Modulating Band Gap of Boron Doping in Amorphous Carbon Nano-Film
Abstract
1. Introduction
2. Experimental
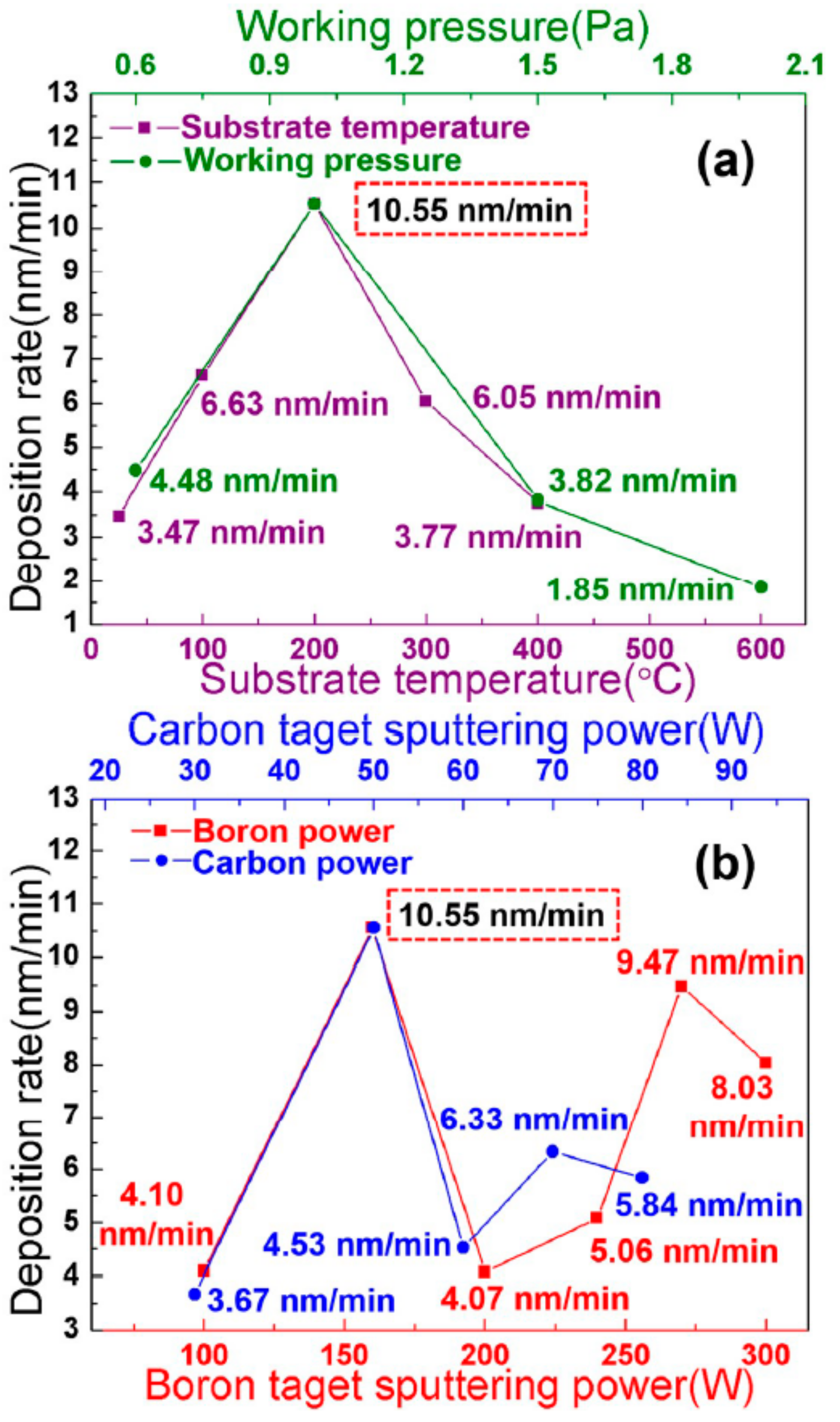
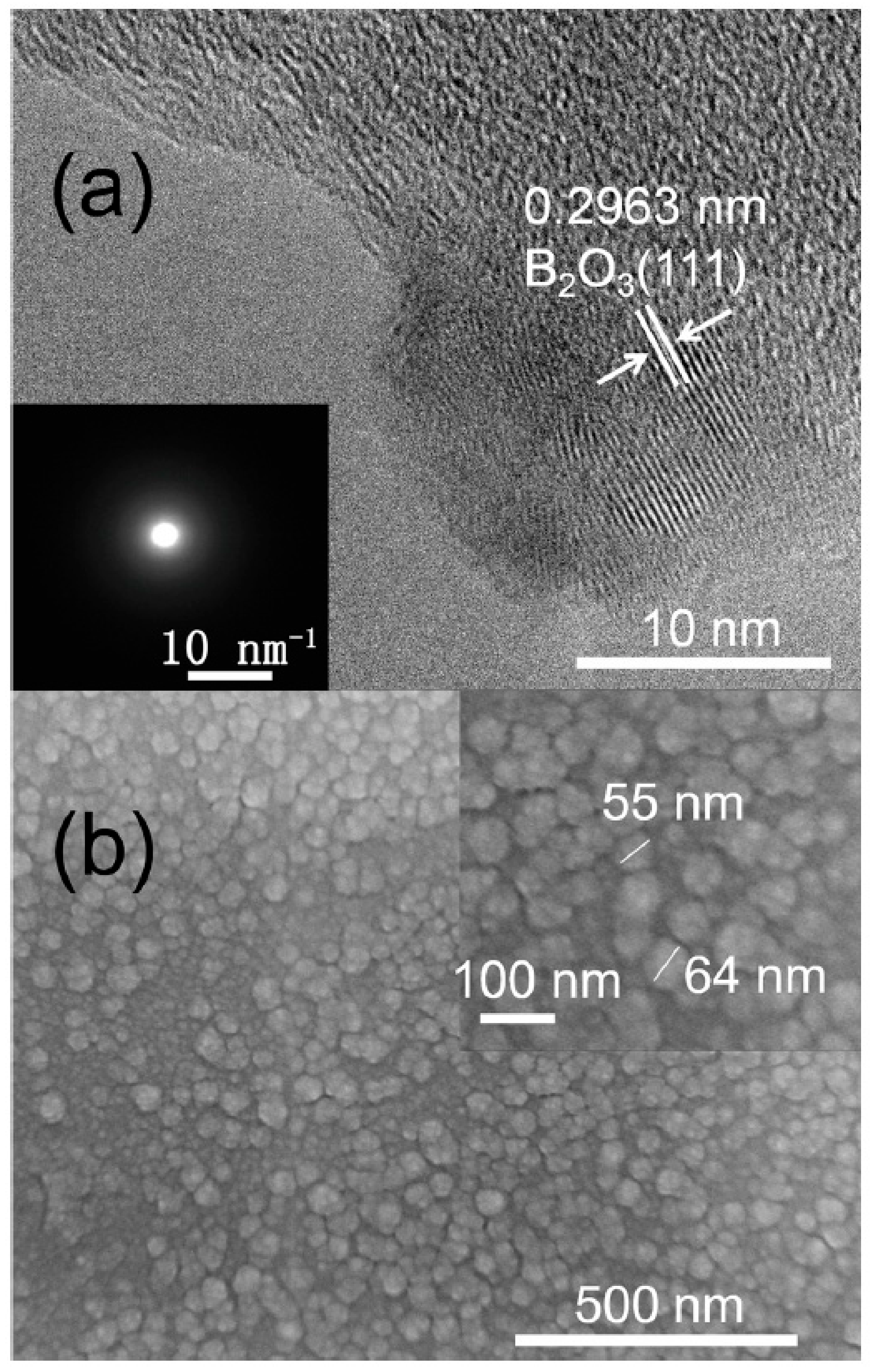
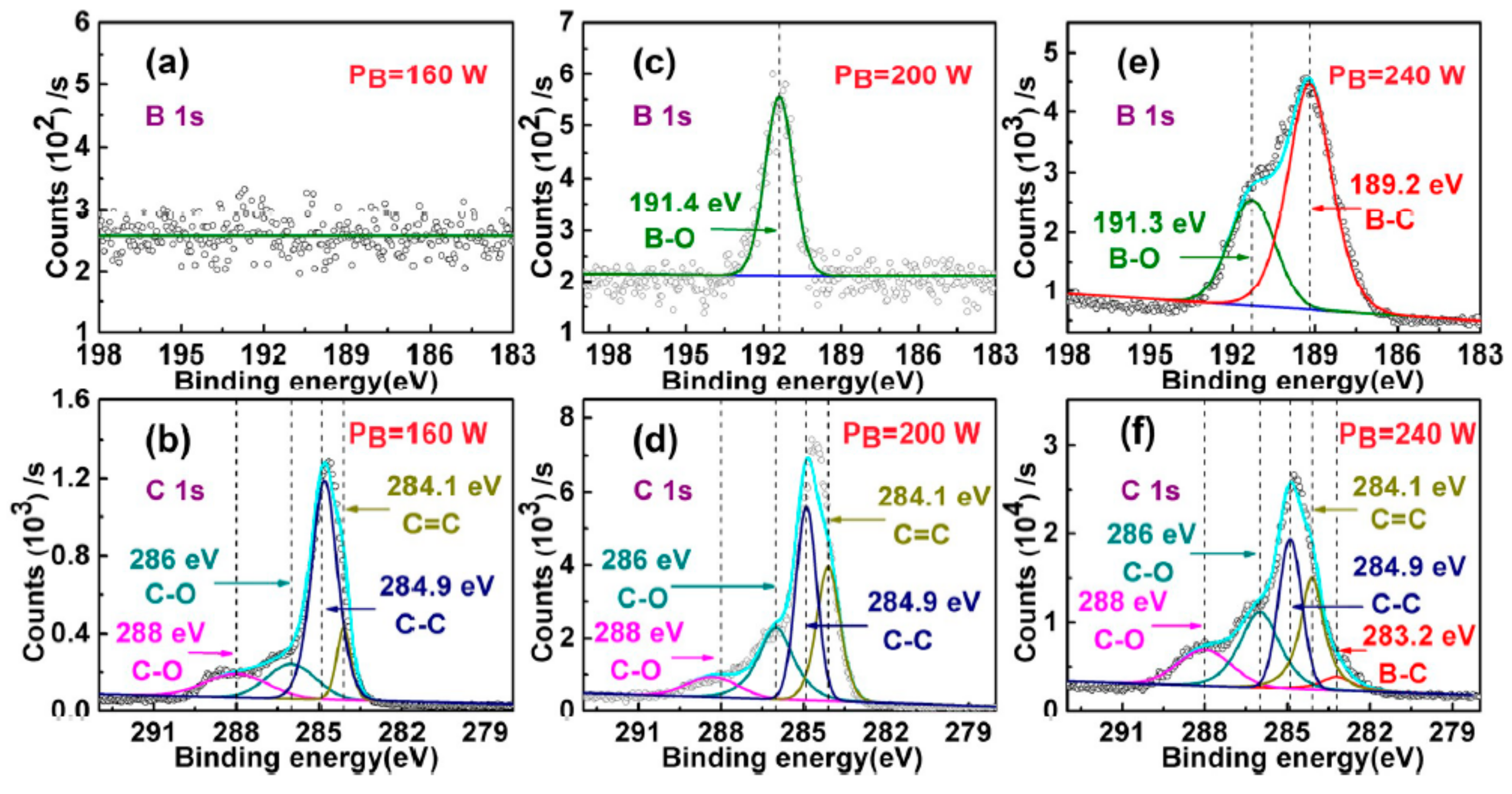
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Myung, H. Synthesis and mechanical properties of amorphous carbon films by closed-field unbalanced magnetron sputtering. Mater. Lett. 2004, 58, 1513–1516. [Google Scholar] [CrossRef]
- Rusop, M.; Rusop, M.; Subban, R.Y.; Kamarulzaman, N.; Wui, W.T. Amorphous Carbon Based Solar Cell: Fabrication and Characterization. AIP Conf. Proc. 2010, 1217, 385–390. [Google Scholar] [CrossRef]
- Zhai, Z.; Shen, H.; Chen, J. Fast growth of conductive amorphous carbon films by HFCVD with filament temperature control. Mater. Lett. 2018, 228, 293–296. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, H.; Li, M.; Meng, Q.; Gao, K.; Lu, X.; Liu, B. The Effect of ZrO2 Nanoparticles on the Microstructure and Properties of Sintered WC-Bronze-Based Diamond Composites. Materials 2016, 9, 343. [Google Scholar] [CrossRef]
- Baker, P.A.; Catledge, S.A.; Harris, S.B.; Ham, K.J.; Chen, W.C.; Chen, C.C.; Vohra, Y.K. Computational Predictions and Microwave Plasma Synthesis of Superhard Boron-Carbon Materials. Materials 2018, 11, 1279. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.U.A.; Grambole, D.; Silva, S.R.P. Studies of carbon ion self-implantation into hydrogenated amorphous carbon films. Diam. Relat. Mater. 2000, 9, 675–679. [Google Scholar] [CrossRef]
- Theye, M.-L.; Paret, V. Spatial organization of the sp2-hybridized carbon atoms and electronic density of states of hydrogenated amorphous carbon films. Carbon 2002, 40, 1153–1166. [Google Scholar] [CrossRef]
- Pradhan, D.; Sharon, M. Opto-electrical properties of amorphous carbon thin film deposited from natural precursor camphor. Appl. Surf. Sci. 2007, 253, 7004–7010. [Google Scholar] [CrossRef]
- Rusop, M.; Omer, A.M.M.; Adhikari, S.; Adhikary, S.; Uchida, H.; Soga, T.; Jimbo, T.; Umeno, M. The Optical Characteristics Of Nitrogen-Doped Amorphous Carbon Thin-Films Grown On Novel Heat Tolerant Flexible Plastic Substrates By A Newly Developed Microwave Surface Wave Plasma Chemical Vapor Deposition Method. Surf. Rev. Lett. 2004, 11, 585–589. [Google Scholar] [CrossRef]
- Baker, P.A.; Goodloe, D.R.; Vohra, Y.K. Morphological Transition in Diamond Thin-Films Induced by Boron in a Microwave Plasma Deposition Process. Materials 2017, 10, 1305. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Chen, Z.Y.; Luo, E.Z.; Cheung, W.Y.; Zhao, J.P.; Wang, X.; Xu, J.B.; Wong, S.P.; Wilson, I.H. Optical and electrical properties of nitrogen incorporated amorphous carbon films. J. Appl. Phys. 2000, 87, 2874–2879. [Google Scholar] [CrossRef]
- Podder, J.; Rusop, M.; Soga, T.; Jimbo, T. Boron doped amorphous carbon thin films grown by r.f. PECVD under different partial pressure. Diam. Relat. Mater. 2005, 14, 1799–1804. [Google Scholar] [CrossRef]
- Ronning, C.; Griesmeier, U.; Gross, M.; Hofsgss, H.C.; Downing, R.G.; Lamaze, G.P. Conduction processes in boron- and nitrogen-doped diamond-like carbon films prepared by mass-separated ion beam deposition. Diam. Relat. Mater. 1995, 4, 666–672. [Google Scholar] [CrossRef]
- Tan, M.; Zhu, J.; Han, J.; Gao, W.; Niu, L.; Lu, J. Chemical analysis and vibrational properties of boronated tetrahedral amorphous carbon films. Diam. Relat. Mater. 2007, 16, 1739–1745. [Google Scholar] [CrossRef]
- Chhowalla, M.; Yin, Y.; Amaratunga, G.A.J.; McKenzie, D.R.; Frauenheim, T. Highly tetrahedral amorphous carbon films with low stress. Appl. Phys. Lett. 1996, 69, 2344–2346. [Google Scholar] [CrossRef]
- Carey, J.D.; Silva, S.R.P. Disorder, clustering, and localization effects in amorphous carbon. Phys. Rev. B 2004, 70, 235417. [Google Scholar] [CrossRef]
- Vishwakarma, P.N.; Subramanyam, S.V. Hopping conduction in boron doped amorphous carbon films. J. Appl. Phys. 2006, 100, 113702. [Google Scholar] [CrossRef]
- Tian, X.M.; Rusop, M.; Hayashi, Y.; Soga, T.; Jimbo, T.; Umeno, M. Photovoltaic characteristics of boron doped amorphous carbon films deposited by pulsed laser deposition using graphite target. Mol. Cryst. Liq. Cryst. 2002, 386, 73–80. [Google Scholar] [CrossRef]
- Tan, M.; Zhu, J.; Han, J.; Han, X.; Niu, L.; Chen, W. Stress evolution of tetrahedral amorphous carbon upon boron incorporation. Scr. Mater. 2007, 57, 141–144. [Google Scholar] [CrossRef]
- Schenk, A.; Winter, B.; Lutterloh, C.; Biener, J.; Schubert, U.A.; Küppers, J. The influence of boron doping on the structure and thermal decomposition of ultrathin C/B:H films. J. Appl. Phys. 1995, 77, 6006–6014. [Google Scholar] [CrossRef]
- He., X.-M.; Walter., K.C.; Nastasi, M. Plasma-immersion ion-processed boron-doped diamond-like carbon films. J. Phys. Condens. Matter 2000, 12, L183–L189. [Google Scholar]
- Tan, M.; Zhu, J.; Han, J.; Niu, L.; Lu, J.; Chen, W. Relative fraction of sp3 bonding in boron incorporated amorphous carbon films determined by X-ray photoelectron spectroscopy. Mater. Res. Bull. 2008, 43, 1670–1678. [Google Scholar] [CrossRef]
- Morales-Acevedo, A. Effective absorption coefficient for graded band-gap semiconductors and the expected photocurrent density in solar cells. Sol. Energy Mater. Sol. Cells 2009, 93, 41–44. [Google Scholar] [CrossRef]
- Merel, P.; Tabbal, M.; Chaker, M.; Moisa, S.; Margot, J. Direct evaluation of the sp3 content in diamond-like-carbon films by XPS. Appl. Surf. Sci. 1998, 136, 105–110. [Google Scholar] [CrossRef]
- Cao, H.; Qi, F.; Ouyang, X.; Zhao, N.; Zhou, Y.; Li, B.; Luo, W.; Liao, B.; Luo, J. Effect of Ti Transition Layer Thickness on the Structure, Mechanical and Adhesion Properties of Ti-DLC Coatings on Aluminum Alloys. Materials 2018, 11, 1742. [Google Scholar] [CrossRef] [PubMed]
- Popescu, A.; Stan, G.; Duta, L.; Nita, C.; Popescu, C.; Surdu, V.-A.; Husanu, M.-A.; Bita, B.; Ghisleni, R.; Himcinschi, C.; et al. The Role of Ambient Gas and Pressure on the Structuring of Hard Diamond-Like Carbon Films Synthesized by Pulsed Laser Deposition. Materials 2015, 8, 3284–3305. [Google Scholar] [CrossRef]
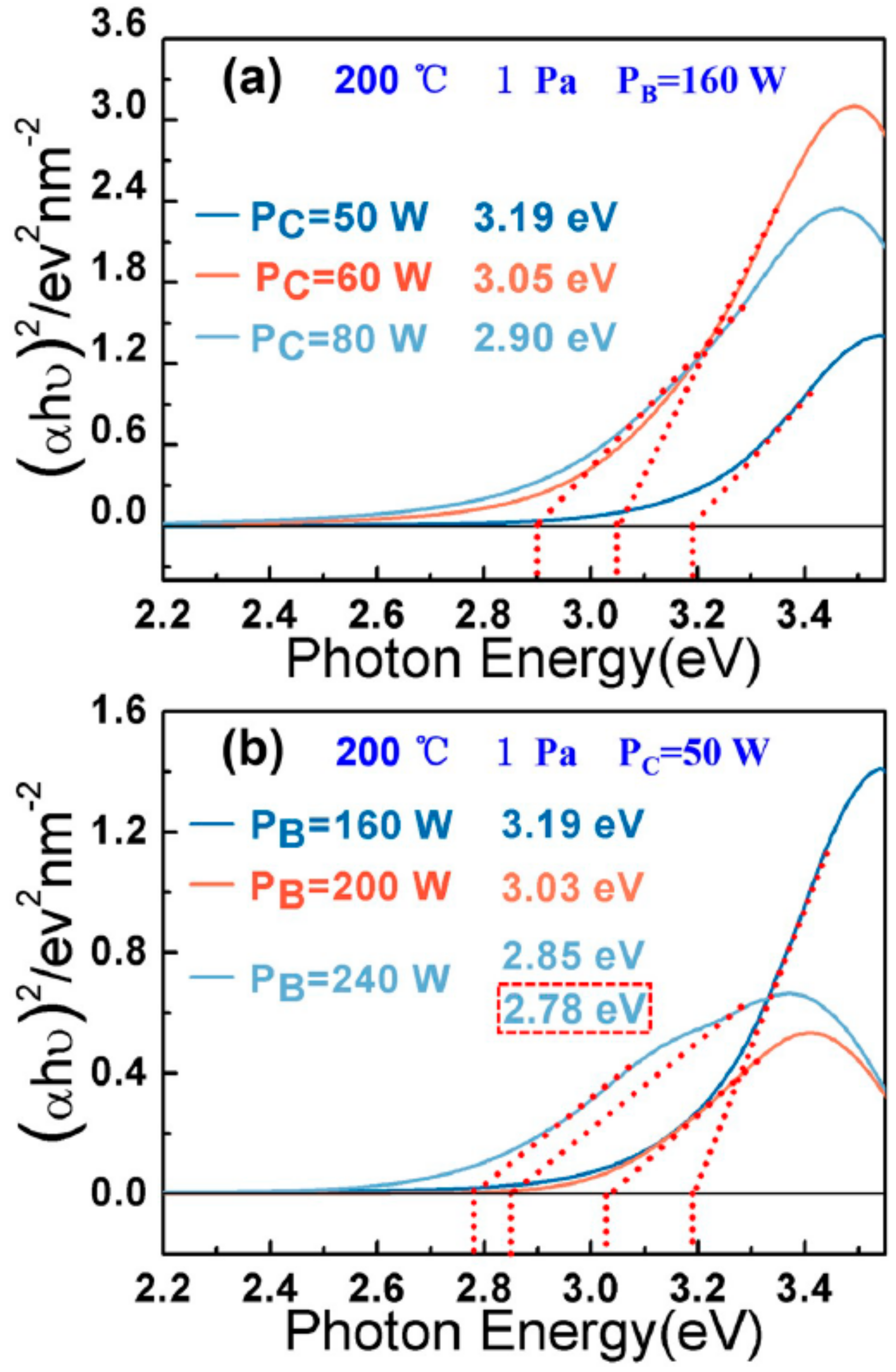
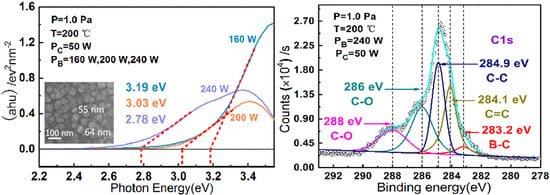
| Sample | PB | PC | T | Pa | B–O | C–O | C=C | C–C | B–C | Eopt | ID/IG |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 160 | 50 | 200 | 1.0 | - | 286; 288 | 284.1 | 284.9 | - | 3.19 | 57% |
| 2 | 200 | 50 | 200 | 1.0 | 191.4 | 286; 287.8 | 284.1 | 284.9 | - | 3.03 | 59% |
| 3 | 240 | 50 | 200 | 1.0 | 191.3 | 286; 288 | 284.1 | 284.9 | 189.2; 283.2 | 2.78 | 61% |
| 4 | 160 | 80 | 200 | 1.0 | 191.3 | 286; 288 | 284.1 | 284.9 | - | 2.90 | 64% |
| 5 | 160 | 50 | 200 | 2.0 | - | 286.2; 288.2 | 284.1 | 284.9 | - | 3.08 | 57% |
| 6 | 160 | 50 | 300 | 1.0 | 191.4 | 286; 288 | 284.1 | 284.9 | 189.2; 283.2 | 3.16 | 59% |
| 7 | 160 | 50 | 400 | 1.0 | 191.2 | 286; 288 | 284.1 | 284.9 | 189.2; 283.2 | 3.15 | 61% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, R.; Tao, Q.; Lian, M.; Feng, X.; Liu, J.; Ye, M.; Wang, X.; Dong, S.; Cui, T.; Zhu, P. Modulating Band Gap of Boron Doping in Amorphous Carbon Nano-Film. Materials 2019, 12, 1780. https://doi.org/10.3390/ma12111780
Zhu R, Tao Q, Lian M, Feng X, Liu J, Ye M, Wang X, Dong S, Cui T, Zhu P. Modulating Band Gap of Boron Doping in Amorphous Carbon Nano-Film. Materials. 2019; 12(11):1780. https://doi.org/10.3390/ma12111780
Chicago/Turabian StyleZhu, Rui, Qiang Tao, Min Lian, Xiaokang Feng, Jiayu Liu, Meiyan Ye, Xin Wang, Shushan Dong, Tian Cui, and Pinwen Zhu. 2019. "Modulating Band Gap of Boron Doping in Amorphous Carbon Nano-Film" Materials 12, no. 11: 1780. https://doi.org/10.3390/ma12111780
APA StyleZhu, R., Tao, Q., Lian, M., Feng, X., Liu, J., Ye, M., Wang, X., Dong, S., Cui, T., & Zhu, P. (2019). Modulating Band Gap of Boron Doping in Amorphous Carbon Nano-Film. Materials, 12(11), 1780. https://doi.org/10.3390/ma12111780