Adsorptive Removal of Methylene Blue from Aquatic Environments Using Thiourea-Modified Poly(Acrylonitrile-co-Acrylic Acid)
Abstract
1. Introduction
2. Experimental
2.1. Materials and Chemicals
2.2. Instrumentation
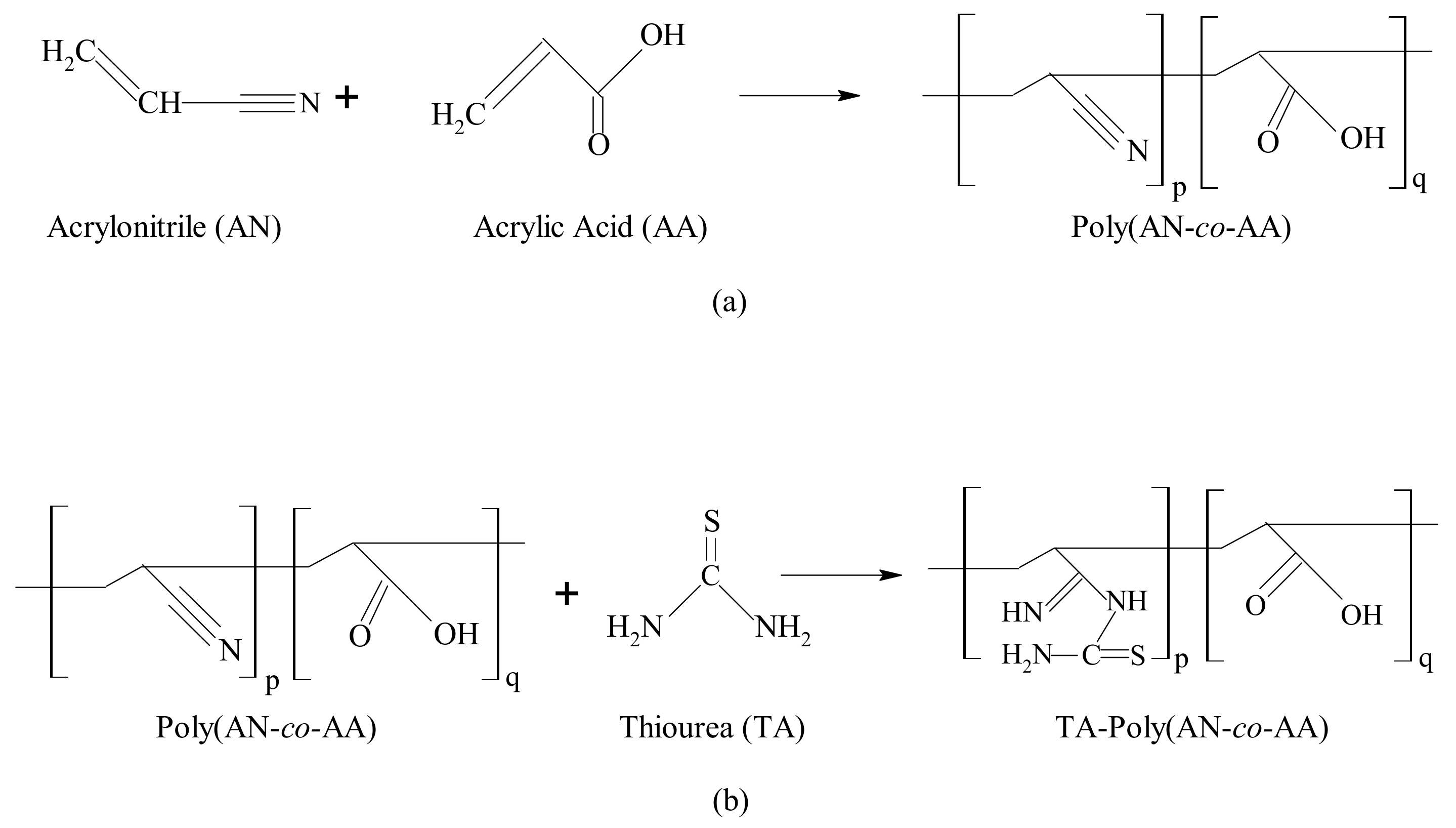
2.3. Synthesis of TA-poly(AN-co-AA)
2.4. Batch Adsorption Study
3. Results and Discussion
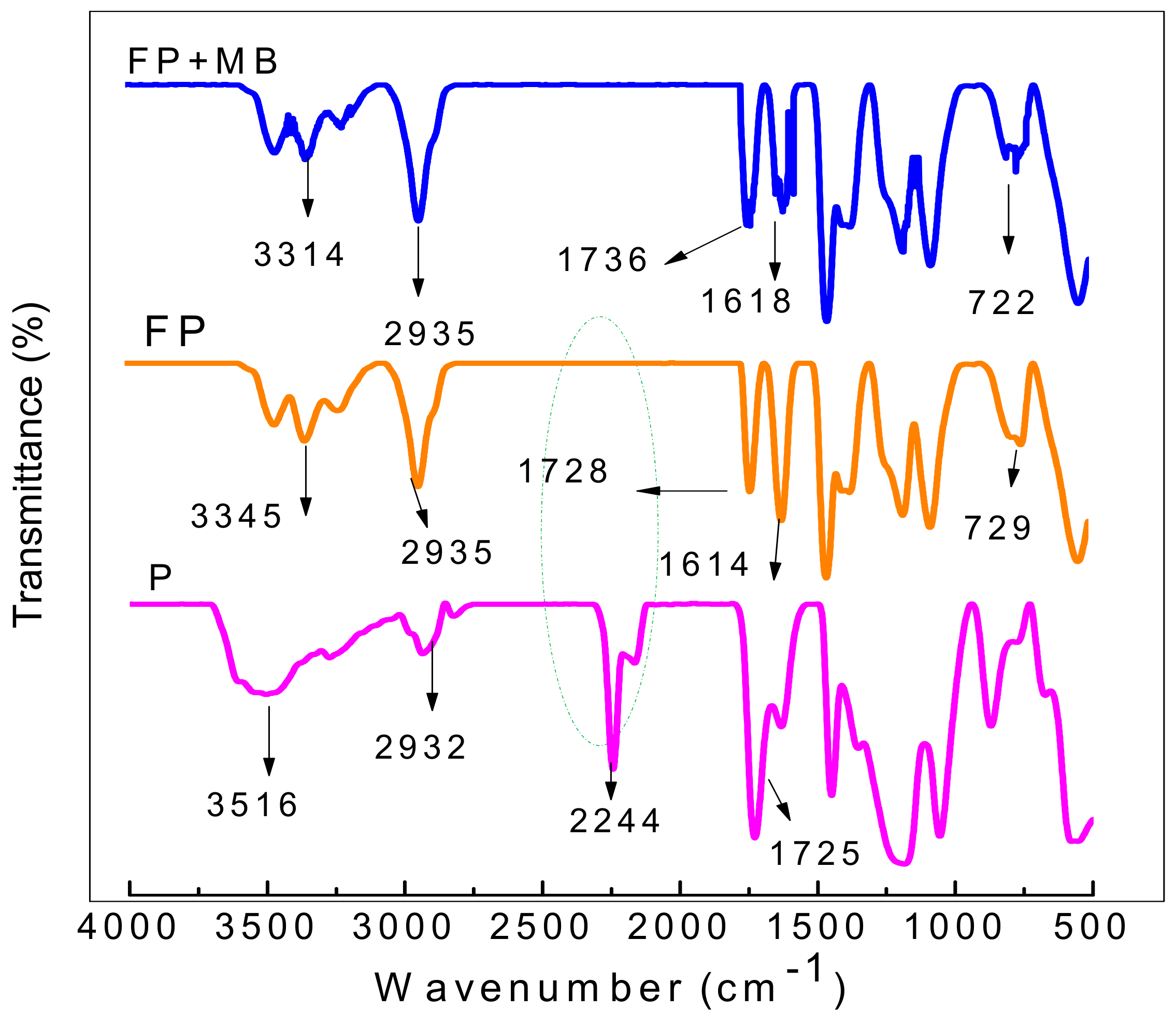
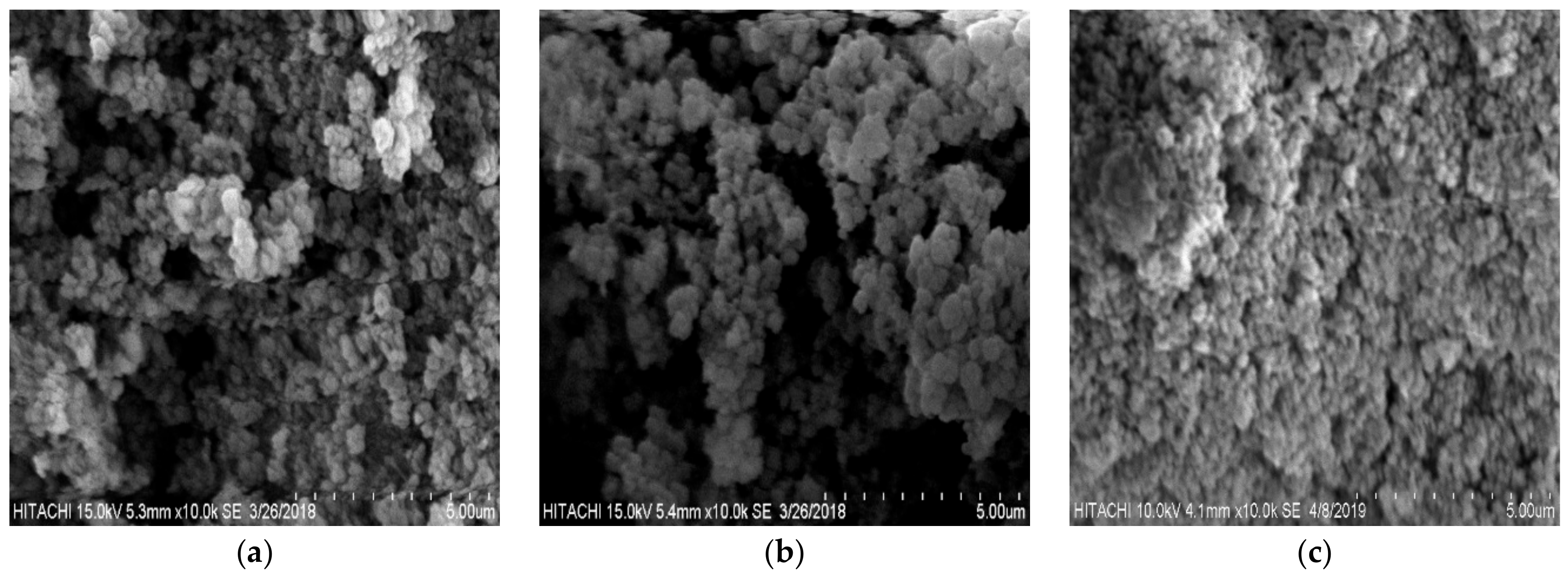
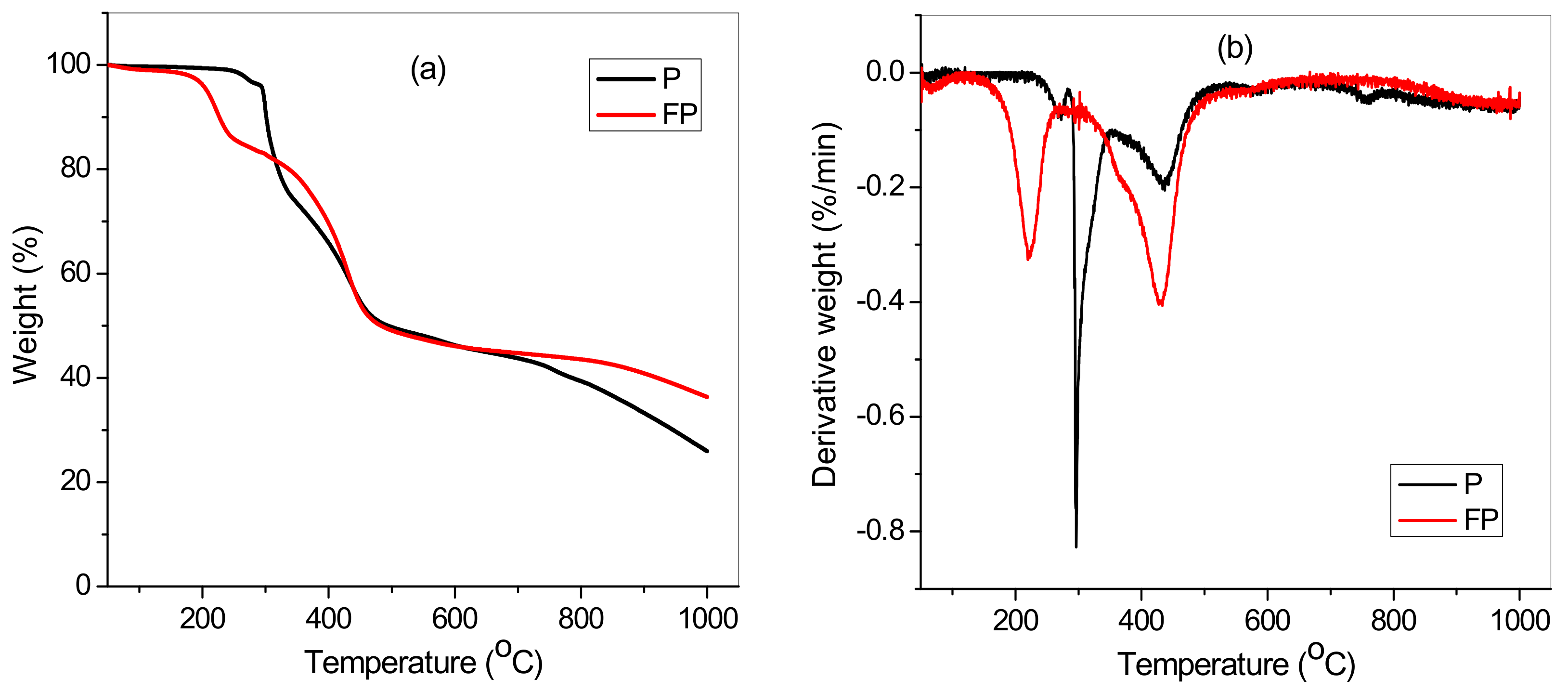
3.1. Characterization of TA Functionalized poly(AN-co-AA) Adsorbent
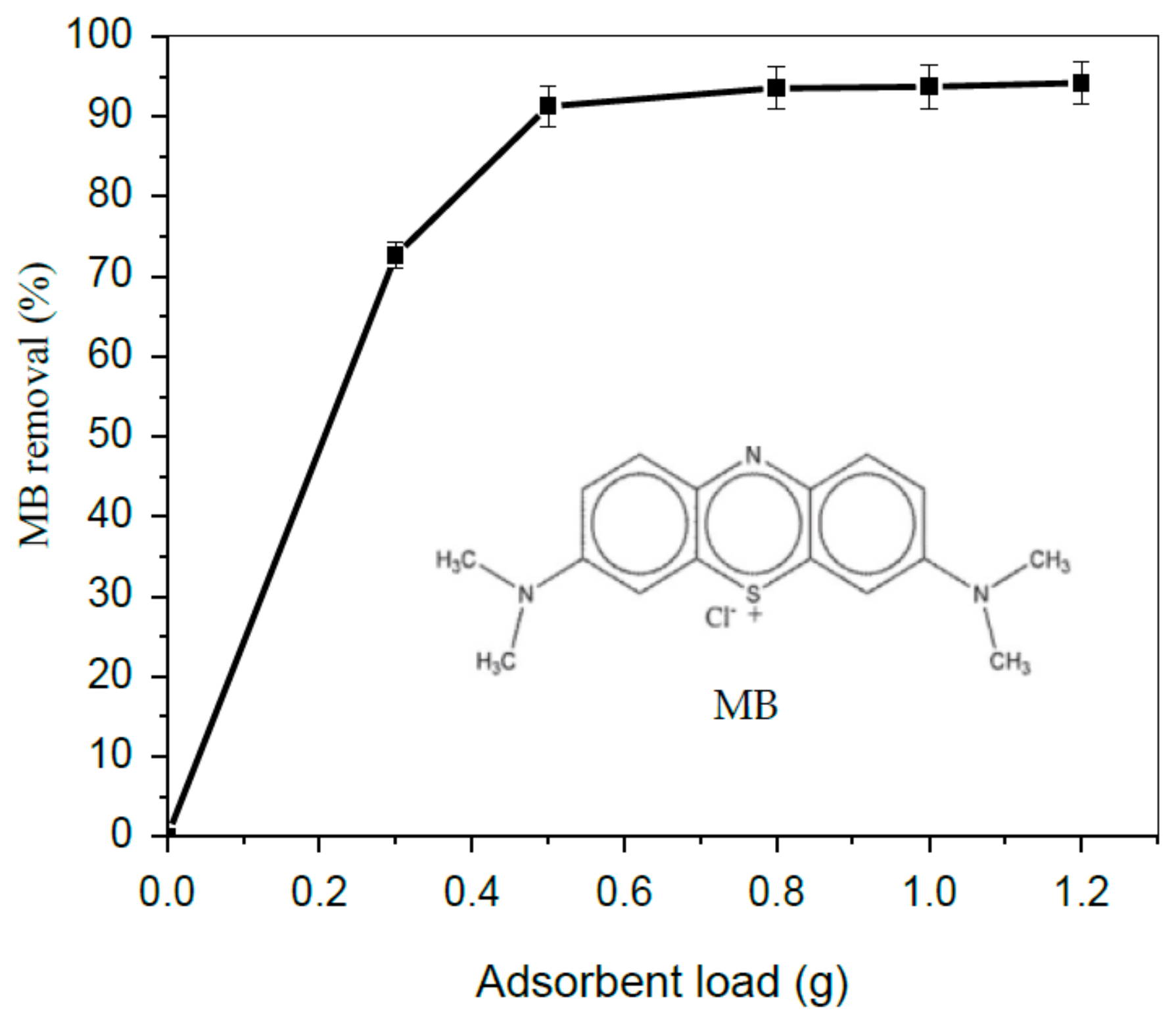
3.2. Effect TA-poly(AN-co-AA) Dose
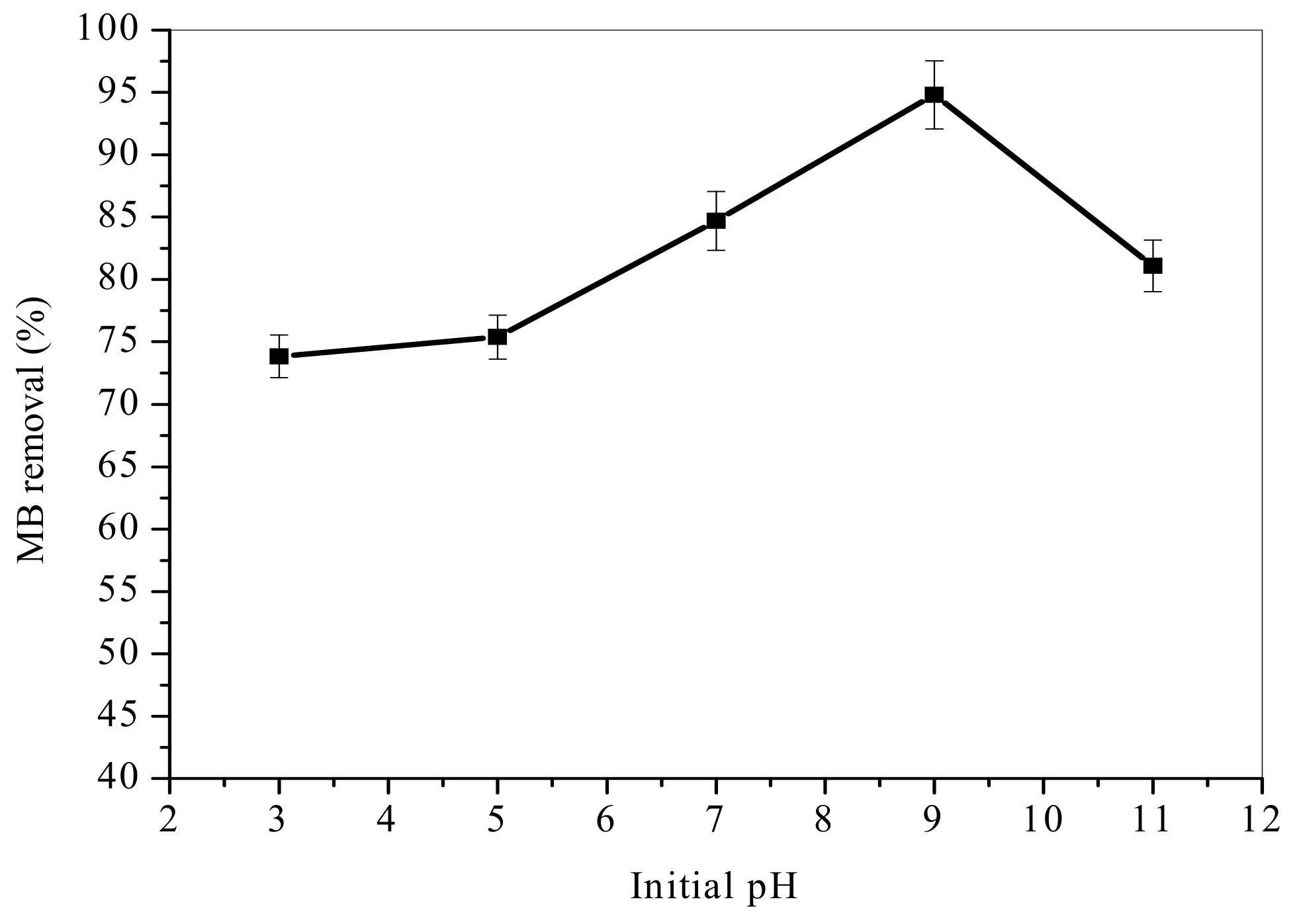
3.3. Effect of Initial pH
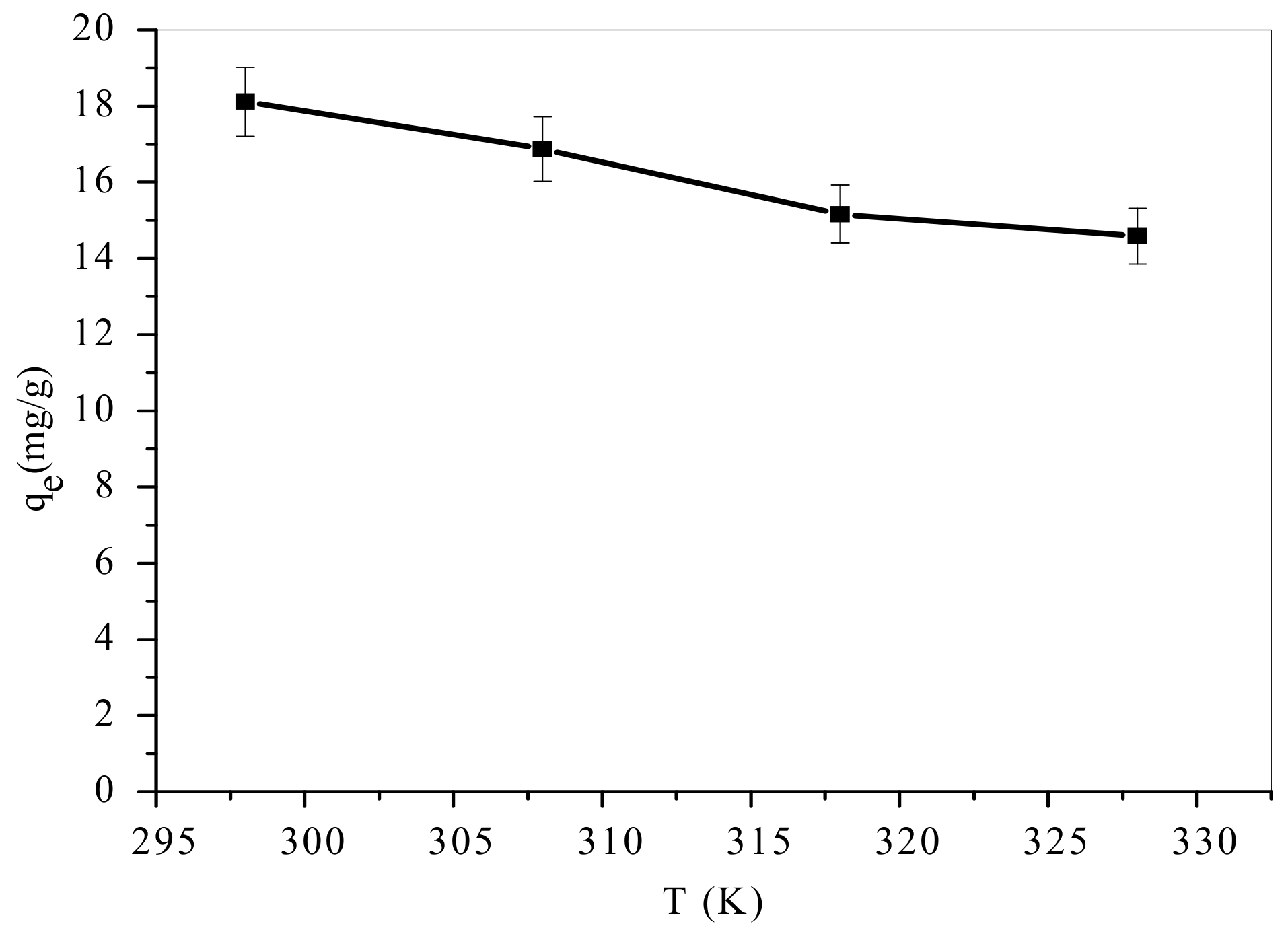
3.4. Influence of Temperature
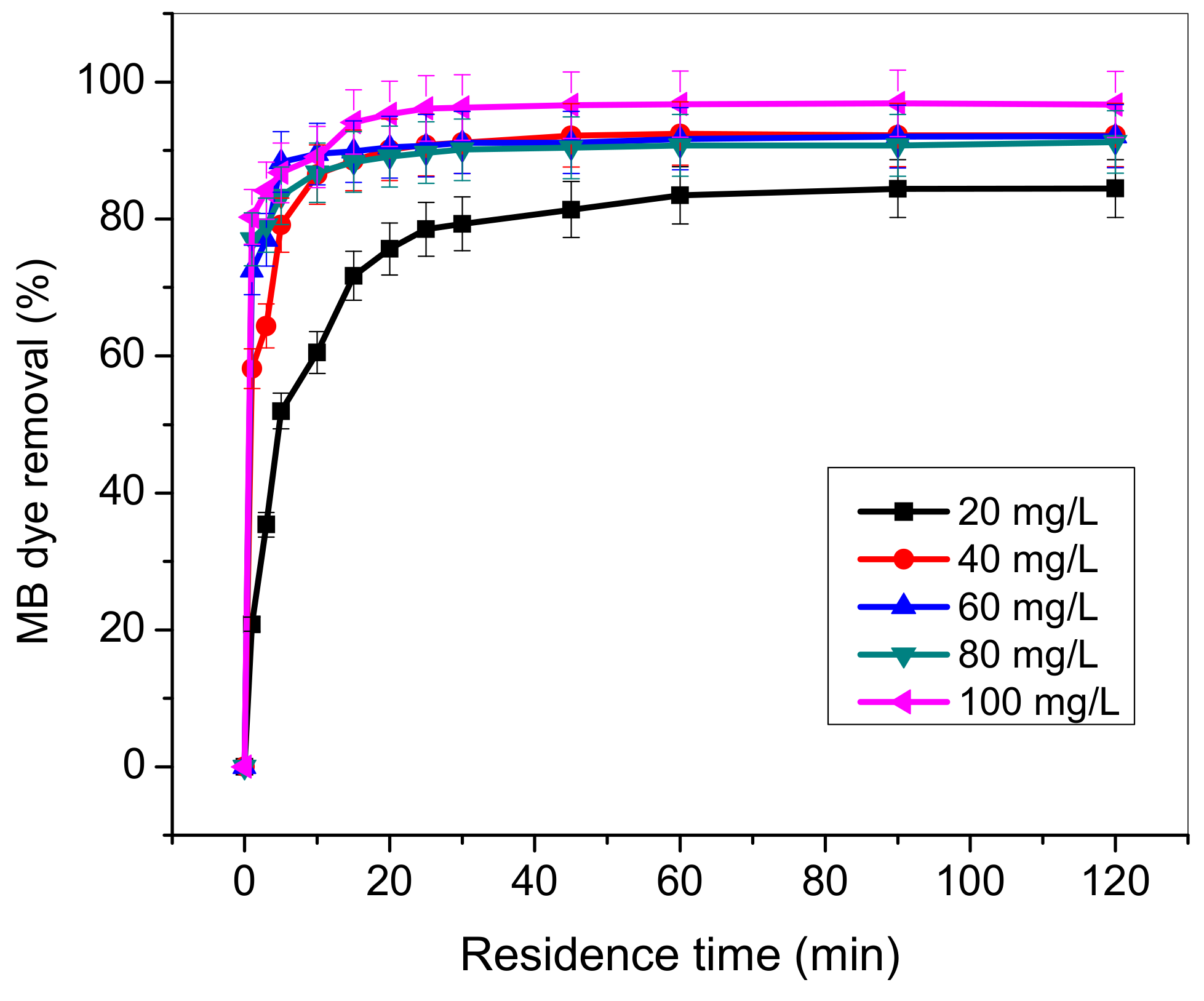
3.5. Influence of Residence Time and MB Concentration
3.6. Kinetic Studies
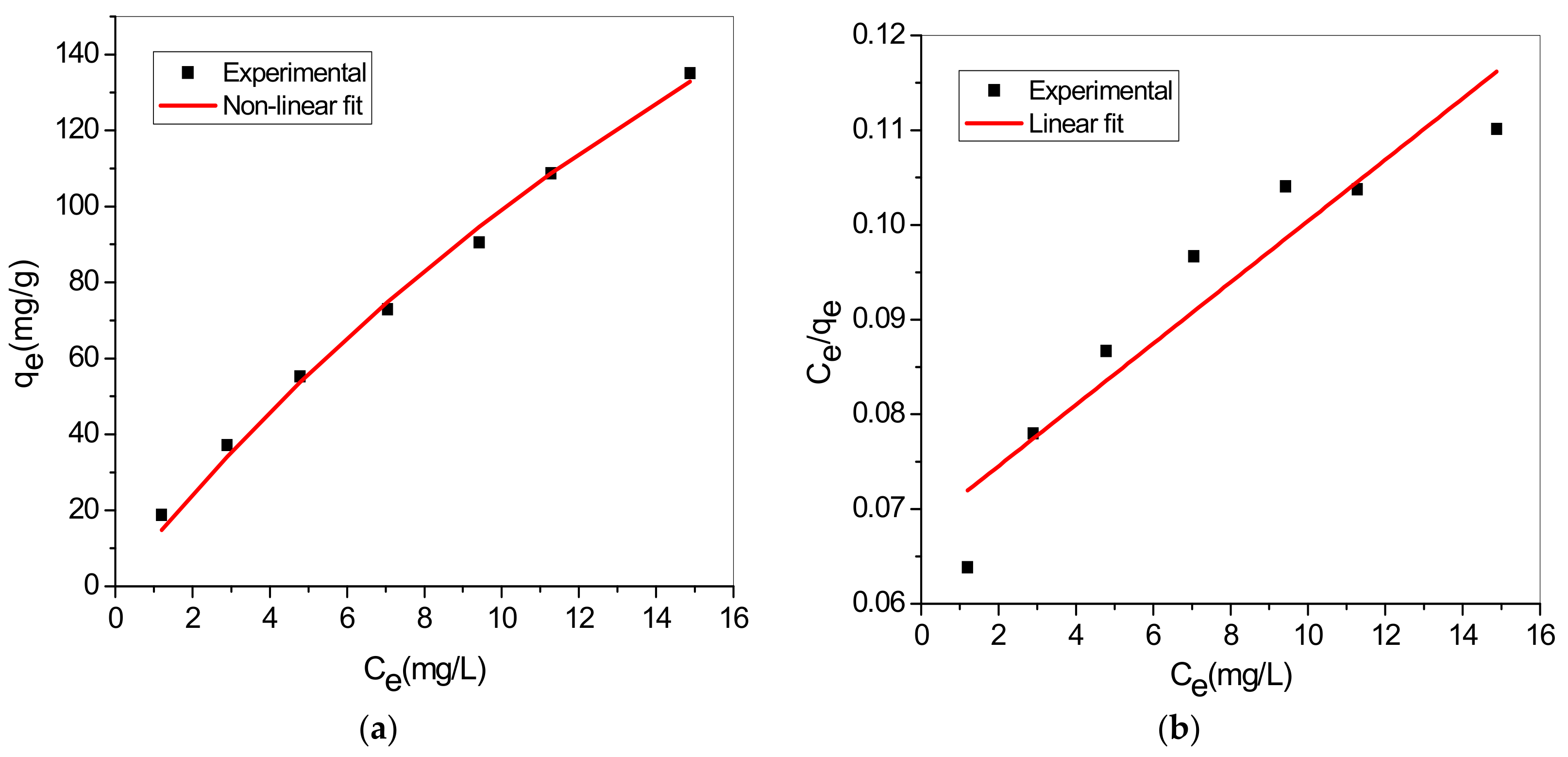
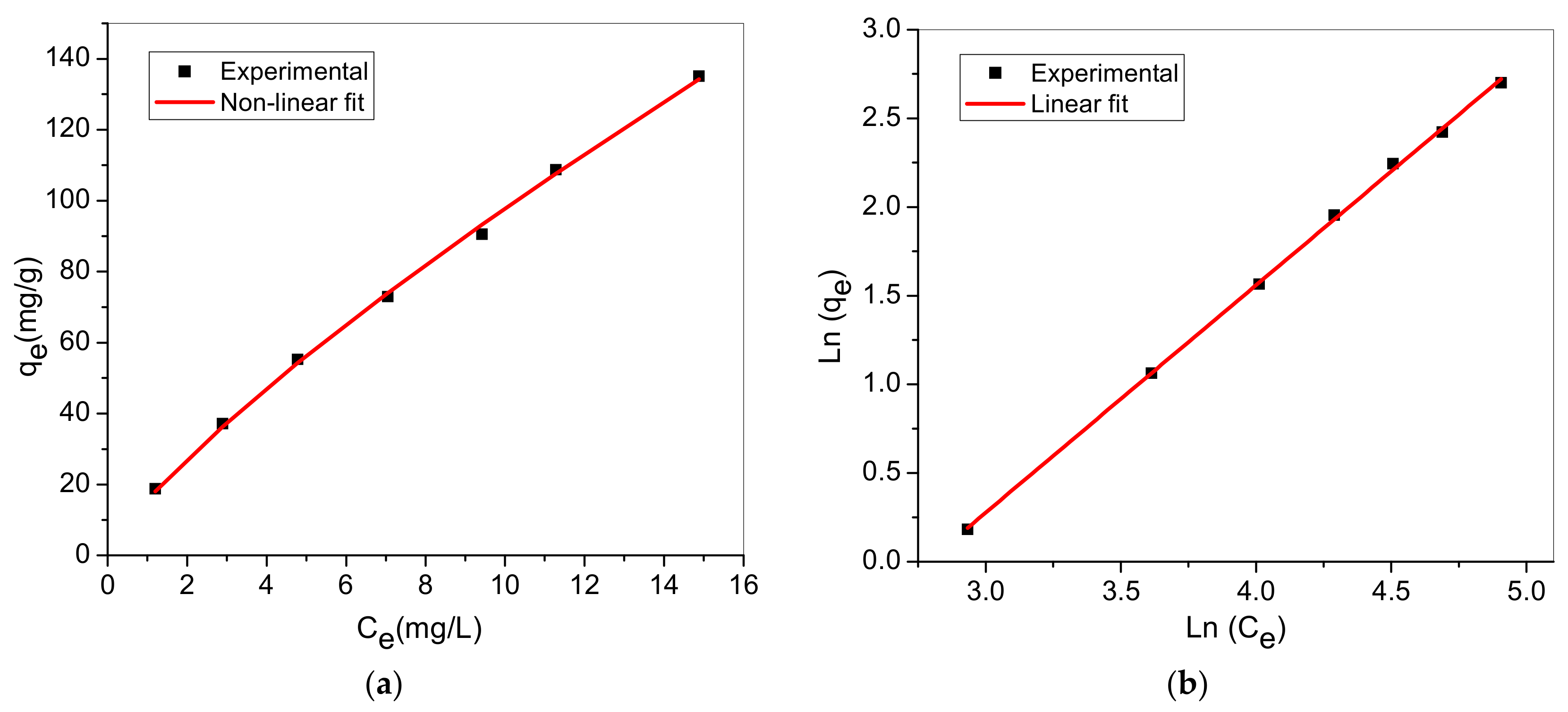
3.7. Adsorption Isotherm
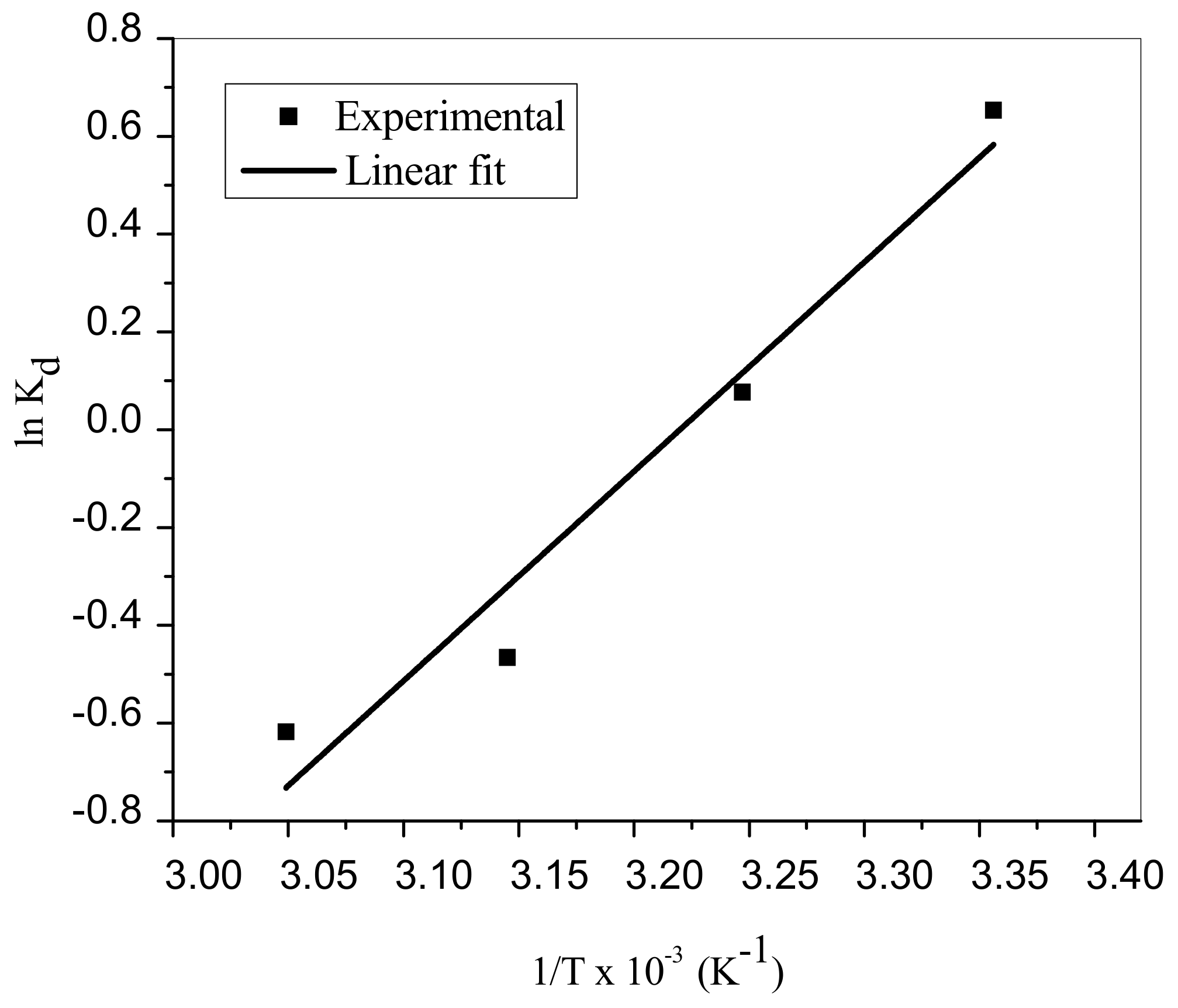
3.8. Thermodynamic Analyses
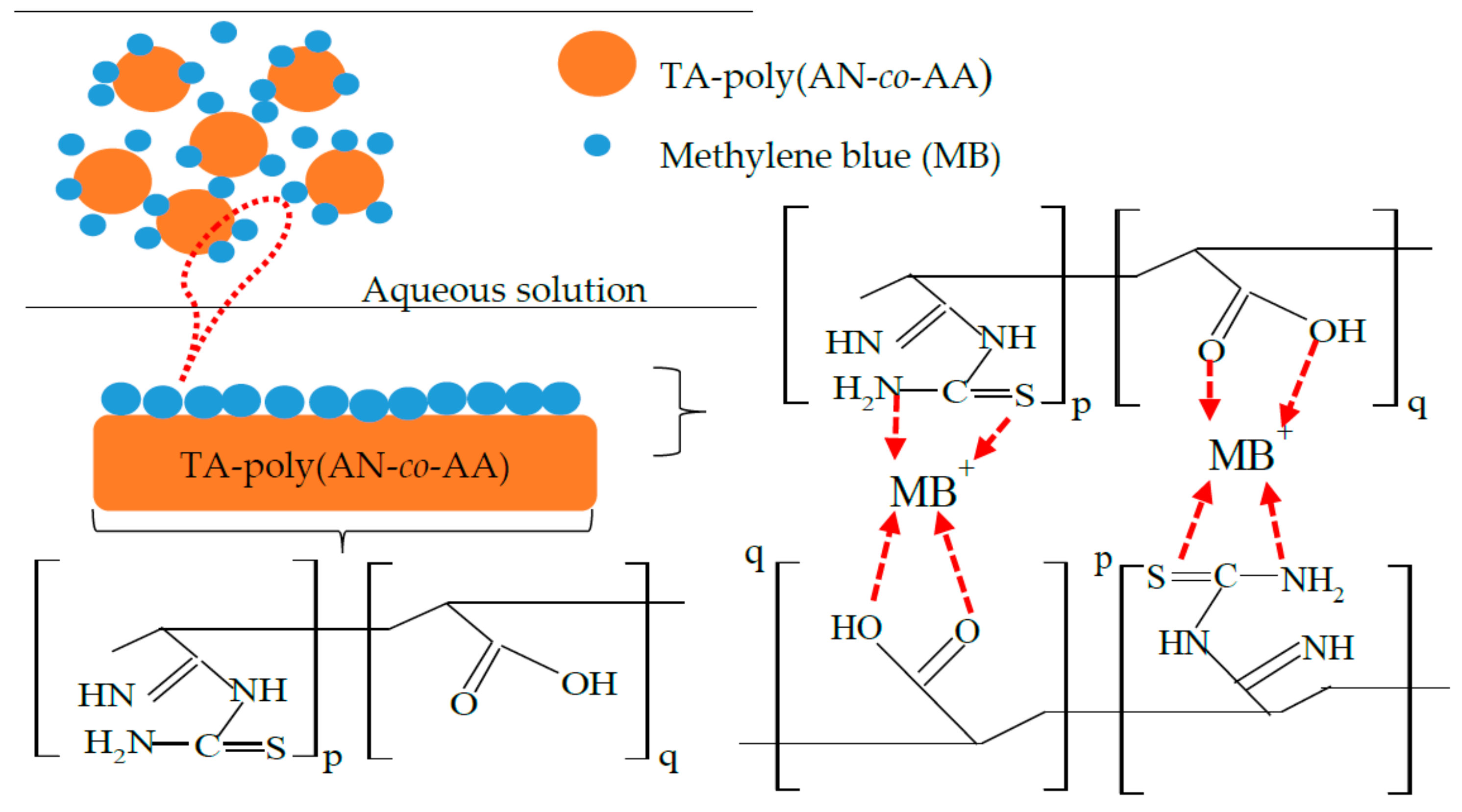
3.9. Mechanism of Adsorption
3.10. Regeneration and Reusability Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alaya, M.N.; Girgis, B.S.; Mourad, W.E. Activated Carbon from Some Agricultural Wastes Under Action of One-Step Steam Pyrolysis. J. Porous Mater. 2000, 7, 509–517. [Google Scholar] [CrossRef]
- Gupta, V.K. Suhas Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Hayati, B.; Mahmoodi, N.M.; Arami, M.; Mazaheri, F. Dye Removal from Colored Textile Wastewater by Poly (propylene imine) Dendrimer: Operational Parameters and Isotherm Studies. Clean Soil Air Water 2011, 39, 673–679. [Google Scholar] [CrossRef]
- Hayati, B.; Mahmoodi, N.M.; Maleki, A. Dendrimer-titania nanocomposite: Synthesis and dye-removal capacity. Res. Chem. Intermed. 2015, 41. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, M.; Huang, K.; Liu, Z. Textile dyeing wastewater treatment. Treat. Text. Effl. 2011, 91–116. [Google Scholar]
- Alaba, P.A.; Oladoja, N.A.; Sani, Y.M.; Bolarinwa, O.; Yakub, I.; Felix, S. Insight into wastewater decontamination using polymeric adsorbents. J. Environ. Chem. Eng. 2018, 6, 1651–1672. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Optimized and functionalized paper sludge activated with potassium fluoride for single and binary adsorption of reactive dyes. J. Ind. Eng. Chem. 2014, 20, 830–840. [Google Scholar] [CrossRef]
- Sengupta, R.; Chakraborty, S.; Bandyopadhyay, S.; Dasgupta, S.; Mukhopadhyay, R.; Auddy, K.; Deuri, S. A Short Review on Rubber/Clay Nanocomposites With Emphasis on Mechanical Properties. Engineering 2007, 47, 21–25. [Google Scholar] [CrossRef]
- Türgay, O.; Ersöz, G.; Atalay, S.; Forss, J.; Welander, U. The treatment of azo dyes found in textile industry wastewater by anaerobic biological method and chemical oxidation. Sep. Purif. Technol. 2011, 79, 26–33. [Google Scholar] [CrossRef]
- Vijayalakshmidevi, S.R.; Muthukumar, K. Improved biodegradation of textile dye effluent by coculture. Ecotoxicol. Environ. Saf. 2015, 114. [Google Scholar] [CrossRef]
- Natarajan, S.; Bajaj, H.C.; Tayade, R.J. Recent advances based on the synergetic effect of adsorption for removal of dyes from waste water using photocatalytic process. J. Environ. Sci. 2017, 65, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Naje, A.S.; Chelliapan, S.; Zakaria, Z.; Ajeel, M.A. A review of electrocoagulation technology for the treatment of textile wastewater. Rev. Chem. Eng. 2016, 1–30. [Google Scholar] [CrossRef]
- Sadaf, S.; Bhatti, H.N.; Nausheen, S.; Amin, M. Application of a novel lignocellulosic biomaterial for the removal of Direct Yellow 50 dye from aqueous solution: Batch and column study. J. Taiwan Inst. Chem. Eng. 2015, 47, 160–170. [Google Scholar] [CrossRef]
- Vieira, M.L.G.; Esquerdo, V.M.; Nobre, L.R.; Dotto, G.L.; Pinto, L.A.A. Glass beads coated with chitosan for the food azo dyes adsorption in a fixed bed column. J. Ind. Eng. Chem. 2014, 20, 3387–3393. [Google Scholar] [CrossRef]
- Atar, N.; Olgun, A.; Wang, S.; Liu, S. Adsorption of Anionic Dyes on Boron Industry Waste in Single and Binary Solutions Using Batch and Fixed-Bed Systems. J. Chem. Eng. Data. 2011, 508–516. [Google Scholar] [CrossRef]
- Varaprasad, K.; Jayaramudu, T.; Sadiku, E.R. Removal of dye by carboxymethyl cellulose, acrylamide and graphene oxide via a free radical polymerization process. Carbohydr. Polym. 2017, 164, 186–194. [Google Scholar] [CrossRef]
- Gupta, V.K.; Pathania, D.; Kothiyal, N.C.; Sharma, G. Polyaniline zirconium (IV) silicophosphate nanocomposite for remediation of methylene blue dye from waste water. J. Mol. Liq. 2014, 190, 139–145. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, J.; Fu, J.; Wang, M.; Wang, X.; Han, R.; Xu, Q. Adsorption of methylene blue onto poly(cyclotriphosphazene-co-4,4′-sulfonyldiphenol) nanotubes: Kinetics, isotherm and thermodynamics analysis. J. Hazard. Mater. 2014, 273, 263–271. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, X.; Huang, H.; Zhang, M.; Nie, C.; Lu, T.; Zhao, W.; Zhao, C. Core@shell poly (acrylic acid) microgels/polyethersulfone beads for dye uptake from wastewater. J. Environ. Chem. Eng. 2017, 5, 1732–1743. [Google Scholar] [CrossRef]
- Dalvand, A.; Nabizadeh, R.; Reza, M.; Khoobi, M. Modeling of Reactive Blue 19 azo dye removal from colored textile wastewater using L-arginine-functionalized Fe3O4 nanoparticles: Optimization, reusability, kinetic and equilibrium studies. J. Magn. Magn. Mater. 2016, 404, 179–189. [Google Scholar] [CrossRef]
- Zare, E.N.; Motahari, A.; Sillanpää, M. Nanoadsorbents based on conducting polymer nanocomposites with main focus on polyaniline and its derivatives for removal of heavy metal ions/dyes: A review. Environ. Res. 2018, 162. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.; Paliwal, K.; Gupta, A.K. Acrylonitrile-Acrylic Acids Copolymers; Synthesis and Characterization. J. Appl. Polym. Sci. 1993, 49, 823–833. [Google Scholar] [CrossRef]
- Yun, J.I.; Bhattarai, S.; Yun, Y.S.; Lee, Y.S. Synthesis of thiourea-immobilized polystyrene nanoparticles and their sorption behavior with respect to silver ions in aqueous phase. J. Hazard. Mater. 2018, 344, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.J.V.E.; da Silva Filho, E.C.; Melo, M.A.; Airoldi, C. Modified coupling agents based on thiourea, immobilized onto silica. Thermodynamics of copper adsorption. Surf. Sci. 2009, 603, 2200–2206. [Google Scholar] [CrossRef]
- Adeyi, A.A.; Jamil, S.N.A.M.; Abdullah, L.C.; Choong, T.S.Y. Adsorption of Malachite Green Dye from Liquid Phase Using Hydrophilic Thiourea-Modified Poly (acrylonitrile-co-acrylic acid): Kinetic and Isotherm Studies. J. Chem. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Zahri, N.A.M.; Jamil, S.N.A.M.; Abdullah, L.C.; Yaw, T.C.S.; Mobarekeh, M.N.; Huey, S.J.; Rapeia, N.S.M. Improved method for preparation of amidoxime modified poly(acrylonitrile-co-acrylic acid): Characterizations and adsorption case study. Polymers (Basel) 2015, 7, 1205–1220. [Google Scholar] [CrossRef]
- Morcali, M.H.; Zeytuncu, B. Investigation of adsorption parameters for platinum and palladium onto a modified polyacrylonitrile-based sorbent. Int. J. Miner. Process. 2015, 137, 52–58. [Google Scholar] [CrossRef]
- Huang, F.; Xu, Y.; Liao, S.; Yang, D.; Hsieh, Y.; Wei, Q. Preparation of Amidoxime Polyacrylonitrile Chelating Nanofibers and Their Application for Adsorption of Metal Ions. Materials 2013, 6, 969–980. [Google Scholar] [CrossRef]
- Hameed, K.S.; Muthirulan, P.; Sundaram, M.M. Adsorption of chromotrope dye onto activated carbons obtained from the seeds of various plants: Equilibrium and kinetics studies. Arabian J. Chem. 2017, 10, S2225–S2233. [Google Scholar] [CrossRef]
- Akbari, S.; Kish, M.H.; Entezami, A.A. Copolymer of acrylonitrile/acrylic acid film dendrigrafted with citric acid: Host/guest properties of dendrigraft/dye complexes in relation to acrylic acid content. Iran. Polym. J. (Engl. Ed.) 2011, 20, 539–549. [Google Scholar]
- Moghadam, S.S.; Bahrami, S.H. Copolymerization of acrylonitrile-acrylic acid in DMF-water mixture. Iran. Polym. J. (Engl. Ed.) 2005, 14, 1032–1041. [Google Scholar]
- Galicia-Aguilar, J.A.; Santamaría-Juárez, J.D.; López-Badillo, M.; Sánchez-Cantú, M.; Varela-Caselis, J.L. Synthesis and characterization of AN/EGDMA-based adsorbents for phenol adsorption. React. Funct. Polym. 2017, 117, 112–119. [Google Scholar] [CrossRef]
- Huang, J.; Turner, S.R. Recent advances in alternating copolymers: The synthesis, modification, and applications of precision polymers. Polymer 2017, 116, 572–586. [Google Scholar] [CrossRef]
- Mahmoodi, N.M. Nickel Ferrite Nanoparticle: Synthesis, Modification by Surfactant and Dye Removal Ability. Ind. Crop. Product. 2015, 42, 119–125. [Google Scholar] [CrossRef]
- Bhunia, P.; Chatterjee, S.; Rudra, P.; De, S. Chelating polyacrylonitrile beads for removal of lead and cadmium from wastewater. Sep. Purif. Technol. 2018, 193, 202–213. [Google Scholar] [CrossRef]
- Rahaman, M.S.A.; Ismail, A.F.; Mustafa, A. A review of heat treatment on polyacrylonitrile fiber. Polym. Degr. Stab. 2007, 92, 1421–1432. [Google Scholar] [CrossRef]
- Hatton, F.L.; Malmström, E.; Carlmark, A. Tailor-made copolymers for the adsorption to cellulosic surfaces. Eur. Polym. J. 2015, 65, 325–339. [Google Scholar] [CrossRef]
- Catherine, H.N.; Ou, M.H.; Manu, B.; Shih, Y. Adsorption mechanism of emerging and conventional phenolic compounds on graphene oxide nanoflakes in water. Sci. Total Environ. 2018, 635, 629–638. [Google Scholar] [CrossRef]
- Akter, N.; Hossain, M.A.; Hassan, M.J.; Amin, M.K.; Elias, M.; Rahman, M.M.; Asiri, A.M.; Siddiquey, I.A.; Hasnat, M.A. Amine modified tannin gel for adsorptive removal of Brilliant Green dye. J. Environ. Chem. Eng. 2016, 4, 1231–1241. [Google Scholar] [CrossRef]
- Belhouchat, N.; Zaghouane-boudiaf, H.; Viseras, C. Removal of anionic and cationic dyes from aqueous solution with activated organo-bentonite/sodium alginate encapsulated beads. Appl. Clay Sci. 2017, 135, 9–15. [Google Scholar] [CrossRef]
- Jana, S.; Ray, J.; Mondal, B.; Pradhan, S.S.; Tripathy, T. pH responsive adsorption/desorption studies of organic dyes from their aqueous solutions by katira gum-cl-poly (acrylic acid-co-N-vinyl imidazole) hydrogel. Colloid. Surf. A 2018, 553, 472–486. [Google Scholar] [CrossRef]
- Mallakpour, S.; Rashidimoghadam, S. Poly (vinyl alcohol)/Vitamin C-multi walled carbon nanotubes composites and their applications for removal of methylene blue: Advanced comparison between linear and nonlinear forms of adsorption isotherms and kinetics models. J. Polym. 2019, 160, 115–125. [Google Scholar] [CrossRef]
- Mishra, A.K.; Agrawal, N.R.; Das, I. Synthesis of water dispersible dendritic amino acid modified polythiophenes as highly effective adsorbent for removal of methylene blue. J. Environ. Chem. Eng. 2017, 5, 4923–4936. [Google Scholar] [CrossRef]
- Viana, F.; Dutra, A.; Pires, B.C.; Nascimento, T.A. Functional polyaniline/multiwalled carbon nanotube composite as an e ffi cient adsorbent material for removing pharmaceuticals from aqueous media. J. Environ. Manag. 2018, 221, 28–37. [Google Scholar]
- Huang, Z.; Li, Y.; Chen, W.; Shi, J.; Zhang, N.; Wang, X.; Li, Z.; Gao, L.; Zhang, Y. Modified bentonite adsorption of organic pollutants of dye wastewater. Mater. Chem. Phys. 2017, 202, 266–276. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Alothman, Z.A.; Ahamad, T. Adsorptive performance of MOF nanocomposite for methylene blue and malachite green dyes: Kinetics, isotherm and mechanism. J. Environ. Manag. 2018, 223, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Sandeep, N. Effect of aligned magnetic field on liquid thin film flow of magnetic-nanofluids embedded with graphene nanoparticles. Adv. Powd. Technol. 2017, 28, 865–875. [Google Scholar] [CrossRef]
- Zhang, D.S.X.; Liu, Y.W.T. Kinetic mechanism of competitive adsorption of disperse dye and anionic dye on fly ash. Int. J. Environ. Sci. Technol. 2013, 799–808. [Google Scholar]
- Joseph, N.T.; Chinonye, O.E.; Philomena, I.K.; Christian, A.C.; Elijah, O.C. Isotherm and kinetic modeling of adsorption of dyestuffs onto kola nut (Cola acuminata) shell activated carbon. J. Chem. Technol. Metallurg. 2016, 51, 188–201. [Google Scholar]
- Nethaji, S.; Sivasamy, A.; Mandal, A.B. Adsorption isotherms, kinetics and mechanism for the adsorption of cationic and anionic dyes onto carbonaceous particles prepared from Juglans regia shell biomass. Int. J. Environ. Sci. Technol. 2013, 10, 231–242. [Google Scholar] [CrossRef]
- Huo, Y.; Wu, H.; Wang, Z.; Wang, F.; Liu, Y.; Feng, Y.; Zhao, Y. Preparation of core/shell nanocomposite adsorbents based on amine polymer-modified magnetic materials for the effi cient adsorption of anionic dyes. Colloid. Surf. A 2018, 549, 174–183. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Ghezelbash, M.; Shabanian, M.; Aryanasab, F.; Saeb, M.R. Efficient removal of cationic dyes from colored wastewaters by dithiocarbamate-functionalized graphene oxide nanosheets: From synthesis to detailed kinetics studies. J. Taiwan Inst. Chem. Eng. 2017, 81, 239–246. [Google Scholar] [CrossRef]
- Jayalakshmi, R.; Jeyanthi, J. Simultaneous removal of binary dye from textile effluent using cobalt ferrite-alginate nanocomposite: Performance and mechanism. Microchem. J. 2019, 145, 791–800. [Google Scholar]
- Ahamad, K.U.; Singh, R.; Baruah, I.; Choudhury, H.; Sharma, M.R. Equilibrium and kinetics modeling of fl uoride adsorption onto activated alumina, alum and brick powder. Groundw. Sustain. Dev. 2018, 7, 452–458. [Google Scholar] [CrossRef]
- Gehlot, G.; Verma, S.; Sharma, S.; Mehta, N. Adsorption Isotherm Studies in the Removal of Malachite Green Dye from Aqueous Solution by Using Coal Fly Ash. Int. J. Chem. Stud. 2015, 3, 42–44. [Google Scholar]
- Jarrah, N. Competitive adsorption isotherms of rhodium 6G and methylene blue on activated carbon prepared from residual fuel oil. J. Environ. Chem. Eng. 2017, 5, 4319–4326. [Google Scholar] [CrossRef]
- Abdel-Ghani, N.T.; Rawash, E.S.A.; El-Chaghaby, G.A. Equilibrium and kinetic study for the adsorption of p-nitrophenol from wastewater using olive cake based activated carbon. Glob. J. Environ. Sci. Manag. 2016, 2, 11–18. [Google Scholar]
- Fu, J.; Chen, Z.; Wu, X.; Wang, M.; Wang, X.; Zhang, J.; Zhang, J. Hollow poly (cyclotriphosphazene-co-phloroglucinol) microspheres: An effective and selective adsorbent for the removal of cationic dyes from aqueous solution. Chem. Eng. J. 2015, 281, 42–52. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Z.; Wang, M.; Liu, S.; Zhang, J.; Zhang, J.; Han, R.; Xu, Q. Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): Kinetics, isotherm, thermodynamics and mechanism analysis. Chem. Eng. J. 2015, 259, 53–61. [Google Scholar] [CrossRef]
- Li, C.; Xiong, Z.; Zhang, J.; Wu, C. The Strengthening Role of the Amino Group in Metal—Organic Framework MIL-53 (Al) for Methylene Blue and Malachite Green Dye Adsorption. J. Chem. Eng. Data. 2015, 60, 3414–3422. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Zhang, Y.; Tang, S.; Guo, C.; Wu, J.; Lau, R. Anionic and cationic dyes adsorption on porous poly-melamine-formaldehyde polymer. Chem. Eng. Res. Des. 2016, 114, 258–267. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.; Chao, H. Thermodynamic parameters of cadmium adsorption onto orange peel calculated from various methods: A comparison study. Biochem. Pharmacol. 2016, 4, 2671–2682. [Google Scholar] [CrossRef]
- Rodeghero, E.; Martucci, A.; Cruciani, G.; Sarti, E.; Cavazzini, A.; Costa, V.; Bagatin, R.; Pasti, L. Detailed Investigation of Thermal Regeneration of High-silica ZSM-5 Zeolite through in situ Synchrotron X-ray Powder Diffraction and Adsorption Studies. J. Phys. Chem. C 2017, 121, 17958–17968. [Google Scholar] [CrossRef]
- Amer, W.A.; Omran, M.M.; Ayad, M.M. Acid-free synthesis of polyaniline nanotubes for dual removal of organic dyes from aqueous solutions. Colloid. Surf. A 2019, 562, 203–212. [Google Scholar] [CrossRef]
| Sample/Element | C (wt.%) | H (wt.%) | N (wt.%) | S (wt.%) |
|---|---|---|---|---|
| Poly(AN-co-AA) | 54.19 | 5.27 | 20.83 | 2.51 |
| TA-poly(AN-co-AA) | 61.94 | 5.62 | 25.06 | 3.09 |
| Samples | Surface Area (m2/g) | Mean Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| Poly(AN-co-AA) (P) | 22.99 | 0.241 | 41.60 |
| TA-Poly(AN-co-AA) (FP) | 26.31 | 0.158 | 47.23 |
| Models | Parameters | ||||
|---|---|---|---|---|---|
| PFO | (mg/L) | (min−1) | (mg/g) | (mg/g) | |
| 20 | 0.0484 | 3.76 | 1.32 | 0.3348 | |
| 40 | 0.0445 | 7.42 | 1.45 | 0.4390 | |
| 60 | 0.0737 | 11.04 | 2.99 | 0.6677 | |
| 80 | 0.0489 | 14.59 | 2.54 | 0.4466 | |
| 100 | 0.0673 | 18.12 | 4.86 | 0.8413 | |
| PSO | (mg/L) | (mg/(g·min)) | (mg/g) | (mg/g) | |
| 20 | 0.0559 | 3.76 | 3.89 | 0.9991 | |
| 40 | 0.0738 | 7.42 | 7.51 | 0.9997 | |
| 60 | 0.0492 | 11.04 | 11.22 | 0.9995 | |
| 80 | 0.0430 | 14.59 | 14.75 | 0.9996 | |
| 100 | 0.0341 | 18.12 | 18.36 | 0.9996 | |
| Elovich | (mg/L) | (g/mg) | (mg/(g·min)) | ||
| 20 | 1.5032 | 2.73 | 0.9502 | ||
| 40 | 1.3492 | 242.33 | 0.9422 | ||
| 60 | 1.1062 | 3297.24 | 0.9371 | ||
| 80 | 1.1106 | 106999.96 | 0.9291 | ||
| 100 | 0.9058 | 165776.93 | 0.9553 | ||
| IPD | (mg/L) | (mg/(g·min1/2)) | (mg/g) | ||
| 20 | 0.2719 | 1.45 | 0.7225 | ||
| 40 | 0.3025 | 4.86 | 0.7140 | ||
| 60 | 0.3467 | 8.02 | 0.8357 | ||
| 80 | 0.3978 | 11.06 | 0.8413 | ||
| 100 | 0.4845 | 13.84 | 0.8533 | ||
| Isotherm | Nonlinear | Linear | Parameters |
|---|---|---|---|
| Langmuir | : monolayer capacity : constant | ||
| Freundlich | : Freundlich constant : adsorption intensity |
| Model | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| Parameters | (L/mg) | (mg/g) | (L/mg) | |||
| Non-linear | 0.0290 | 440.81 | 0.9891 | 15.561 | 1.253 | 0.9952 |
| Linear | 0.0476 | 308.64 | 0.8712 | 0.028 | 1.283 | 0.9993 |
| Adsorbents | References | ||
|---|---|---|---|
| Linear | Nonlinear | ||
| Poly(acrylic acid)/polyethersulfone composite | 84.82 | 129.01 | [19] |
| Poly(cyclotriphosphazene-co-phloroglucinol) | 50.7 | - | [58] |
| Polydopamine microspheres | 161.29 | - | [59] |
| Amino group in metal organic frameworks (MIL-53(Al)-NH2) | - | 188.6 | [60] |
| Poly-melamine-formaldehyde polymer | 80.8 | - | [61] |
| Glutaraldehyde-crosslinked poly(vinyl alcohol)/vitamin C-CNTs composite | 16.84 | 18.36 | [42] |
| Dithiocarbamate-functionalized graphene oxide | 137 | - | [52] |
| TA-poly(AN-co-AA) | 308.64 | 440.82 | This work |
| Temperature (K) | Parameters | |||
|---|---|---|---|---|
| 298 | 1.9220 | −1.443 | −35.588 | −114.58 |
| 308 | 1.0793 | −0.297 | - | - |
| 318 | 0.6274 | 0.848 | - | - |
| 328 | 0.5388 | 1.994 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeyi, A.A.; Jamil, S.N.A.M.; Abdullah, L.C.; Choong, T.S.Y.; Lau, K.L.; Abdullah, M. Adsorptive Removal of Methylene Blue from Aquatic Environments Using Thiourea-Modified Poly(Acrylonitrile-co-Acrylic Acid). Materials 2019, 12, 1734. https://doi.org/10.3390/ma12111734
Adeyi AA, Jamil SNAM, Abdullah LC, Choong TSY, Lau KL, Abdullah M. Adsorptive Removal of Methylene Blue from Aquatic Environments Using Thiourea-Modified Poly(Acrylonitrile-co-Acrylic Acid). Materials. 2019; 12(11):1734. https://doi.org/10.3390/ma12111734
Chicago/Turabian StyleAdeyi, Abel Adekanmi, Siti Nurul Ain Md Jamil, Luqman Chuah Abdullah, Thomas Shean Yaw Choong, Kia Li Lau, and Mohammad Abdullah. 2019. "Adsorptive Removal of Methylene Blue from Aquatic Environments Using Thiourea-Modified Poly(Acrylonitrile-co-Acrylic Acid)" Materials 12, no. 11: 1734. https://doi.org/10.3390/ma12111734
APA StyleAdeyi, A. A., Jamil, S. N. A. M., Abdullah, L. C., Choong, T. S. Y., Lau, K. L., & Abdullah, M. (2019). Adsorptive Removal of Methylene Blue from Aquatic Environments Using Thiourea-Modified Poly(Acrylonitrile-co-Acrylic Acid). Materials, 12(11), 1734. https://doi.org/10.3390/ma12111734







