Human Osteoblast Cell Behaviour on Titanium Discs Treated with Argon Plasma
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Cell Culture
2.3. Cell Viability Analysis
2.4. Morphological Analysis and Mitochondrial Energy Balance
2.5. Statistical Analysis
3. Results
3.1. Cell Viability
3.2. Morphological Analysis of Cells
3.3. Analysis of Mitochondrial Energy Balance
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Esposito, M.; Ardebili, Y.; Worthington, H.V. Interventions for replacing missing teeth: different types of dental implants. In Cochrane Database of Systematic Reviews; John Wiley & Sons: Hoboken, NJ, USA, 2014; Volume 7. [Google Scholar]
- Baró, A.M.; García, N.; Miranda, R.; Vázquez, L.; Aparicio, C.; Olivé, J.; Lausmaa, J. Characterization of surface roughness in titanium dental implants measured with scanning tunnelling microscopy at atmospheric pressure. Biomaterials. 1986, 7, 463–466. [Google Scholar] [CrossRef]
- Bagno, A.; Di Bello, C. Surface treatments and roughness properties of Ti-based biomaterials. J. Mater. Sci. Mater. Med. 2004, 15, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 1—Review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar]
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Fox, C.H.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef]
- Kang, B.S.; Sul, Y.T.; Oh, S.J.; Lee, H.J.; Albrektsson, T. XPS, AES and SEM analysis of recent dental implants. Acta Biomater. 2009, 5, 2222–2229. [Google Scholar] [CrossRef]
- Hayashi, R.; Ueno, T.; Migita, S.; Tsutsumi, Y.; Doi, H.; Ogawa, T.; Hanawa, T.; Wakabayashi, N. Hydrocarbon Deposition Attenuates Osteoblast Activity on Titanium. J. Dent. Res. 2014, 93, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, M.; Canullo, L.; Donnarumma, G.; Caputo, P.; Nastri, L.; Guida, L. Bacterial inactivation/sterilization by argon plasma treatment on contaminated titanium implant surfaces: In vitro study. Med. Oral Patol. Oral Cir. Bucal. 2016, 21, e118–e121. [Google Scholar] [CrossRef] [PubMed]
- Cools, P.; Geyter, N.D.; Vanderleyden, E.; Dubruel, P.; Morent, R. Surface Analysis of Titanium Cleaning and Activation Processes: Non-thermal Plasma Versus Other. Plasma Chem. Plasma Process. 2014, 34, 917–932. [Google Scholar] [CrossRef]
- Ogawa, T.; Nishimura, I. Different bone integration profiles of turned and acid-etched implants associated with modulated expression of extracellular matrix genes. Int. J. Oral Maxillofac. Implant 2003, 18, 200–210. [Google Scholar]
- Roy, M.; Pompella, A.; Kubacki, J.; Szade, J.; Roy, R.A.; Hedzelek, W. Photofunctionalization of titanium: An alternative explanation of its chemical-physical mechanism. PLoS ONE 2016, 11, e0157481. [Google Scholar] [CrossRef] [PubMed]
- Henningsen, A.; Smeets, R.; Hartjen, P.; Heinrich, O.; Heuberger, R.; Heiland, M.; Precht, C.; Cacaci, C. Photofunctionalization and non-thermal plasma activation of titanium surfaces. Clin Oral Investig. 2018, 22, 1045–1054. [Google Scholar] [CrossRef]
- Yoshihara, C.; Ueno, T.; Chen, P.; Tsutsumi, Y.; Hanawa, T.; Wakabayashi, N. Inverse response of osteoblasts and fibroblasts to growth on carbon-deposited titanium surfaces. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 106, 1869–1877. [Google Scholar] [CrossRef]
- Canullo, L.; Genova, T.; Wang, H.L.; Carossa, S.; Mussano, F. Plasma of Argon Increases Cell Attachment and Bacterial Decontamination on Different Implant Surfaces. Int. J. Oral Maxillofac. Implants 2017, 32, 1315–1323. [Google Scholar] [CrossRef]
- Canullo, L.; Genova, T.; Tallarico, M.; Gautier, G.; Mussano, F.; Botticelli, D. Plasma of Argon Affects the Earliest Biological Response of Different Implant Surfaces. J. Dent. Res. 2016, 95, 566–573. [Google Scholar] [CrossRef]
- Rizo-Gorrita, M.; Luna-Oliva, I.; Serrera-Figallo, M.A.; Torres-Lagares, D. Superficial Characteristics of Titanium after Treatment of Chorreated Surface, Passive Acid, and Decontamination with Argon Plasma. J. Funct. Biomater. 2018, 9, 71. [Google Scholar] [CrossRef]
- ImageJ User Guide 1.46r. Available online: https://imagej.nih.gov/ij/docs/guide/user-guide.pdf (accessed on 2 October 2012).
- Hirota, M.; Ikeda, T.; Sugita, Y.; Ishijima, M.; Hirota, S.; Ogawa, T. Impaired osteoblastic behavior and function on saliva-contaminated titanium and its restoration by UV treatment. Mater. Sci. Eng. C. 2019, 100, 165–177. [Google Scholar] [CrossRef]
- Roy, M.; Pompella, A.; Kubacki, J.; Piosik, A.; Psiuk, B.; Klimontko, J.; Szade, J.; Roy, R.A.; Hedzelek, W. Photofunctionalization of dental zirconia oxide: Surface modification to improve bio-integration preserving crystal stability. Colloids Surf. B Biointerfaces 2017, 156, 194–202. [Google Scholar] [CrossRef]
- Alves, C.; Guerra Neto, C.L.B.; Morais, G.H.S.; Da Silva, C.F.; Hajek, V. Nitriding of titanium disks and industrial dental implants using hollow cathode discharge. Surf. Coat. Tech. 2005, 194, 196–202. [Google Scholar] [CrossRef]
- Choi, S.H.; Jeong, W.S.; Cha, J.Y.; Lee, J.H.; Yu, H.S.; Choi, E.H.; Kim, K.M.; Hwang, C.J. Time-dependent effects of ultraviolet and nonthermal atmospheric pressure plasma on the biological activity of titanium. Sci. Rep. 2016, 6, 33421. [Google Scholar] [CrossRef]
- Smeets, R.; Henningsen, A.; Heuberger, R.; Hanisch, O.; Schwarz, F.; Precht, C. Influence of UV irradiation and cold atmospheric pressure plasma on zirconia surfaces: an in vitro study. Int. J. Oral Maxillofac. Implants 2019, 34, 329–336. [Google Scholar] [CrossRef]
- Henningsen, A.; Smeets, R.; Heuberger, R.; Jung, O.T.; Hanken, H.; Heiland, M.; Cacaci, C.; Precht, C. Changes in surface characteristics of titanium and zirconia after surface treatment with ultraviolet light or non-thermal plasma. Eur. J. Oral Sci. 2018, 126, 126–134. [Google Scholar] [CrossRef]
- Coelho, P.G.; Giro, G.; Teixeira, H.S.; Marin, C.; Witek, L.; Thompson, V.P.; Tovar, N.; Silva, N.R.F.A. Argon-based atmospheric pressure plasma enhances early bone response to rough titanium surfaces. J. Biomed. Mater. Res. Part A. 2012, 100A, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- De Queiroz, J.D.; Leal, A.M.; Terada, M.; Agnez-Lima, L.F.; Costa, I.; Pinto, N.C.; De Medeiros, S.R. Surface modification by argon plasma treatment improves antioxidant defense ability of CHO-k1 cells on titanium surfaces. Toxicol. In Vitro 2014, 28, 381–387. [Google Scholar] [CrossRef]
- Tavares, J.C.M.; Cornélio, D.A.; Da Silva, N.B.; Bezerra de Moura, C.E.; De Queiroz, J.D.F.; Sá, J.C.; Junior, C.A.; De Medeiros, S.R.B. Effect of titanium surface modified by plasma energy source on genotoxic response in vitro. Toxicology. 2009, 262, 138–145. [Google Scholar] [CrossRef]
- Seon, G.M.; Seo, H.J.; Kwon, S.Y.; Lee, M.H.; Kwon, B.J.; Kim, M.S.; Koo, M.A.; Park, B.J.; Park, J.C. Titanium surface modification by using microwave-induced argon plasma in various conditions to enhance osteoblast biocompatibility. Biomater. Res. 2015, 19, 13. [Google Scholar] [CrossRef]
- Pistilli, R.; Genova, T.; Canullo, L.; Faga, M.G.; Terlizzi, M.E.; Gribaudo, G.; Mussano, F. Effect of Bioactivation on Traditional Surfaces and Zirconium Nitride: Adhesion and Proliferation of Preosteoblastic Cells and Bacteria. Int. J. Oral Maxillofac. Implants 2018, 33, 1247–1254. [Google Scholar] [CrossRef]
- Naujokat, H.; Harder, S.; Schulz, L.Y.; Wiltfang, J.; Flörke, C.; Açil, Y. Surface conditioning with cold argon plasma and its effect on the osseointegration of dental implants in miniature pigs. J. Craniomaxillofac. Surg. 2019, 47, 484–490. [Google Scholar] [CrossRef]
- Duske, K.; Jablonowski, L.; Koban, I.; Matthes, R.; Holtfreter, B.; Sckell, A. Cold atmospheric plasma in combination with mechanical treatment improves osteoblast growth on biofilm covered titanium discs. Biomaterials 2015, 52, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, H.S.; Marin, C.; Witek, L.; Freitas, A., Jr.; Silva, N.R.F.; Lilin, T.; Tovar, N.; Janal, M.N.; Coelho, P.G. Assessment of a chair-side argon-based non-thermal plasma treatment on the surface characteristics and integration of dental implants with textured surfaces. J. Mech. Behav. Biomed. Mater. 2012, 9, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Garcia, B.; Camacho, F.; Peñarrocha, D.; Tallarico, M.; Perez, S.; Canullo, L. Influence of plasma cleaning procedure on the interaction between soft tissue and abutments: a randomized controlled histologic study. Clin. Oral Implants Res. 2017, 28, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Penarrocha, D.; Micarelli, C.; Massidda, O.; Bazzoli, M. Hard tissue response to argon plasma cleaning/sterilisation of customised titanium abutments versus 5-second steam cleaning: results of a 2-year post-loading follow-up from an explanatory randomised controlled trial in periodontally healthy patients. Eur. J. Oral Implantol. 2013, 6, 251–260. [Google Scholar] [PubMed]
- Salido, M.; Vilches-Perez, J.I.; Gonzalez, J.L.; Vilches, J. Mitochondrial bioenergetics and distribution in living human osteoblasts grown on implant surfaces. Histol Histopathol. 2009, 24, 1275–1286. [Google Scholar]
- Johansson, C.B.; Han, C.H.; Wennerberg, A.; Albrektsson, T. A quantitative comparison of machined commercially pure titanium and titanium-aluminum-vanadium implants in rabbit bone. Int. J. Oral Maxillofac. Implants 1998, 13, 315–321. [Google Scholar] [PubMed]
- Shah, F.A.; Trobos, M.; Thomsen, P.; Palmquist, A. Commercially pure titanium (cp-Ti) versus titanium alloy (Ti6Al4V) materials as bone anchored implants - Is one truly better than the other? Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.C.; Rodrigues, R.C.; Rosa, A.L.; Ribeiro, R.F. Experimental titanium alloys for dental applications. J. Prosthet. Dent. 2014, 112, 1448–1460. [Google Scholar] [CrossRef]
- Czekanska, E.M.; Stoddart, M.J.; Richards, R.G.; Hayes, J.S. In search of an osteoblast cell model for in vitro research. Eur. Cell Mater. 2012, 9, 1–17. [Google Scholar] [CrossRef]
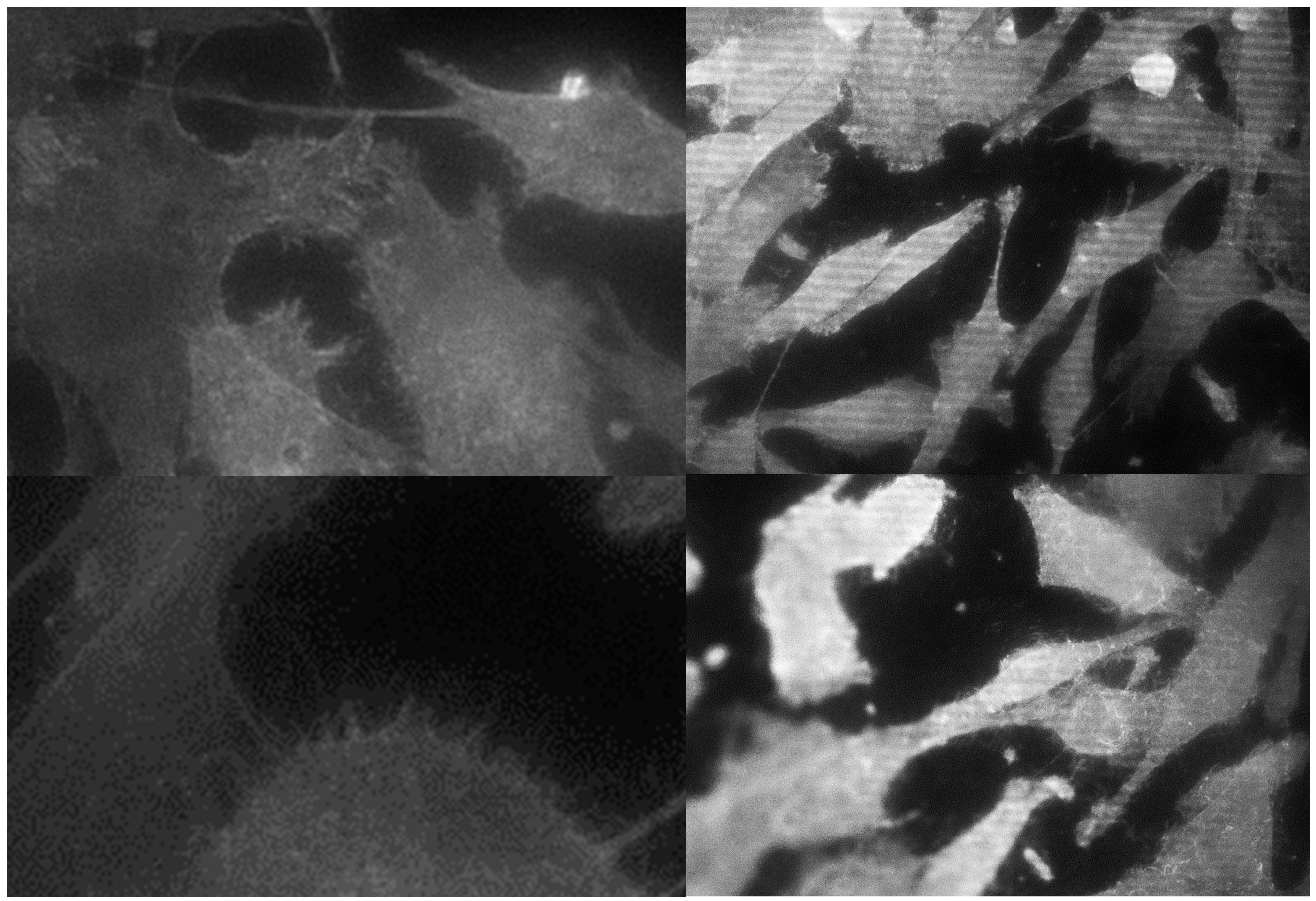
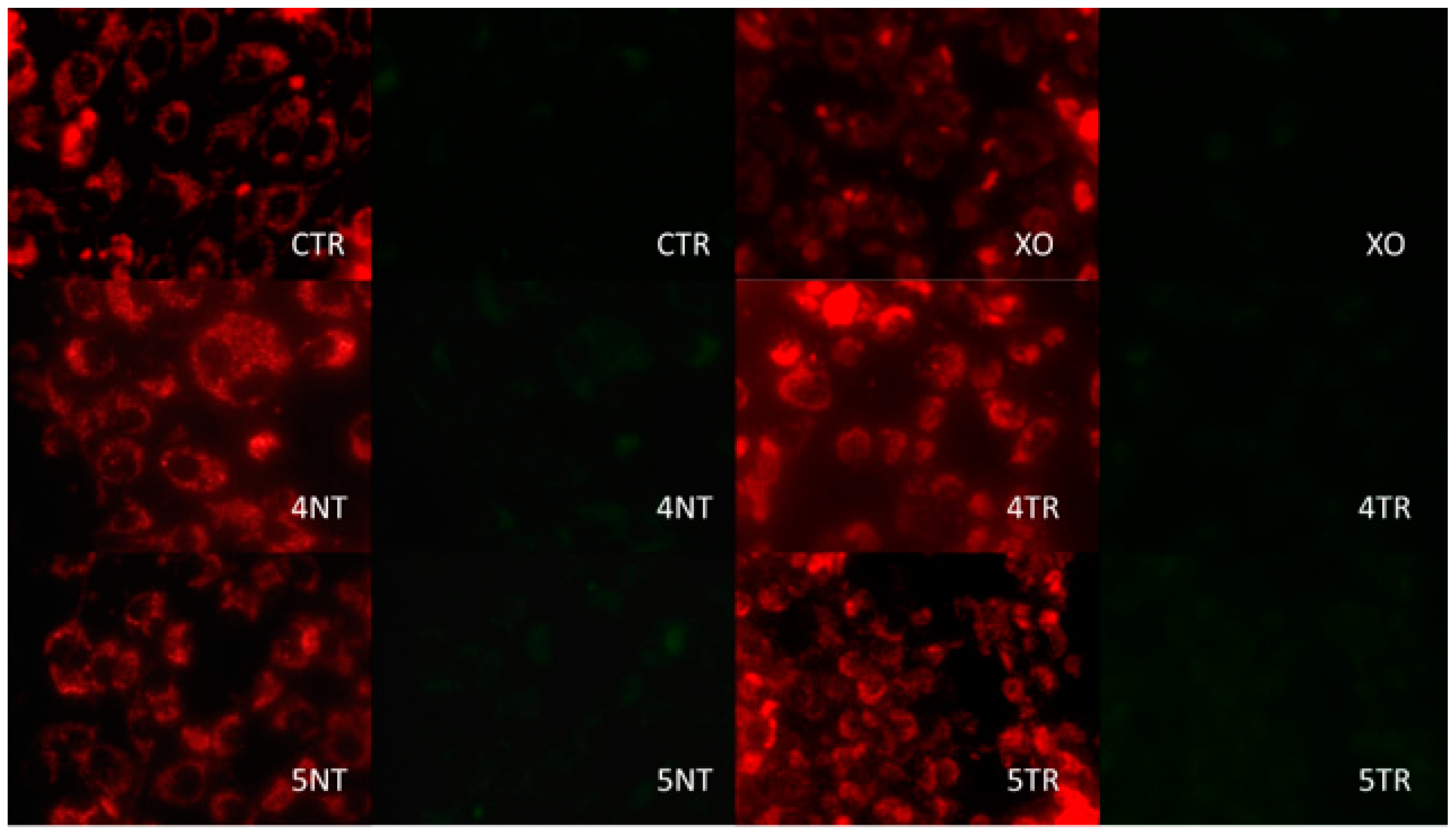
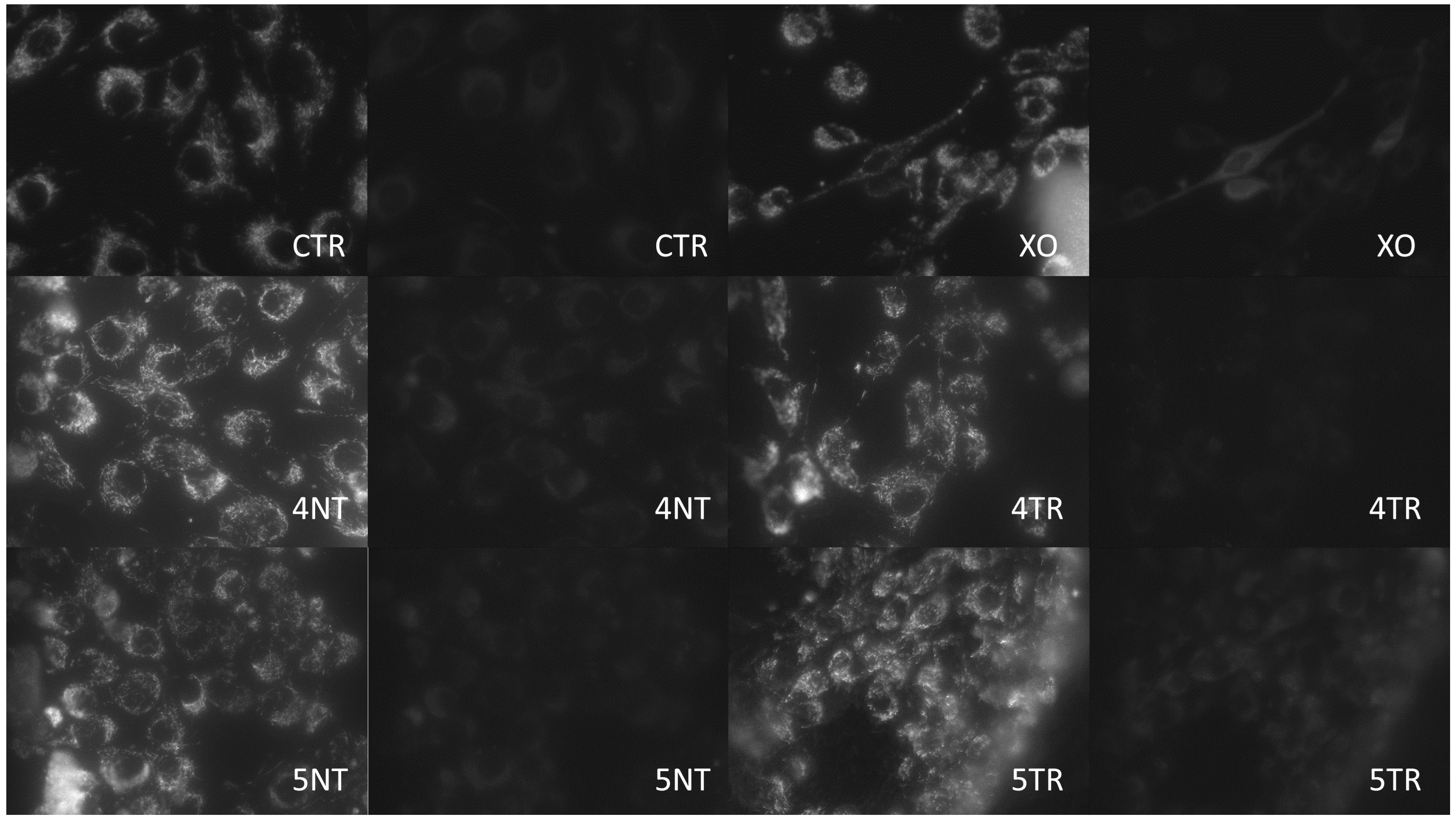
| Sample | Titanium IV (GR4NT) | Titanium IV (GR4TR) | Titanium V (GR5NT) | Titanium V (GR5TR) |
|---|---|---|---|---|
| 6 h | 38.7 ± 2.5% ab | 79.8 ± 15.2% a | 53.3 ± 4.0% | 87.6 ± 6.3% b |
| 24 h | 47.3 ± 8.1% ab | 79.1 ± 1.1% a | 77.0 ± 11.0% c | 91.3 ± 3.0% bc |
| 48 h | 86.0 ± 19.3% | 77.2 ± 2.5% | 93.0 ± 5.2% | 105.4 ± 3.5% |
| Cell Parameter | Titanium IV (GR4NT) | Titanium IV (GR4TR) | Titanium V (GR5NT) | Titanium V (GR5TR) |
|---|---|---|---|---|
| AREA (µm2) | 430 ± 208 a | 1212 ± 412 b | 525 ± 184 c | 1474 ± 425 abc |
| CIRCULARITY | 0.42 ± 0.02 ab | 0.38 ± 0.03 c | 0.30 ± 0.03 acd | 0.34 ± 0.01 b |
| Titanium IV (GR4NT) | Titanium IV (GR4TR) | Titanium V (GR5NT) | Titanium V (GR5TR) | Control (CTR) | Xanthine Oxidase (XO) | |
|---|---|---|---|---|---|---|
| JC 1 | 4.56 ± 0.58 abcd | 11.88 ± 0.60 a | 13.40 ± 1.04 be | 10.93 ± 2.66 cef | 13.2 ± 1.7 df | 3.7 ± 0.5 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Blanco, C.; Rizo-Gorrita, M.; Luna-Oliva, I.; Serrera-Figallo, M.-Á.; Torres-Lagares, D.; Gutiérrez-Pérez, J.-L. Human Osteoblast Cell Behaviour on Titanium Discs Treated with Argon Plasma. Materials 2019, 12, 1735. https://doi.org/10.3390/ma12111735
González-Blanco C, Rizo-Gorrita M, Luna-Oliva I, Serrera-Figallo M-Á, Torres-Lagares D, Gutiérrez-Pérez J-L. Human Osteoblast Cell Behaviour on Titanium Discs Treated with Argon Plasma. Materials. 2019; 12(11):1735. https://doi.org/10.3390/ma12111735
Chicago/Turabian StyleGonzález-Blanco, Carolina, María Rizo-Gorrita, Irene Luna-Oliva, María-Ángeles Serrera-Figallo, Daniel Torres-Lagares, and José-Luis Gutiérrez-Pérez. 2019. "Human Osteoblast Cell Behaviour on Titanium Discs Treated with Argon Plasma" Materials 12, no. 11: 1735. https://doi.org/10.3390/ma12111735
APA StyleGonzález-Blanco, C., Rizo-Gorrita, M., Luna-Oliva, I., Serrera-Figallo, M.-Á., Torres-Lagares, D., & Gutiérrez-Pérez, J.-L. (2019). Human Osteoblast Cell Behaviour on Titanium Discs Treated with Argon Plasma. Materials, 12(11), 1735. https://doi.org/10.3390/ma12111735







