Basic Blue Dye Adsorption from Water Using Polyaniline/Magnetite (Fe3O4) Composites: Kinetic and Thermodynamic Aspects
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of PANI
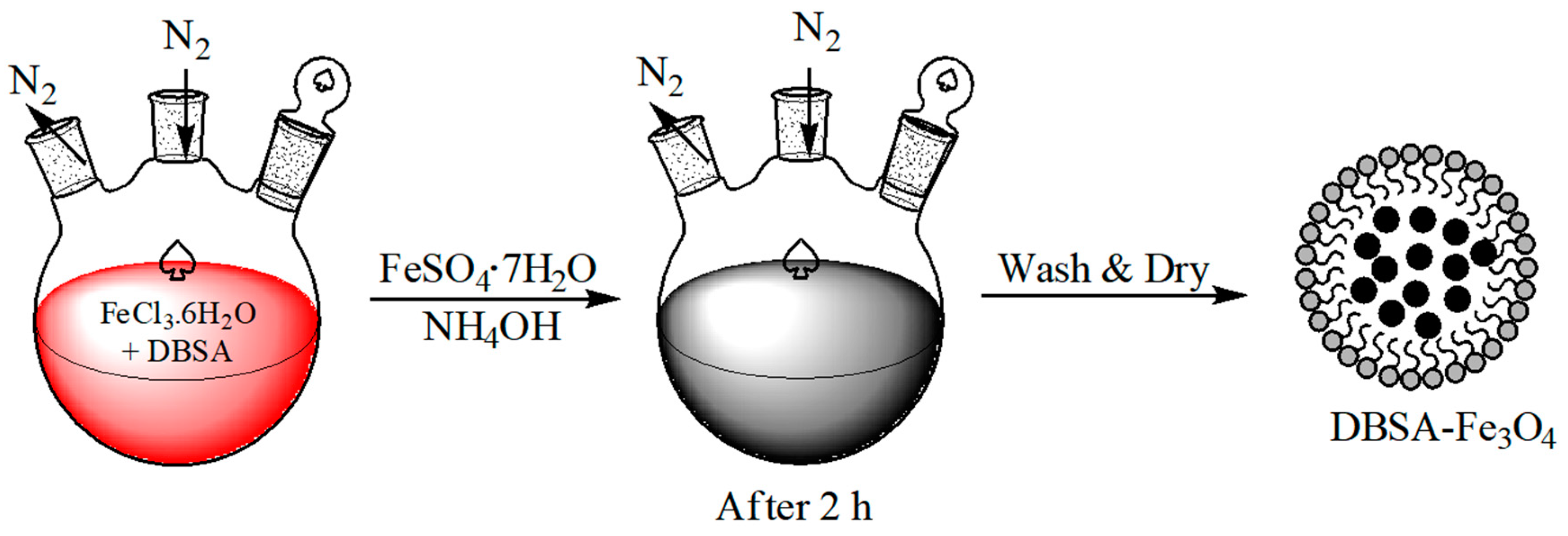
2.3. Synthesis of Fe3O4
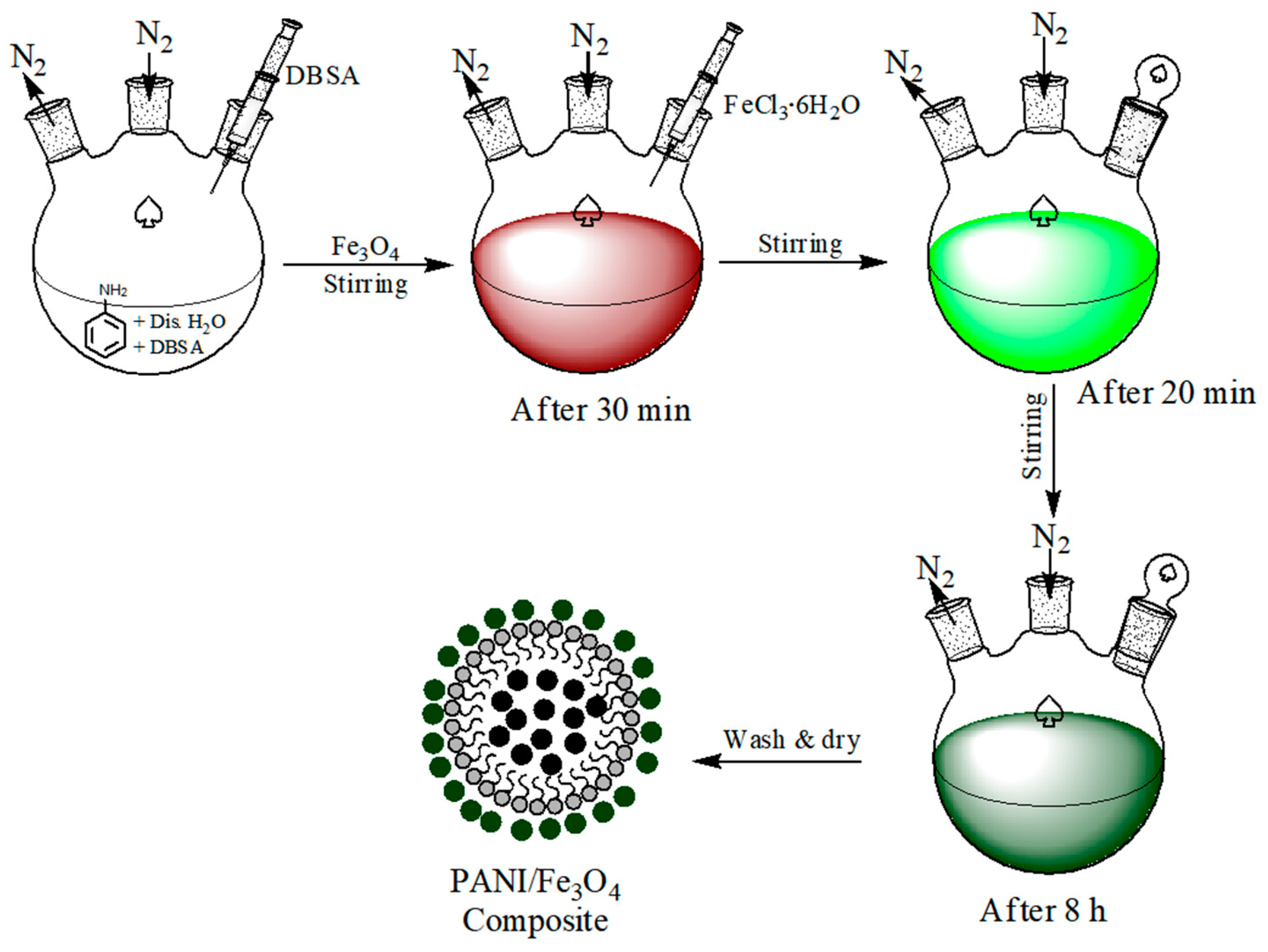
2.4. Synthesis of PANI/Fe3O4 Composites
2.4.1. Batch Adsorption Study for Removal of BB3 Dye
2.4.2. Characterization
3. Results
3.1. Characterization
3.1.1. SEM Study
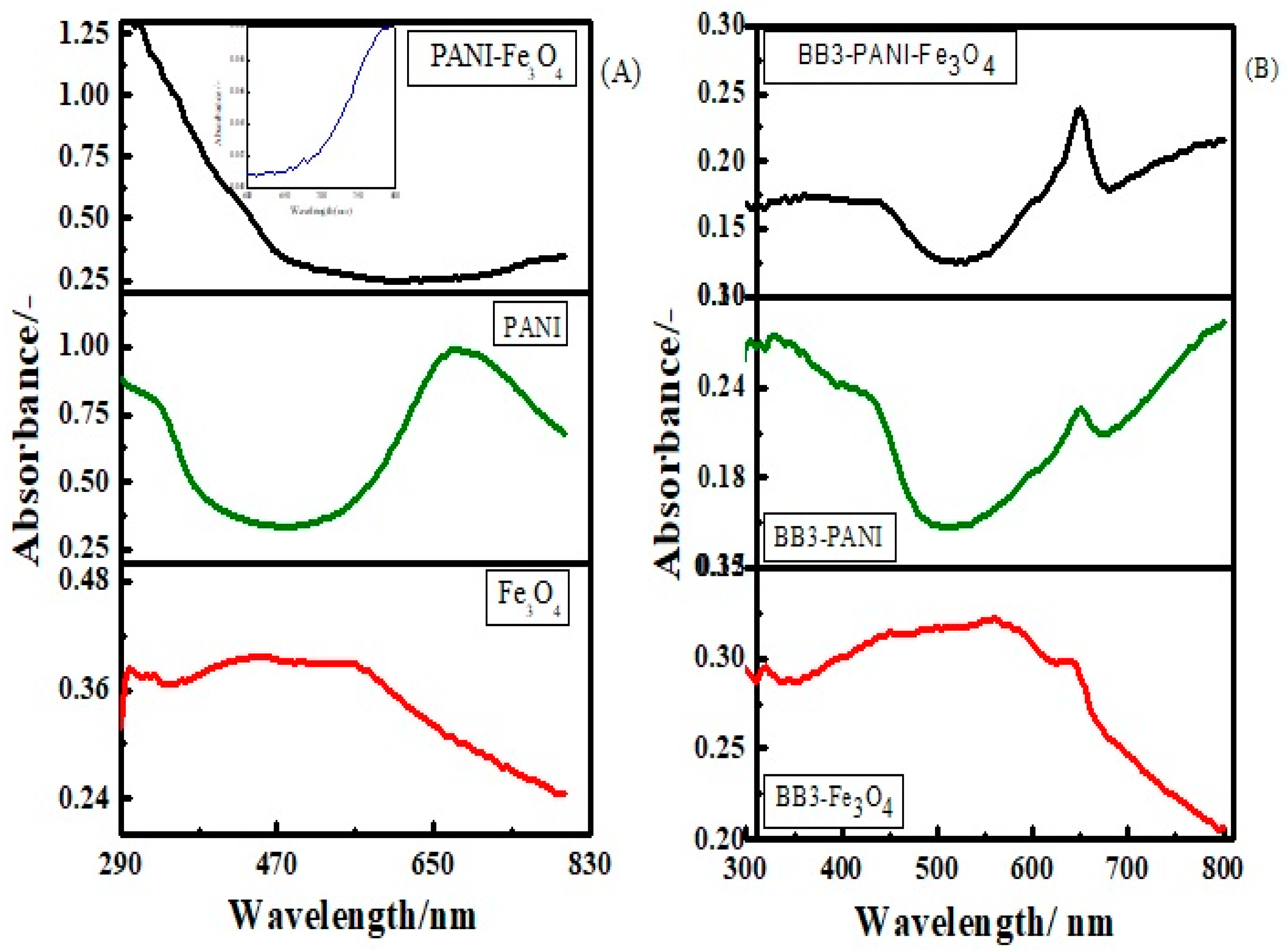
3.1.2. UV-Vis Spectroscopic Study
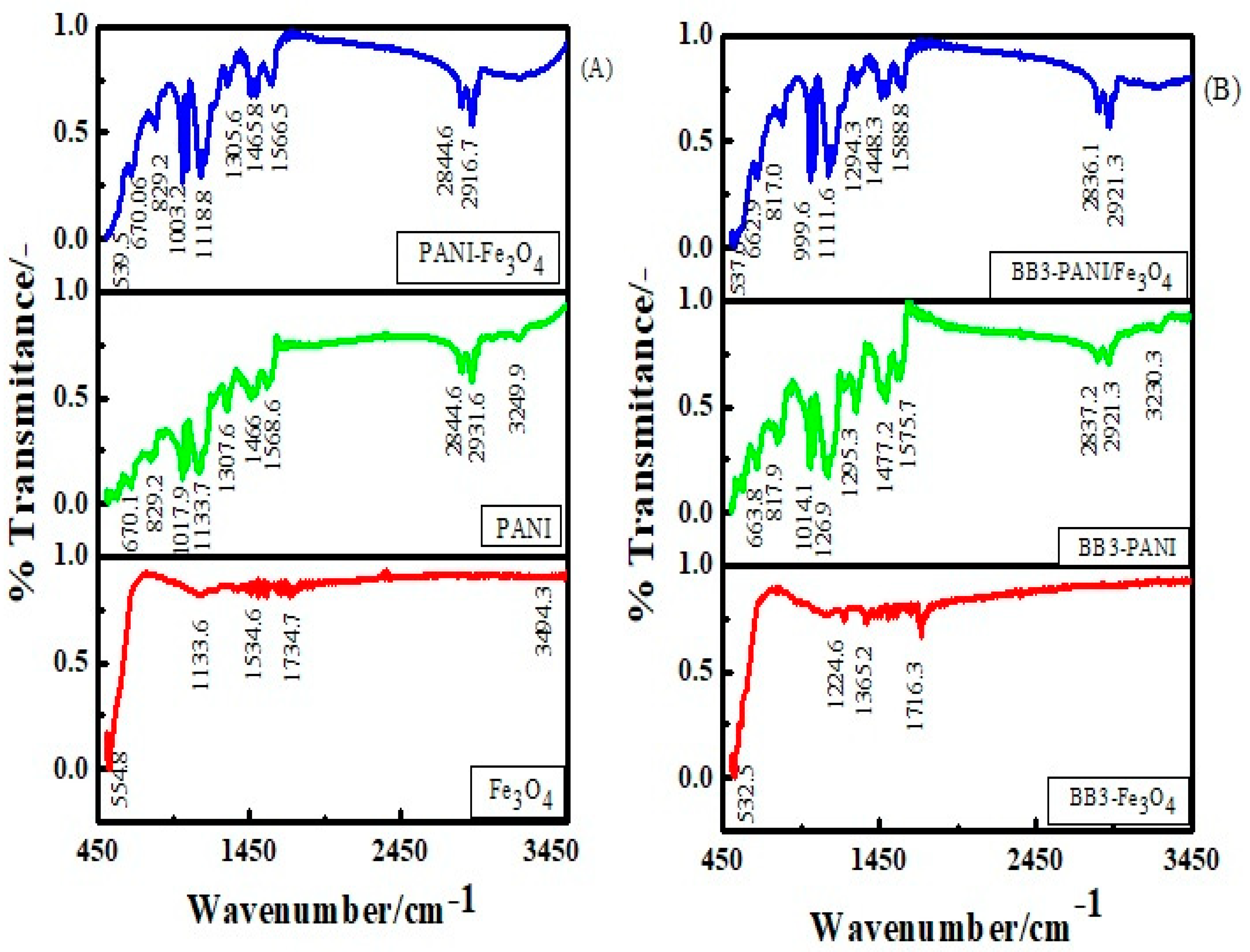
3.1.3. FTIR Spectroscopy
3.1.4. EDX Spectroscopy
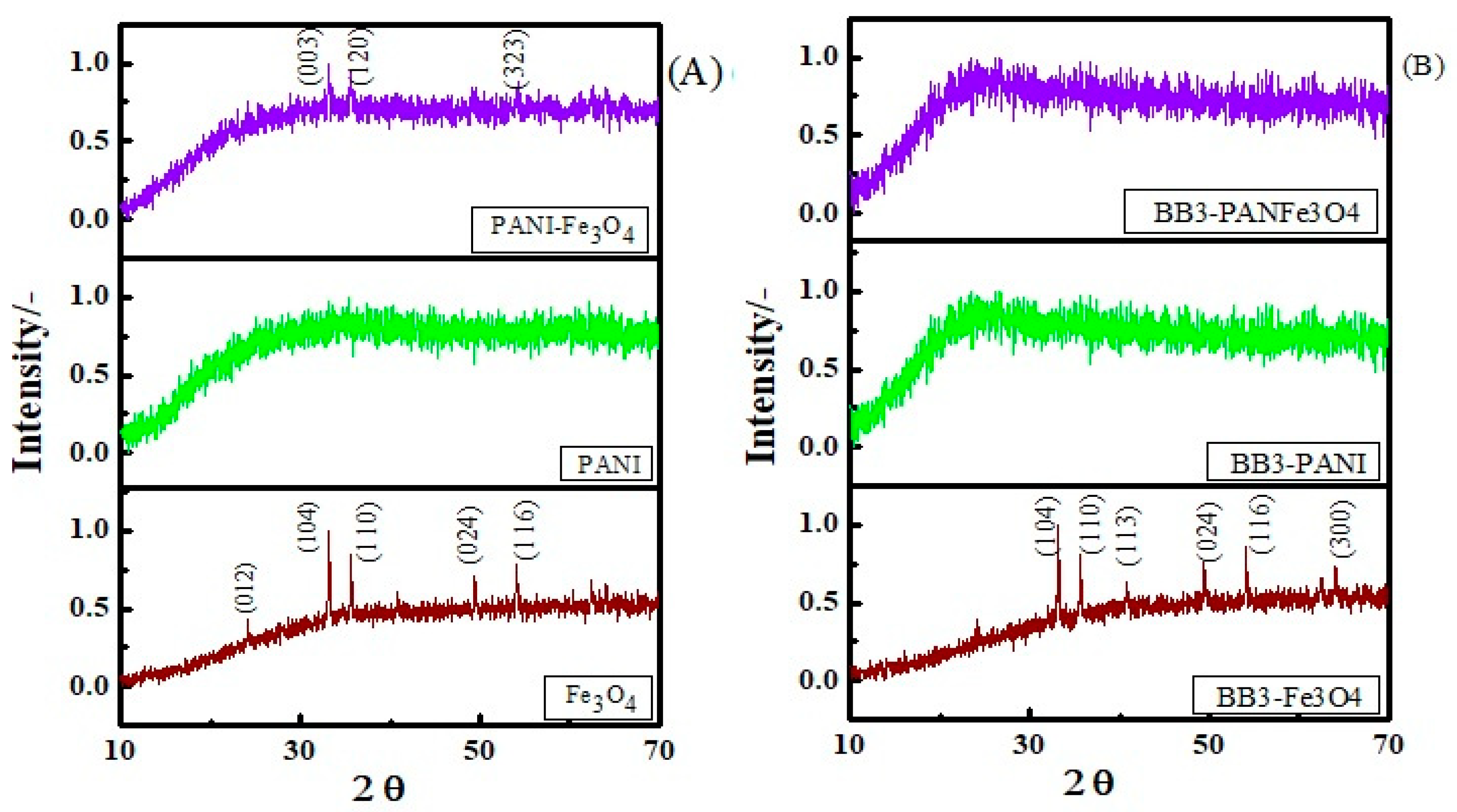
3.1.5. XRD Study
3.1.6. Surface Area Analysis
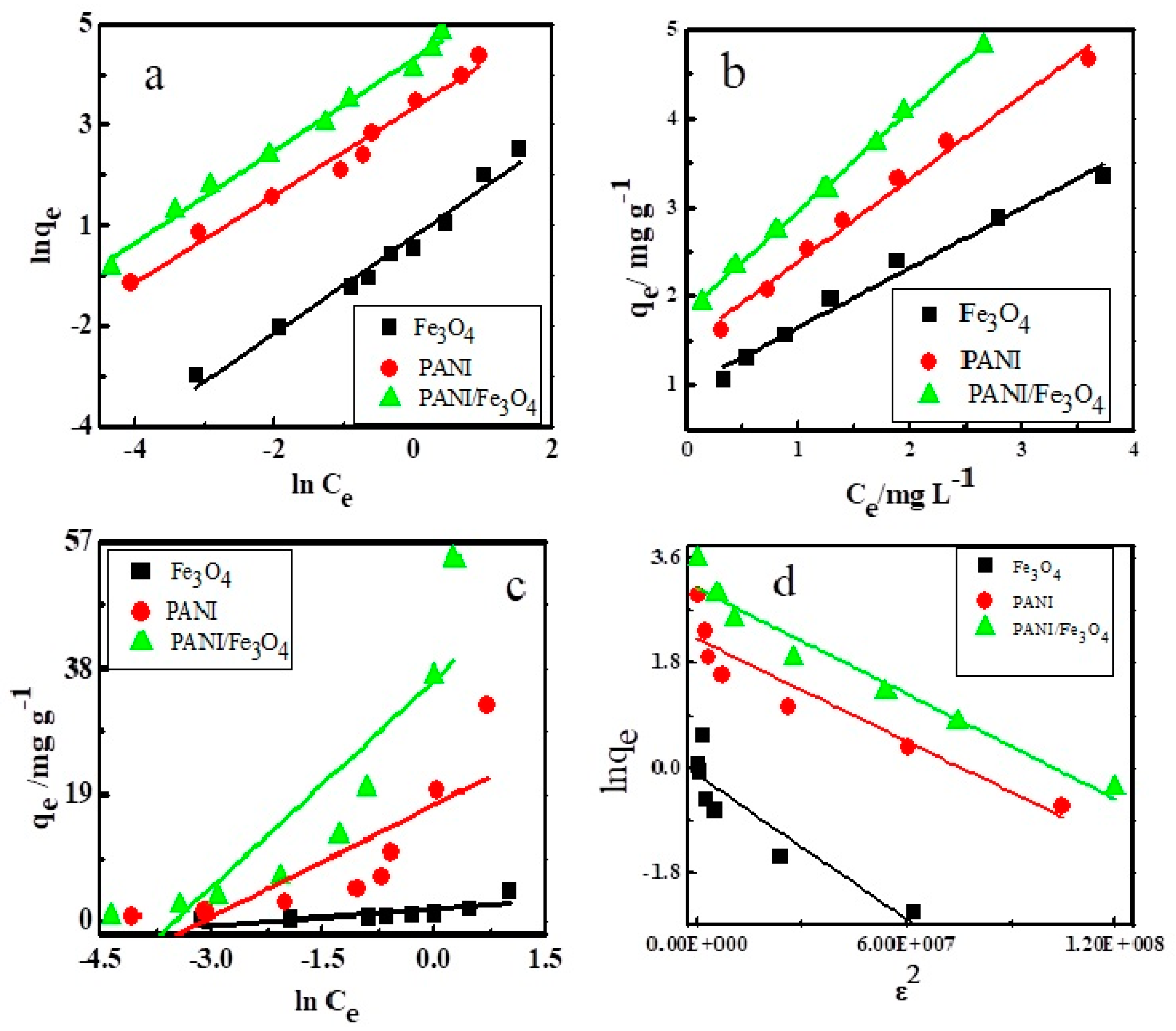
3.2. Equilibrium Study
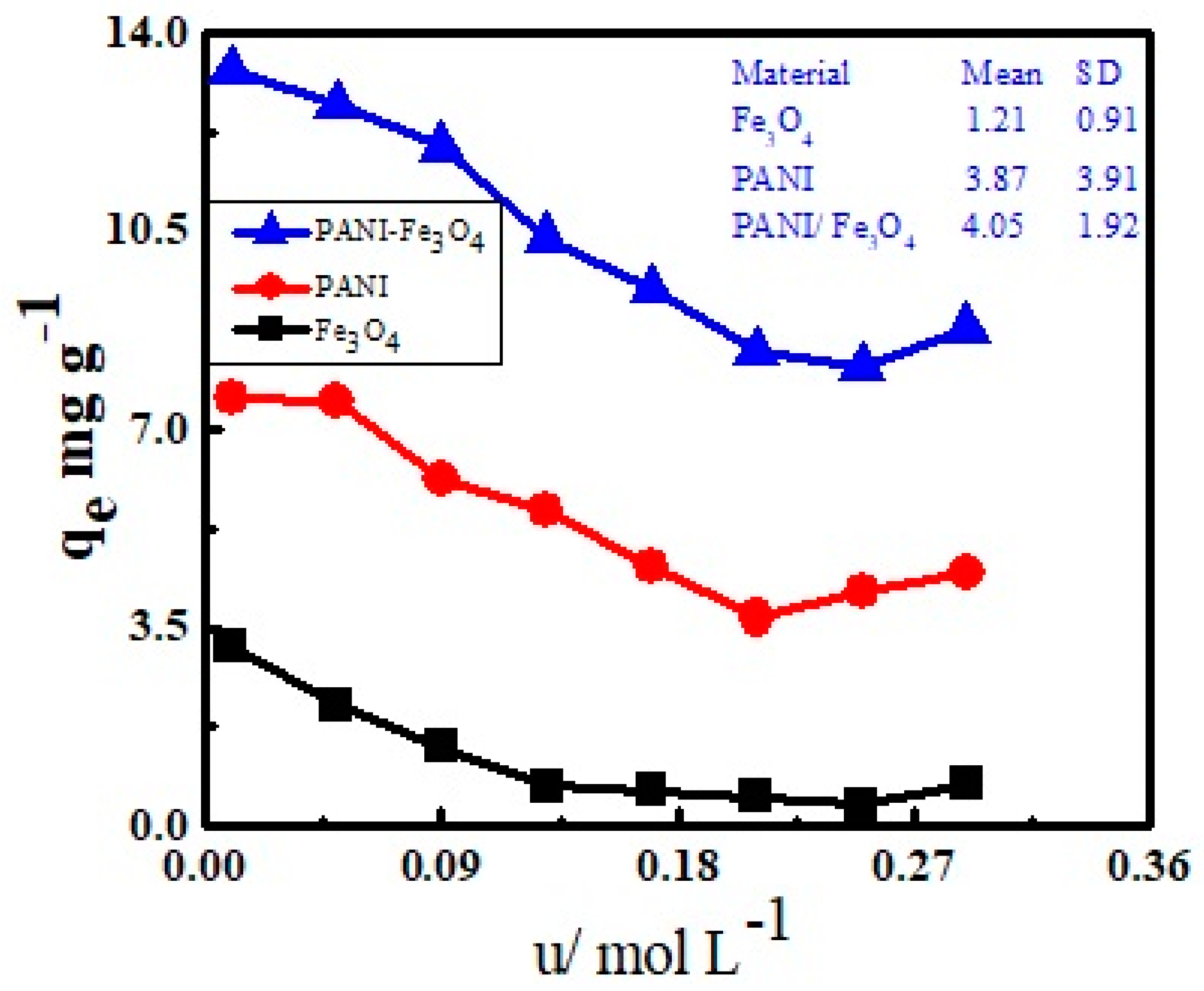
3.3. Effect of Ionic Strength
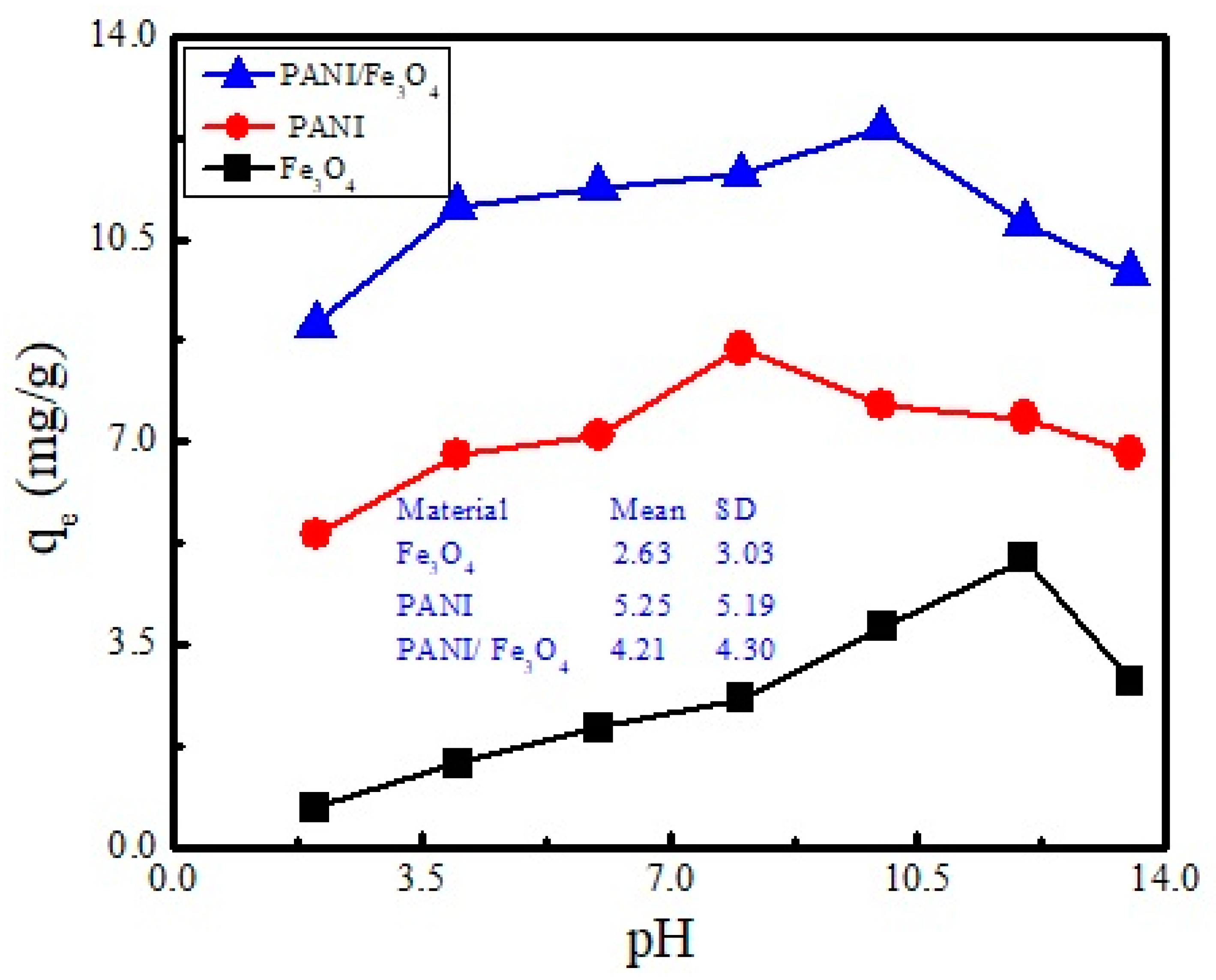
3.4. Effect of pH
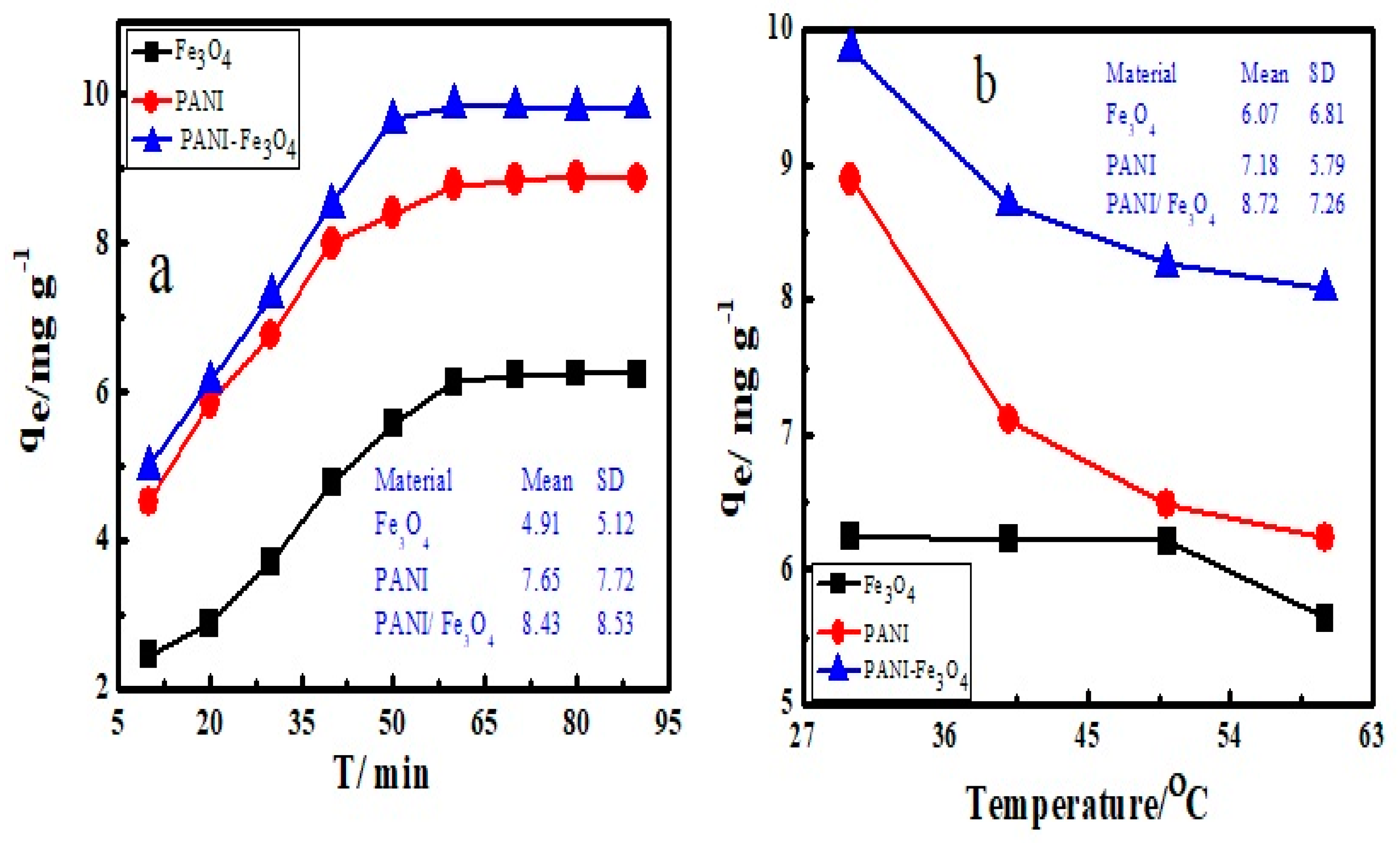
3.5. Effect of Contact Time and Temperature
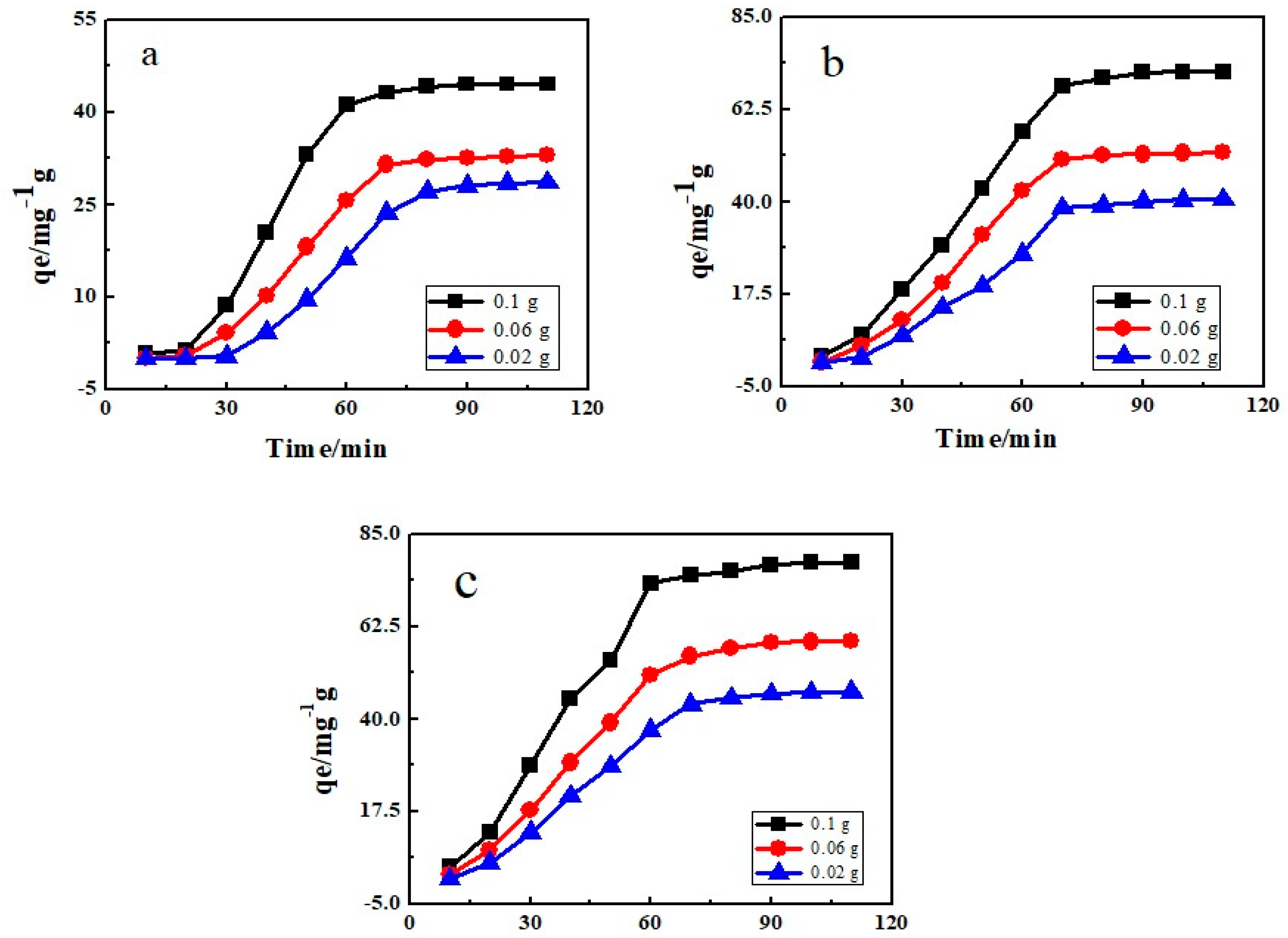
3.6. Effect of Adsorbent Dose
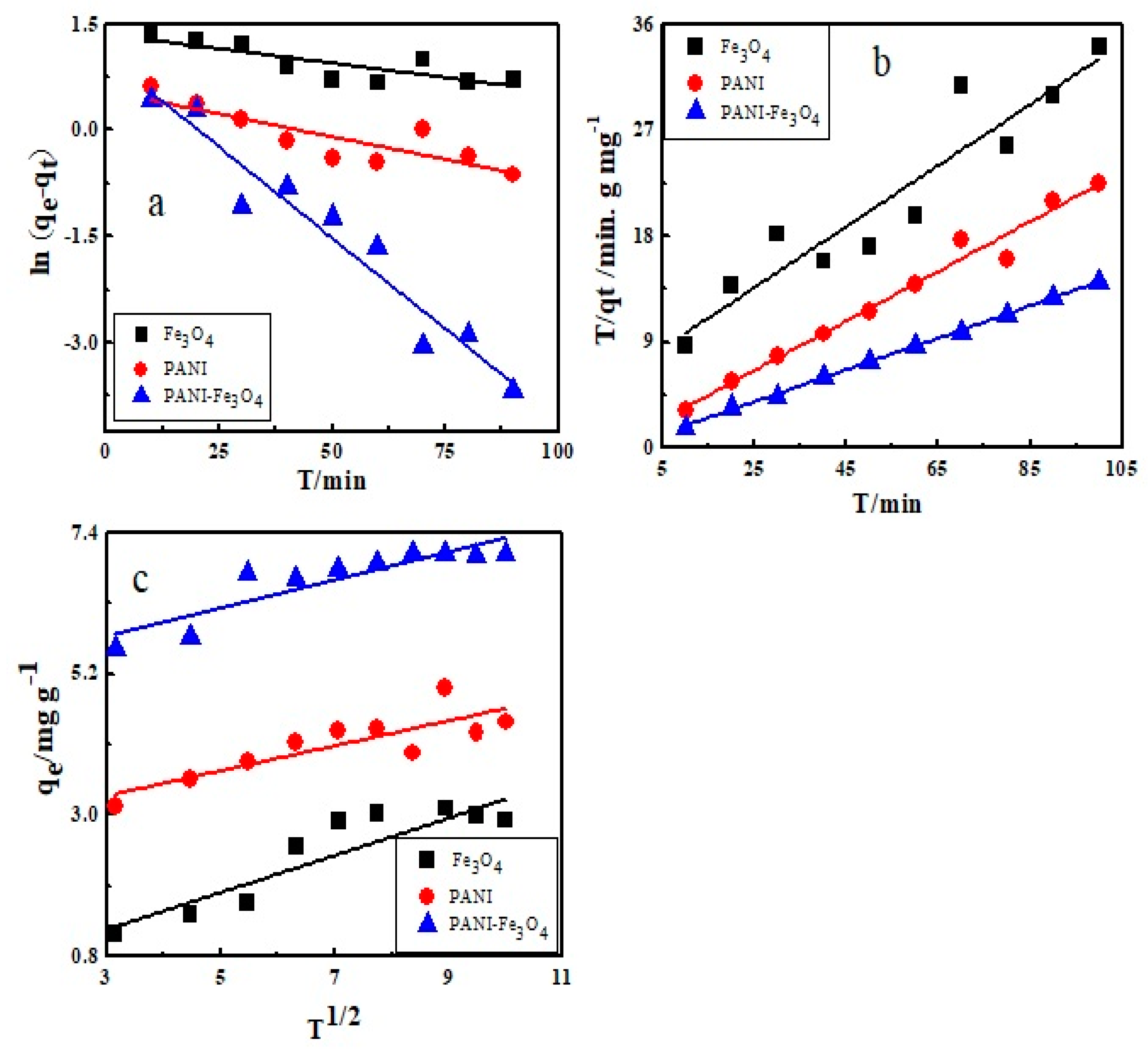
3.7. Kinetic Study
3.8. Mechanism of Adsorption
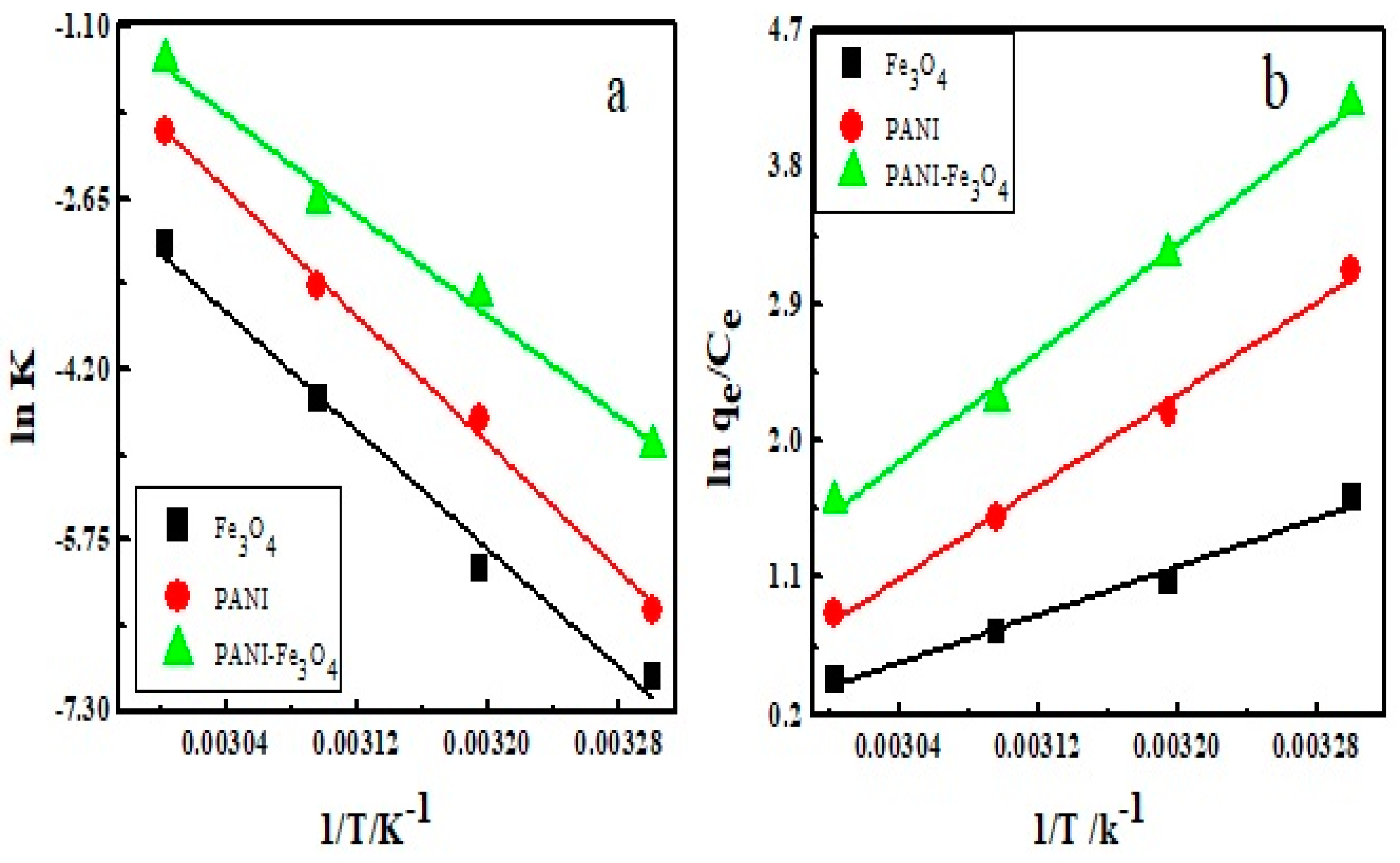
3.9. Calculation of Thermodynamic Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ayad, M.M.; El-Nasr, A.A. Adsorption of Cationic Dye (Methylene Blue) from Water Using Polyaniline Nanotubes Base. J. Phys. Chem. C 2010, 114, 14377–14383. [Google Scholar] [CrossRef]
- Gonga, J.L.; Wanga, B.; Zenga, G.M.; Yanga, C.P.; Niua, C.G.; Niua, Q.Y.; Zhoua, W.J.; Liangb, Y. Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as adsorbent. J. Hazard. Mater. 2009, 164, 1517–1522. [Google Scholar] [CrossRef]
- Bukallah, S.B.; Rauf, M.A.; AlAli, S.S. Removal of Methylene Blue from aqueous solution by adsorption on sand. Dyes Pigm. 2007, 74, 85–87. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Haghighi, R. Chemical catalytic reaction and biological oxidation for treatment of non-biodegradable textile effluent. J. Chem. Eng. 2003, 95, 163–169. [Google Scholar] [CrossRef]
- Blanco, M.; Martinez, A.; Marcaide, A.; Aranzabe, E.; Aranzabe, A. Heterogeneous Fenton Catalyst for the Efficient Removal of Azo Dyes in Water. Am. J. Anal. Chem. 2014, 5, 490–499. [Google Scholar] [CrossRef]
- Amin, N.K. Removal of direct blue-106 dye from aqueous solution using new activated carbons developed from pomegranate peel: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2009, 165, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Darvishmanesh, S.; Pethica, B.A.; Sundaresan, S. Forward osmosis using draw solutions manifesting liquid-liquid phase Separation. Desalination 2017, 421, 23–31. [Google Scholar] [CrossRef]
- Konicki, W.; Aleksandrzak, M.; Mijowska, E. Equilibrium, kinetic and thermodynamic studies on adsorption of cationic dyes from aqueous solutions using graphene oxide. Chem. Eng. Res. Des. 2017, 123, 35–49. [Google Scholar] [CrossRef]
- Zhao, B.; Xiao, W.; Shang, Y.; Zhu, H.; Han, R. Adsorption of light green anionic dye using cationic surfactant-modified peanut husk in batch mode. Arab. J. Chem. 2017, 10, S3595–S3602. [Google Scholar] [CrossRef]
- Ali, A.F.; Kovo, A.S.; Adetunji, S.A. Methylene Blue and Brilliant Green Dyes Removal from Aqueous Solution Using Agricultural Wastes Activated Carbon. J. Encaps. Adsorpt. Sci. 2017, 7, 95–107. [Google Scholar] [CrossRef]
- Bonou1, S.A.S.; Sagbo, E.; Charvillat, S.O.C.; Nissan, B.B.; Cazalbou, S. Adsorption of Textile Dyes on the Shells of Snails Achatina achatina and Lanistes varicus Acclimatized in Benin: Influence of Their Heating Treatment. J. Environ. Prot. 2018, 9, 158–174. [Google Scholar] [CrossRef]
- Lin, L.; Zhai, S.R.; Xiao, Z.Y.; Song, Y.; Song, X.W. Dye adsorption of mesoporous activated carbons produced from NaOH-pretreated rice husks. Bioresour. Technol. 2013, 136, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Sahal, T.K.; Bhoumik, N.C.; Karmaker, S.; Ahmed, M.G.; Ichikawa, H.; Fukumori., Y. Adsorption of Methyl Orange onto Chitosan from Aqueous Solution. J. Water Resour. Prot. 2010, 2, 898–906. [Google Scholar] [CrossRef]
- Muinde, V.M.; Onyari, J.M.; Wamalwa, B.; Wabomba, J.; Nthumbi, R.M. Adsorption of Malachite Green from Aqueous Solutions onto Rice Husks: Kinetic and Equilibrium Studies. J. Environ. Prot. 2017, 8, 215–230. [Google Scholar] [CrossRef]
- Fernandez, M.E.; Nunell, G.V.; Bonelli, P.R.; Cukierman, A.L. Activated carbon developed from orange peels: Batch and dynamic competitive adsorption of basic dyes. Ind. Crop. Prod. 2014, 62, 437–445. [Google Scholar] [CrossRef]
- Zhang, G.L.; Deng, H.; Sun, P. Dyes adsorption using a synthetic carboxymethyl cellulose-acrylic acid Adsorbent. J. Environ. Sci. 2014, 26, 1203–1211. [Google Scholar] [CrossRef]
- Krishnani, K.K.; Srinives, S.; Mohapatra, B.C.; Boddu, V.M.; Hao, J.; Meng, X.; Mulchandani, A. Hexavalent chromium removal mechanism using conducting polymers. J. Hazard. Mater. 2013, 252, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Bilal, S.; Farooq, S.; Shah, H.A.A.; Holze, R. Improved solubility, conductivity, thermal stability and corrosion protection properties of poly(o-toluidine. synthesized via chemical polymerization. Synth. Met. 2014, 197, 144–153. [Google Scholar] [CrossRef]
- Kaloni, T.P.; Giesbrecht, P.K.; Schreckenbach, G.; Freund, M.S. Polythiophene: From Fundamental Perspectives to Applications. Chem. Mater. 2017, 29, 10248–10283. [Google Scholar] [CrossRef]
- Bai, H.; Chen, Q.; Li, C.; Lu, C.; Shi, G. Electrosynthesis of polypyrrole/sulfonated polyaniline composite films and their applications for ammonia gas sensing. Polymer 2007, 48, 4015–4020. [Google Scholar] [CrossRef]
- Shih, Y.C.; Ke, C.Y.; Yu, C.J.; Lu, C.Y.; Tseng, W.L. Combined Tween 20-Stabilized Gold Nanoparticles and Reduced Graphite Oxide−Fe3O4 Nanoparticle Composites for Rapid and Efficient Removal of Mercury Species from a Complex Matrix. ACS Appl. Mater. Interface 2014, 6, 17437–17445. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Q.; Feng, J.; Yan, W. Synthesis of PPy-modified TiO2 composite in H2SO4 solution and its novel adsorption characteristics for organic dyes. Chem. Eng. J. 2013, 225, 766–775. [Google Scholar] [CrossRef]
- El-Naggar, I.M.; Zakaria, E.S.; Ali, M.; Khali, M.; El-Shahat, M.F. Removal of Cesium on Polyaniline Titanotungstate as Composite Ion Exchanger. Adv. Chem. Eng. Sci. 2012, 2, 166–179. [Google Scholar] [CrossRef]
- Khuspe, G.D.; Navale, S.T.; Chougule, M.A.; Sen, S.; Agawane, G.L.; Kim, J.H.; Patil, V.B. Facile method of synthesis of polyaniline-SnO2 hybrid nano composites: Microstructural, optical and electrical transport properties. Synth. Met. 2013, 178, 1–9. [Google Scholar] [CrossRef]
- Vivekanandan, J.; Ponnusamy, V.; Mahudeswaraand, A.; Vijayanand, P.S. Synthesis, characterization and conductivity study of polyaniline prepared by chemical oxidative and electrochemical methods. Arch. Appl. Sci. Res. 2011, 3, 147–153. [Google Scholar]
- Jamadade, V.S.; Dhawale, D.S.; Lokhande, C.D. Studies on electro synthesized leucoemeraldine, emeraldine and pernigraniline forms of polyaniline films and their super capacitive behavior. Synth. Met. 2010, 160, 955–960. [Google Scholar] [CrossRef]
- Zaragoza, C.E.A.; Hernández, E.A.; Estrada, M.; Kobayashi, T. Synthesis of diphenylamine-co-aniline copolymers in emulsified systems using a reactive surfactant as the emulsifying agent and aniline monomer. Synth. Met. 2016, 214, 5–13. [Google Scholar] [CrossRef]
- Bian, C.; Yu, A.; Wu, H. Fibriform polyaniline/nano-TiO2 composite as an electrode material for aqueous redox supercapacitors. Electrochem. Commun. 2009, 11, 266–269. [Google Scholar] [CrossRef]
- Gemeay, A.H.; El-Sharkawy, R.G.; Mansour, I.A.; Zaki, A.B. Catalytic activity of polyaniline/MnO2 composites towards the oxidative decolorization of organic dyes. Appl. Catal. B Environ. 2008, 80, 106–115. [Google Scholar] [CrossRef]
- Huang, X.; Wang, G.; Yang, M.; Guo, W.; Gao, H. Synthesis of polyaniline-modified Fe3O4/SiO2/TiO2 composite microspheres and their photocatalytic application. Mater. Lett. 2011, 65, 2887–2890. [Google Scholar] [CrossRef]
- Jing, S.; Xing, S.; Yu, L.; Wu, Y.; Zhao, C. Synthesis and characterization of Ag/polyaniline core–shell nanocomposites based on silver nanoparticles colloid. Mater. Lett. 2007, 61, 2794–2797. [Google Scholar] [CrossRef]
- Ji, Y.; Qin, C.; Niu, H.; Sun, L.; Jin, Z.; Bai, X. Electrochemical and electro chromic behaviors of polyaniline-graphene oxide composites on the glass substrate/Ag nano-film electrodes prepared by vertical target pulsed laser deposition. Dyes Pigm. 2015, 117, 72–82. [Google Scholar] [CrossRef]
- Liu, P.; Huang, Y.; Zhang, X. Synthesis, characterization and excellent electromagnetic wave absorption properties of graphene@CoFe2O4@polyaniline nanocomposites. Synth. Met. 2015, 201, 76–81. [Google Scholar] [CrossRef]
- Janaki, V.; Oh, B.; Shanthi, K.; Lee, K.; Ramasamy, A.K.; Kamala, S. Polyaniline/chitosan composite: An eco-friendly polymer for enhanced removal of dyes from aqueous solution. Synth. Met. 2012, 162, 974–980. [Google Scholar] [CrossRef]
- Sultana, S.; Zain, K.M. Synthesis and characterization of copper ferrite nanoparticles doped polyaniline. J. Alloy. Compd. 2012, 535, 44–49. [Google Scholar] [CrossRef]
- Neuberger, T.; Schöpf, B.; Hofmann, H.; Hofmann, M.; Rechenberg, B. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J. Magn. Magn. Mater. 2005, 293, 483–496. [Google Scholar] [CrossRef]
- Khurshid, H.; Hadjipanayis, C.; Chen, H.W.; Li, H.; Mao, H.; Machaidze, R.; Tzitzios, V.; Hadjipanay, G.C. Core/shell structured iron/iron-oxide nanoparticles as excellent MRI contrast enhancement agents. J. Magn. Magn. Mater. 2013, 331, 17–20. [Google Scholar] [CrossRef]
- Wang, G.; Chang, Y.; Wang, L.; Wei, Z.; Kang, J.; Sang, L.; Dong, X.; Chen, G.; Wang, H.; Qi, H. Preparation and characterization of PVPI-coated Fe3O4 nanoparticles as an MRI contrast agent. J. Magn. Magn. Mater. 2013, 340, 57–60. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef]
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005, 100, 1–11. [Google Scholar] [CrossRef]
- Do, S.H.; Jo, Y.H.; Park, J.Y.; Hong, S.H. As3+ removal by Ca–Mn–Fe3O4 with and without H2O2: Effects ofcalcium oxide in Ca–Mn–Fe3O4. J. Hazard. Mater. 2014, 280, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Irene, M.C.; Chen, G. Fast Removal and Recovery of Cr(VI) Using Surface-Modified Jacobsite (MnFe2O4) Nanoparticles. Langmuir 2005, 21, 11173–11179. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, A.; Sohrabi, M.; Kargari, A. A review of methods for synthesis of nano structured metals with emphasis on iron compounds. Chem. Pap. 2007, 61, 151–170. [Google Scholar] [CrossRef]
- Amer, M.A.; Meaz, T.M.; Attalah, S.S.; Ghoneim, A.I. Structural and magnetic characterization of the Mg0.2_xSrxMn0.8Fe2O4 nanoparticles. J. Magn. Magn. Mater. 2014, 363, 60–65. [Google Scholar] [CrossRef]
- Lam, U.T.; Mammucari, R.; Suzuki, K.; Foster, N.R. Processing of iron oxide nanoparticles by supercritical fluids. Ind. Eng. Chem. Res. 2008, 47, 599–614. [Google Scholar] [CrossRef]
- Teja, A.S.; Koh, P.Y. Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Majewski, P.; Thierry, B. Functionalized magnetite nanoparticles-synthesis, properties, and bio-applications. Solid State Mater. Sci. 2007, 32, 203–215. [Google Scholar] [CrossRef]
- Jia, Z.; Yujun, W.; Yangcheng, L.; Jingyu, M.; Guangsheng, L. In situ preparation of magnetic chitosan/Fe3O4 composite nanoparticles in tiny pools of water-in-oil microemulsion. React. Funct. Polym. 2006, 66, 1552–1558. [Google Scholar] [CrossRef]
- Racuciu, M.; Creanga, D.E.; Airinei, A. Citric-acid coated magnetite nanoparticles for biological Applications. Eur. Phys. J. E 2006, 21, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Shena, J.; Shahida, S.; Amuraa, I.; Sarihana, A.; Tiana, M.; Emanuelsson, E.A. Enhanced adsorption of cationic and anionic dyes from aqueous solutions by polyacid doped polyaniline. Synth. Met. 2018, 245, 151–159. [Google Scholar] [CrossRef]
- Ahmadi, S.; Chia, C.H.; Zakaria, S.; Saeedfar, K.; Asim, N. Synthesi of Fe3O4 nanocrystals using hydrothermal approach. J. Magn. Magn. Mater. 2012, 324, 4147–4150. [Google Scholar] [CrossRef]
- Shreepathi, S.; Holze, R. Spectro electrochemical Investigations of Soluble Polyaniline Synthesized via New Inverse Emulsion Pathway. Chem. Mater. 2005, 17, 4078–4085. [Google Scholar] [CrossRef]
- Samania, M.R.; Borghei, S.M.; Olad, A.; Chaichi, M. Removal of chromium from aqueous solution using polyaniline-Poly ethylene glycol composites. Hazard. Mater. 2010, 184, 248–254. [Google Scholar] [CrossRef]
- Wai, P.B.S.; Kuramoto, N. Development and Investigation of Polyaniline Micro/nanocomposites that Possess Moderate Conductivity, Dielectric and Magnetic Properties. Polymer 2008, 40, 25–32. [Google Scholar] [CrossRef]
- Liu, Y.; Drew, M.G.B.; Cao, F.L. A comparative study of Fe3O4/polyaniline composites with octahedral and microspherical inorganic kernels. J. Mater. Sci. 2014, 49, 3694–3704. [Google Scholar] [CrossRef]
- Eskizeybek, V.; Sar, F.; Gülce, H.; Gülce, A.; Avc, A. Preparation of the new polyaniline/ZnO nano composite and its photocatalytic activity for degradation of methylene blue and malachite green dyes under UV and natural sun lights irradiations. Appl. Catal. B 2012, 119, 197–206. [Google Scholar] [CrossRef]
- Tung, L.M.; Cong, N.X.; Huy, L.T.; Lan, N.T.; Phan, V.N.; Hoa, N.Q.; Vinh, L.K.; Thinh, N.V.; Tai, L.T.; Ngo, D.T.; Mølhave, K.; Tran Quang Huy, T.Q.; Le, A.T.; et al. Synthesis, Characterizations of Superparamagnetic Fe3O4–Ag Hybrid Nanoparticles and Their Application for Highly Effective Bacteria Inactivation. J. Nanosci. Nanotechnol. 2016, 16, 5902–5912. [Google Scholar] [CrossRef]
- Bachan, N.; Asha, A.; Jeyarani, W.J.; Kumar, D.A.; Shyla, J.M. A Comparative Investigation on the Structural, Optical and Electrical Properties of SiO2–Fe3O4 Core–Shell Nanostructures with Their Single Components. Acta Metall. Sin. Engl. Lett. 2015, 28, 1317–1325. [Google Scholar] [CrossRef]
- Ullah, R.; Bowmaker, G.A.; Laslau, C.; Zujovic, Z.D.; Ali, K.; Shah, A.H.A.; Sejdic, J.T. Synthesis of polyaniline by using CuCl2 as oxidizing agent. Synth. Met. 2014, 198, 203–211. [Google Scholar] [CrossRef]
- Aphesteguy, J.C.; Jacobo, S.E. Synthesis of a soluble polyaniline ferrite composite: Magnetic and electric properties. J. Mater. Sci. 2007, 42, 7062–7068. [Google Scholar] [CrossRef]
- Huang, J.; Li, Q.; Wang, Y.; Dong, L.; Xie, H.; Wang, J.; Xiong, C. Fluxible Nanoclusters of Fe3O4 Nanocrystals Embedded Polyaniline by Macromolecule-Induced Self-Assembly. Langmuir 2013, 29, 10223–10228. [Google Scholar] [CrossRef] [PubMed]
- Khataee, A.R.; Mirzajani, O. UV/peroxydisulfate oxidation of C. I. Basic Blue 3: Modeling of key factors by artificial neural network. Desalination 2010, 251, 64–69. [Google Scholar] [CrossRef]
- Roychowdhury, A.; Pati, S.P.; Mishra, A.K.; Kumar, S.; Das, D. Magnetically addressable fluorescent Fe3O4/ZnO nanocomposites: Structural, optical and magnetization studies. J. Phys. Chem. Solids 2013, 74, 811–818. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Yamini, Y.; Dayeni, M.; Seidi, S. Removal of Methylene Blue and Neutral Red from Aqueous Solutions by Surfactant-Modified Magnetic Nanoparticles as Highly Efficient Adsorbent. Environ. Prog. Sustain. Energy 2015, 34, 1683–1693. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Barkhi, M.; Zare, D.; Davoodi, D.; Tabatabaei, M. Preparation and Characterization of CTAB-Coated Fe3O4 Nanoparticles. Nano-Metal Chem. 2012, 42, 644–648. [Google Scholar] [CrossRef]
- Cao, C.; Xiao, L.; Chen, C.; Shi, X.; Cao, Q.; Gao, L. In situ preparation of magnetic Fe3O4/chitosan nanoparticles via a novel reduction–precipitation method and their application in adsorption of reactive azo dye. Powder Technol. 2014, 260, 90–97. [Google Scholar] [CrossRef]
- Ayad, M.; Hefnawy, G.E.; Zaghlol, S. Facile synthesis of polyaniline nanoparticles; its adsorption behavior. J. Chem. Eng. 2013, 217, 460–465. [Google Scholar] [CrossRef]
- Umare, S.S.; Shambharkar, B.H.; Ningthoujam, R.S. Synthesis and characterization of polyaniline–Fe3O4 nanocomposite: Electrical conductivity, magnetic, electrochemical studies. Synth. Met. 2010, 160, 1815–1821. [Google Scholar] [CrossRef]
- Zhan, J.; Zhang, H.; Zhub, G. Magnetic photocatalysts of cenospheres coated with Fe3O4/TiO2 core/shell nanoparticles decorated with Ag nanopartilces. Ceram. Int. 2014, 40, 8547–8559. [Google Scholar] [CrossRef]
- Yang, Y.; Qi, S. Preparation of pyrrole with iron oxide precipitated on the surface of graphite nanosheet. J. Magn. Magn. Mater. 2012, 324, 2380–2387. [Google Scholar] [CrossRef]
- Shariati, S.; Faraji, M.; Yamini, Y.; Rajabi, A.A. Fe3O4 magnetic nanoparticles modified with sodium dodecyl sulfate for removal of safranin O dye from aqueous solutions. Desalination 2011, 270, 160–165. [Google Scholar] [CrossRef]
- Absalan, G.; Asadi, M.; Kamran, S.; Sheikhian, L.; Goltz, D.G. Removal of reactive red-120 and 4-(2-pyridylazo. resorcinol from aqueous samples by Fe3O4 magnetic nanoparticles using ionic liquid as modifier. J. Hazard. Mater. 2011, 192, 476–484. [Google Scholar] [CrossRef]
- Deshpande, N.G.; Gudagea, Y.G.; Sharma, R.; Vyas, J.C.; Kim, J.B.; Lee, Y.P. Studies on tin oxide-intercalated polyaniline nanocomposite for ammonia gas sensing applications. Sens. Actuators B 2009, 138, 76–84. [Google Scholar] [CrossRef]
- Jang, J.H.; Lim, H.B. Characterization and analytical application of surface modified magnetic nanoparticles. Microchem. J. 2010, 94, 148–158. [Google Scholar] [CrossRef]
- Xuan, S.; Wang, F.; Lai, J.M.Y.; Sham, K.W.Y.; Xiang, J.Y.; Lee, W.S.F.; Yu, J.C.; Cheng, C.H.K.; Leung, K.C. Synthesis of Biocompatible, Mesoporous Fe3O4 Nano/Microspheres with Large Surface Area for Magnetic Resonance Imaging and Therapeutic Applications. Appl. Mater. Interfaces 2011, 3, 237–244. [Google Scholar] [CrossRef]
- Etim, U.J.; Umoren, S.A.; Eduok, U.M. Coconut coir dust as a low cost adsorbent for the removal of cationic dye from aqueous solution. J. Saudi Chem. Soc. 2016, 20, S67–S76. [Google Scholar] [CrossRef]
- Salem, M.A. The role of polyaniline salts in the removal of direct blue 78 from aqueous solution: A kinetic study. React. Funct. Polym. 2010, 70, 707–714. [Google Scholar] [CrossRef]
- Rauf, M.A.; Bukallah, S.B.; Hamour, F.A.; Nasir, A.S. Adsorption of dyes from aqueous solutions onto sand and their kinetic behavior. J. Chem. Eng. 2008, 137, 238–243. [Google Scholar] [CrossRef]
- Sharma, P.; Das, M.R. Removal of a Cationic Dye from Aqueous Solution Using Graphene Oxide Nanosheets: Investigation of Adsorption Parameters. J. Chem. Eng. Data 2013, 58, 151–158. [Google Scholar] [CrossRef]
- Abdelwahab, O. Evaluation of the use of loofa activated carbons as potential adsorbents for aqueous solutions containing dye. Desalination 2008, 222, 357–367. [Google Scholar] [CrossRef]
- Zreig, M.A.; Rudra, R.P.; Dickinson, W.T.; Evans, L. Effect of surfactants on sorption of atrazine by soil. J. Contam. Hydrol. 1999, 36, 249–263. [Google Scholar] [CrossRef]
- Patil, M.R.; Khairnar, S.D.; Shrivastava, V.S. Synthesis, characterisation of polyaniline–Fe3O4 magnetic nanocomposite and its application for removal of an acid violet 19 dye. Appl. Nanosci. 2016, 6, 495–502. [Google Scholar] [CrossRef]
- Ouazene, N.; Lounis, A. Adsorption characteristics of CI Basic Blue 3 from aqueous solution onto Alleppo pine-tree sawdust. Color. Technol. 2011, 127, 21–27. [Google Scholar]
- Ong, S.T.; Lee, C.K.; Zainal, Z. A cmparison of sorption and photodegradation study in the removal of basic and reactive dyes. Aust. J. Basic Appl. Sci. 2009, 3, 3408–3416. [Google Scholar]
- Bangash, F.K.; Manaf, A. Dyes removal from aqueous solution using wood activated charcoal of bombax cieba tree. J. Chin. Chem. Soc. 2005, 52, 489–494. [Google Scholar] [CrossRef]
- Wong, S.Y.; Tan, Y.P.; Abdullah, A.H.; Ong, S.T. The removal of basic and reactive dyes using quartenised sugar cane bagasse. J. Phys. Sci. 2009, 20, 59–74. [Google Scholar]
- Chu, H.C.; Chen, K.M. Reuse of activated sludge biomass: I. Removal of basic dyes from wastewater biomass. Process Biochem. 2002, 37, 595–600. [Google Scholar] [CrossRef]
- Marungrueng, K.; Pavasant, P. Removal of basic dye (Astrazon Blue FGRL. using macroalga Caulerpa lentillifera. J. Environ. Manag. 2006, 78, 268–274. [Google Scholar] [CrossRef]
- Barsa, A.; Buha, R.; Dulman, V. Removal of Basic Blue 3 by sorption onto weak acid acrylic resin. J. Appl. Polym. Sci. 2009, 113, 607–614. [Google Scholar] [CrossRef]
- Vasanth, K.; Sivanesan, S. Isotherm parameters for basic dyes onto activated carbon: Comparison of linear and non-linear method. J. Hazard. Mater. 2006, 129, 147–150. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, D.; Zhang, G.; Cai, C.; Zhang, C.; Qiu, G.; Zheng, K.; Wu, Z. Adsorption of methylene blue from aqueous solution onto multiporous palygorskite modified by ion beam bombardment: Effect of contact time, temperature, pH and ionic strength. Appl. Clay Sci. 2013, 83, 137–143. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, T.; Ye, X.; Li, Q.; Guo, M.; Liu, H.; Wu, Z. Dye adsorption by resins: Effect of ionic strength on hydrophobic and electrostatic interactions. Chem. Eng. J. 2013, 228, 392–397. [Google Scholar] [CrossRef]
- Xu, D.; Tan, X.L.; Chen, C.L.; Wang, X.K. Adsorption of Pb(II) from aqueous solution to MX-80 bentonite: Effect of pH, ionic strength, foreign ions and temperature. Appl. Clay Sci. 2008, 41, 37–46. [Google Scholar] [CrossRef]
- Lian, L.; Guo, L.; Guo, C. Adsorption of Congo red from aqueous solutions onto Ca-bentonite. J. Hazard. Mater. 2009, 16, 126–131. [Google Scholar] [CrossRef]
- Mahanta, D.; Madras, G.; Radhakrishnan, S.; Patil, S. Adsorption of Sulfonated Dyes by Polyaniline Emeraldine Salt and Its Kinetics. J. Phys. Chem. B 2008, 112, 10153–10157. [Google Scholar] [CrossRef]
- Ai, L.; Jianga, J.; Zhang, R. Uniform polyaniline microspheres: A novel adsorbent for dye removal from aqueous solution. Synth. Met. 2010, 160, 762–767. [Google Scholar] [CrossRef]
- Muller-Dethlefs, K.; Hobza, P. Noncovalent interactions: A challenge for experiment and theory. Chem. Rev. 2000, 100, 143–167. [Google Scholar] [CrossRef]
- Wang, J.; Deng, B.; Chen, H.; Wang, X.O.; Zheng, J.Z. Removal of aqueous Hg(II. by polyaniline: Sorption characteristics and mechanisms. Environ. Sci. Technol. 2009, 43, 5223–5228. [Google Scholar] [CrossRef]
- Ahmad, R.; Kumar, R. Conducting Polyaniline/Iron Oxide Composite: A Novel Adsorbent for the Removal of Amido Black 10B. J. Chem. Eng. Data 2010, 55, 3489–3493. [Google Scholar] [CrossRef]
- Yang, C.; Du, J.; Peng, Q.; Qiao, R.; Chen, W.; Xu, C.; Shuai, Z.; Gao, M. Polyaniline/Fe3O4 Nanoparticle Composite: Synthesis and Reaction Mechanism. J. Phys. Chem. B 2009, 113, 5052–5058. [Google Scholar] [CrossRef]
- Fernandes, A.N.; Almedia, C.A.P.; Debacher, N.A.; Sierra, M.D.S. Isotherm and thermodynamic data of adsorption of methylene blue from aqueous solution onto peat. J. Mol. Struct. 2010, 982, 62–65. [Google Scholar] [CrossRef]
- Gupta, V.K.; Pathania, D.; Kothiyal, N.C.; Sharma, G. Polyaniline zirconium (IV) silicophosphate nanocomposite for remediation of methylene blue dye from waste water. J. Mol. Liq. 2014, 190, 139–145. [Google Scholar] [CrossRef]
- Ai, L.; Li, M.; Li, L. Adsorption of Methylene Blue from Aqueous Solution with Activated Carbon/Cobalt Ferrite/Alginate Composite Beads: Kinetics, Isotherms, and Thermodynamics. J. Chem. Eng. Data 2011, 56, 3475–3483. [Google Scholar] [CrossRef]


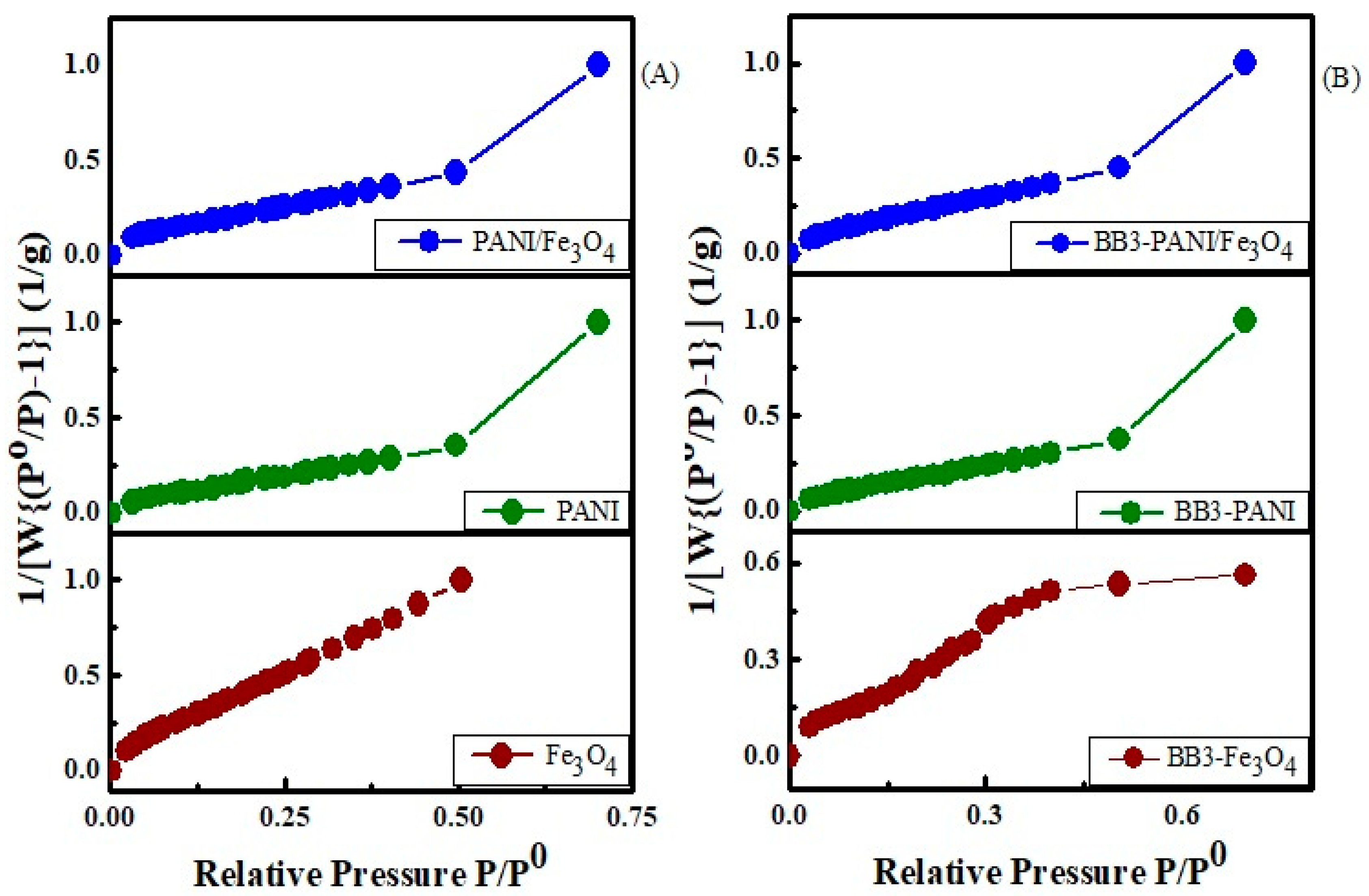
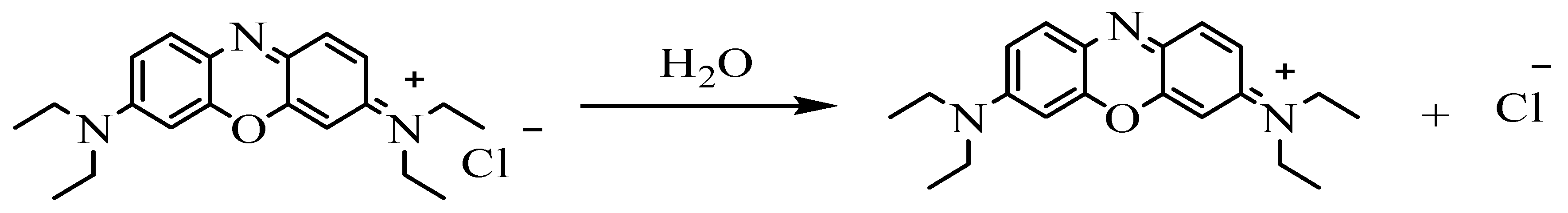
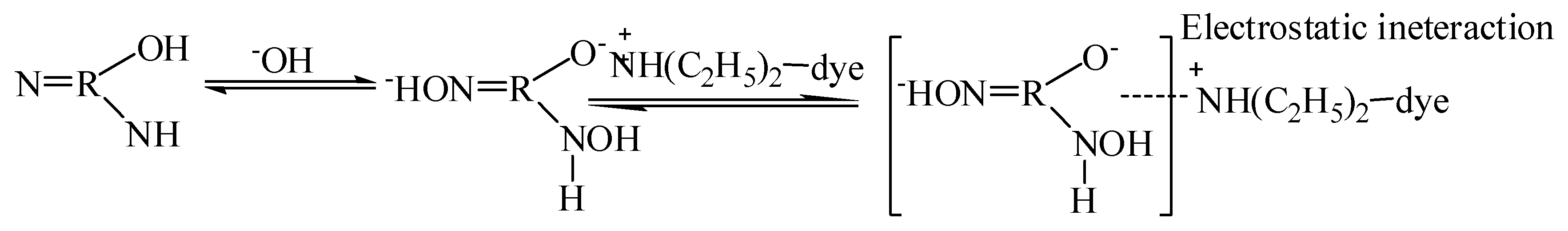
| Observations | Sample | BJH Average Pore Radius (Å) | BJH Pore Volume (cc/g) | Surface Area (m2/g) |
|---|---|---|---|---|
| Before adsorption | Fe3O4 | 14.879 | 0.033 | 65.818 |
| PANI | 15.500 | 0.021 | 70.263 | |
| PANI/Fe3O4 | 14.951 | 0.062 | 99.759 | |
| After adsorption | Fe3O4 | 14.864 | 0.023 | 46.608 |
| PANI | 14.822 | 0.020 | 46.698 | |
| PANI/Fe3O4 | 14.944 | 0.046 | 53.196 |
| Adsorbents | Freundlich | Langmuir | Tempkin | D-R | ||||
|---|---|---|---|---|---|---|---|---|
| Fe3O4 | 1/n | 0.9593 | qmax | 7.474 | β | −0.8096 | qs | 0.888 |
| Kf | 1.312 | KL | 0.0911 | KT | 8.565 | Eads | 0.899 | |
| R2 | 0.9755 | RL | 0.1210 | R2 | 0.5048 | R2 | 0.8036 | |
| - | - | R2 | 0.9788 | - | - | - | - | |
| PANI | 1/n | 0.8673 | qmax | 47.977 | β | −5.626 | qs | 9.183 |
| Kf | 16.912 | KL | 0.0141 | KT | 22.26 | Eads | 0.999 | |
| R2 | 0.9797 | RL | 0.4710 | R2 | 0.6196 | R2 | 0.8620 | |
| - | - | R2 | 0.9849 | - | - | - | - | |
| PANI/Fe3O4 | 1/n | 0.9112 | qmax | 78.13 | β | −10.372 | qs | 20.54 |
| Kf | 44.719 | KL | 0.0071 | KT | 33.04 | Eads | 0.897 | |
| R2 | 0.9911 | RL | 0.6410 | R2 | 0.7514 | R2 | 0.9437 | |
| - | - | R2 | 0.9985 | - | - | - | - | |
| Adsorbents | pH | T (°C) | Maximum Adsorption (mg/g) | Refs. |
|---|---|---|---|---|
| Aleppo pine-tree sawdust | 7 | 20 | 65.4 | [83] |
| Ethylenediamine modified rice husk | 4.7 | 25 | 3.29 | [84] |
| Wood activated Charcoal | 7 | 10–50 | 0.59–0.64 | [85] |
| Quartinized sugarcane bagass | 6–8 | 20 | 37.59 | [86] |
| Activated sludge biomass | - | 20 | 36.5 | [87] |
| Palm fruit bunch particles | - | - | 91.33 | [88] |
| CM-60 weak acid acrylic resin | 5.5 | 17–50 | 34.36–59.53 | [89] |
| Activated Carbon | 8 | 32 | 406 | [90] |
| Fe3O4 | 12 | 30 | 7.474 | Present study |
| PANI | 8 | 30 | 47.97 | Present study |
| PANI/Fe3O4 | 10 | 30 | 78.13 | Present study |
| Pseudo 1st Order | Pseudo 2nd Order | Intra Particle Diffusion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adsorbents | K1 (min−1) | qe (mg g−1) | R2 | K2 (g mg−1 min−1) | qe (mg g−1) | R2 | Kd (g mg−1 min−1/2) | C (mg g−1) | R2 | ||
| Fe3O4 | −0.0081 | 3.859 | 0.644 | 0.259 | 7.145 | 0.873 | 0.2887 | 0.353 | 0.745 | ||
| PANI | −0.0128 | 1.733 | 0.688 | 0.211 | 44.50 | 0.979 | 0.1946 | 2.712 | 0.679 | ||
| PANI/Fe3O4 | −0.0513 | 2.869 | 0.932 | 0.136 | 76.71 | 0.999 | 0.2192 | 5.122 | 0.777 | ||
| Adsorbents | (kJ/mol) | (kJ/mol) | (kJ/(mol·K)) | Ea (kJ/mol) |
|---|---|---|---|---|
| Fe3O4 | −04.05 | −32.84 | −0.095 | 11.14 |
| PANI | −07.78 | −62.93 | −0.182 | 11.97 |
| PANI/Fe3O4 | −10.63 | −74.26 | −0.210 | 09.94 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad, A.; Shah, A.-u.-H.A.; Bilal, S.; Rahman, G. Basic Blue Dye Adsorption from Water Using Polyaniline/Magnetite (Fe3O4) Composites: Kinetic and Thermodynamic Aspects. Materials 2019, 12, 1764. https://doi.org/10.3390/ma12111764
Muhammad A, Shah A-u-HA, Bilal S, Rahman G. Basic Blue Dye Adsorption from Water Using Polyaniline/Magnetite (Fe3O4) Composites: Kinetic and Thermodynamic Aspects. Materials. 2019; 12(11):1764. https://doi.org/10.3390/ma12111764
Chicago/Turabian StyleMuhammad, Amir, Anwar-ul-Haq Ali Shah, Salma Bilal, and Gul Rahman. 2019. "Basic Blue Dye Adsorption from Water Using Polyaniline/Magnetite (Fe3O4) Composites: Kinetic and Thermodynamic Aspects" Materials 12, no. 11: 1764. https://doi.org/10.3390/ma12111764
APA StyleMuhammad, A., Shah, A.-u.-H. A., Bilal, S., & Rahman, G. (2019). Basic Blue Dye Adsorption from Water Using Polyaniline/Magnetite (Fe3O4) Composites: Kinetic and Thermodynamic Aspects. Materials, 12(11), 1764. https://doi.org/10.3390/ma12111764








