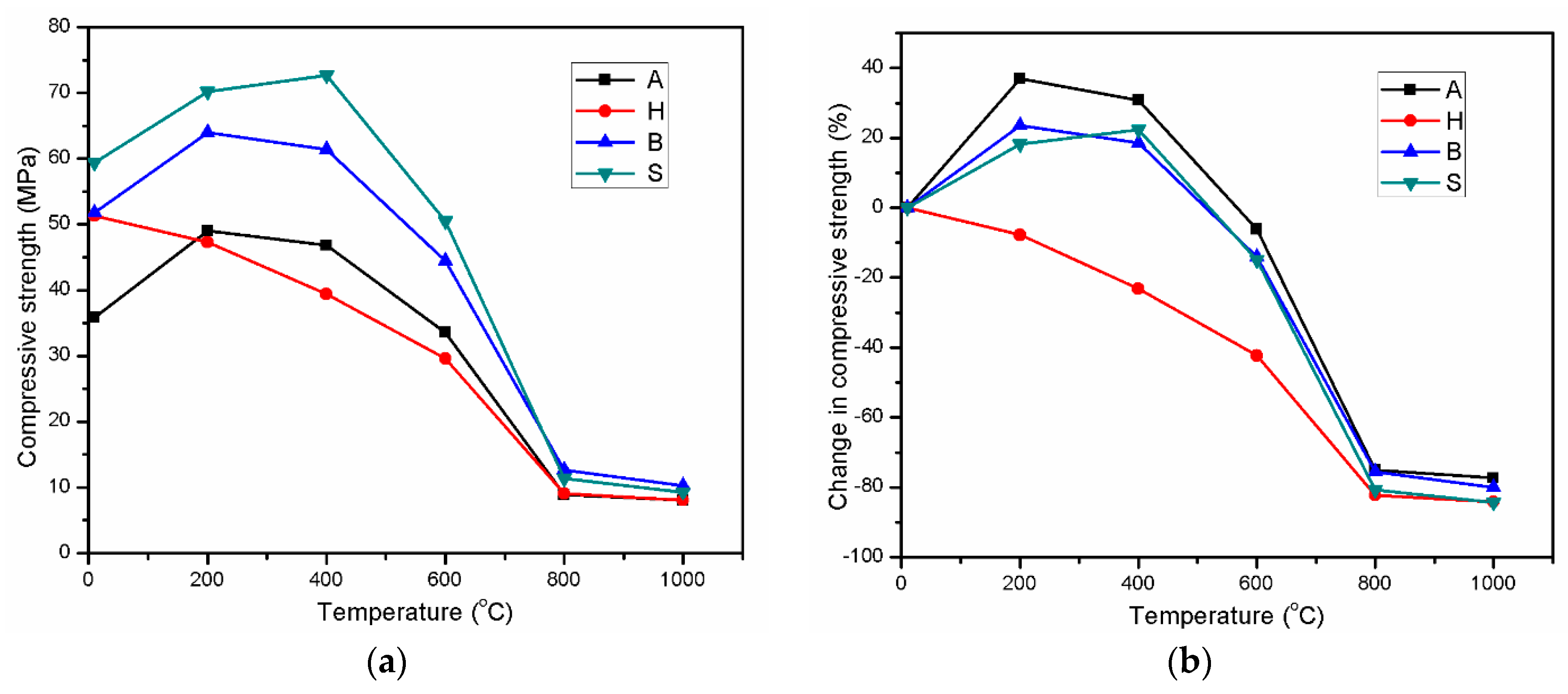
3.2. Residual Compressive Strength
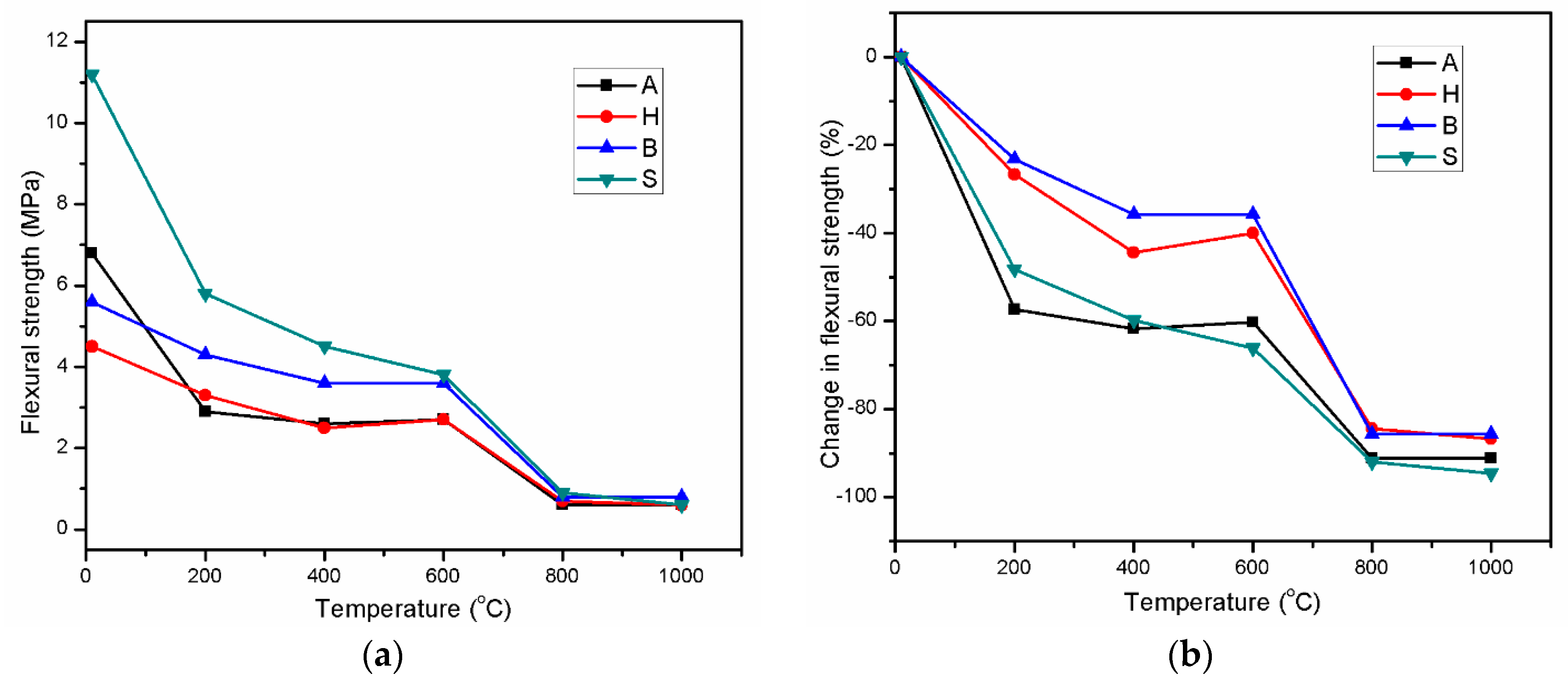
3.3. Residual Flexural Strength
3.4. Residual Tensile Strength
3.5. Thermogravimetric Analysis (TGA)
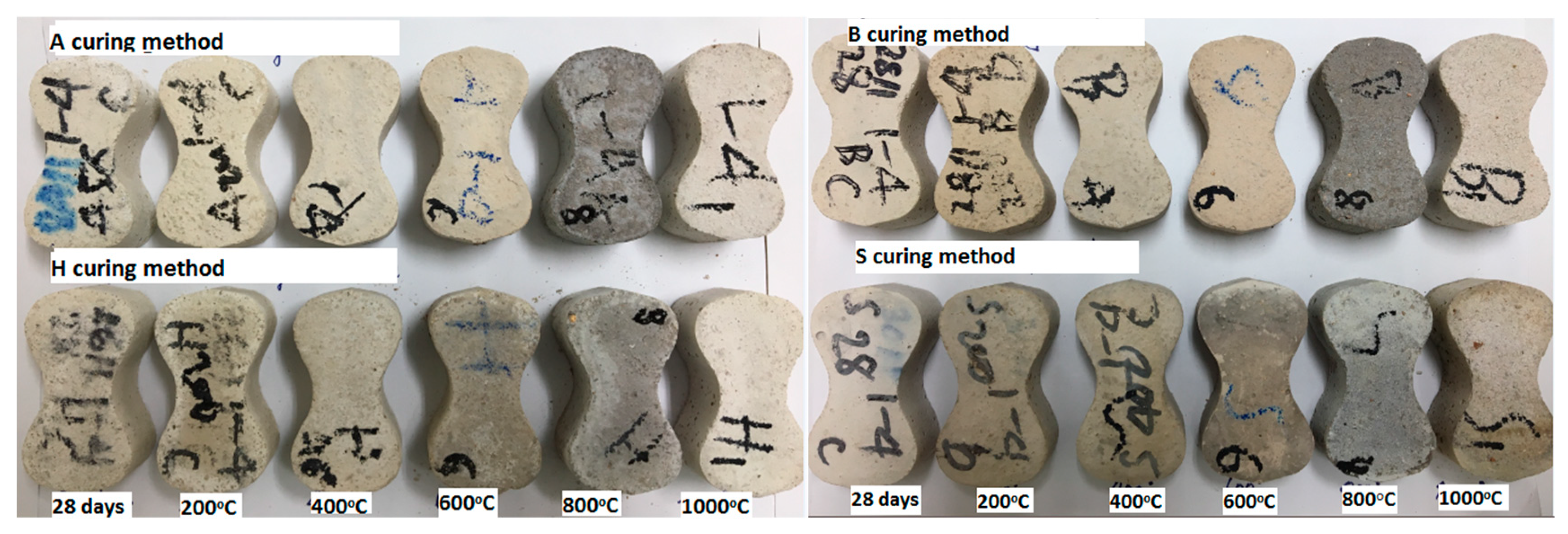
3.6. Microstructural Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Roy, D.M. Alkali activated cements, Opportunities and challenges. Cem. Concr. Res. 1999, 29, 249–254. [Google Scholar] [CrossRef]
- Gebregziabiher, B.S.; Thomas, R.J.; Peethamparan, S. Temperature and activator effect on early-age reaction kinetics of alkali-activated slag binders. Constr. Build. Mater. 2016, 113, 783–793. [Google Scholar] [CrossRef]
- Gebregziabiher, B.S.; Thomas, R.; Peethamparan, S. Very early-age reaction kinetics and microstructural development in alkali-activated slag. Cem. Concr. Compos. 2015, 55, 91–102. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review Part 1. Historical background, terminology, reaction mechanisms and hydration products. Constr. Build. Mater. 2008, 22, 1305–1314. [Google Scholar] [CrossRef]
- Wang, S.; Scrivener, K.L. Hydration products of alkali-activated slag. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Alkali activation of Australian slag cements. Cem. Concr. Res. 1999, 29, 113–120. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, J.G.; Puertas, F. Alkali-Activated Slag Mortars Mechanical Strength Behavior. Cem. Concr. Res. 1999, 29, 1313–1321. [Google Scholar] [CrossRef]
- Collins, F.G.; Sanjayan, J.G. Workability and mechanical properties of alkali activated slag concrete. Cem. Concr. Res. 1999, 29, 455–458. [Google Scholar] [CrossRef]
- Chi, M. Effects of dosage of alkali-activated solution and curing conditions on the properties and durability of alkali-activated slag concrete. Constr. Build. Mater. 2012, 35, 240–245. [Google Scholar] [CrossRef]
- Aydin, S.; Baradan, B. Effect of activator type and content on properties of alkali-activated slag mortars. Compos. Part B Eng. 2014, 57, 166–172. [Google Scholar] [CrossRef]
- Haha, M.B.; Le Saout, G.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Shi, Z.; Shi, C.; Wan, S.; Ou, Z. Effect of alkali dosage on alkali-silica reaction in sodium hydroxide activated slag mortars. Constr. Build. Mater. 2017, 143, 16–23. [Google Scholar] [CrossRef]
- Ye, H.; Cartwright, C.; Rajabipour, F.; Radli, A. Understanding the drying shrinkage performance of alkali-activated slag mortars ska. Cem. Concr. Res. 2017, 76, 13–24. [Google Scholar] [CrossRef]
- Krizan, D.; Zivanovic, B. Effects of dosage and modulus of water glass on early hydration of alkali-slag cements. Cem. Concr. Res. 2002, 32, 1181–1188. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Effect of elevated temperature curing on properties of alkali-activated slag concrete. Cem. Concr. Res. 2000, 30, 1367–1374. [Google Scholar] [CrossRef]
- Aydin, S.; Baradan, B. Mechanical and microstructural properties of heat cured alkali-activated slag mortars. Mater. Des. 2012, 35, 374–383. [Google Scholar] [CrossRef]
- Altan, E.; Erdoǧan, S.T. Alkali activation of a slag at ambient and elevated temperatures. Cem. Concr. Compos. 2012, 34, 131–139. [Google Scholar] [CrossRef]
- Chi, M.C.; Chang, J.J.; Huang, R. Strength and drying shrinkage of alkali-activated slag paste and mortar. Adv. Civ. Eng. 2012, 2012. [Google Scholar] [CrossRef]
- Colins, F.; Sanjayan, J.G. Effect of pore size distribution on drying shrinking of alkali-activated slag concrete. Cem. Concr. Compos. 2000, 8846, 1401–1406. [Google Scholar] [CrossRef]
- Jumppanen, U.-M.; Diederichs, U.; Hinrichsmeyer, K. Material Properties of F-Concrete at High Temperatures; Valtion Teknillinen Tutkimuskeskus: Espoo, Finland, 1986; p. 60. [Google Scholar]
- Guerrieri, M.; Sanjayan, J.; Collins, F. Residual strength properties of sodium silicate alkali activated slag paste exposed to elevated temperatures. Mater. Struct. 2010, 43, 765–773. [Google Scholar] [CrossRef]
- Rashad, A.M.; Bai, Y.; Basheer, P.A.M.; Collier, N.C.; Milestone, N.B. Chemical and mechanical stability of sodium sulfate activated slag after exposure to elevated temperature. Cem. Concr. Res. 2012, 42, 333–343. [Google Scholar] [CrossRef]
- Zuda, L.; Rovnaník, P.; Bayer, P.; Černý, R. Thermal properties of alkali-activated slag subjected to high temperatures. J. Build. Phys. 2007, 30, 337–350. [Google Scholar] [CrossRef]
- Zuda, L.; Pavlík, Z.; Rovnaníková, P.; Bayer, P.; Černý, R. Properties of alkali activated aluminosilicate material after thermal load. Int. J. Thermophys. 2006, 27, 1250–1263. [Google Scholar] [CrossRef]
- Zuda, L.; Rovnaník, P.; Bayer, P.; Černý, R. Effect of high temperatures on the properties of alkali activated aluminosilicate with electrical porcelain filler. Int. J. Thermophys. 2008, 29, 693–705. [Google Scholar] [CrossRef]
- Rashad, A.M.; Sadek, D.M.; Hassan, H.A. An investigation on blast-furnace stag as fine aggregate in alkali-activated slag mortars subjected to elevated temperatures. J. Clean. Prod. 2016, 112, 1086–1096. [Google Scholar] [CrossRef]
- Guerrieri, M.; Sanjayan, J.; Collins, F. Residual compressive behavior of alkali-activated concrete exposed to elevated temperatures. Fire Mater. 2009, 33, 51–62. [Google Scholar] [CrossRef]
- Aslani, F.; Asif, Z. Properties of Ambient-Cured Normal and Heavyweight Geopolymer Concrete Exposed to high temperatures. Materials 2019. [Google Scholar] [CrossRef] [PubMed]
- Türker, H.T.; Balçikanli, M.; Durmuş, I.H.; Özbay, E.; Erdemir, M. Microstructural alteration of alkali activated slag mortars depend on exposed high temperature level. Constr. Build. Mater. 2016, 104, 169–180. [Google Scholar] [CrossRef]
- ASTM 303–305—Standard Practice for Mechanical Mixing of Hydraulic Cement Pastes and Mortars; ASTM: West Conshohocken, PA, USA, 2004.
- ASTM C109-02—Standard Test Method for Compressive Strength of Hydraulic Cement Mortars; ASTM: West Conshohocken, PA, USA, 2002.
- ASTM C348-02—Standard Test Method for Flexural Strength of Hydraulic-Cement Mortars; ASTM: West Conshohocken, PA, USA, 2002.
- ASTM C190-85—Standard Test Method for Tensile Strength of Hydraulic Cement Mortars; ASTM: West Conshohocken, PA, USA, 1985.
- Rashad, A.M.; Zeedan, S.R.; Hassan, A.A. Influence of the activator concentration of sodium silicate on the thermal properties of alkali-activated slag pastes. Constr. Build. Mater. 2016, 102, 811–820. [Google Scholar] [CrossRef]
- Duran Atiş, C.; Bilim, C.; Çelik, Ö.; Karahan, O. Influence of activator on the strength and drying shrinkage of alkali-activated slag mortar. Constr. Build. Mater. 2009, 23, 548–555. [Google Scholar] [CrossRef]
- Kahraman, S.; Altindag, R. A brittleness index to estimate fracture toughness. Int. J. Rock Mech. Min. Sci. 2004, 41, 343–348. [Google Scholar] [CrossRef]
- Gunsallus, K.T.; Kulhawy, F.H. A comparative evaluation of rock strength measures. Int. J. Rock Mech. Min. Sci. Geomech. Abstr. 1984, 21, 233–248. [Google Scholar] [CrossRef]
- Gu, Y.M.; Fang, Y.H.; You, D.; Gong, Y.F.; Zhu, C.H. Properties and microstructure of alkali-activated slag cement cured at below- and about-normal temperature. Constr. Build. Mater. 2015, 79, 1–8. [Google Scholar] [CrossRef]
- Tran, T.T.; Kwon, H. Influence of activator Na2O concentration on residual strengths of alkali-activated slag mortar upon exposure to elevated temperatures. Materials 2018, 11. [Google Scholar] [CrossRef]
- Wardhono, A.; Gunasekara, C.; Law, D.W.; Setunge, S. Comparison of long term performance between alkali activated slag and fly ash geopolymer concretes. Constr. Build. Mater. 2017, 143, 272–279. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Kodur, V.; Wu, B.; Cao, L.; Wang, F. Thermal behavior and mechanical properties of geopolymer mortar after exposure to elevated temperatures. Constr. Build. Mater. 2016, 109, 17–24. [Google Scholar] [CrossRef]
- Lecomte, I.; Henrist, C.; Li, M.; Maseri, F.; Rulmont, A.; Cloots, R. (Micro)-structural comparison between geopolymers, alkali-activated slag cement and Portland cement. J. Eur. Ceram. Soc. 2006, 26, 3789–3797. [Google Scholar] [CrossRef]
- Jia, Z.; Yang, Y.; Yang, L.; Zhang, Y.; Sun, Z. Hydration products, internal relative humidity and drying shrinkage of alkali activated slag mortar with expansion agents. Constr. Build. Mater. 2018, 158, 198–207. [Google Scholar] [CrossRef]
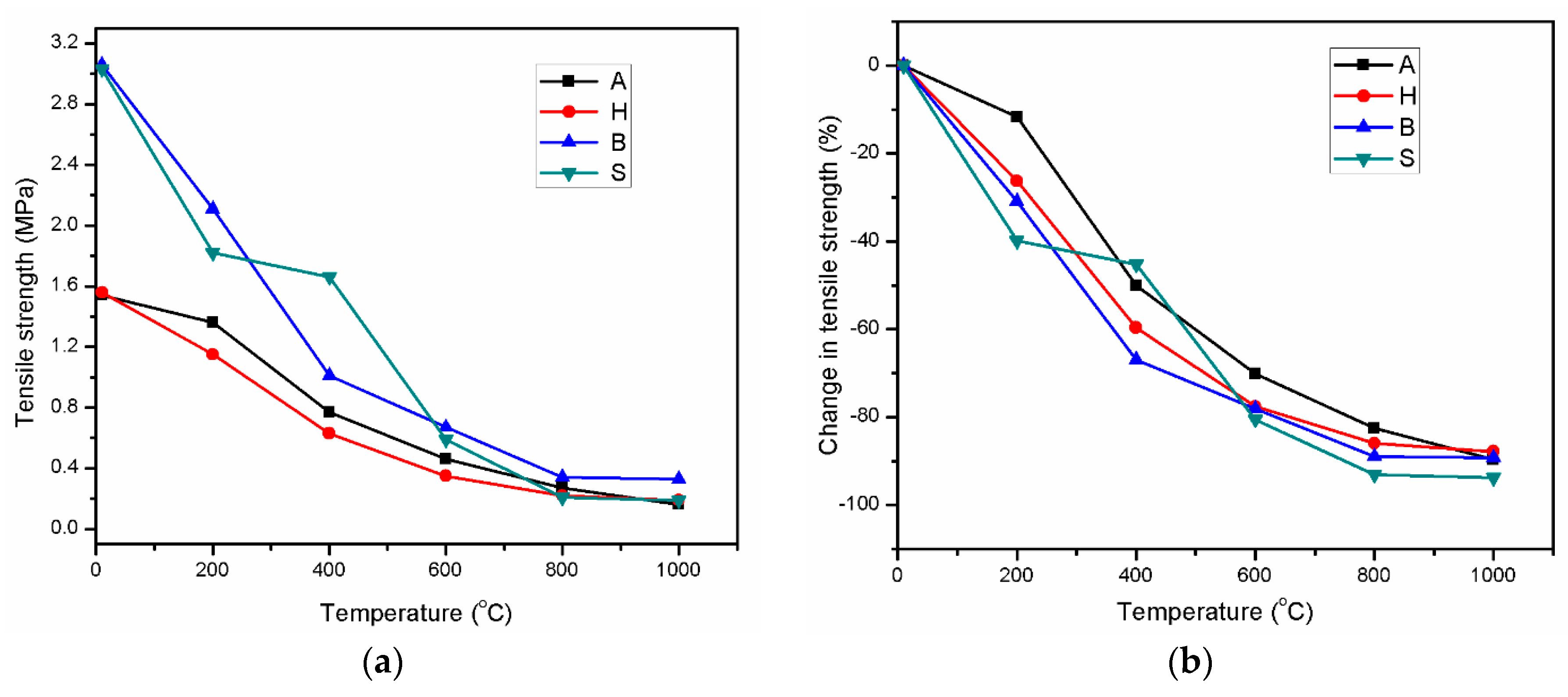

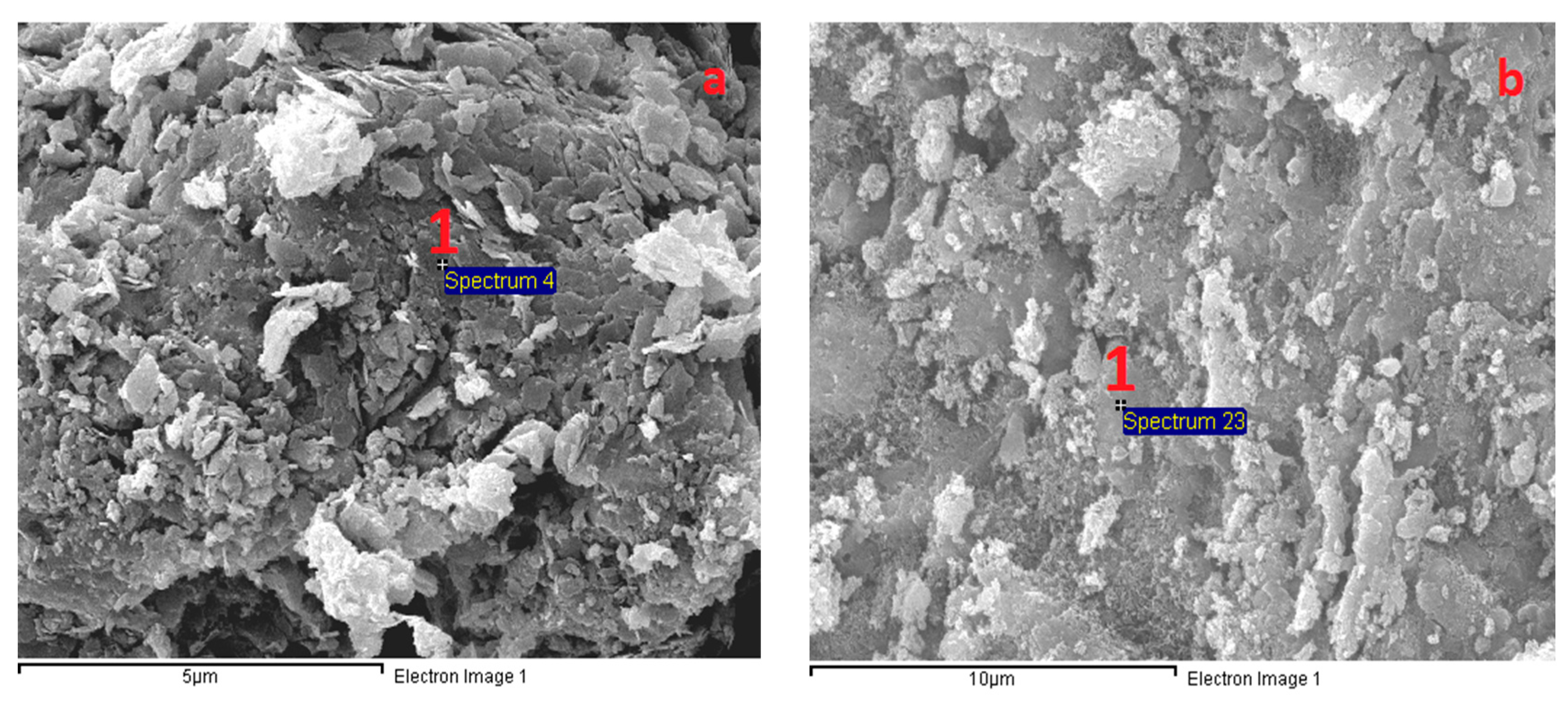




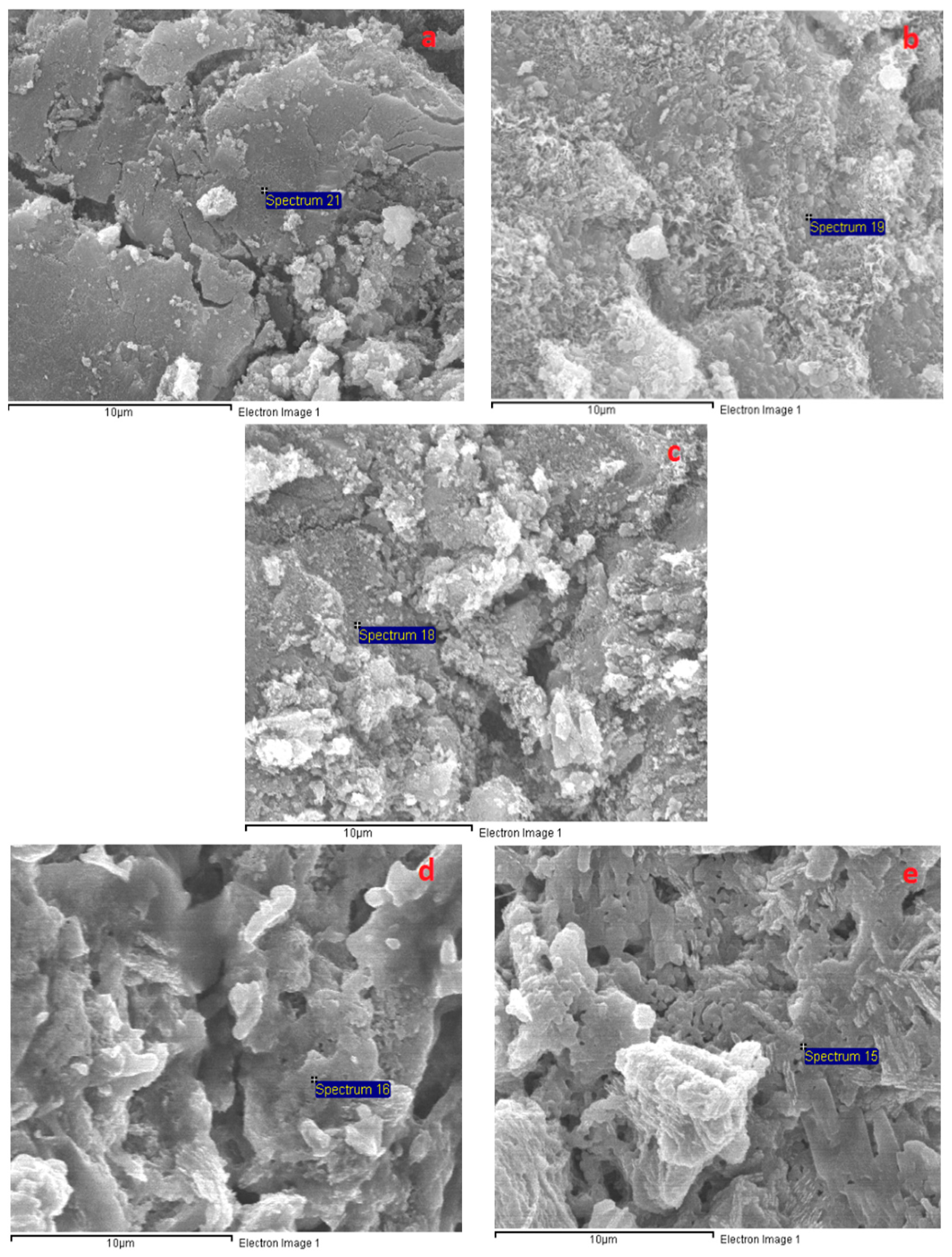
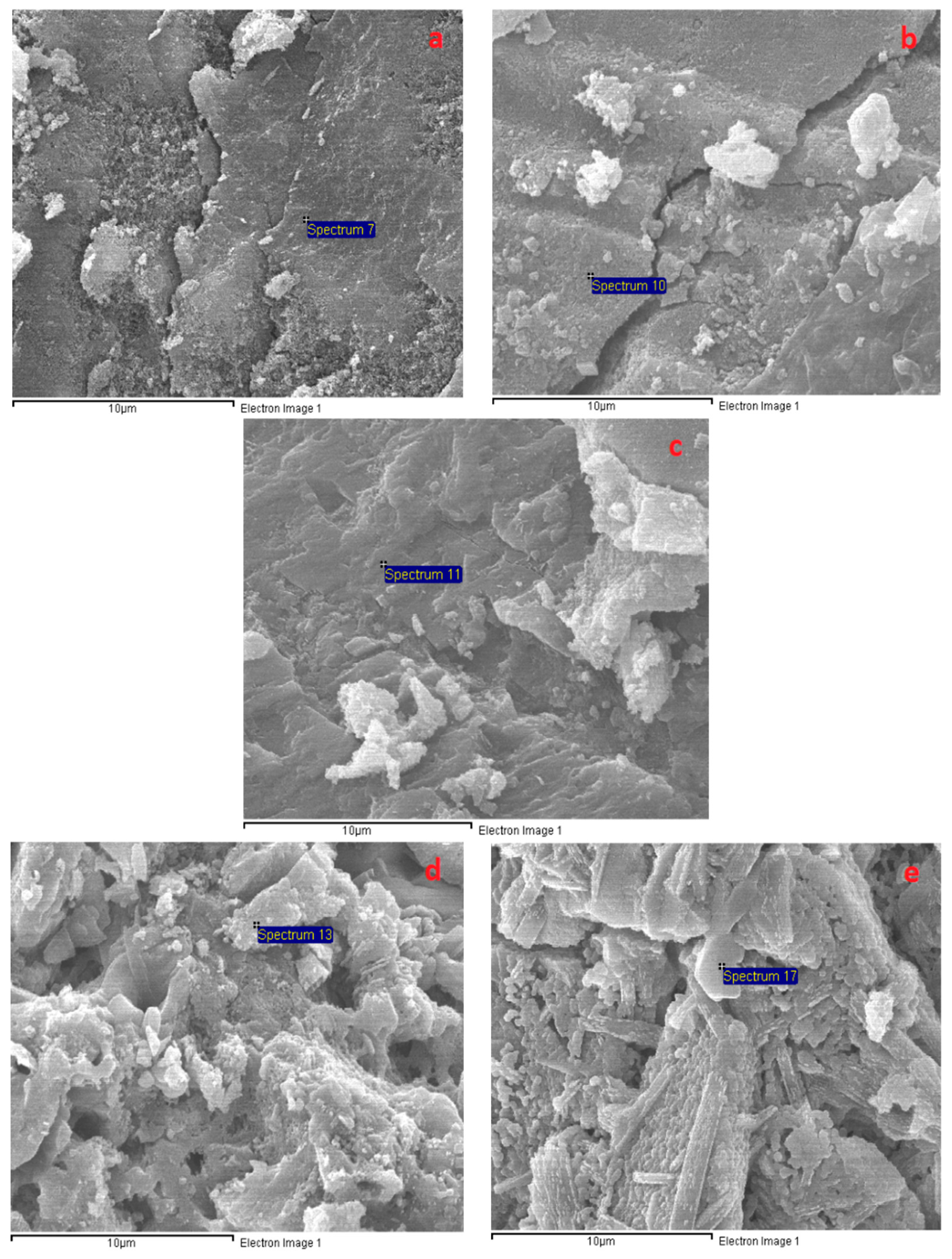

| Oxide | SiO2 | CaO | Al2O3 | Fe2O3 | MgO | SO3 | Na2O | K2O | LOI |
|---|---|---|---|---|---|---|---|---|---|
| (%) | 33.81 | 41.24 | 15.19 | 0.41 | 5.54 | 2.51 | 0.25 | 0.61 | 0.18 |
| Specimen | Compressive Strength (CS) (MPa) | Flexural Strength (FS) (MPa) | Tensile Strength (TS) (MPa) | FS/CS | Φ * (Radian) |
|---|---|---|---|---|---|
| A | 35.77 | 6.82 | 1.54 | 0.1907 | 0.6786 |
| H | 51.32 | 4.5 | 1.56 | 0.0877 | 0.6964 |
| B | 51.81 | 5.62 | 3.06 | 0.1085 | 0.659 |
| S | 59.36 | 11.19 | 3.03 | 0.1885 | 0.6685 |
| Specimen | Temperature | Compressive Strength (CS) (MPa) | Flexural Strength (FS) (MPa) | Tensile Strength (TS) (MPa) | FS/CS | Φ * (Radian) |
|---|---|---|---|---|---|---|
| A | 200 | 48.97 | 2.92 | 1.36 | 0.0596 | 0.7005 |
| 400 | 46.84 | 2.64 | 0.77 | 0.0564 | 0.7206 | |
| 600 | 33.63 | 2.71 | 0.46 | 0.0806 | 0.7264 | |
| 800 | 8.92 | 0.64 | 0.27 | 0.0717 | 0.6966 | |
| 1000 | 8.05 | 0.61 | 0.16 | 0.0758 | 0.7140 | |
| H | 200 | 47.32 | 3.28 | 1.15 | 0.0693 | 0.7062 |
| 400 | 39.39 | 2.52 | 0.63 | 0.0640 | 0.7215 | |
| 600 | 29.57 | 2.7 | 0.35 | 0.0913 | 0.7306 | |
| 800 | 9.07 | 0.7 | 0.22 | 0.0772 | 0.7063 | |
| 1000 | 8.07 | 0.65 | 0.19 | 0.0805 | 0.7075 | |
| B | 200 | 64.04 | 4.28 | 2.11 | 0.0668 | 0.6926 |
| 400 | 61.44 | 3.55 | 1.01 | 0.0578 | 0.7206 | |
| 600 | 44.49 | 3.58 | 0.67 | 0.0805 | 0.7234 | |
| 800 | 12.65 | 0.8 | 0.34 | 0.0632 | 0.7019 | |
| 1000 | 10.32 | 0.81 | 0.33 | 0.0785 | 0.6941 | |
| S | 200 | 70.17 | 5.81 | 1.82 | 0.0828 | 0.7035 |
| 400 | 72.71 | 4.53 | 1.66 | 0.0623 | 0.7087 | |
| 600 | 50.53 | 3.82 | 0.59 | 0.0756 | 0.7309 | |
| 800 | 11.44 | 0.93 | 0.21 | 0.0813 | 0.7168 | |
| 1000 | 9.31 | 0.58 | 0.19 | 0.0623 | 0.7130 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.T.; Kang, H.; Kwon, H.-M. Effect of Heat Curing Method on the Mechanical Strength of Alkali-Activated Slag Mortar after High-Temperature Exposure. Materials 2019, 12, 1789. https://doi.org/10.3390/ma12111789
Tran TT, Kang H, Kwon H-M. Effect of Heat Curing Method on the Mechanical Strength of Alkali-Activated Slag Mortar after High-Temperature Exposure. Materials. 2019; 12(11):1789. https://doi.org/10.3390/ma12111789
Chicago/Turabian StyleTran, Tai Thanh, Hyuk Kang, and Hyug-Moon Kwon. 2019. "Effect of Heat Curing Method on the Mechanical Strength of Alkali-Activated Slag Mortar after High-Temperature Exposure" Materials 12, no. 11: 1789. https://doi.org/10.3390/ma12111789
APA StyleTran, T. T., Kang, H., & Kwon, H.-M. (2019). Effect of Heat Curing Method on the Mechanical Strength of Alkali-Activated Slag Mortar after High-Temperature Exposure. Materials, 12(11), 1789. https://doi.org/10.3390/ma12111789





