Recent Progress on Organic Electrodes Materials for Rechargeable Batteries and Supercapacitors
Abstract
1. Introduction
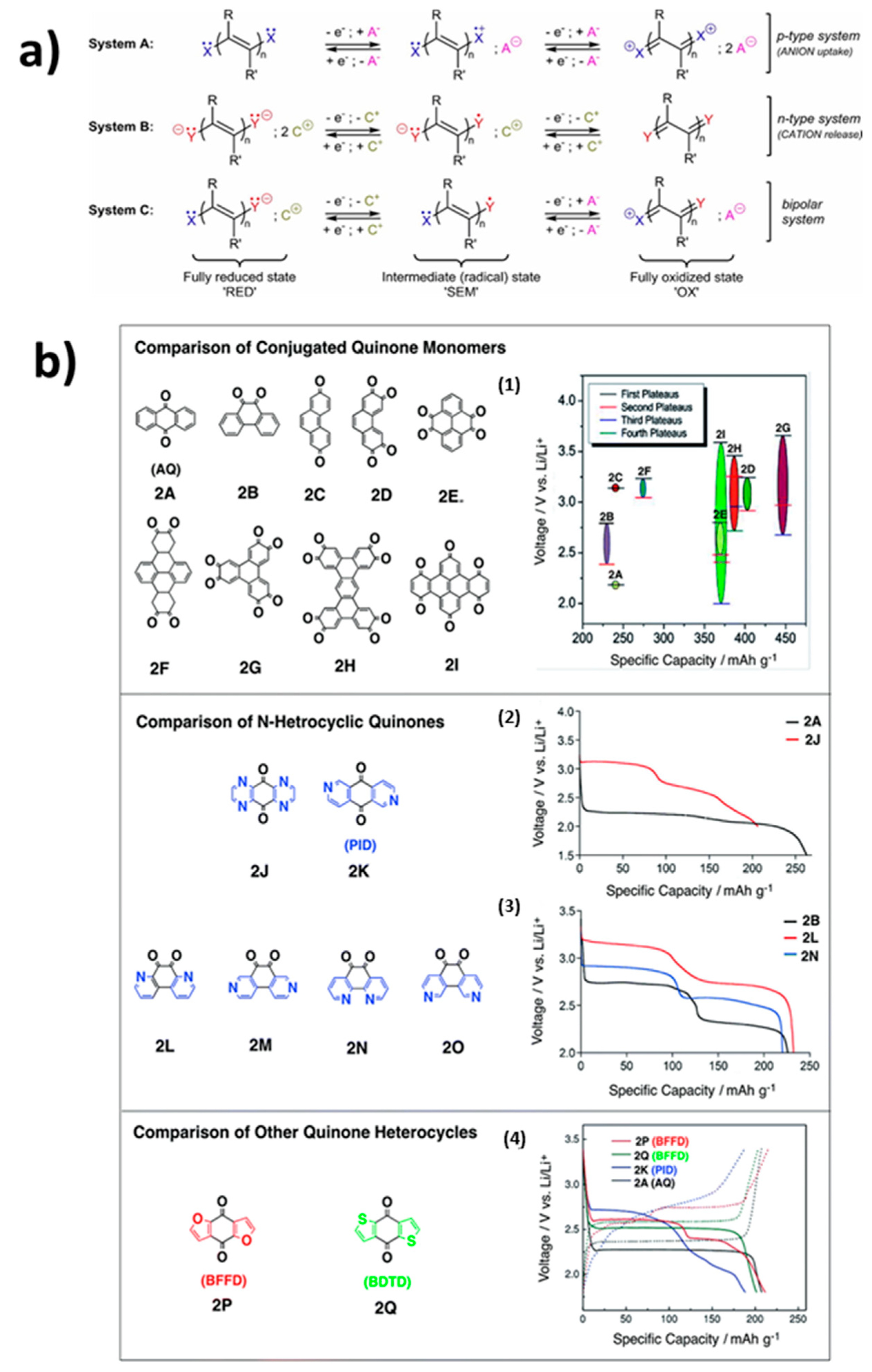
2. Quinones
2.1. Adjustment of the Redox Potential
2.2. Reduction of the Solubility
2.2.1. Benzoquinones
2.2.2. Anthraquinones
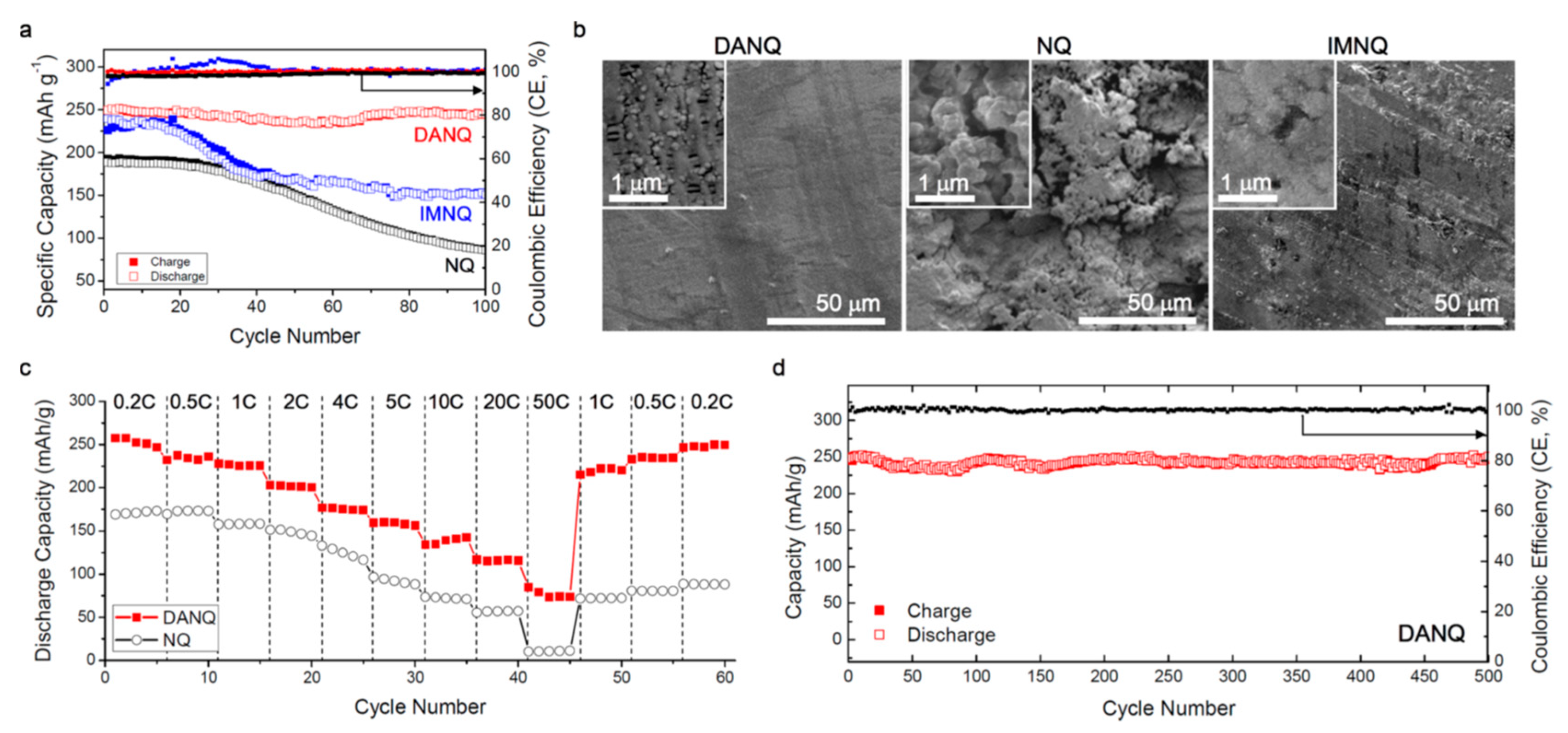
2.2.3. Naphtoquinones Derivatives
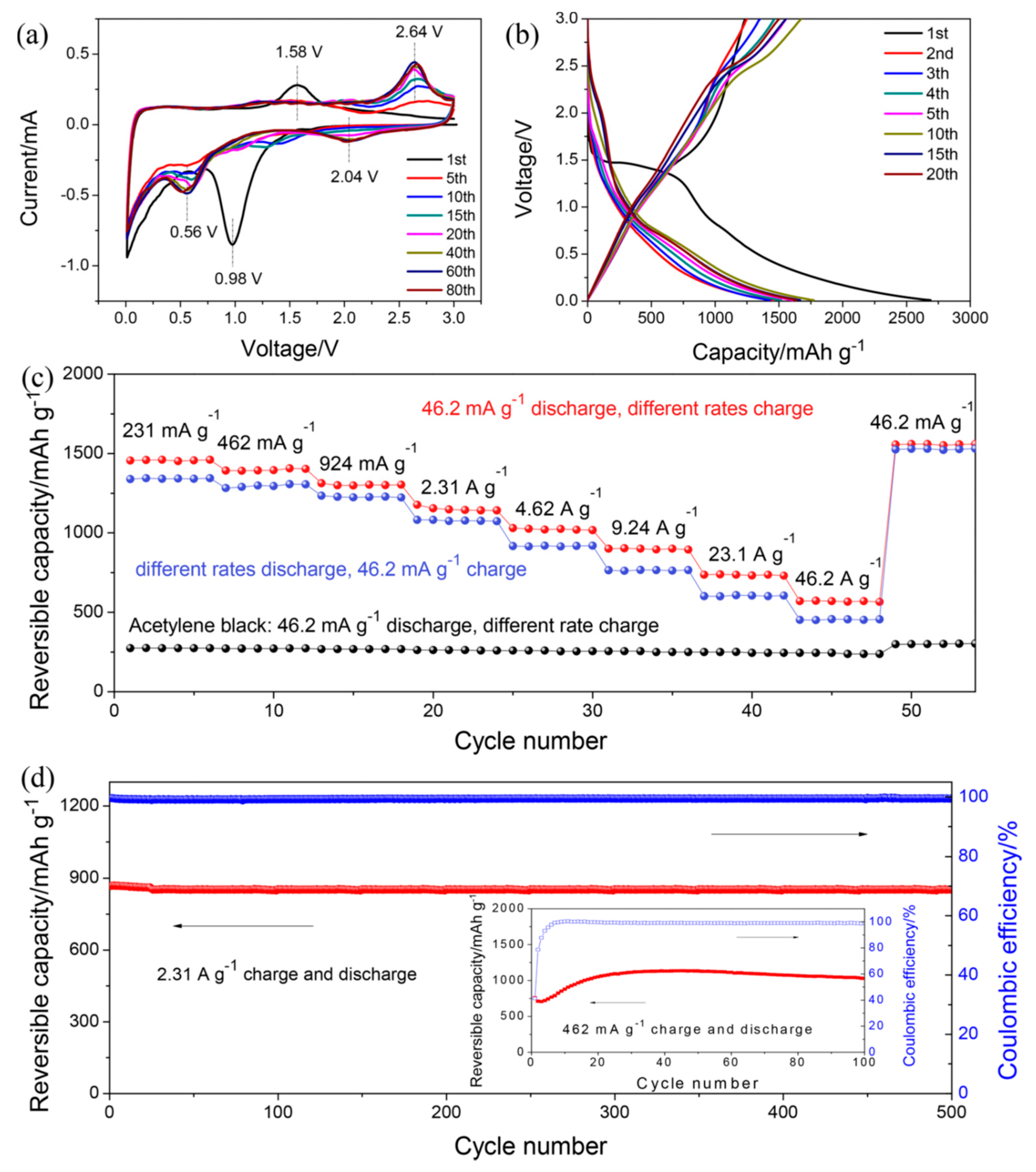
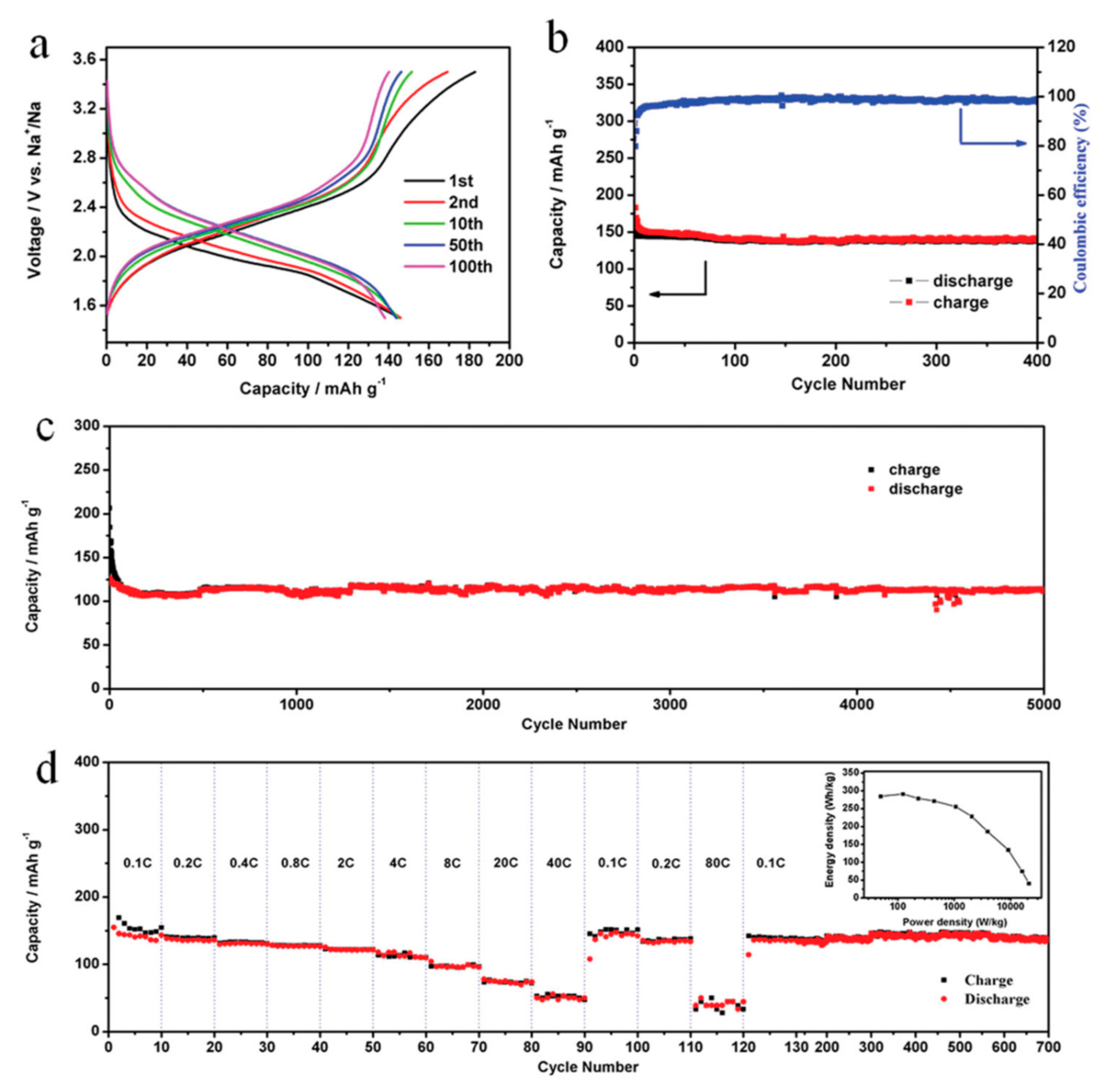
2.3. Conjugated Compounds with n Quinone Units
2.4. Carboxyl Based Materials
2.5. Modifications by Cyanide Functional Groups
2.6. Quinone Based Flow Batteries
2.7. Quinone Based Supercapacitors
3. Imides-Dianhydrides
4. Salts
4.1. Quinone Based Salts
4.2. Azo Based Salts
5. Nitroxide Radical Polymers
6. Ferrocene Based Materials
7. Poly Fluorenylethynylene
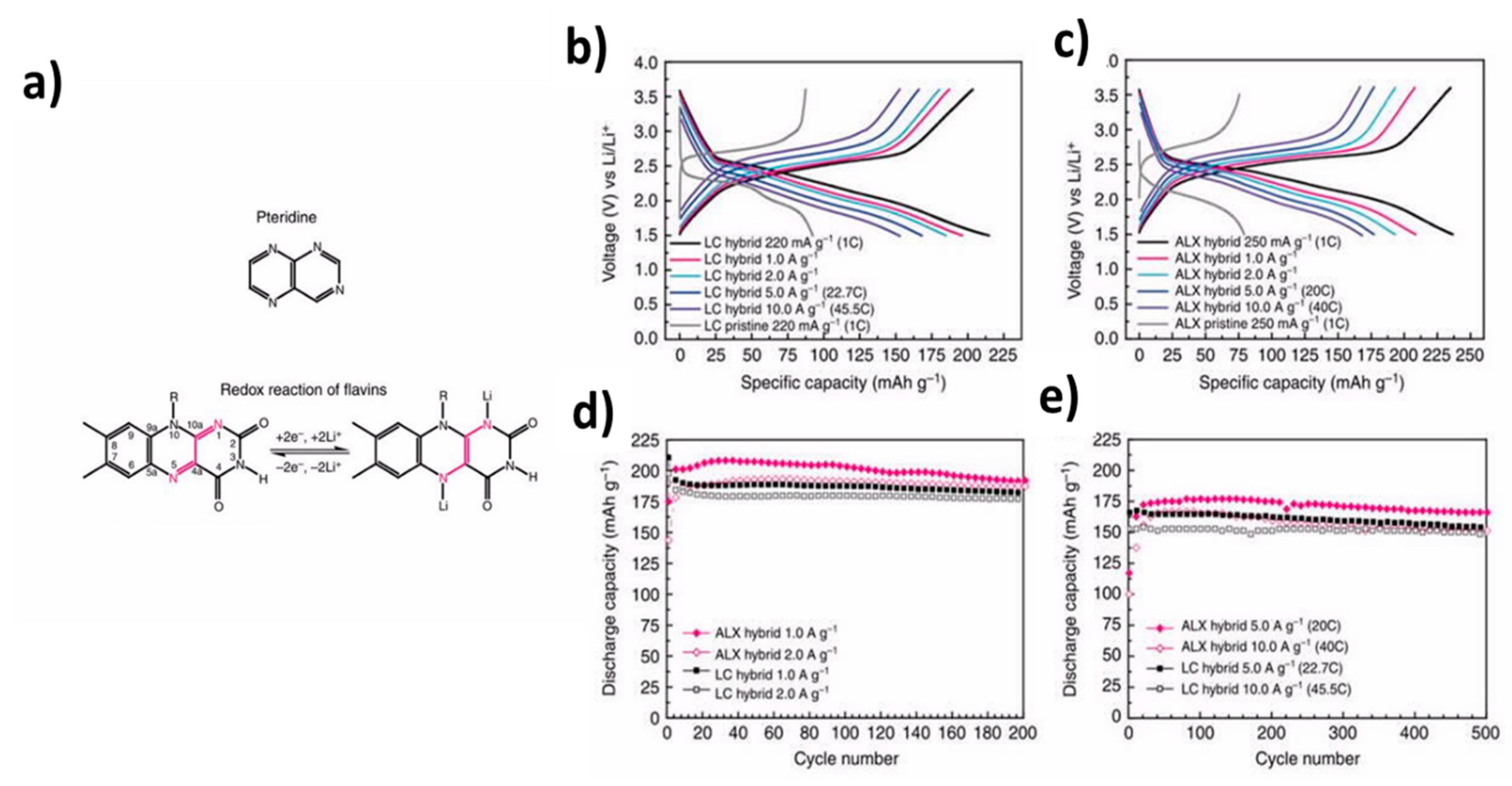
8. Flavin Compounds
9. Tetrathiafulvalene Derivatives
10. Porous Organic Skeletons
11. Organic Molecule-Polymer Hybridization
12. Super Lithiation in Organic Electrodes
13. Carbon Organic Molecule Composites
13.1. In-Situ Carbon Incorporation
13.2. Carbon Nanotubes Organic Molecules
13.3. Graphene Organic Molecules Composites
14. Carbon Radical Organic Polymers
15. Dual Ion Batteries
16. Lithium-Sulfur Batteries
17. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Julien, C.M.; Mauger, A.; Vijh, A.; Zaghib, K. Lithium Batteries: Science and Technology; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Mauger, A.; Julien, C.M. Critical review on lithium-ion batteries: Are they safe? Sustainable? Ionics 2017, 23, 1933–1947. [Google Scholar] [CrossRef]
- Report of the Agence de l’Environnement et de la Maîtrise de l’Energie (ADEME). “Elaboration selon les principes des ACV des bilans énergétiques, des émidssions de gaz à effet de serre et des autres impacts environnementaux induits par l’ensemble des filières de véhicules électriques et de véhicules thermiques, VP de semgment B (citadine polyvalente) et VUL à l’horizon 2012 et 2020”. Available online: http://www.ademe.fr/sites/default/files/assets/documents/90511_acv-comparative-ve-vt-rapport.pdf (accessed on 15 November 2013). (In French).
- Nishide, H.; Oyaizu, K. Towards flexible batteries. Science 2008, 319, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.-M. Researchers must find a sustainable way of providing the power our modern lifestyles demand. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Chen, H.; Armand, M.; Demailly, G.; Dolhem, F.; Poizot, P.; Tarascon, J.M. From biomass to a renewable LixC6O6 organic electrode for sustainable Li-ion batteries. Chem. Sus. Chem. 2008, 1, 348–355. [Google Scholar] [CrossRef]
- Chen, H.; Armand, M.; Courty, M.; Jiang, M.; Grey, C.P.; Dolhem, F.; Tarascon, J.M.; Poizot, P. Lithium salt of tetrahydroxybenzoquinone: Toward the development of a sustainable Li-ion battery. J. Am. Chem. Soc. 2009, 131, 8984–8988. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Yamashita, E.; Baniulis, D.; Cramer, W.A. Quinone-dependent proton transfer pathways in the photosynthetic cytochrome b6f complex. Proc. Natl. Acad. Sci. USA 2013, 110, 4297–4302. [Google Scholar] [CrossRef]
- Liang, Y.; Jing, Y.; Gheytani, S.; Lee, K.Y.; Liu, P.; Facchetti, A.; Yao, Y. Universal quinone electrodes for long cycle life aqueous rechargeable batteries. Nat. Mater. 2017, 16, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Mauger, A.; Julien, C.M.; Paollela, A.; Armand, M.; Zaghib, K. A comprehensive review of lithium salts and beyond for rechargeable batteries: Progress and perspectives. Mater. Sci. Eng. R 2018, 34, 1–21. [Google Scholar] [CrossRef]
- Deuchert, K.; Hünig, S. Multistage organic redox systems-A general structural principle. Angew. Chem. Int. Ed. 1978, 17, 875–886. [Google Scholar] [CrossRef]
- Gottis, S.; Barrès, A.L.; Dolhem, F.; Poizot, P. Voltage gain in lithiated enolate-based organic cathode materials by isomeric effect. ACS Appl. Mater. Interfaces 2014, 6, 10870–10876. [Google Scholar] [CrossRef]
- Armand, M.; Grugeon, S.; Vezin, H.; Laruelle, S.; Ribière, P.; Poizot, P.; Tarascon, J.M. Conjugated dicarboxylate anodes for Li ion batteries. Nat. Mater. 2009, 8, 120−125. [Google Scholar] [CrossRef]
- Abouimrane, A.; Weng, W.; Eltayeb, H.; Cui, Y.; Niklas, J.; Poluektov, O.; Amine, K. Sodium insertion in carboxylate based materials and their application in 3.6 V full sodium cells. Energy Environ. Sci. 2012, 5, 9632–9638. [Google Scholar] [CrossRef]
- Renault, S.; Mihali, V.A.; Brandell, D. Optimizing the electrochemical performance of water-soluble organic Li-ion battery electrodes. Electrochem. Commun. 2013, 34, 174–176. [Google Scholar] [CrossRef]
- Fédèle, L.; Sauvage, F.; Bois, J.; Tarascon, J.M.; Bécuwe, M. Lithium insertion/de-insertion properties of π-extended naphthyl-based dicarboxylate electrode synthesized by freeze-drying. J. Electrochem. Soc. 2014, 161, A46–A52. [Google Scholar] [CrossRef]
- Renault, S.; Geng, J.; Dolhem, F.; Poizot, P. Evaluation of polyketones with N-cyclic structure as electrode material for electrochemical energy storage: Case of pyromellitic diimide dilithium salt. Chem. Commun. 2011, 47, 2414–2416. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Damien, D.; Nagarajan, K.; Shaijumon, M.M.; Hariharan, M. Perylene-polyimide-based organic electrode materials for rechargeable lithium batteries. J. Phys. Chem. Lett. 2013, 4, 3192–3197. [Google Scholar] [CrossRef]
- Wu, H.; Wang, K.; Meng, Y.; Lua, K.; Wei, Z. An organic cathode material based on a polyimide/CNT nanocomposite for lithium ikon batteries. J. Mater. Chem. A 2013, 1, 6366–6372. [Google Scholar] [CrossRef]
- Renault, S.; Gottis, S.; Courty, M.; Chauvet, O.; Dolhem, F.; Poizot, P. A green Li−organic battery working as a fuel cell in case of emergency. Energy Environ. Sci. 2013, 6, 2124–2133. [Google Scholar] [CrossRef]
- Liang, Y.; Tao, Z.; Chen, J. Organic electrode materials for rechargeable lithium batteries. Adv. Energy Mater. 2012, 2, 742–769. [Google Scholar] [CrossRef]
- Song, Z.; Zhou, H. Towards sustainable and versatile energy storage devices: An overview of organic electrode materials. Energy Environ. Sci. 2013, 6, 2280–2301. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, P.; Yang, S.; Tao, Z.; Chen, J. Fused heteroaromatic organic compounds for High-power electrodes of rechargeable lithium batteries. Adv. Energy Mater. 2013, 3, 600–605. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Zhang, K.; Zhu, Z.; Tao, Z.; Chen, J. Organic Li4C8H2O6 nanosheets for lithium-ion batteries. Nano Lett. 2013, 13, 4404–4409. [Google Scholar] [CrossRef]
- Genorio, B.; Pirnat, K.; Cerc-Korosec, R.; Dominko, R.; Gaberscek, M. Electroactive organic molecules immobilized onto solid nanoparticles as a cathode material for lithium-ion batteries. Angew. Chem. Inter. Ed. 2010, 49, 7222–7224. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, T.; Reiman, K.H.; Grossel, M.C.; Owen, J.R. Poly(2,5-dihydroxy-1,4-benzoquinone-3,6-methylene): A new organic polymer as positive electrode material for rechargeable lithium batteries. J. Power Sources 2003, 119, 316–320. [Google Scholar] [CrossRef]
- Suga, T.; Pu, Y.-J.; Kasatori, S.; Nishide, H. Cathode- and anode-active poly(nitroxylstyrene)s for rechargeable batteries: P- and n-type redox switching via substituent effects. Macromolecules 2007, 40, 3167–3173. [Google Scholar] [CrossRef]
- Häringer, D.; Novák, P.; Haas, O.; Piro, B.; Pham, M.-C. Poly(5-amino-1,4-naphthoquinone), a novel lithium-inserting electroactive polymer with high specific charge. J. Electrochem. Soc. 1999, 146, 2393–2396. [Google Scholar] [CrossRef]
- Casado, N.; Hernandez, G.; Sardon, H.; Mecerreyes, D. Current trends in redox polymers for energy and medicine. Prog. Polym. Sci. Suppl. C 2016, 52, 107–135. [Google Scholar] [CrossRef]
- Muench, S.; Wild, A.; Friebe, C.; Häupler, B.; Janoschka, T.; Schubert, U.S. Polymer-Based Organic batteries. Chem. Rev. 2016, 116, 9438–9484. [Google Scholar] [CrossRef]
- Casado, N.; Hernandez, G.; Veloso, A.; Devaraj, S.; Mecerreyes, D.; Armand, M. PEDOT radical polymer with synergetic redox and electrical properties. ACS Macro Lett. 2016, 5, 59–64. [Google Scholar] [CrossRef]
- Gracia, R.; Mecerreyes, D. Polymers with redox properties: Materials for batteries, biosensors and more. Polym. Chem. 2013, 4, 2206–2214. [Google Scholar] [CrossRef]
- Häupler, B.; Wild, A.; Schubert, U.S. Carbonyls: Powerful organic materials for secondary batteries. Adv. Energy Mater. 2015, 5, 1402034. [Google Scholar] [CrossRef]
- Huang, W.; Zhu, Z.; Wang, L.; Wang, S.; Li, H.; Tao, Z.; Shi, J.; Guan, L.; Chen, J. Quasi-solid-state rechargeable lithium-ion batteries with a calix(4)quinone cathode and gel polymer electrolyte. Angew. Chem. Int. Ed. 2013, 52, 9162–9166. [Google Scholar] [CrossRef]
- Vlad, A.; Arnould, K.; Ernould, B.; Sieuw, L.; Rolland, J.; Gohy, J.-F. Exploring the potential of polymer battery cathodes with electrically conductive molecular backbone. J. Mater. Chem. A 2015, 3, 11189–11193. [Google Scholar] [CrossRef]
- Song, Z.; Zhan, H.; Zhou, Y. Anthraquinone based polymer as high performance cathode material for rechargeable lithium batteries. Chem. Commun. 2009, 448–450. [Google Scholar] [CrossRef]
- Song, Z.; Qian, Y.; Liu, X.; Zhang, T.; Zhu, Y.; Yu, H.; Otani, M.; Zhou, H. A quinone-based oligomeric lithium salt for superior Li–organic batteries. Energy Environ. Sci. 2014, 7, 4077–4086. [Google Scholar] [CrossRef]
- Song, Z.; Qian, Y.; Zhang, T.; Otani, M.; Zhou, H. Poly(benzoquinonyl sulfide) as a high-energy organic cathode for rechargeable Li and Na batteries. Adv. Sci. 2015, 2, 1500124. [Google Scholar] [CrossRef] [PubMed]
- Nokami, T.; Matsuo, T.; Inatomi, Y.; Hojo, N.; Tsukagoshi, T.; Yoshizawa, H.; Shimizu, A.; Kuramoto, H.; Komae, K.; Tsuyama, H.; et al. Polymer-bound pyrene-4,5,9,10-tetraone for fast-charge and -discharge lithium-ion batteries with high capacity. J. Am. Chem. Soc. 2012, 134, 19694–19700. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Harada, D.; Oyaizu, K.; Nishide, H. Aqueous electrochemistry of poly(vinylanthraquinone) for anode-active materials in high-density and rechargeable polymer/air batteries. J. Am. Chem. Soc. 2011, 133, 19839–19843. [Google Scholar] [CrossRef]
- Kolek, M.; Otteny, F.; Schmidt, P.; Muck-Lichtenfeld, C.; Einholz, C.; Becking, J.; Schleicher, E.; Winter, M.; Bieker, P.; Esser, B. Ultra-high cycling stability of poly(vinylphenothiazine) as a battery cathode material resulting from π–π interactions. Energy Environ. Sci. 2017, 10, 2334–2341. [Google Scholar]
- Han, X.; Chang, C.; Yuan, L.; Sun, T.; Sun, J. Aromatic carbonyl derivative polymers as high-performance Li-ion storage materials. Adv. Mater. 2007, 19, 1616–1621. [Google Scholar] [CrossRef]
- Geng, J.; Bonnet, J.P.; Renault, S.; Dolhem, F.; Poizot, P. Evaluation of polyketones with N-cyclic structure as electrode material for electrochemical energy storage: Case of tetraketopiperazine unit. Energy Environ. Sci. 2010, 3, 1929–1933. [Google Scholar] [CrossRef]
- Song, Z.; Zhan, H.; Zhou, Y. Polyimides: Promising energy-storage materials. Angew. Chem. Int. Ed. 2010, 49, 8444–8448. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Araki, M.; Senoh, H.; Yamazaki, S.I.; Sakai, T.; Yasuda, K. Indigo dye as a positive-electrode material for rechargeable lithium batteries. Chem. Lett. 2010, 39, 950–952. [Google Scholar] [CrossRef]
- Yao, M.; Senoh, H.; Yamazaki, S.I.; Siroma, Z.; Sakai, T.; Yasuda, K. High-capacity organic positive-electrode material based on a benzoquinone derivative for use in rechargeable lithium batteries. J. Power Sources 2010, 195, 8336–8340. [Google Scholar] [CrossRef]
- Yao, M.; Senoh, H.; Sakai, T.; Kiyobayashi, T. 5,7,12,14-Pentacenetetrone as a high-capacity organic positive-electrode material for use in rechargeable lithium batteries. Int. J. Electrochem. Sci. 2011, 6, 2905–2911. [Google Scholar]
- Liang, Y.; Zhang, P.; Chen, J. Function-oriented design of conjugated carbonyl compound electrodes for high energy lithium batteries. Chem. Sci. 2013, 4, 1330–1337. [Google Scholar] [CrossRef]
- Lv, M.; Zhang, F.; Wu, Y.; Chen, M.; Yao, C.; Nan, J.; Shu, D.; Zeng, R.; Zeng, H.; Chou, S.-L. Heteroaromatic organic compound with conjugated multi-carbonyl as cathode material for rechargeable lithium batteries. Sci. Rep. 2016, 6, 23515. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.; Grugeon, S.; Mentre, O.; Laruelle, S.; Tarascon, J.M.; Wudl, F. Ethoxycarbonyl-based organic electrode for Li-batteries. J. Am. Chem. Soc. 2010, 132, 6517–6523. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.H.; Kim, H.; Goddard, W.A., III; Kang, K. The predicted crystal structure of Li4C6O6, an organic cathode material for Li-ion batteries, from first-principles multi-level computational methods. Energy Environ. Sci. 2011, 4, 4938–4941. [Google Scholar] [CrossRef]
- Miroshnikov, M.; Divya, K.P.; Babu, G.; Meiyazhagan, A.; Arava, L.M.R.; Ajayan, P.M.; John, G. Power from nature: Designing green battery materials from electroactive quinone derivatives and organic polymers. J. Mater. Chem. A 2016, 4, 12370–12386. [Google Scholar] [CrossRef]
- Vadehra, G.S.; Maloney, R.P.; Garcia-Garibay, M.A.; Dunn, B. Naphthalene diimide based materials with adjustable redox potentials: Evaluation for organic lithium-ion batteries. Chem. Mater. 2014, 26, 7151–7157. [Google Scholar] [CrossRef]
- Nakahara, K.; Iwasa, S.; Satoh, M.; Morioka, Y.; Iriyama, J.; Suguro, M.; Hasegawa, E. Rechargeable batteries with organic radical cathodes. Chem. Phys. Lett. 2002, 359, 351–354. [Google Scholar] [CrossRef]
- Li, J.; Zhan, H.; Zhou, L.; Deng, S.; Li, Z.; Zhou, Y. Aniline-based polyorganodisulfide redox system of high energy for secondary lithium batteries. Electrochem. Commun. 2004, 6, 515–519. [Google Scholar] [CrossRef]
- Sarukawa, T.; Yamaguchi, S.; Oyama, N. Electrochemical behaviors of hexathiadipentalene ring, dithiol ring, and methyl sulfide groups on anthracene derivatives confined on electrode. J. Electrochem. Soc. 2010, 157, F196–F201. [Google Scholar] [CrossRef]
- Zeng, R.-H.; Li, X.-P.; Qiu, Y.-C.; Li, W.-S.; Yi, J.; Lu, D.-S.; Tan, C.-L.; Xu, M.-Q. Synthesis and properties of a lithium-organic coordination compound as lithium-inserted material for lithium ion batteries. Electrochem. Commun. 2010, 12, 1253–1256. [Google Scholar] [CrossRef]
- Morita, Y.; Nishida, S.; Murata, T.; Moriguchi, M.; Ueda, A.; Satoh, M.; Arifuku, K.; Sato, K.; Takui, T. Organic tailored batteries materials using stable open-shell molecules with degenerate frontier orbitals. Nat. Mater. 2011, 10, 947–951. [Google Scholar] [CrossRef]
- Hin, J.Y.; Yamada, T.; Yoshikawa, T.H.; Awaga, K.; Shinokubo, H. An antiaromatic electrode-active material enabling high capacity and stable performance of rechargeable batteries. Angew. Chem. Int. Ed. 2014, 53, 3096–3101. [Google Scholar]
- Akaushi, K.; Nickerl, G.; Wisser, F.M.; Nishio-Hamane, D.; Hosono, E.; Zhou, H.; Kaskel, S.; Eckert, J. An energy storage principle using bipolar porous polymeric frameworks. Angew. Chem. Int. Ed. 2012, 51, 7850–7854. [Google Scholar] [CrossRef]
- Hanyu, Y.; Honma, I. Rechargeable quasi-solid state lithium battery with organic crystalline cathode. Sci. Rep. 2012, 2, 453. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, G.; Ku, K.; Yoon, K.; Jung, S.-K.; Lim, H.-D.; Kang, K. Recent progress in organic electrodes for Li and Na rechargeable batteries. Adv. Mater. 2018, 1704682. [Google Scholar] [CrossRef] [PubMed]
- Stolar, M.; Baumgartner, T. Organic n-type materials for charge transport and charge storage applications. Phys. Chem. Chem. Phys. 2013, 15, 9007–9024. [Google Scholar] [CrossRef] [PubMed]
- Schon, T.B.; McAllister, B.T.; Li, P.F.; Seferos, D.S. The rise of organic electrode materials for energy storage. Chem. Soc. Rev. 2016, 45, 6345–6404. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Q. Recent progress in rechargeable lithium batteries with organic materials as promising electrodes. J. Mater. Chem. A 2016, 4, 7091–7106. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, C.; Zhang, L.; Zhou, Y.; Yu, G. Molecular engineering of organic electroactive materials for redox flow batteries. Chem. Soc. Rev. 2018, 47, 69–103. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Hager, M.D.; Schubert, U.S. Photo-rechargeable electric energy storage systems. Adv. Energy Mater. 2016, 6, 1500369. [Google Scholar] [CrossRef]
- Wang, H.; Li, F.; Zhu, B.; Guo, L.; Yang, Y.; Hao, R.; Wang, H.; Liu, Y.; Wang, W.; Guo, X.; et al. Flexible integrated electrical cables based on biocomposites for synchronous energy transmission and storage. Adv. Funct. Mater. 2016, 26, 3472–3479. [Google Scholar] [CrossRef]
- Hu, P.; Wang, H.; Yang, Y.; Yang, J.; Lin, J.; Guo, L. Renewable-biomolecule-based full lithium-ion batteries. Adv. Mater. 2016, 28, 3486–3492. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, K.; Niibori, Y.; Takahashi, A.; Nishide, H. BODIPY-sensitized photocharging of anthraquinone-populated polymer layers for organic photorechargeable air battery. J. Inorg. Organomet. Polym. 2012, 23, 243–250. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Hao, R.; Guo, L. Transition-metal-free biomolecule-based flexible asymmetric supercapacitors. Small 2016, 12, 4683–4689. [Google Scholar] [CrossRef]
- Son, E.J.; Kim, J.H.; Kim, K.; Park, C.B. Quinone and its derivatives for energy harvesting and storage materials. J. Mater. Chem. A 2016, 4, 11179–11202. [Google Scholar] [CrossRef]
- Anjos, D.M.; McDonough, J.K.; Perre, E.; Brown, G.M.; Overbury, S.H.; Gogotsi, Y.; Presser, V. Pseudocapacitance and performance stability of quinone-coated carbon onions. Nano Energy 2013, 2, 702–712. [Google Scholar] [CrossRef]
- Campbell, P.G.; Merrill, M.D.; Wood, B.C.; Montalvo, E.; Worsley, M.A.; Baumann, T.F.; Biener, J. Battery/supercapacitor hybrid via non-covalent functionalization of graphene macro-assemblies. J. Mater. Chem. A 2014, 2, 17764–17770. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, Y.K.; Lee, H.; Lee, S.B.; Park, H.S. Superior pseudocapacitive behavior of confined lignin nanocrystals for renewable energy-storage materials. Chem. Sus. Chem. 2014, 7, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, M.; Weingarth, D.; Presser, V. Quinone-decorated onion-like carbon/carbon fiber hybrid electrodes for high-rate supercapacitor applications. Chem. Electro. Chem. 2015, 2, 1117–1127. [Google Scholar] [CrossRef]
- Chambers, J.Q. Electrochemistry of quinones. In The Chemistry of the Quinonoid Compounds, 1st ed.; Patai, S., Ed.; John Wiley & Sons: London, UK, 1974; Volume 1, pp. 737–791. [Google Scholar]
- Shim, Y.B.; Park, S.M. Spectroelectrochemical studies of p-benzoquinone reduction in aqueous media. J. Electroanal. Chem. 1997, 425, 201–207. [Google Scholar] [CrossRef]
- Namazian, M.; Almodarresieh, H.A.; Noorbala, M.R.; Zare, H.R. DFT calculation of electrode potentials for substituted quinones in aqueous solution. Chem. Phys. Lett. 2004, 396, 424–428. [Google Scholar] [CrossRef]
- Cape, J.L.; Bowman, M.K.; Kramer, D.M. Computation of the redox and protonation properties of quinones: Towards the prediction of redox cycling natural products. Phytochemistry 2006, 67, 1781–1788. [Google Scholar] [CrossRef]
- Park, M.; Shin, D.-S.; Ryu, J.; Choi, M.; Park, N.; Hong, S.Y.; Cho, J. Organic-catholyte-containing flexible rechargeable lithium batteries. Adv. Mater. 2015, 27, 5141–5146. [Google Scholar] [CrossRef]
- Zhu, Z.; Hong, M.; Guo, D.; Shi, J.; Tao, Z.; Chen, J. All-solid-state lithium organic battery with composite polymer electrolyte and pillar-quinone cathode. J. Am. Chem. Soc. 2014, 136, 16461–16464. [Google Scholar] [CrossRef]
- Yokoji, T.; Matsubara, H.; Satoh, M. Rechargeable organic lithium-ion batteries using electron-deficient benzoquinones as positive-electrode materials with high discharge voltages. J. Mater. Chem. A 2014, 2, 19347–19354. [Google Scholar] [CrossRef]
- Kwon, J.E.; Hyun, C.-S.; Ryu, Y.J.; Lee, J.; Min, D.J.; Park, M.J.; An, B.-K.; Park, S.Y. Triptycene-based quinone molecules showing multi-electron redox reactions for large capacity and high energy organic cathode materials in Li-ion batteries. J. Mater. Chem. A 2018, 6, 3134–3140. [Google Scholar] [CrossRef]
- Wu, D.; Xie, Z.; Zhou, Z.; Shen, P.; Chen, Z. Designing high-voltage carbonyl-containing polycyclic aromatic hydrocarbon cathode materials for Li-ion batteries guided by Clar’s theory. J. Mater. Chem. A 2015, 3, 19137–19143. [Google Scholar] [CrossRef]
- Kim, K.C.; Liu, T.; Lee, S.W.; Jang, S.S. First-principles density functional theory modeling of Li binding: Thermodynamics and redox properties of quinone derivatives for lithium-ion batteries. J. Am. Chem. Soc. 2016, 138, 2374–2382. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Burgos, K.; Burkhardt, S.E.; Rodríguez-Calero, G.G.; Hennig, R.G.; Abruña, H.C.D. Theoretical studies of carbonyl-based organic molecules for energy storage applications: The heteroatom and substituent effect. J. Phys. Chem. C 2014, 118, 6046–6051. [Google Scholar] [CrossRef]
- Karlsson, C.; Jämstorp, E.; Stromme, M.; Sjödin, M. Computational electrochemistry study of 16 isoindole-4,7-diones as candidates for organic cathode materials. J. Phys. Chem. C 2012, 116, 3793–3801. [Google Scholar] [CrossRef]
- Pan, B.; Huang, J.; Feng, Z.; Zeng, L.; He, M.; Zhang, L.; Vaughey, J.T.; Bedzyk, M.J.; Fenter, P.; Zhang, Z.; et al. Polyanthraquinone-based organic cathode for high-performance rechargeable magnesium-ion batteries. Adv. Energy Mater. 2016, 6, 1600140. [Google Scholar] [CrossRef]
- Yoshida, H.; Uotani, N.; Saida, Y. Polymer having isoindole structure. US Patent 4,833,231, 23 May 1989. [Google Scholar]
- Pan, B.; Zhou, D.; Huang, J.; Zhang, L.; Burrell, A.K.; Vaughey, J.T.; Zhang, Z.; Liao, C. 2,5-Dimethoxy-1,4-benzoquinone (DMBQ) as organic cathode for rechargeable magnesium-ion batteries. J. Electrochem. Soc. 2016, 163, A580–A583. [Google Scholar] [CrossRef]
- Lee, M.; Hong, J.; Kim, H.; Lim, H.-D.; Cho, S.B.; Kang, K.; Park, C.B. Organic nanohybrids for fast and sustainable energy storage. Adv. Mater. 2014, 26, 2558–2565. [Google Scholar] [CrossRef] [PubMed]
- Pirnat, K.; Dominko, R.; Cerc-Korosec, R.; Mali, G.; Genorio, B.; Gaberscek, M. Electrochemically stabilised quinone based electrode composites for Li-ion batteries. J. Power Sources 2012, 199, 308–314. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, W.; Wang, A.; Yuan, K.; Chen, S.; Yang, Y. A novel polyquinone cathode material for rechargeable lithium batteries. J. Power Sources 2013, 233, 23–27. [Google Scholar] [CrossRef]
- Shimizu, A.; Kuramoto, H.; Tsujii, Y.; Nokami, T.; Inatomi, Y.; Hojo, N.; Suzuki, H.; Yoshida, J.-I. Introduction of two lithiooxycarbonyl groups enhances cyclability of lithium batteries with organic cathode materials. J. Power Sources 2014, 260, 211–217. [Google Scholar] [CrossRef]
- Kim, H.; Kwon, J.E.; Lee, B.; Hong, J.; Lee, M.; Park, S.Y. High energy organic cathode for sodium rechargeable batteries. Chem. Mater. 2015, 27, 7258–7264. [Google Scholar] [CrossRef]
- Jing, Y.; Liang, Y.; Gheytani, S.; Yao, Y. Cross-conjugated oligomeric quinones for high performance organic batteries. Nano Energy 2017, 37, 46–52. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.; Huang, Z.; Yang, X.; Li, W.; Yu, L.C.; Zeng, R.; Luo, Y.; Chou, S.L. C10H4O2S2/graphene composite as a cathode material for sodium-ion batteries. J. Mater. Chem. A 2016, 4, 18409–18415. [Google Scholar] [CrossRef]
- Song, Z.; Xu, T.; Gordin, M.L.; Jiang, Y.-B.; Bae, I.-T.; Xiao, Q.; Zhan, H.; Liu, J.; Wang, D. Polymer–graphene nanocomposites as ultrafast-charge and -discharge cathodes for rechargeable lithium batteries. Nano Lett. 2012, 12, 2205–2211. [Google Scholar] [CrossRef] [PubMed]
- Senoh, H.; Yao, M.; Sakaebe, H.; Yasuda, K.; Siroma, Z. A two-compartment cell for using soluble benzoquinone derivatives as active materials in lithium secondary batteries. Electrochim. Acta 2011, 56, 10145–10150. [Google Scholar] [CrossRef]
- Yokoji, T.; Kaeyama, Y.; Matsubara, H. High-capacity organic cathode active materials of 2,2’-bis-p-benzoquinone derivatives for rechargeable batteries. J. Mater. Chem. A 2016, 4, 5457–5466. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, L.; Zhao, Q.; Li, F.; Chen, J. An insoluble benzoquinone-based organic cathode for use in rechargeable lithium-ion batteries. Angew. Chem. Int. Ed. 2017, 56, 12561–12565. [Google Scholar] [CrossRef]
- Bachman, J.E.; Curtiss, L.A.; Assary, R.S. Investigation of the redox chemistry of anthraquinone derivatives using density functional theory. J. Phys. Chem. A 2014, 118, 8852−8860. [Google Scholar] [CrossRef]
- Wan, W.; Lee, H.; Yu, X.; Wang, C.; Nam, K.-W.; Yang, X.-Q.; Zhou, H. Tuning the electrochemical performances of anthraquinone organic cathode materials for Li-ion batteries through the sulfonic sodium functional group. RSC Adv. 2014, 4, 19878–19882. [Google Scholar] [CrossRef]
- Zeng, R.; Xing, L.; Qiu, Y.; Wang, Y.; Huang, W.; Li, W.; Yang, S. Polycarbonyl(quinonyl) organic compounds as cathode materials for sustainable lithium ion batteries. Electrochim. Acta 2014, 146, 447–454. [Google Scholar] [CrossRef]
- Song, Z.; Qian, Y.; Gordin, M.L.; Tang, D.; Xu, T.; Otani, M.; Zhan, H.; Zhou, H.; Wang, D. Polyanthraquinone as a reliable organic electrode for stable and fast lithium storage. Angew. Chem. Int. Ed. 2015, 54, 13947–13951. [Google Scholar] [CrossRef]
- Xu, W.; Read, A.; Koech, P.K.; Hu, D.; Wang, C.; Xiao, J.; Padmaperuma, A.B.; Graff, G.L.; Liu, J.; Zhang, J.G. Factors affecting the battery performance of anthraquinone-based organic cathode materials. J. Mater. Chem. 2012, 22, 4032–4039. [Google Scholar] [CrossRef]
- Deng, W.; Liang, X.; Wu, X.; Qian, J.; Cao, Y.; Ai, X.; Feng, J.; Yang, H. A low cost, all- organic Na-ion battery based on polymeric cathode and anode. Sci. Rep. 2013, 3, 2671. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.; Liang, Y.; Rodriguez-Perez, I.A.; Yao, Y.; Ji, X. Poly(anthraquinonyl sulfide) cathode for potassium-ion batteries. Electrochem. Commun. Suppl. C 2016, 71, 5–8. [Google Scholar] [CrossRef]
- Lee, W.; Suzuki, S.; Miyayama, M. Electrochemical properties of poly(anthraquinonyl sulfide)/graphene sheets composites as electrode materials for electrochemical capacitors. Nanomaterials 2014, 4, 599–611. [Google Scholar] [CrossRef]
- Vizintin, A.; Bitenc, J.; Kopac, L.A.; Pirnat, K.; Grdadolnik, J.; Stare, J.; Randon-Vitanova, A.; Dominko, R. Probing electrochemical reactions in organic cathode materials via in operando infrared spectroscopy. Nat. Commun. 2018, 9, 661. [Google Scholar] [CrossRef]
- Bitenc, J.; Pirnat, K.; Bancic, T.; Gaberscek, M.; Genorio, B.; Randon-Vitanova, A.; Dominko, R. Anthraquinone-based polymer as cathode in rechargeable magnesium batteries. ChemSusChem 2015, 8, 4128–4132. [Google Scholar] [CrossRef]
- Gomez, I.; Leonet, O.; Blazquez, J.A.; Grande, H.J.; Mecerreyes, D. Poly(anthraquinonyl sulfides): High capacity redox polymers for energy storage. ACS Macro Lett. 2018, 7, 419–424. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.; Park, M.J. Long-life, high-rate lithium- organic batteries based on naphthoquinone derivatives. Chem. Mater. 2016, 28, 2408–2416. [Google Scholar] [CrossRef]
- Jaffe, A.; Saldivar Valdes, A.; Karunadasa, H.I. Quinone-functionalized carbon black cathodes for lithium batteries with high power densities. Chem. Mater. 2015, 27, 3568–3571. [Google Scholar] [CrossRef]
- Tian, B.; Ding, Z.; Ning, G.-H.; Tang, W.; Peng, C.; Liu, B.; Su, J.; Su, C.; Loh, K.P. Amino group enhanced phenazine derivatives as electrode materials for lithium storage. Chem. Commun. 2017, 53, 2914–2917. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P. Electrolytes for solid-state lithium rechargeable batteries: Recent advances and perspectives. Chem. Soc. Rev. 2014, 40, 2525–2540. [Google Scholar] [CrossRef]
- Cao, D.; Kou, Y.; Liang, J.; Chen, Z.; Wang, L.; Meier, H. A facile and efficient preparation of pillararenes and a pillarquinone. Angew. Chem. Int. Ed. 2009, 48, 9721–9723. [Google Scholar] [CrossRef]
- Lao, K.; Yu, C.A. computational study of unique properties of pillar[n]quinones: Self-assembly to tubular structures and potential applications as electron acceptors and anion recognizers. J. Comput. Chem. 2011, 32, 2716–2726. [Google Scholar] [CrossRef]
- Zhu, Z.; Guo, D.; Tao, Z.; Chen, J. Pillar[5]quinone as cathode material for quasi-solid-state rechargeable lithium batteries. J. Sci. China Chem. 2014, 44, 1175–1180. [Google Scholar]
- Hanyu, Y.; Sugimoto, T.; Ganbe, Y.; Masuda, A.; Honma, I. Multielectron redox compounds for organic cathode quasi-solid state lithium battery. J. Electrochem. Soc. 2014, 161, A6–A9. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, W.; Carter, M.; Zhou, L.; Dai, J.; Fu, K.; Lacey, S.; Li, T.; Wan, J.; Han, X.; et al. Organic electrode for non-aqueous potassium-ion batteries. Nano Energy 2015, 18, 205–211. [Google Scholar] [CrossRef]
- Luo, W.; Allen, M.; Raju, V.; Ji, X. An Organic pigment as a high-performance cathode for sodium-ion batteries. Adv. Energy Mater. 2014, 4, 1400554. [Google Scholar] [CrossRef]
- Banda, H.; Damien, D.; Nagarajan, K.; Hariharan, M.; Shaijumon, M.M. A polyimide based all- organic sodium ion battery. J. Mater. Chem. A 2015, 3, 10453–10458. [Google Scholar] [CrossRef]
- Han, X.; Qing, G.; Sun, J.; Sun, T. How many lithium ions can be inserted onto fused C6 aromatic ring systems. Angew. Chem. Int. Ed. 2012, 51, 5147–5151. [Google Scholar] [CrossRef] [PubMed]
- Fédèle, L.; Sauvage, F.; Gottis, S.; Davoisne, C.; Salager, E.; Chotard, J.-N.; Becuwe, M. 2D-layered lithium carboxylate based on biphenyl core as negative electrode for organic lithium-ion batteries. Chem. Mater. 2017, 29, 546–554. [Google Scholar] [CrossRef]
- Choi, A.; Kim, Y.K.; Kim, T.K.; Kwon, M.S.; Lee, K.T.; Moon, H.R. 4,4’-Biphenyldicarboxylate sodium coordination compounds as anodes for Na-ion batteries. J. Mater. Chem. A 2014, 2, 14986–14993. [Google Scholar] [CrossRef]
- Luo, C.; Huang, R.; Kevorkyants, R.; Pavanello, M.; He, H.; Wang, C. Self-assembled organic nanowires for high power density lithium ion batteries. Nano Lett. 2014, 14, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, Y.; Pan, L.; Shi, Y.; Yue, Z.; Shi, Y.; Yu, G. Understanding the size-dependent sodium storage properties of Na2C6O6-based organic electrodes for sodium-ion batteries. Nano Lett. 2016, 16, 3329–3334. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Fang, Y.G.; Xu, Y.; Liang, L.Y.; Zhou, H.P.; Zhao, M.; Lei, Y. Manipulation of disodium rhodizonate: Factors for fast-charge ad fast-discharge sodium-ion batteries with long-term cyclability. Adv. Funct. Mater. 2016, 26, 1777–1786. [Google Scholar] [CrossRef]
- Lee, M.; Hong, J.; Lopez, J.; Sun, Y.; Feng, D.; Lim, K.; Chueh, W.C.; Toney, M.F.; Cui, Y.; Bao, Z.N. High-performance sodium–organic battery by realizing four-sodium storage in disodium rhodizonate. Nat. Energy 2017, 2, 861–868. [Google Scholar] [CrossRef]
- Wan, F.; Wu, X.-L.; Guo, J.-Z.; Li, J.-Y.; Zhang, J.-P.; Niu, L.; Wang, R.-S. Nanoeffects promote the electrochemical properties of organic Na2C8H4O4 as anode material for sodium-ion batteries. Nano Energy 2015, 13, 450–457. [Google Scholar] [CrossRef]
- Ogihara, N.; Yasuda, T.; Kishida, Y.; Ohsuna, T.; Miyamoto, K.; Ohba, N. Organic dicarboxylate negative electrode materials with remarkably small strain for high-voltage bipolar batteries. Angew. Chem. Int. Ed. 2014, 53, 11467–11472. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, Q.; Zhou, A.; Liu, X.; Li, J. Porous Li2C8H4O4 coated with N-doped carbon by using CVD as an anode material for Li-ion batteries. J. Mater. Chem. A 2014, 2, 5696–5702. [Google Scholar] [CrossRef]
- Park, Y.; Shin, D.-S.; Woo, S.H.; Choi, N.S.; Shin, K.H.; Oh, S.M.; Lee, K.T.; Hong, S.Y. Sodium terephthalate as an organic anode material for sodium ion batteries. Adv. Mater. 2012, 24, 3562–3567. [Google Scholar] [CrossRef] [PubMed]
- Talapaneni, S.N.; Hwang, T.H.; Sang, H.J.; Buyukcakir, O.; Choi, J.W.; Coskun, A. Elemental-sulfur-mediated facile synthesis of a covalent triazine framework for high-performance lithium-sulfur batteries. Angew. Chem. Int. Ed. 2016, 55, 3106. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Y.; Wang, J.W.; Zheng, Y.H.; Li, J.; Han, X.G.; He, G.; Du, Y.P. Organic thiocarboxylate electrodes for a room-temperature sodium-ion battery delivering an ultrahigh capacity. Angew. Chem. Int. Ed. 2017, 56, 15334–15338. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Zhu, Z.; Hu, Z.; Zhao, Q.; Chen, J. All organic sodium-ion batteries with Na4C8H2O6. Angew. Chem. Int. Ed. 2014, 53, 5892–5896. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, H.; Mou, C.; Cui, Q.; Deng, Q.; Li, J. Dicarboxylate CaC8H4O4 as a high-performing anode for Li-ion batteries. Nano Res. 2015, 8, 523–532. [Google Scholar] [CrossRef]
- Wang, L.; Mou, C.; Sun, Y.; Liu, W.; Deng, Q.; Li, J. Structure-property of metal organic frameworks calcium terephthalates anodes of lithium-ion batteries. Electrochem. Acta 2015, 173, 235–241. [Google Scholar] [CrossRef]
- Wang, L.; Mou, C.; Wu, B.; Xue, J.; Li, J. Alkaline earth metal terephthalates MC8H4O4 (M=Ca, Sr, Ba) as Anodes for Lithium Ion Batteries. Electrochim. Acta 2016, 196, 118–124. [Google Scholar] [CrossRef]
- Xue, J.; Fan, C.; Deng, Q.; Zhao, M.; Wang, L.; Zhou, A.; Li, J. Silver terephthalate (Ag2C8H4O4) offering in-situ formed metal/organic nanocomposite as the highly efficient organic anode in Li-ion and Na-ion batteries. Electrochim. Acta 2016, 219, 418–424. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, J.; Hu, Y.-S.; Li, H.; Zhou, Z.; Armand, M.; Chen, L. Disodium terephthalate (Na2C8H4O4) as high performance anode material for low-cost room-temperature sodium-ion battery. Adv. Energy Mater. 2012, 2, 962–965. [Google Scholar] [CrossRef]
- Sk, M.A.; Manzhos, S. Exploring the sodium storage mechanism in disodium terephthalate as anode for organic battery using density-functional theory calculations. J. Power Sources 2016, 324, 572–581. [Google Scholar] [CrossRef]
- Chen, Y.; Lüder, J.; Manzhos, S. Disodium pyridine dicarboxylate vs disodium terephthalate as anode materials for organic Na ion batteries: Effect of molecular structure on voltage from the molecular modeling perspective. MRS Adv. 2017, 2, 3231–3235. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Deng, Y.; Qu, Q.; Zheng, X.; Zhang, J.; Liu, G.; Battaglia, V.S.; Zheng, H. Ultrahigh-capacity organic anode with high-rate capability and long cycle life for lithium-ion batteries. ACS Energy Lett. 2017, 2, 2140–2148. [Google Scholar] [CrossRef]
- Chen, Y.; Manzhos, S. A computational study of lithium interaction with tetracyanoethylene (TCNE) and tetracyanoquinodimethane molecules. Phys. Chem. Chem. Phys. 2016, 18, 1470–1477. [Google Scholar] [CrossRef]
- Chen, Y.; Manzhos, S. Comparative computational study of lithium and sodium insertion in van der Waals and covalent tetracyanoethylene (TCNE)-based crystals as promising materials for organic lithium and sodium ion batteries. Phys. Chem. Chem. Phys. 2016, 18, 8874–8880. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Manzhos, S. Li storage on TCNE and TCNE-(doped)-graphene complexes: A computational study. MRS Proc. 2014. [Google Scholar] [CrossRef]
- Banda, H.; Damien, D.; Nagarajan, K.; Raj, A.; Hariharan, M.; Shaijumon, M.M. Twisted perylene diimides with tunable redox properties for organic sodium-ion batteries. Adv. Energy Mater. 2017, 7, 1701316. [Google Scholar] [CrossRef]
- Nishida, S.; Yamamoto, Y.; Takui, T.; Morita, Y. Organic rechargeable batteries with tailored voltage and cycle performance. Chem. Sus. Chem. 2013, 6, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Eisenach, L.; Aziz, M.J. Cycling Analysis of a Quinone-Bromide Redox Flow Battery. J. Electrochem. Soc. 2016, 163, A5057–A5063. [Google Scholar] [CrossRef]
- Huskinson, B.; Marshak, M.P.; Gerhardt, M.R.; Aziz, M.J. Cycling of a quinone-bromide flow battery for large-scale electrochemical energy storage. ECS Trans. 2014, 61, 27–30. [Google Scholar] [CrossRef]
- Huskinson, B.; Nawar, S.; Gerhardt, M.R.; Aziz, M.J. Novel Quinone-based couples for flow batteries. ECS Trans. 2013, 53, 101–105. [Google Scholar] [CrossRef]
- Chen, Q.; Gerhardt, M.R.; Hartle, L.; Aziz, M.J. A quinone-bromide flow battery with 1 W/cm2 power density. J. Electrochem. Soc. 2016, 163, A5010–A5013. [Google Scholar] [CrossRef]
- Zaghib, K.; Mauger, A.; Julien, C.M. Rechargeable lithium batteries for energy storage in smart grids. In Rechargeable Lithium Batteries: From Fundamentals to Applications; Franco, A.A., Ed.; Woodhead Publ.: Amlsterdam, The Netherlands, 2015. [Google Scholar]
- Huskinson, B.; Marshak, M.P.; Suh, C.; Er, S.; Gerhardt, M.R.; Galvin, C.J.; Chen, X.; Aspuru-Guzik, A.; Gordon, R.G.; Aziz, M.J. A metal-free organic-inorganic aqueous flow battery. Nature 2014, 505, 195–198. [Google Scholar] [CrossRef]
- Quan, M.; Sanchez, D.; Wasylkiw, M.F.; Smith, D.K. Voltammetry of quinones in unbuffered aqueous solution: Reassessing the roles of proton transfer and hydrogen bonding in the aqueous electrochemistry of quinones. J. Am. Chem. Soc. 2007, 129, 12847–12856. [Google Scholar] [CrossRef]
- Er, S.; Suh, C.; Marshak, M.P.; Aspuru-Guzik, A. Computational design of molecules for an all-quinone redox flow battery. Chem. Sci. 2015, 6, 885–893. [Google Scholar] [CrossRef]
- Lin, K.; Chen, Q.; Gerhardt, M.R.; Tong, L.; Kim, S.B.; Eisenach, L.; Valle, A.W.; Hardee, D.; Gordon, R.G.; Aziz, M.J.; et al. Alkaline quinone flow battery. Science 2015, 349, 1529–1532. [Google Scholar] [CrossRef]
- Yang, B.; Hoober-Burkhardt, L.; Wang, F.; Surya Prakash, G.K.; Narayanan, S.R. An inexpensive aqueous flow battery for large-scale electrical energy storage based on water-soluble organic redox couples. J. Electrochem. Soc. 2014, 161, A1371–A1380. [Google Scholar] [CrossRef]
- Brushett, F.R.; Vaughey, J.T.; Jansen, A.N. An all- organic non-aqueous lithium-ion redox flow battery. Adv. Energy Mater. 2013, 2, 1390–1396. [Google Scholar] [CrossRef]
- Zhou, W.D.; Hernandez-Burgos, K.; Burkhardt, S.E.; Qian, H.L.; Abruna, H.D. Synthesis and electrochemical and computational analysis of two new families of thiophene-carbonyl molecules. J. Phys. Chem. C 2013, 117, 6022–6032. [Google Scholar] [CrossRef]
- Huang, J.; Su, L.; Kowalski, J.A.; Barton, J.L.; Ferrandon, M.; Burrell, A.K.; Brushett, F.R.; Zhang, L. A subtractive approach to molecular engineering of dimethoxybenzene-based redox materials for non-aqueous flow batteries. J. Mater. Chem. A 2015, 3, 14971–14976. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Yi, H.; Wang, X.; Yan, X.; Guo, Z. Anthraquinone on Porous Carbon Nanotubes with Improved Supercapacitor Performance. J. Phys. Chem. C 2014, 118, 8262–8270. [Google Scholar] [CrossRef]
- Cha, I.; Lee, E.J.; Park, H.S.; Kim, J.-H.; Kim, Y.H.; Song, C. Facile electrochemical synthesis of polydopamine-incorporated graphene oxide/PEDOT hybrid thin films for pseudocapacitive behaviors. Synth. Met. 2014, 195, 162–166. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Yu, X.; Bak, B.M.; Pang, C.; Park, H.S. Bio-inspired functionalization and redox charge transfer of graphene oxide sponges for pseudocapacitive electrodes. Carbon 2015, 83, 71–78. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, B.; Liu, C.; Han, N.; Xu, X.; Zhao, F.; Fan, J.; Li, Y. Polyanthraquinone-based nanostructured electrode material capable of high-performance pseudocapacitive energy storage in aprotic electrolyte. Nano Energy 2015, 15, 654–661. [Google Scholar] [CrossRef]
- Algharaibeh, Z.; Liu, X.; Pickup, P.G. An asymmetric anthraquinone-modified carbon/ruthenium oxide supercapacitor. J. Power Sources 2009, 187, 640–643. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, P.; Sun, S. A theoretical method to predict novel organic electrode materials for Na-ion batteries. Comput. Mater. Sci. 2017, 134, 42–47. [Google Scholar] [CrossRef]
- Wu, H.; Shevlin, S.A.; Meng, Q.; Guo, W.; Meng, Y.; Lu, K.; Wei, Z.; Guo, Z. Flexible and binder-free organic cathode for high-performance lithium-ion batteries. Adv. Mater. 2014, 26, 3338–3343. [Google Scholar]
- Bhosale, M.E.; Krishnamoorthy, K. Chemically reduced organic small-molecule-based lithium battery with improved efficiency. Chem. Mater. 2015, 27, 2121–2126. [Google Scholar] [CrossRef]
- Li, L.; Hong, Y.J.; Chen, D.-Y.; Lin, M.-J. Molecular engineering of perylene imides for high-performance lithium batteries: Diels–Alder extension and chiral dimerization. Chem. Eur. J. 2017, 23, 16612–16620. [Google Scholar] [CrossRef] [PubMed]
- Kozma, E.; Catellani, M. Perylene diimides based materials for organic solar cells. Dyes Pigments 2013, 98, 160–179. [Google Scholar] [CrossRef]
- Deng, W.; Shen, Y.; Qian, J.; Cao, Y.; Yang, H. A perylene diimide crystal with high capacity and stable cyclability for Na-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 21095–21099. [Google Scholar] [CrossRef]
- Sasada, Y.; Langford, S.J.; Oyaizu, K.; Nishide, H. Poly(norbornyl-NDIs) as a potential cathode-active material in rechargeable charge storage devices. RSC Adv. 2016, 6, 42911–42916. [Google Scholar] [CrossRef]
- Schon, T.B.; Tilley, A.; Kynaston, E.L.; Seferos, D.S. Three-dimensional arylene diimide frameworks for highly stable lithium ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 15631–15637. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, J.M.; Kim, J.S.; Shim, E.G.; Lee, S.Y. Polyimide/carbon black composite nanocoating layers as a facile surface modification strategy for high-voltage lithium ion cathode materials. J. Mater. Chem. A 2013, 1, 12441–12447. [Google Scholar] [CrossRef]
- Xu, F.; Xia, J.; Shi, W. Anthraquinone-based polyimide cathodes for sodium secondary batteries. Electrochem. Commun. 2015, 60, 117–120. [Google Scholar] [CrossRef]
- Wang, H.G.; Yuan, S.; Ma, D.; Huang, X.; Meng, F.; Zhang, X.B. Tailored aromatic carbonyl derivative polyimides for high-power and long-cycle sodium-organic batteries. Adv. Energy Mater. 2014, 4, 1301651. [Google Scholar] [CrossRef]
- Guo, W.; Yin, Y.-X.; Xin, S.; Guo, Y.G.; Wan, L.-J. Superior radical polymer cathode material with a two-electron process redox reaction promoted by graphene. Energy. Environ. Sci. 2012, 5, 5221–5225. [Google Scholar] [CrossRef]
- Deng, W.; Shen, Y.; Qian, J.; Yang, H. A polyimide anode with high capacity and superior cyclability for aqueous Na-ion batteries. Chem. Commun. 2015, 51, 5097–5099. [Google Scholar] [CrossRef]
- Hernandez, G.; Casado, N.; Coste, R.; Shanmukaraj, D.; Rubatat, L.; Armand, M.; Mecerreyes, D. Redox-active polyimide–polyether block copolymers as electrode materials for lithium batteries. RSC Adv. 2015, 5, 17096–17103. [Google Scholar] [CrossRef]
- Xu, F.; Wang, H.; Lin, J.; Luo, X.; Cao, S.A.; Yang, H. Poly(anthraquinonyl imide) as a high capacity organic cathode material for Na-ion batteries. J. Mater. Chem. A 2016, 4, 11491–11497. [Google Scholar] [CrossRef]
- Xie, J.; Chen, W.; Wang, Z.; Jie, K.C.W.; Liu, M.; Zhang, Q. Synthesis and exploration of Ladder-structured large aromatic dianhydrides as organic cathodes for rechargeable lithium-ion batteries. Chem. Asian J. 2017, 12, 868–876. [Google Scholar] [CrossRef]
- Qin, H.; Song, Z.P.; Zhan, Z.; Zhou, Y.H. Aqueous rechargeable alkali-ion batteries with polyimide anode. J. Power Sources 2014, 249, 367–372. [Google Scholar] [CrossRef]
- Chan, L.; Li, W.; Wang, Y.; Wang, C.; Xia, Y. Polyimide as anode electrode material for rechargeable sodium batteries. RSC Adv. 2014, 4, 25369–25373. [Google Scholar] [CrossRef]
- Bernsmann, F.; Ball, V.; Addiego, F.; Ponche, A.; Michel, M.; de Almeida Gracio, J.J.; Toniazzo, V.; Ruch, D. Dopamine-melanin film deposition depends on the used oxidant and buffer solution. Langmuir 2011, 27, 2819–2825. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, H.; Liang, J.; Tao, Z.; Chen, J. The disodium salt of 2,5-dihydroxy-1,4-benzoquinone as anode material for rechargeable sodium ion batteries. Chem. Commun. 2015, 51, 1446–1448. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Kim, K.C.; Lee, B.; Chen, Z.; Noda, S.; Jang, S.S.; Lee, S.W. Self-polymerized dopamine as an organic cathode for Li- and Na-ion batteries. Energy Environ. Sci. 2017, 10, 205–215. [Google Scholar] [CrossRef]
- Sun, T.; Li, Z.; Wang, H.; Bao, D.; Meng, F.; Zhang, X. A Biodegradable Polydopamine-Derived Electrode Material for High-Capacity and Long-Life Lithium-Ion and Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2016, 55, 10662–10666. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Wu, W.; Chun, S.E.; Whitacre, J.F.; Bettinger, C.J. Catechol-mediated reversible binding of multivalent cations in eumelanin half-cells. Adv. Mater. 2014, 26, 6572–6579. [Google Scholar] [CrossRef]
- Barres, A.L.; Geng, J.; Bonnard, G.; Renault, S.; Gottis, S.; Mentré, O.; Frayret, C.; Dolhem, F.; Poizot, P. High-potential reversible Li deintercalation in a substituted tetrahydroxy-p-benzoquinone Dilithium salt: An experimental and theoretical study. Chem. Eur. J. 2012, 18, 8800–8812. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Nagarajan, S.; Chumyim, P.; Gowda, S.R.; Pradhan, P.; Jadhav, S.R.; Dubey, M.; John, G.; Ajayan, P.M. Lithium storage mechanisms in purpurin based organic lithium ion battery electrodes. Sci. Rep. 2012, 2, 960. [Google Scholar] [CrossRef]
- Luo, C.; Xu, G.-L.; Ji, X.; Hou, S.; Chen, L.; Wang, F.; Jiang, J.; Chen, Z.; Ren, Y.; Amine, K.; et al. Reversible redox chemistry of azo compounds for sodium-ion batteries. Angew. Chem. Int. Ed. 2018, 57, 2879–2883. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Wang, J.; Fan, X.; Zhu, Y.; Han, F.; Suo, L.; Wang, C. Roll-to-roll fabrication of organic nanorod electrodes for sodium ion batteries. Nano Energy 2015, 13, 537–545. [Google Scholar] [CrossRef]
- Lopez-Herraiz, M.; Castillo-Martinez, E.; Carretero-Gonzalez, J.; Carrasco, J.; Rojo, T.; Armand, M. Oligomeric-Schiff bases as negative electrodes for sodium ion batteries: Unveiling the nature of their active redox centers. Energy Environ. Sci. 2015, 8, 3233–3241. [Google Scholar] [CrossRef]
- Lüder, J.; Legrain, F.; Chen, Y.; Manzhos, S. Doping of active electrode materials for electrochemical batteries: An electronic structure perspective. MRS Commun. 2017, 7, 523–540. [Google Scholar] [CrossRef]
- Lüder, J.; Cheow, M.H.; Manzhos, S. Understanding doping strategies in the design of organic electrode materials for Li and Na ion batteries: An electronic structure perspective. Phys. Chem. Chem. Phys. 2017, 19, 13195–13209. [Google Scholar] [CrossRef]
- Luo, C.; Ji, X.; Hou, S.; Eidson, N.; Liang, Y.; Deng, T.; Jiang, J.; Wang, C. Azo compounds derived from electrochemical reduction of nitro compounds for high performance Li-ion batteries. Adv. Mater. 2018, 30, 1706498. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.-A.; Blinco, J.P. Nitroxide radical polymers -a versatile material class for high-tech applications. Polym. Chem. 2018, 9, 1479–1516. [Google Scholar] [CrossRef]
- Suga, T.; Konishi, H.; Nishide, H. Photocrosslinked nitroxide polymer cathode-active materials for application in an organic-based paper battery. Chem. Commun. 2007, 17, 1730–1732. [Google Scholar] [CrossRef] [PubMed]
- Yonekuta, Y.; Susuki, K.; Oyaizu, K.; Honda, K.; Nishide, H. Battery-inspired, nonvolatile, and rewritable memory architecture: A radical polymer-based organic device. J. Amer. Chem. Soc. 2007, 129, 14128–14129. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, K.; Oyaizu, K.; Nishide, H. Organic radical battery approaching practical use. Chem. Lett. 2011, 40, 222–227. [Google Scholar] [CrossRef]
- Kim, J.-K.; Ahn, J.-H.; Cheruvally, G.; Chauhan, G.S.; Choi, J.-W.; Kim, D.-S.; Ahn, H.-J.; Lee, S.-H.; Song, C.-E. Electrochemical properties of rechargeable organic radical battery with PTMA cathode. Metals Mater. Int. 2009, 15, 77–82. [Google Scholar] [CrossRef]
- Kim, J.-K.; Cheruvally, G.; Choi, J.-W.; Ahn, J.-H.; Lee, S.-H.; Choi, D.-S.; Song, C.-E. Effect of radical polymer cathode thickness on the electrochemical performance of organic radical battery. Solid State Ionics 2007, 178, 1546–1551. [Google Scholar] [CrossRef]
- Kim, J.-K.; Cheruvally, G.; Ahn, J.-H.; Seo, Y.-G.; Choi, D.-S.; Lee, S.-H.; Song, C.-E. Organic radical battery with PTMA cathode: Effect of PTMA content on electrochemical properties. J. Ind. Eng. Chem. 2008, 14, 371–376. [Google Scholar] [CrossRef]
- Lopez-Pena, H.A.; Hernández-Munoz, L.S.; Cardoso, J.; González, F.J.; Gonzalez, I.; Frontana, C. Electrochemical and spectroelectrochemical properties of nitroxyl radical species in PTMA, an organic radical polymer. Influence of the microstructure. Electrochem. Commun. 2009, 11, 1369–1372. [Google Scholar] [CrossRef]
- Nakahara, K.; Iriyama, J.; Iwasa, S.; Suguro, M.; Satoh, M.; Cairns, E.J. High-rate capable organic radical cathodes for lithium rechargeable batteries. J. Power Sources 2007, 165, 870–873. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, Y.; Park, S.; Ko, H.; Kim, Y. Encapsulation of organic active materials in carbon nanotubes for application to high-electrochemical-performance sodium batteries. Energy Environ. Sci. 2016, 9, 1264–1269. [Google Scholar] [CrossRef]
- Kim, T.S.; Lim, J.-E.; Oh, M.-S.; Kim, J.-K. Carbon conductor- and binder-free organic electrode for flexible organic rechargeable batteries with high energy density. J. Power Sources 2017, 361, 15–20. [Google Scholar] [CrossRef]
- Nesvadba, P.; Bugnon, L.; Maire, P.; Novak, P. Synthesis of A Novel spirobisnitroxide polymer and its evaluation in an organic radical battery. Chem. Mater. 2010, 22, 783–788. [Google Scholar] [CrossRef]
- Ibe, T.; Frings, R.B.; Lachowicz, A.; Kyo, S.; Nishide, H. Nitroxide polymer networks formed by Michael addition: On site-cured electrode-active organic coating. Chem. Commun. 2010, 46, 3475–3477. [Google Scholar] [CrossRef]
- Kim, J.-K.; Thébault, F.; Heo, M.-Y.; Kim, D.-S.; Hansson, Ö.; Ahn, J.-H.; Johansson, P.; Öhrström, L.; Matic, A.; Jacobsson, P. 2,3,6,7,10,11-Hexamethoxytriphenylene (HMTP): A new organic cathode material for lithium batteries. Electrochem. Commun. 2012, 21, 50–53. [Google Scholar] [CrossRef]
- Montoto, E.C.; Nagarjuna, G.; Hui, J.; Burgess, M.; Sekerak, N.M.; Hernandez-Burgos, K.; Wei, T.S.; Kneer, M.; Grolman, J.; Cheng, K.J.; et al. Redox active colloids as discrete energy storage carriers. J. Am. Chem. Soc. 2016, 138, 13230–13237. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Ye, Y.; Xu, L.; Zhang, C. Synthesis and charge–discharge properties of a ferrocene-containing polytriphenylamine derivative as the cathode of a lithium ion battery. J. Mater. Chem. 2012, 22, 22658–22662. [Google Scholar] [CrossRef]
- Su, C.; Wang, L.; Xu, L.; Zhang, C. Synthesis of a novel ferrocene-contained polypyrrole derivative and its performance as a cathode material for Li-ion batteries. Electrochim. Acta 2013, 104, 302–307. [Google Scholar] [CrossRef]
- Facchetti, A. π-conjugated polymers for organic electronics and photovoltaic cell applications. Chem. Mater. 2011, 23, 733–758. [Google Scholar] [CrossRef]
- Xiang, J.; Burges, R.; Häupler, B.; Wild, A.; Schubert, U.; Ho, C.L.; Wong, W.Y. Synthesis, characterization and charge-discharge studies of ferrocene-containing poly(fluorenylethynylene) derivatives as organic cathode materials. Polymer 2015, 68, 328–334. [Google Scholar] [CrossRef]
- Lee, M.; Hong, J.; Seo, D.-H.; Nam, D.H.; Nam, K.T.; Kang, K.; Park, C.B. Redox cofactor from biological energy transduction as molecularly tunable energy-storage compound. Angew. Chem. Int. Ed. 2013, 52, 8322–8328. [Google Scholar] [CrossRef] [PubMed]
- Hasford, J.J.; Kemnitzer, W.; Rizzo, C.J. Conformational effects on flavin redox chemistry. J. Org. Chem. 1997, 62, 5244–5245. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Z.; Jing, Y.; Rong, Y.; Facchetti, A.; Yao, Y. Electrochemical properties of large-sized pouch-type lithium ion batteries with bio-inspired organic cathode materials. J. Am. Chem. Soc. 2015, 137, 4956–4959. [Google Scholar] [CrossRef]
- Hong, J.; Lee, M.; Lee, B.; Seo, D.-H.; Park, C.B.; Kang, K. Biologically inspired pteridine redox centres for rechargeable batteries. Nat. Commun. 2014, 7, 5335. [Google Scholar] [CrossRef]
- Orita, A.; Verde, M.G.; Sakai, M.; Meng, Y.S. A biomimetic redox flow battery based on flavin mononucleotide. Nat. Commun. 2016, 7, 13230. [Google Scholar] [CrossRef] [PubMed]
- Inatomi, Y.; Hojo, N.; Yamamoto, T.; Watanabe, S.; Misaki, Y. Construction of rechargeable batteries using multifused tetrathiafulvalene systems as cathode materials. ChemPlusChem 2012, 77, 973–976. [Google Scholar] [CrossRef]
- Kato, M.; Senoo, K.-I.; Yao, M.; Misaki, Y. A pentakis-fused tetrathiafulvalene system extended by cyclohexene-1,4-diylidenes: A new positive electrode material for rechargeable batteries utilizing ten electron redox. J. Mater. Chem. A 2014, 2, 6747–6754. [Google Scholar] [CrossRef]
- Iwamoto, S.; Inatomi, Y.; Ogi, D.; Shibayama, S.; Murakami, Y.; Kato, M.; Takahashi, K.; Tanaka, K.; Hojo, N.; Misaki, Y. New tris- and pentakis-fused donors containing extended tetrathiafulvalenes: New positive electrode materials for rechargeable batteries. Belstein J. Org. Chem. 2015, 11, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Avestro, A.J.; Chen, Z.; Sun, J.; Wang, S.; Xiao, M.; Erno, Z.; Algaradah, M.M.; Nassar, M.S.; Amine, K. A rigid naphthalenediimide triangle for organic rechargeable lithium-ion batteries. Adv. Mater. 2015, 27, 2907–2912. [Google Scholar]
- Sakaushi, K.; Hosono, E.; Nickerl, G.; Gemming, T.; Zhou, H.; Kaskel, S.; Eckert, J. Aromatic porous-honeycomb electrodes for a sodium- organic energy storage device. Nat. Commun. 2013, 4, 1485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, X.; Ren, W.; Wang, Y.; Su, F.; Jiang, J.-X. Microporous organic polymer-based lithium ion batteries with improved rate performance and energy density. J. Power Sources 2016, 317, 49–56. [Google Scholar] [CrossRef]
- Feng, X.; Ding, X.; Jiang, D. Covalent organic frameworks. Chem. Soc. Rev. 2012, 41, 6010–6022. [Google Scholar] [CrossRef]
- Yang, D.-H.; Yao, Z.-Q.; Wu, D.; Zhang, Y.-H.; Zhou, Z.; Bu, X.-H. Structure-modulated crystalline covalent organic frameworks as high-rate cathodes for Li-ion batteries. J. Mater. Chem. A 2016, 4, 18621–18627. [Google Scholar] [CrossRef]
- Tian, D.; Zhang, H.-Z.; Zhang, D.-S.; Chang, Z.; Han, J.; Gao, X.-P.; Bu, X.-H. Li-ion storage and gas adsorption properties of porous polyimides (Pis). RSC Adv. 2014, 4, 7506–7510. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Shao, P.; Han, Y.; Gao, X.; Ma, L.; Yuan, S.; Ma, X.; Zhou, J.; Feng, X.; et al. Exfoliation of covalent organic frameworks into few-layer redox-active nanosheets as cathode materials for lithium-ion batteries. J. Am. Chem. Soc. 2017, 139, 4258–4261. [Google Scholar] [CrossRef]
- DeBlase, C.R.; Hernandez-Burgos, K.; Silberstein, K.E.; Rodrıguez-Calero, G.G.; Bisbey, R.P.; Abruna, H.D.; Dichtel, W.R. Rapid and efficient redox processes within 2D covalent organic framework thin films. ACS Nano 2015, 9, 3178–3183. [Google Scholar] [CrossRef]
- Xu, F.; Jin, S.; Zhong, H.; Wu, D.; Yang, X.; Chen, X.; Wei, H.; Fu, R.; Jiang, D. Mesoporous covalent organic frameworks on carbon nanotubes for synergistic lithium-ion battery energy storage. Sci. Rep. 2015, 5, 8225. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Furukawa, K.; Addicoat, M.; Chen, L.; Takahashi, S.; Irle, S.; Nakamura, T.; Jiang, D. Large pore donor-acceptor covalent organic frameworks. Chem. Sci. 2013, 4, 4505–4511. [Google Scholar] [CrossRef]
- Zhang, Y.; Riduan, S.N.; Wang, J. Redox active metal– and covalent organic frameworks for energy storage: Balancing porosity and electrical conductivity. Chem. Eur. J. 2017, 23, 16419–16431. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Zhao, Q.; Wang, J.B.; Pan, Z.; Chen, J. A sulfur heterocyclic quinone cathode and a multifunctional binder for a high-performance rechargeable lithium-ion battery. Angew. Chem. Int. Ed. 2016, 55, 6428–6432. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, H.-S.; Kim, T.-H.; Kim, S.Y.; Song, H.-K. An inter-tangled network of redox-active and conducting polymers as a cathode for ultrafast rechargeable batteries. Phys. Chem. Chem. Phys. 2014, 16, 5295–5300. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jamal, R.; Wang, Y.; Yang, L.; Liu, F.; Abdiryim, T. Functionalization of graphene oxide and its composite with poly(3,4-ethylenedioxythiophene) as electrode material for supercapacitors. Nanoscale Res. Lett. 2015, 10, 370. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Cho, J.; Moore, J.S.; Park, H.S.; Braun, P.V. High-Performance Mesostructured Organic Hybrid Pseudocapacitor Electrodes. Adv. Funct. Mater. 2016, 26, 903–910. [Google Scholar] [CrossRef]
- DeBlase, C.R.; Silberstein, K.E.; Truong, T.-T.; Abruna, H.D.; Dichtel, W.R. β-ketoenamine-linked covalent organic frameworks capable of pseudocapacitive energy storage. J. Am. Chem. Soc. 2013, 135, 16821–16824. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, Y.; Sun, M.; Qian, J.; Cao, Y.; Ai, X.; Yang, H. Self-doped polypyrrole with ionizable sodium sulfonate as a renewable cathode material for sodium ion batteries. Chem. Commun. 2013, 49, 11370–11372. [Google Scholar] [CrossRef]
- Zhao, R.; Zhu, L.; Cao, Y.; Ai, X.; Yang, H.X. An aniline-nitroaniline copolymer as a high capacity cathode for Na-ion batteries. Electrochem. Commun. 2012, 21, 36–38. [Google Scholar] [CrossRef]
- Zhou, M.; Xiong, Y.; Cao, Y.; Ai, X.; Yang, H. Electroactive organic anion-doped polypyrrole as a low cost and renewable cathode for sodium-ion batteries. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 114–118. [Google Scholar] [CrossRef]
- Ghosh, A.; Mitra, S. In situ surface coating of squaric acid with conductive polyaniline for a high-capacity and sustainable lithium battery anode. Chem. Electro. Chem 2018, 5, 159–165. [Google Scholar] [CrossRef]
- Han, X.; Yi, F.; Sun, T.; Sun, S. Synthesis and electrochemical performance of Li and Ni 1,4,5,8-naphthalene tetracarboxylates as anodes for Li-ion batteries. Electrochem. Commun. 2012, 25, 136–139. [Google Scholar] [CrossRef]
- Lee, H.H.; Park, Y.; Shin, K.-H.; Lee, K.T.; Hong, S.Y. Abnormal excess capacity of conjugated dicarboxylates in lithium-ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 19118–19126. [Google Scholar] [CrossRef]
- Wu, J.; Rui, X.; Wang, C.; Pei, W.-B.; Lau, R.; Yan, Q.; Zhang, Q. Nanostructured conjugated ladder polymers for stable and fast lithium storage anodes with high-capacity. Adv. Energy Mater. 2015, 5, 1402189. [Google Scholar] [CrossRef]
- Wu, J.; Rui, X.; Long, G.; Chen, W.; Yan, Q.; Zhang, Q. Pushing up lithium storage through nanostructured polyazaacene analogues as anode. Angew. Chem. Int. Ed. 2015, 54, 7354–7358. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Liu, X.; Li, Z.; Feng, W. Metal dicarboxylates: New anode materials for lithium-ion batteries with good cycling performance. Dalton Trans. 2015, 44, 9909–9914. [Google Scholar] [CrossRef]
- Lee, H.H.; Park, Y.; Kim, S.H.; Yeon, S.-H.; Kwak, S.K.; Lee, K.T.; Hong, S.Y. Mechanistic studies of transition metal-terephthalate coordination complexes upon electrochemical lithiation and delithiation. Adv. Funct. Mater. 2015, 25, 4859–4866. [Google Scholar] [CrossRef]
- Renault, S.; Oltean, V.A.; Araujo, C.M.; Grigoriev, A.; Edström, K.; Brandell, D. Superlithiation of organic electrode materials: The case of dilithium benzenedipropiolate. Chem. Mater. 2016, 28, 1920–1926. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, C.; Zhao, Q.; Niu, Z.; Chen, J. High-performance organic lithium batteries with an ether-based electrolyte and 9,10-anthraquinone (AQ)/CMK-3 cathode. Adv. Sci. 2015, 2, 1500018. [Google Scholar] [CrossRef]
- Ishii, Y.; Tashiro, K.; Hosoe, K.; Al-Zubaidi, A.; Kawasaki, S. Electrochemical lithium-ion storage properties of quinone molecules encapsulated in single-walled carbon nanotubes. Phys. Chem. Chem. Phys. 2016, 18, 10411–10418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, H.; Tang, Q.; Sun, M.; Wang, G. Activated hierarchical porous carbon@π-conjugated polymer composite as cathode for high-performance lithium storage. J. Solid State Electrochem. 2016, 20, 2169–2177. [Google Scholar] [CrossRef]
- Vlad, A.; Rolland, J.; Hauffman, G.; Ernould, B.; Gohy, J.F. Melt-polymerization of TEMPO methacrylates with nano carbons enables superior battery materials. Chem. Sus. Chem. 2015, 8, 1692–1696. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Scheers, J.; Ahn, J.-H.; Johansson, P.; Matic, A.; Jacobsson, P. Nano-fibrous polymer films for organic rechargeable batteries. J. Mater. Chem. A 2013, 1, 2426–2430. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, J. Review—Advanced carbon-supported organic electrode materials for lithium (sodium)-ion batteries. J. Electrochem. Soc. 2015, 162, A2393–A2405. [Google Scholar] [CrossRef]
- Bachman, J.C.; Kavian, R.; Graham, D.J.; Kim, D.Y.; Noda, S.; Nocera, D.G.; Shao-Horn, Y.; Lee, S.W. Electrochemical polymerization of pyrene derivatives on functionalized carbon nanotubes for pseudocapacitive electrodes. Nat. Commun. 2015, 6, 7040. [Google Scholar] [CrossRef] [PubMed]
- Charrier, G.; Desrues, A.; Barchasz, C.; Leroy, J.; Cornut, R.; Jousselme, B.; Campidelli, S. Covalently functionalized carbon nanotubes as stable cathode materials of lithium/organic batteries. J. Mater. Chem. A 2016, 4, 15036–15040. [Google Scholar] [CrossRef]
- Yan, X.; Ye, H.; Wu, X.-L.; Zheng, Y.-P.; Wan, F.; Liu, M.; Zhang, X.-H.; Zhang, J.-P.; Guo, Y.-G. Three-dimensional carbon nanotube networks enhanced sodium trimesic: A new anode material for sodium ion batteries and Na-storage mechanism revealed by ex situ studies. J. Mater. Chem. A 2017, 5, 16622–16629. [Google Scholar] [CrossRef]
- Choi, W.; Ohtani, S.; Oyaizu, K.; Nishide, H.; Geckeler, K.E. Radical polymer-wrapped SWNTs at a molecular level: High-rate redox mediation through a percolation network for a transparent charge-storage material. Adv. Mater. 2011, 23, 4440–4443. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, Q.; Shao, Q.; Li, Q.; Gao, B.; Duan, Q.; Wang, H.G. Free-standing and flexible organic cathode based on aromatic carbonyl compound/carbon nanotube composite for lithium and sodium organic batteries. J. Colloid Int. Sci. 2018, 517, 72–79. [Google Scholar] [CrossRef]
- Suga, T.; Ohshiro, H.; Sugita, S.; Oyaizu, K.; Nishide, H. Emerging n-type redox-active radical polymer for a totally organic polymer-based rechargeable battery. Adv. Mater. 2009, 21, 1627–1630. [Google Scholar] [CrossRef]
- Wu, H.P.; Yang, Q.; Meng, Q.H.; Ahmad, A.; Zhang, M.; Zhu, L.Y.; Liu, Y.G.; Wei, Z.X. A polyimide derivative containing different carbonyl groups for flexible lithium ion batteries. J. Mater. Chem. A 2016, 4, 2115–2121. [Google Scholar] [CrossRef]
- Meng, Y.; Wu, H.; Zhang, Y.; Wei, Z. A flexible electrode based on a three-dimensional graphene network-supported polyimide for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 10842–10846. [Google Scholar] [CrossRef]
- Wu, H.; Meng, Q.; Yang, Q.; Zhang, M.; Lu, K.; Wei, Z. Large-area polyimide/SWCNT nanocable cathode for flexible lithium-ion batteries. Adv. Mater. 2015, 27, 6504–6510. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-X. A dispersion-corrected DFT study on adsorption of battery active materials anthraquinone and its derivatives on monolayer graphene and h-BN. J. Mater. Chem. A 2014, 2, 8910–8917. [Google Scholar] [CrossRef]
- Yu, Y.-X. Binding energy and work function of organic electrode materials phenanthraquinone, pyromellitic dianhydride and their derivatives adsorbed on graphene. ACS Appl. Mater. Interfaces 2014, 6, 16267–16275. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, Q.; Wang, G.; Li, F.; Chen, J. Enhanced adsorption of carbonyl molecules on graphene via π-Li-π interaction: A first-principle study. Sci. China Mater. 2017, 60, 674–680. [Google Scholar] [CrossRef]
- Yang, G.; Bu, F.; Huang, Y.; Zhang, Y.; Shakir, I.; Xu, Y. In situ growth and wrapping of aminoanthraquinone nanowires in 3D graphene framework as foldable organic cathode for lithium-ion batteries. ChemSusChem 2017, 10, 3419–3426. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Ning, G.-H.; Su, J.; Zhong, G.; Tang, W.; Tian, B.; Su, C.; Yu, D.; Zu, L.; Yang, J.; et al. Reversible multi-electron redox chemistry of π-conjugated N-containing heteroaromatic molecule-based organic cathodes. Nat. Energy 2017, 2, 17074. [Google Scholar] [CrossRef]
- Ai, W.; Zhou, W.; Du, Z.; Sun, C.; Yang, J.; Chen, Y.; Sun, Z.; Feng, S.; Zhao, J.; Dong, X.; et al. Toward high energy organic cathodes for Li-ion batteries: A case study of vat dye/graphene composites. Adv. Funct. Mater. 2017, 27, 1603603. [Google Scholar] [CrossRef]
- Ai, W.; Du, Z.; Fan, Z.; Jiang, J.; Wang, Y.; Zhang, H.; Xie, L.; Huang, W.; Yu, T. Chemically engineered graphene oxide as high performance cathode materials for Li-ion batteries. Carbon 2014, 76, 148–154. [Google Scholar] [CrossRef]
- Ahmad, A.; Wu, H.; Guo, Y.; Meng, Q.; Meng, Y.; Lu, K.; Liu, L.; Wei, Z. A graphene supported polyimide nanocomposite as a high performance organic cathode material for lithium ion batteries. RSC Adv. 2016, 6, 33287–33294. [Google Scholar] [CrossRef]
- Wang, H.-G.; Li, W.; Liu, D.-P.; Feng, X.-L.; Wang, J.; Yang, X.-Y.; Zhang, X.-B.; Zhu, Y.; Zhang, Y. Flexible electrodes for sodium-ion batteries: Recent progress and perspectives. Adv. Mater. 2017, 29, 1703012. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, K.; Liu, J.; Zhong, X.; Duan, X.; Shakir, M.I.; Xu, Y. Three-dimensional graphene/polyimide composite-derived flexible high-performance organic cathode for rechargeable lithium and sodium batteries. J. Mater. Chem. A 2017, 5, 2710–2716. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Yang, G.; Bu, F.; Li, K.; Shakir, I.; Xu, Y. Dispersion−assembly approach to synthesize three-dimensional graphene/polymer composite aerogel as a powerful organic cathode for rechargeable Li and Na batteries. ACS Appl. Mater. Interfaces 2017, 9, 15549–15556. [Google Scholar] [CrossRef]
- Deng, W.; Qian, J.; Cao, Y.; Ai, X.; Yang, H. Graphene-wrapped Na2C12H6O4 Nanoflowers as high performance anodes for sodium-ion batteries. Small 2016, 12, 583–587. [Google Scholar] [CrossRef]
- Su, Y.; Liu, Y.; Wu, D.; Zuhang, X.; Zhang, F.; Feng, X. Compact coupled graphene and porous polyaryltriazine-derived frameworks as high performance cathodes for lithium-ion batteries. Angew. Chem. Int. Ed. 2015, 54, 1812–1816. [Google Scholar] [CrossRef]
- Luo, C.; Zhu, Y.; Xu, Y.; Liu, Y.; Gao, T.; Wang, J.; Wang, C. Graphene oxide wrapped croconic acid disodium salt for sodium ion battery electrodes. J. Power Sources 2014, 250, 372–378. [Google Scholar] [CrossRef]
- Li, A.; Feng, Z.; Sun, Y.; Shang, L.; Xu, L. Porous organic polymer/RGO composite as high performance cathode for half and full sodium ion batteries. J. Power Sources 2017, 343, 424–430. [Google Scholar] [CrossRef]
- Amin, K.; Meng, Q.; Ahmad, A.; Cheng, M.; Zhang, M.; Mao, L.; Lu, K.; Wei, Z. A carbonyl compound-based flexible cathode with superior rate performance and cyclic stability for flexible lithium-ion batteries. Adv. Mater. 2018, 30, 1703868. [Google Scholar] [CrossRef]
- Genorio, B.; Lu, W.; Dimiev, A.M.; Zhu, Y.; Raji, A.R.O.; Novosel, B.; Alemany, L.B.; Tour, J.M. In situ intercalation replacement and selective functionalization of graphene nanoribbon stacks. ACS Nano 2012, 6, 4231–4240. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Ruan, G.; Genorio, B.; Zhu, Y.; Novosel, B.; Peng, Z.; Tour, J.M. Functionalized graphene nanoribbons via anionic polymerization initiated by alkali metal-intercalated carbon nanotubes. ACS Nano 2013, 7, 2669–2675. [Google Scholar] [CrossRef] [PubMed]
- Pirnat, K.; Bitenc, J.; Jerman, I.; Dominko, R.; Genorio, B. Redox-active functionalized graphene nanoribbons as electrode material for Li-ion batteries. ChemElectroChem 2014, 1, 2131–2137. [Google Scholar] [CrossRef]
- Wang, H.; Hu, P.F.; Yang, J.; Gong, G.M.; Guo, L.; Chen, X.D. Renewable-Juglone-based high-performance sodium-ion batteries. Adv. Mater. 2015, 27, 2348–2354. [Google Scholar] [CrossRef] [PubMed]
- Mosch, H.L.; Höppener, S.; Paulus, R.M.; Schröter, B.; Schubert, U.S.; Ignaszak, A. The correlation of the binding mechanism of the polypyrrole–carbon capacitive interphase with electrochemical stability of the composite electrode. Phys. Chem. Chem. Phys. 2015, 17, 13323–13332. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.; Zhou, X.; Yu, C.; Wu, S.; Shen, J. Highly dispersed carbon nanotube/polypyrrole core/shell composites with improved electrochemical capacitive performance. J. Mater. Chem. A 2013, 1, 15230–15234. [Google Scholar] [CrossRef]
- Shi, K.; Zhitomirsky, I. Fabrication of polypyrrole-coated carbon nanotubes using oxidant-surfactant nanocrystals for supercapacitor electrodes with high mass loading and enhanced performance. ACS Appl. Mater. Interfaces 2013, 5, 13161–13170. [Google Scholar] [CrossRef]
- Martinez, C.R.; Iverson, B.L. Rethinking the term “pi-stacking”. Chem. Sci. 2012, 3, 2191–2201. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Jung, Y.C.; So, H.H.; Cho, J.W. Polypyrrole coated carbon nanotubes: Synthesis, characterization, and enhanced electrical properties. Synth. Met. 2007, 157, 374–379. [Google Scholar] [CrossRef]
- Lin, C.-H.; Lee, J.-T.; Yang, D.-R.; Chen, H.-W.; Wu, S.-T. Nitroxide radical polymer/carbon-nanotube-array electrodes with improved C-rate performance in organic radical batteries. RSC Adv. 2015, 5, 33044–33048. [Google Scholar] [CrossRef]
- Sukegawa, T.; Sato, K.; Oyaizu, K.; Nishide, H. Efficient charge transport of a radical polyether/SWCNT composite electrode for an organic radical battery with high charge-storage density. RSC Adv. 2015, 5, 15448–15452. [Google Scholar] [CrossRef]
- Ernould, B.; Devos, M.; Bourgeois, J.-P.; Rolland, J.; Vlad, A.; Gohy, J.-F. Grafting of a redox polymer onto carbon nanotubes for high capacity battery materials. J. Mater. Chem. A 2015, 3, 8832–8839. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, I.A.; Ji, X. Anion hosting cathodes in dual-ion batteries. ACS Energy Lett. 2017, 2, 1762–1770. [Google Scholar]
- Rodríguez-Pérez, I.A.; Jian, Z.; Waldenmaier, P.K.; Palmisano, J.W.; Chandrabose, R.S.; Wang, X.; Lerner, M.M.; Carter, R.G.; Ji, X. A hydrocarbon cathode for dual-ion batteries. ACS Energy Lett. 2016, 1, 719–723. [Google Scholar]
- Koshika, K.; Chikushi, N.; Sano, N.; Oyaizu, K.; Nishide, H. A TEMPO-substituted polyacrylamide as a new cathode material: An organic rechargeable device composed of polymer electrodes and aqueous electrolyte. Green Chem. 2010, 12, 1573–1575. [Google Scholar] [CrossRef]
- Sano, N.; Tomita, W.; Hara, S.; Min, C.M.; Lee, J.S.; Oyaizu, K.; Nishide, H. Polyviologen hydrogel with high-rate capability for anodes toward an aqueous electrolyte-type and organic-based rechargeable device. ACS Appl. Mater. Interfaces 2013, 5, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Suga, T.; Sugita, S.; Ohshiro, H.; Oyaizu, K.; Nishide, H. p- and n-type bipolar redox-active radical polymer: Toward totally organic polymer-based rechargeable devices with variable configuration. Adv. Mater. 2011, 23, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Speer, M.E.; Kolek, M.; Jassoy, J.J.; Heine, J.; Winter, M.; Bieker, P.M.; Esser, B. Thianthrene-functionalized polynorbornenes as high-voltage materials for organic cathode-based dual-ion batteries. Chem. Commun. 2015, 51, 15261–15264. [Google Scholar] [CrossRef]
- Wild, A.; Strumpf, M.; Häupler, B.; Hager, M.D.; Schubert, U.S. All- organic battery composed of thianthrene- and TCAQ-based polymers. Adv. Energy Mater. 2017, 7, 1601415. [Google Scholar] [CrossRef]
- Häupler, B.; Burges, R.; Janoschka, T.; Jähnert, T.; Wild, A.; Schubert, U.S. PolyTCAQ in organic batteries: Enhanced capacity at constant cell potential using two-electron-redox-reactions. J. Mater. Chem. A 2014, 2, 8999–9001. [Google Scholar] [CrossRef]
- Deunf, É.; Jiménez, P.; Guyomard, D.; Dolhem, F.; Poizot, P. A dual-ion battery using diamino-rubicene as anion-inserting positive electrode material. Electrochem. Commun. 2016, 72, 64–68. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, H.; Kim, C.-S.; Zaghib, K.; Mauger, A.; Julien, C.M. Advances in lithium-sulfur batteries. Mater. Sci. Eng. R 2017, 121, 1–29. [Google Scholar] [CrossRef]
- Griebel, J.J.; Li, G.; Glass, R.S.; Char, K.; Pyun, J. Kilogram scale inverse vulcanization of elemental sulfur to prepare high capacity polymer electrodes for Li/S batteries. J. Polym. Sci. Part A Polym. Chem. 2014, 53, 173–177. [Google Scholar] [CrossRef]
- Chung, W.J.; Griebel, J.J.; Kim, E.T.; Yoon, H.; Simmonds, A.G.; Ji, H.J.; Dirlam, P.T.; Glass, R.S.; Wie, J.J.; Nguyen, N.A.; et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 2013, 5, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, X.; Seo, M.H.; Li, M.; Ma, L.; Yuan, Y.; Wu, T.; Wang, S.; Lu, J.; Chen, Z. Chemisorption of polysulfides through redox reactions with organic molecules for lithium–sulfur batteries. Nat. Commun. 2018, 9, 705. [Google Scholar] [CrossRef]
- Song, J.; Xu, T.; Gordin, M.L.; Zhu, P.; Lv, D.; Jiang, Y.-B.; Chen, Y.; Duan, Y.; Wang, D. Nitrogen-doped mesoporous carbon promoted chemical adsorption of sulfur and fabrication of high-areal-capacity sulfur cathode with exceptional cycling stability for lithium-sulfur batteries. Adv. Funct. Mater. 2014, 24, 1243–1250. [Google Scholar] [CrossRef]
- Deng, C.; Wang, Z.; Wang, S.; Yu, J. Inhibition of polysulfide diffusion in lithium–sulfur batteries: Mechanism and improvement strategies. J. Mater. Chem. A 2019. [Google Scholar] [CrossRef]
- Wei, S.; Ma, L.; Hendrickson, K.E.; Tu, Z.; Archer, L.A. Metal–sulfur battery cathodes based on PAN–sulfur composites. J. Am. Chem. Soc. 2015, 137, 12143–12152. [Google Scholar] [CrossRef]
- Poonam; Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Special Issue Vanadium Redox Flow Battery and its Applications, MDPI. Available online: https://www.mdpi.com/journal/batteries/special_issues/Vanadium_Redox_Flow_Battery_Its_Applications (accessed on 31 December 2018).

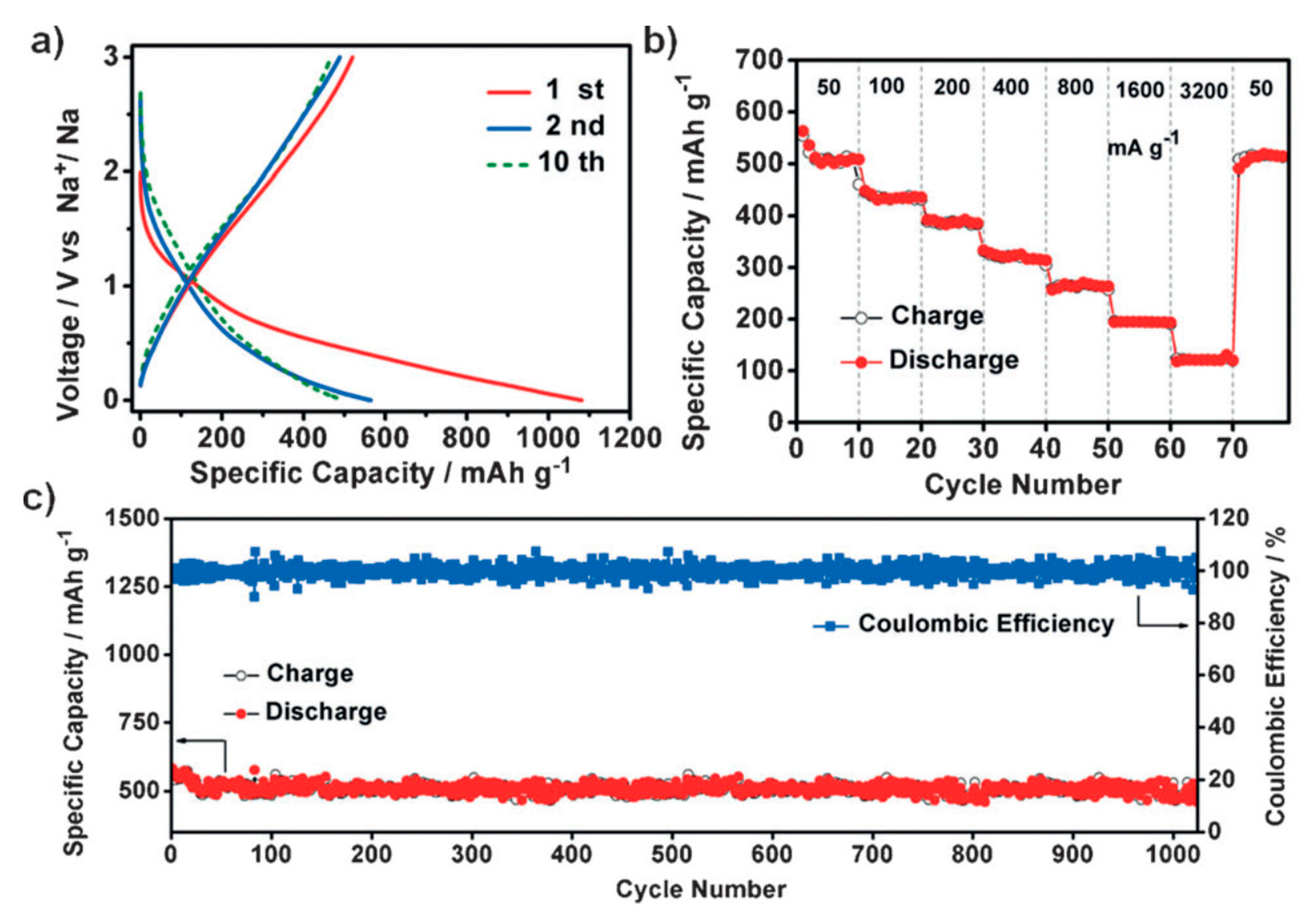
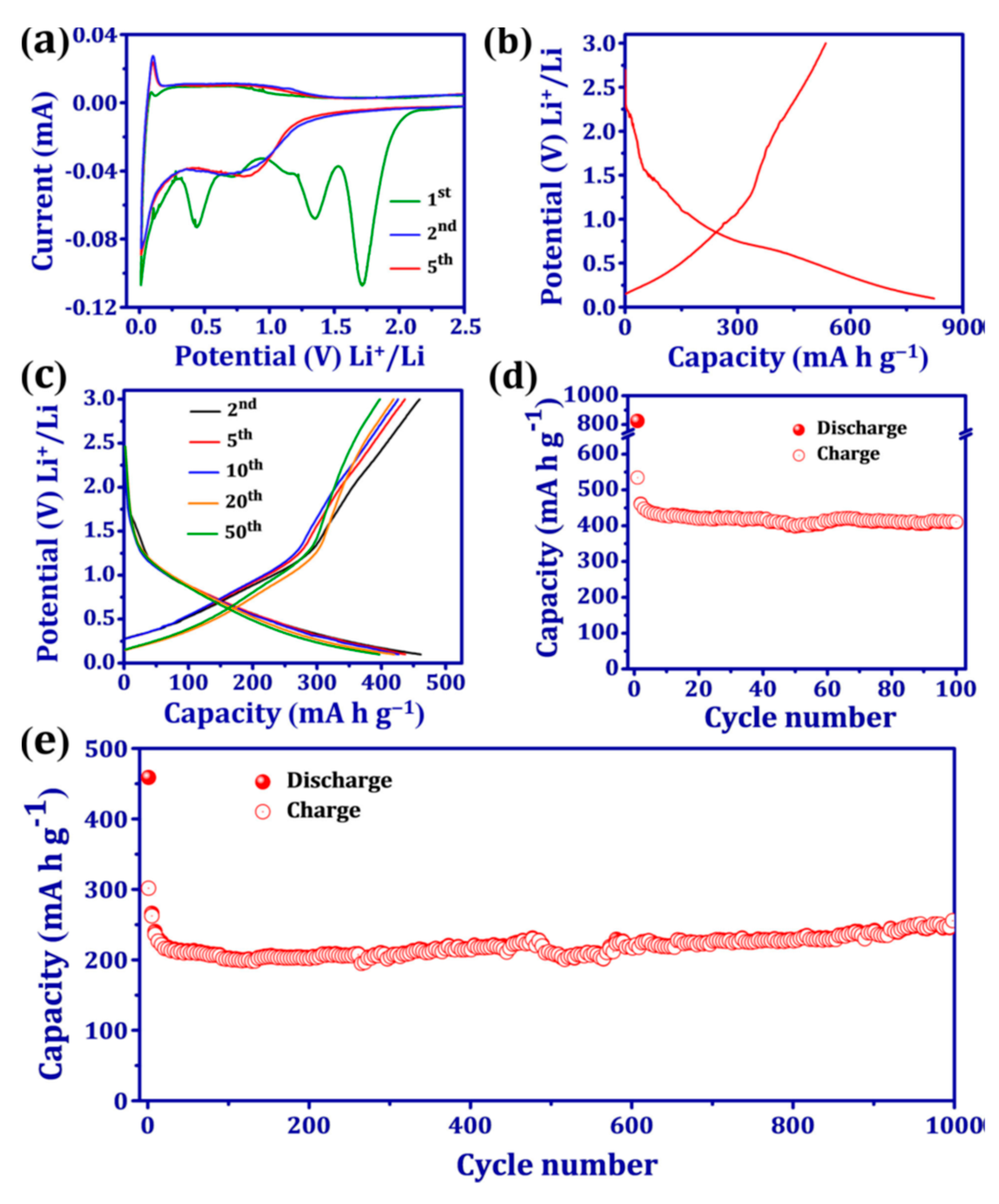
| Substituents | Calculated Redox Potential (V vs. SHE) | ||
|---|---|---|---|
| R2 | R5 | R6 | |
| Me | CN | CN | 0.096 |
| Me | OH | OH | 0.273 |
| Me | CF3 | H | −0.080 |
| pH | Anode | Charge Carrier * | Reduction Potential *,† (V vs. SHE) | Specific Capacity * (mAh g−1) | Cathode | Specific Energy (Wh kg−1) | Energy Density ‡ (Wh L−1) | Cycling Stability a) |
|---|---|---|---|---|---|---|---|---|
| −1 | PTO | H+ | 0.51 | 395 | PbO2 | 76 | 161 | 96%@1500 (1200 h) |
| −1 | Pb | −0.34 | 129 | PbO2 | 78 | 171 | 80%@240 (4500 h) § | |
| −1 | AC | H+ | 0.48 | 50 | PbO2 | 38 | 37 | 83%@3000 (5500 h) |
| 3~4 | PPTO | Mg2+ | 0.04 | 144 | CuHCF | 25 | 45 | 66%@1,000 (1600 h) |
| 7 | PPTO | Li+ | −0.06 | 229 | LiMn2O4 | 92 | 208 | 80%@3000 (3500 h) |
| 7 | PPTO | Na+ | −0.07 | 201 | Na3V2(PO4)3 | 30 | 80 | 79%@80 (150 h) |
| 7 | LiTi2(PO4)3 | Li+ | −0.52 | 103 | LiMn2O4 | 90 | 243 | 89%@1200 (1600 h) |
| 7 | Polyimide | Li+ | −0.19 | 160 | LiMn2O4 | 89 | 186 | 70%@50,000 (950 h) |
| 13 | PPTO | Li+ | −0.06 | 195 | LiCoO2 | 66 | 180 | 83%@700 (1200 h) |
| 15 | PAQS | K+ | −0.60 | 200 | Ni(OH)2 | 79 | 138 | 88%@1350 (2300 h) |
| 15 | MmH | H+ | −0.81 | 300 | Ni(OH)2 | 180 | 597 | 80%@1300 (n/a) |
| 15 | Zn | OH− | −1.19 | 500 | Ni(OH)2 | 290 | 714 | 80%@300 (800 h) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauger, A.; Julien, C.; Paolella, A.; Armand, M.; Zaghib, K. Recent Progress on Organic Electrodes Materials for Rechargeable Batteries and Supercapacitors. Materials 2019, 12, 1770. https://doi.org/10.3390/ma12111770
Mauger A, Julien C, Paolella A, Armand M, Zaghib K. Recent Progress on Organic Electrodes Materials for Rechargeable Batteries and Supercapacitors. Materials. 2019; 12(11):1770. https://doi.org/10.3390/ma12111770
Chicago/Turabian StyleMauger, Alain, Christian Julien, Andrea Paolella, Michel Armand, and Karim Zaghib. 2019. "Recent Progress on Organic Electrodes Materials for Rechargeable Batteries and Supercapacitors" Materials 12, no. 11: 1770. https://doi.org/10.3390/ma12111770
APA StyleMauger, A., Julien, C., Paolella, A., Armand, M., & Zaghib, K. (2019). Recent Progress on Organic Electrodes Materials for Rechargeable Batteries and Supercapacitors. Materials, 12(11), 1770. https://doi.org/10.3390/ma12111770









