Development and Characterization of Mechanically Durable Silicone-Polythiourethane Composites Modified with Tetrapodal Shaped ZnO Particles for the Potential Application as Fouling-Release Coating in the Marine Sector
Abstract
1. Introduction
2. Materials and Experiments
2.1. Materials
2.2. Sample Preparation
2.3. Instrumentations
3. Results and Discussion
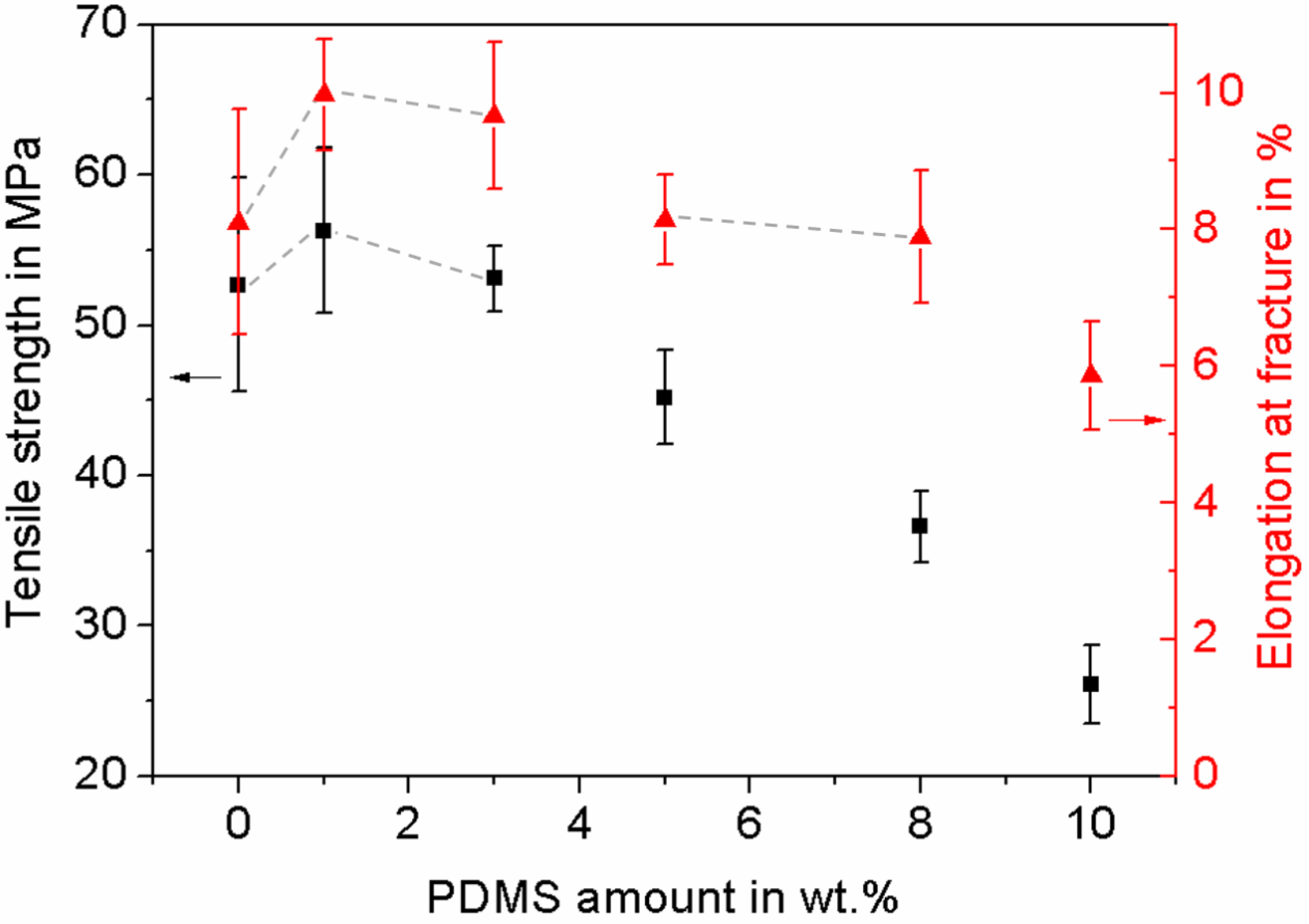
3.1. Characterization of the Influence of the PDMS Amount on Mechanical Properties of the PTU/PDMS Polymer Blend
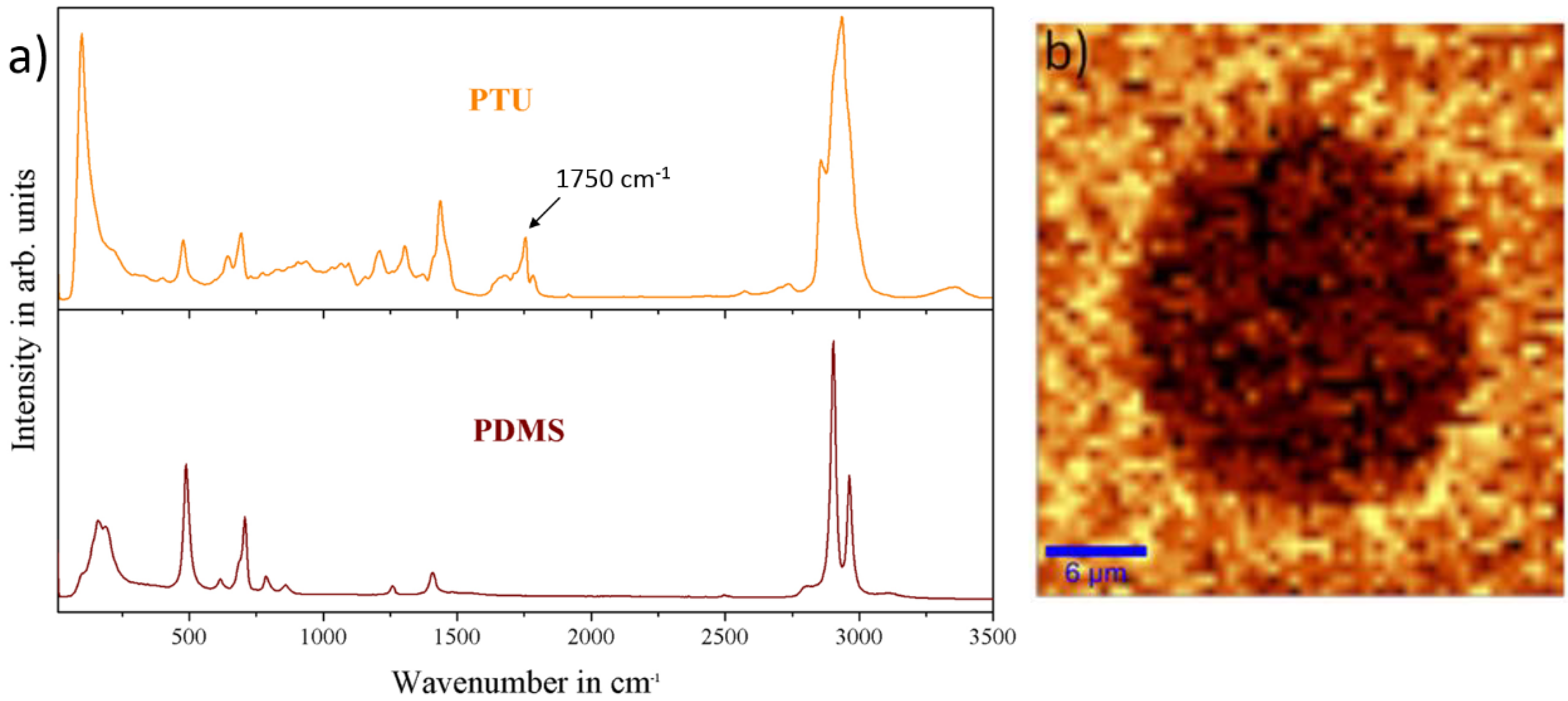
3.2. Physico-Chemical Characterization of the PTU/PDMS Polymer Blend
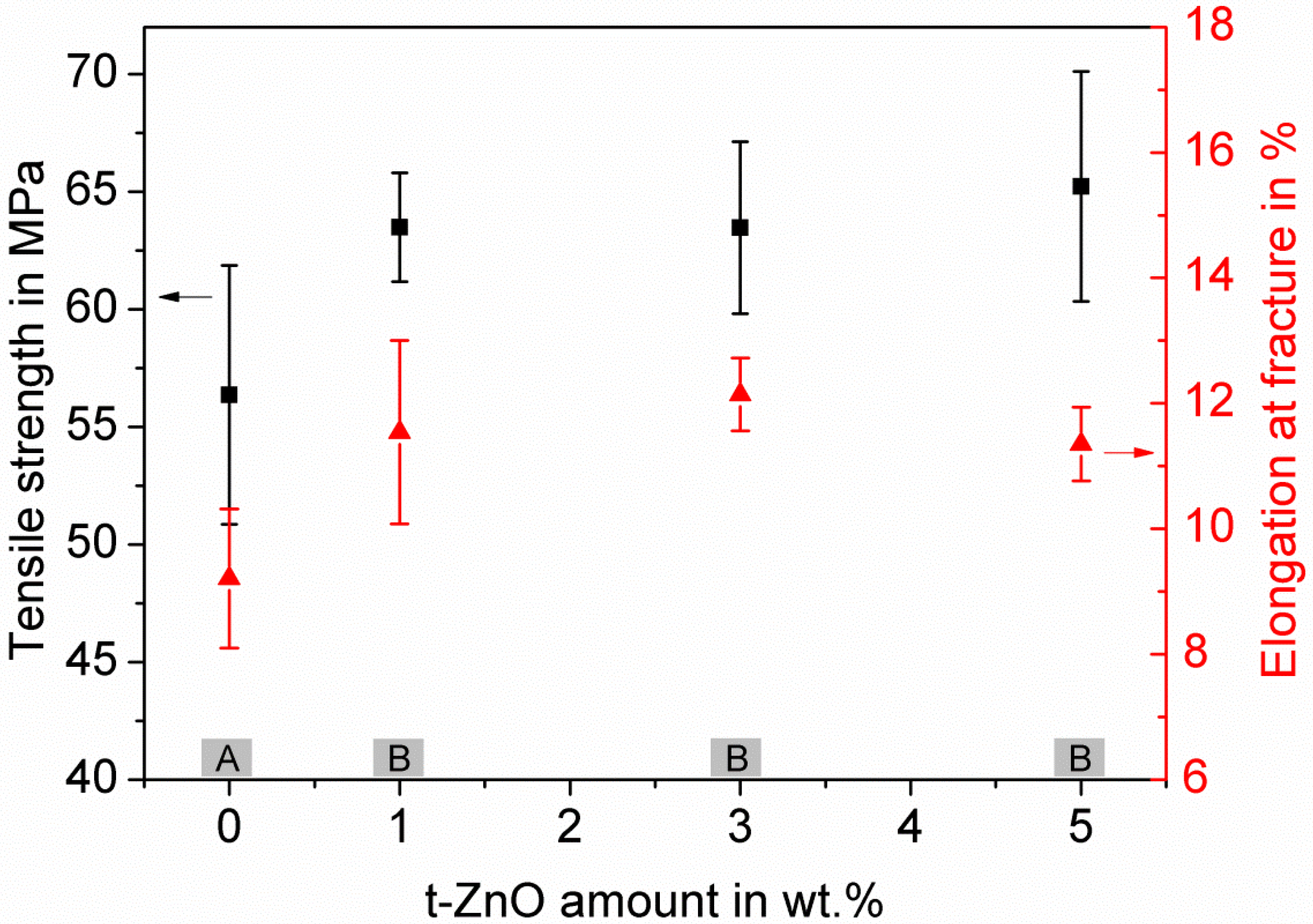
3.3. Characterization of the Influence of the Addition of t-ZnO Particles on Mechanical Properties of the PTU/PDMS Polymer Blend
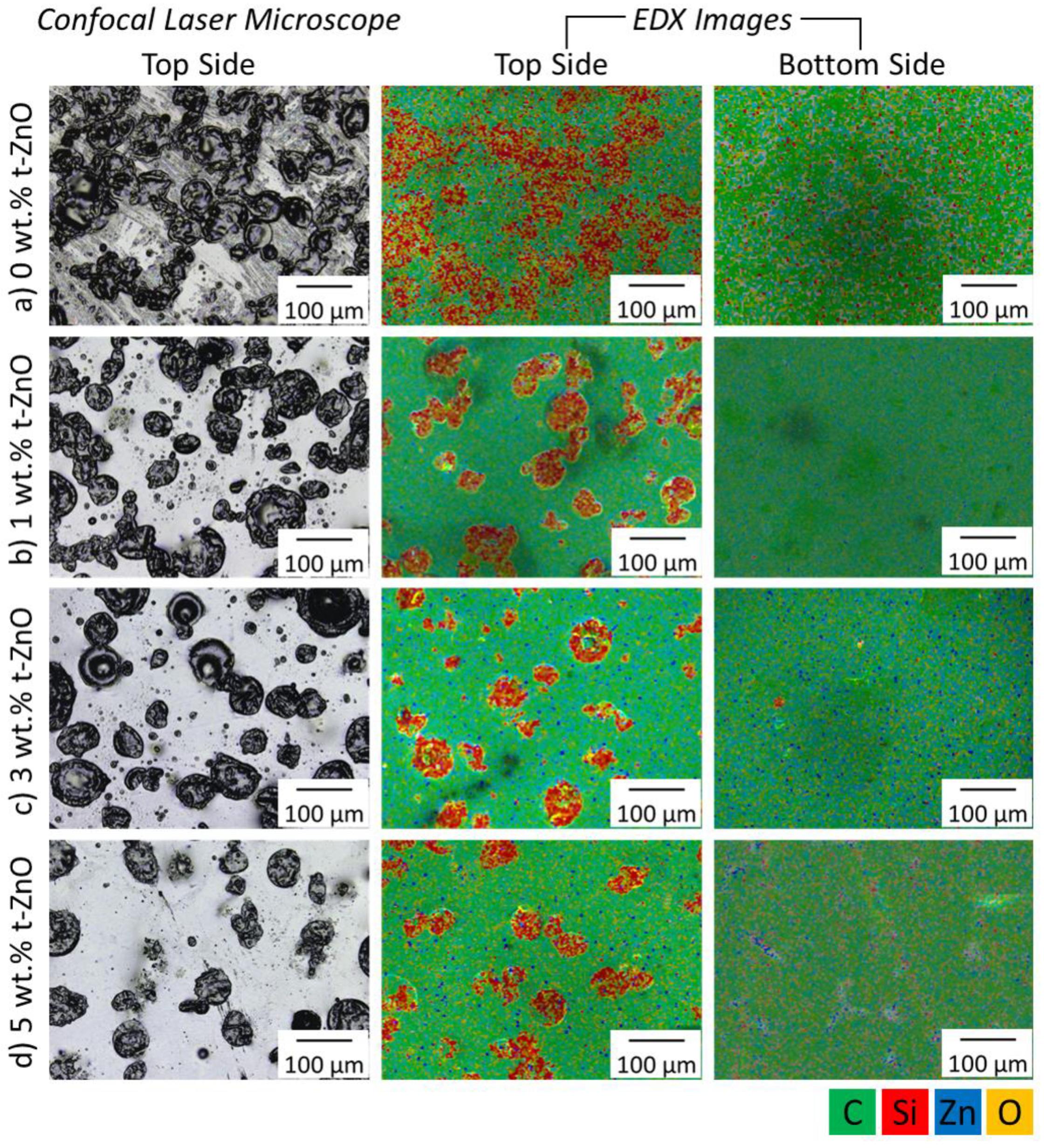
3.4. Effect of t-ZnO Addition on PDMS Microdomain Formation
3.5. Influence of t-ZnO Addition on Surface Wettability of the PTU/PDMS Polymer Blend
3.6. Characterization of the Fouling-Release Properties of the Composites by Peel-Off Test
3.7. Fouling Release Behavior in Terms of Barnacles Removing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, W.P.; Wang, Y.; Tian, R.M.; Bougouffa, S.; Yang, B.; Cao, H.L.; Zhang, G.; Wong, Y.H.; Batang, Z.; Al-Suweilem, A.; et al. Species Sorting during biofilm assembly by artificial substrates deployed in a cold seep system. Sci. Rep. 2014, 4, 6647. [Google Scholar] [CrossRef] [PubMed]
- Dafforn, K.A.; Lewis, J.A.; Johnston, E.L. Antifouling strategies: History and regulation, ecological impacts and mitigation. Mar. Pollut. Bull. 2011, 62, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Wahl, M. Marine epibiosis. I. Fouling and antifouling: Some basic aspects. Mar. Ecol. Prog. Ser. 1989, 58, 175–189. [Google Scholar] [CrossRef]
- Cao, S.; Wang, J.; Chen, H.; Chen, D. Progress of marine biofouling and antifouling technologies. Chin. Sci. Bull. 2011, 56, 598–612. [Google Scholar] [CrossRef]
- Carteau, D.; Vallée-Réhel, K.; Linossier, I.; Quiniou, F.; Davy, R.; Compère, C.; Delbury, M.; Faÿ, F. Development of environmentally friendly antifouling paints using biodegradable polymer and lower toxic substances. Prog. Org. Coat. 2013, 77, 485–493. [Google Scholar] [CrossRef]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology: Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Abarzua, S.; Jakubowski, S. Biotechnological investigation for the prevention of biofouling. I. Biological and biochemical principles for the prevention of biofouling. Mar. Ecol. Prog. Ser. 1995, 123, 301–312. [Google Scholar] [CrossRef]
- Rosenhahn, A.; Schilp, S.; Kreuzer, H.J.; Grunze, M. The role of “inert” surface chemistry in marine biofouling prevention. Phys. Chem. Chem. Phys. 2010, 12, 4275–4286. [Google Scholar] [CrossRef] [PubMed]
- Page, H.M.; Dugan, J.E.; Piltz, F. Fouling and antifouling in oil and other offshore industries. In Biofouling; Dürr, S., Thomason, J.C., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2010; pp. 252–266. [Google Scholar] [CrossRef]
- Flemming, H.C. Biofouling in Water Systems—Cases, Causes and Countermeasures. Appl. Microbiol. Biotechnol. 2002, 59, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Wake, H.; Takahashi, H.; Takimoto, T.; Takayanagi, H.; Ozawa, K.; Kadoi, H.; Okochi, M.; Matsunaga, T. Development of an Electrochemical Antifouling System for Seawater Cooling Pipelines of Power Plants Using Titanium. Biotechnol. Bioeng. 2006, 95, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.S.; Shenashen, M.A.; El-Safty, S.A.; Higazy, S.A.; Selim, M.M.; Isago, H.; Elmarakbi, A. Recent Progress in Marine Foul-Release Polymeric Nanocomposite Coatings. Prog. Mater. Sci. 2017, 87, 1–32. [Google Scholar] [CrossRef]
- Gittens, J.E.; Smith, T.J.; Suleiman, R.; Akid, R. Current and Emerging Environmentally-Friendly Systems for Fouling Control in the Marine Environment. Biotechnol. Adv. 2013, 31, 1738–1753. [Google Scholar] [CrossRef] [PubMed]
- Alzieu, C. Environmental Impact of TBT: The French Experience. Sci. Total Environ. 2000, 258, 99–102. [Google Scholar] [CrossRef]
- Yonehara, Y.; Yamashita, H.; Kawamura, C.; Itoh, K. A New Antifouling Paint Based on a Zinc Acrylate Copolymer. Prog. Org. Coat. 2001, 42, 150–158. [Google Scholar] [CrossRef]
- Fang, J.; Kelarakis, A.; Wang, D.; Giannelis, E.P.; Finlay, J.A.; Callow, M.E.; Callow, J.A. Fouling Release Nanostructured Coatings Based on PDMS-Polyurea Segmented Copolymers. Polymer 2010, 51, 2636–2642. [Google Scholar] [CrossRef]
- Guo, H.; Ma, Y.; Sun, P.; Cui, S.; Qin, Z.; Liang, Y. Self-Cleaning and Antifouling Nanofiltration Membranes-Superhydrophilic Multilayered Polyelectrolyte/CSH Composite Films Towards Rejection of Dyes. RSC Adv. 2015, 5, 63429–63438. [Google Scholar] [CrossRef]
- Fürstner, R.; Barthlott, W.; Neinhuis, C.; Walzel, P. Wetting and Self-Cleaning Properties of Artificial Superhydrophobic Surfaces. Langmuir 2005, 21, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Shi, L.; Li, J.; Xin, Y.; Yang, T.; Guo, Z. Transparent Superhydrophobic/Superhydrophilic Coatings for Self-Cleaning and Anti-Fogging. Appl. Phys. Lett. 2012, 101, 033701. [Google Scholar] [CrossRef]
- Genzer, J.; Efimenko, K. Recent Development in Superhydrophobic Surfaces and Their Relevance to Marine Fouling: A Review. Biofouling 2006, 22, 339–360. [Google Scholar] [CrossRef] [PubMed]
- Sommer, S.; Abdullah, E.; Webster, D.C.; Stafslien, S.J.; Daniels, J.; VanderWal, L.J.; Thompson, S.E.M.; Callow, M.E.; Callow, J.A. A Preliminary Study on the Properties of Fouling-Release Performance Siloxane-Polyurethane Coatings Prepared from Poly(Dimethylsiloxane)(PDMS) Macromers. Biofouling 2010, 26, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Yilgör, E.; Yilgör, I. Silicone Containing Copolymers: Synthesis, Properties and Applications. Prog. Polym. Sci. 2014, 39, 1165–1195. [Google Scholar] [CrossRef]
- Rahman, M.M.; Chun, H.-H. Waterborne Polysiloxan-Urethane-Urea for Potentialmarine Coatings. J. Coat. Technol. Res. 2011, 8, 389–399. [Google Scholar] [CrossRef]
- Majumdar, P.; Webster, D.C. Preparation of Siloxane-Urethane Coatings Having Spontaneously Formed Stable Biphasic Microtopograpical Surfaces. Macromolecules 2005, 38, 5857–5859. [Google Scholar] [CrossRef]
- Majumdar, P.; Webster, D.C. Surface Microtopography in Siloxane-Polyurethane Thermosets: The Influence of Siloxane and Extent of Reaction. Polymer 2007, 48, 7499–7509. [Google Scholar] [CrossRef]
- Galhenage, T.P.; Hoffman, D.; Silbert, S.D.; Stafslien, S.J.; Daniels, J.; Miljkovic, T.; Finlay, J.A.; Franco, S.C.; Clare, A.S.; Nedved, B.T.; et al. Fouling-Release Performance of Silicone Oil-Modified Siloxane-Polyurethane Coatings. ACS Appl. Mater. Interfaces 2016, 8, 29025–29036. [Google Scholar] [CrossRef] [PubMed]
- Gapeeva, A.; Hölken, I.; Adelung, R.; Baum, M.; Ag, P. characterization of a polydimethylsiloxane- polythiourethane polymer blend with potential as fouling-release coating. In Proceedings of the 2017 IEEE 7th International Conference Nanomaterials: Application & Properties (NAP), Odessa, Ukraine, 10–15 September 2017; pp. 1–5. [Google Scholar] [CrossRef]
- Rath, S.K.; Chavan, J.G.; Sasane, S.; Srivastava, A.; Patri, M.; Samui, A.B.; Chakraborty, B.C.; Sawant, S.N. Coatings of PDMS-Modified Epoxy via Urethane Linkage: Segmental Correlation Length, Phase Morphology, Thermomechanical and Surface Behavior. Prog. Org. Coat. 2009, 65, 366–374. [Google Scholar] [CrossRef]
- Dou, Q.; Wang, C.; Cheng, C.; Han, W.; Thüne, P.C.; Ming, W. PDMS-Modified Polyurethane Films with Low Water Contact Angle Hysteresis. Macromol. Chem. Phys. 2006, 207, 2170–2179. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Raju, K.V.S.N. Structural Engineering of Polyurethane Coatings for High Performance Applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Swain, G. Redefining Antifouling Coatings. J. Prot. Coat. Linings 1999, 16, 26–35. [Google Scholar]
- Yuan, B.; Shao, M.; Lu, S.; Wang, B. Source Profiles of Volatile Organic Compounds Associated with Solvent Use in Beijing, China. Atmos. Environ. 2010, 44, 1919–1926. [Google Scholar] [CrossRef]
- Pavlicevic, J.; Spirkova, M.; Bera, O.; Jovicic, M.; Pilic, B.; Balos, S.; Budinski-Simendic, J. The Influence of ZnO Nanoparticles on Thermal and Mechanical Behavior of Polycarbonate-Based Polyurethane Composites. Compos. Part B 2014, 60, 673–679. [Google Scholar] [CrossRef]
- Xu, T.; Xie, C. Tetrapod-like Nano-Particle ZnO/Acrylic Resin Composite and Its Multi-Function Property. Prog. Org. Coat. 2003, 46, 297–301. [Google Scholar] [CrossRef]
- Hölken, I. Mechanically Stable and Environmentally Friendly Polymer/Particle Composites for the Application as Low-Fouling Coating in the Marine Sector. Ph.D. Thesis, Universitätsbibliothek Kiel, Kiel, Germany, 2016. [Google Scholar]
- Hölken, I.; Hoppe, M.; Adelung, R.; Baum, M. Functional Ecofriendly Coatings for Marine Applications. IFMBE Proc. 2015, 55, 250–253. [Google Scholar] [CrossRef]
- Hölken, I.; Hoppe, M.; Mishra, Y.K.; Gorb, S.N.; Adelung, R.; Baum, M. Complex Shaped ZnO Nano- and Microstructure Based Polymer Composites: Mechanically Stable and Environmentally Friendly Coatings for Potential Antifouling Applications. Phys. Chem. Chem. Phys. 2016, 18, 7114–7123. [Google Scholar] [CrossRef] [PubMed]
- Mishra, Y.K.; Kaps, S.; Schuchardt, A.; Paulowicz, I.; Jin, X.; Gedamu, D.; Freitag, S.; Claus, M.; Wille, S.; Kovalev, A.; et al. Fabrication of Macroscopically Flexible and Highly Porous 3D Semiconductor Networks from Interpenetrating Nanostructures by a Simple Flame Transport Approach. Part. Part. Syst. Charact. 2013, 30, 775–783. [Google Scholar] [CrossRef]
- Adamson, A.W. Physical Chemistry of Surfaces; Wiley: New York, NY, USA, 1982; ISBN 10-0471610194. [Google Scholar]
- Owens, D.; Wendt, R. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Brady, R.F. A Fracture Mechanical Analysis of Fouling Release from Nontoxic Antifouling Coatings. Prog. Org. Coat. 2001, 43, 188–192. [Google Scholar] [CrossRef]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Elsevier: Amsterdam, The Netherlands, 1991; ISBN 0-12-451160-0. [Google Scholar]
- Zhao, J.; Rojstaczer, S.R.; Chen, J.; Xu, M.; Gardella, J.A. Effect of Siloxane Segment Length on the Surface Composition of Poly(Imidesiloxane) Copolymers and Its Role in Adhesion. Macromolecules 1999, 32, 455–461. [Google Scholar] [CrossRef]
- Ho, T.; Wynne, K.J.; Nissan, R.A. Polydimethylsiloxane-Urea-Urethane Copolymers with 1,4-Benzenedimethanol as Chain Extender. Macromolecules 1993, 26, 7029–7036. [Google Scholar] [CrossRef]
- Mahoney, C.M.; Gardella, J.A.; Rosenfeld, J.C. Surface Characterization and Adhesive Properties of Poly(Imidesiloxane) Copolymers Containing Multiple Siloxane Segment Lengths. Macromolecules 2002, 35, 5256–5266. [Google Scholar] [CrossRef]
- Chen, X.; Gardella, J.A., Jr.; Ho, T.; Wynne, K.J. Surface Composition of a Series of Dimethylsiloxane Urea Urethane Segmented Copolymers Studied by Electron Spectroscopy for Chemical Analysis. Macromolecules 1995, 28, 1635–1642. [Google Scholar] [CrossRef]
- Lee, Y.; Akiba, I.; Akiyama, S. The Study of Surface Segregation and the Formation of Gradient Domain Structure at the Blend of Poly(Methyl Methacrylate)/Poly(Dimethyl Siloxane) Graft Copolymers and Acrylate Adhesive Copolymers. J. Appl. Polym. Sci. 2003, 87, 375–380. [Google Scholar] [CrossRef]
- Niu, L.N.; Fang, M.; Jiao, K.; Tang, L.H.; Xiao, Y.H.; Shen, L.J.; Chen, J.H. Tetrapod-like Zinc Oxide Whisker Enhancement of Resin Composite. J. Dent. Res. 2010, 89, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-Y.; Zhang, W.-D. Effects of Flower-like ZnO Nanowhiskers on the Mechanical, Thermal and Antibacterial Properties of Waterborne Polyurethane. Polym. Degrad. Stab. 2009, 94, 1103–1109. [Google Scholar] [CrossRef]
- Xu, H.H.K. Whisker-Reinforced Heat-Cured Dental Resin Composites: Effects of Filler Level and Heat-Cure Temperature and Time. J. Dent. Res. 2000, 79, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Bormashenko, E.Y. Physics of Wetting: Phenomena and Applications of Fluids on Surfaces; De Gruyter: Berlin, Germany, 2017; ISBN-10 311044481X. [Google Scholar]
- Zhang, X.M.; Li, L.; Zhang, Y. Study on the Surface Structure and Properties of PDMS/PMMA Antifouling Coatings. Phys. Procedia 2013, 50, 328–336. [Google Scholar] [CrossRef]
- Hoipkemeier-Wilson, L.; Schumacher, J.F.; Carman, M.L.; Gibson, A.L.; Feinberg, A.W.; Callow, M.E.; Finlay, J.A.; Callow, J.A.; Brennan, A.B. Antifouling Potential of Lubricious, Micro-Engineered, PDMS Elastomers against Zoospores of the Green Fouling Alga Ulva (Enteromorpha). Biofouling 2004, 20, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, D.R.; Wenning, B.; Finlay, J.A.; Callow, M.E.; Callow, J.A.; Fischer, D.; Ober, C.K. Amphiphilic Oligopeptides Grafted to PDMS-Based Diblock Copolymers for Use in Antifouling and Fouling Release Coatings. Polym. Adv. Technol. 2015, 26, 829–836. [Google Scholar] [CrossRef]
- Zhao, X.; Su, Y.; Li, Y.; Zhang, R.; Zhao, J.; Jiang, Z. Engineering Amphiphilic Membrane Surfaces Based on PEO and PDMS Segments for Improved Antifouling Performances. J. Memb. Sci. 2014, 450, 111–123. [Google Scholar] [CrossRef]
- Buskens, P.; Wouters, M.; Rentrop, C.; Vroon, Z. A Brief Review of Environmentally Benign Antifouling and Foul-Release Coatings for Marine Applications. J. Coat. Technol. Res. 2013, 10, 29–36. [Google Scholar] [CrossRef]

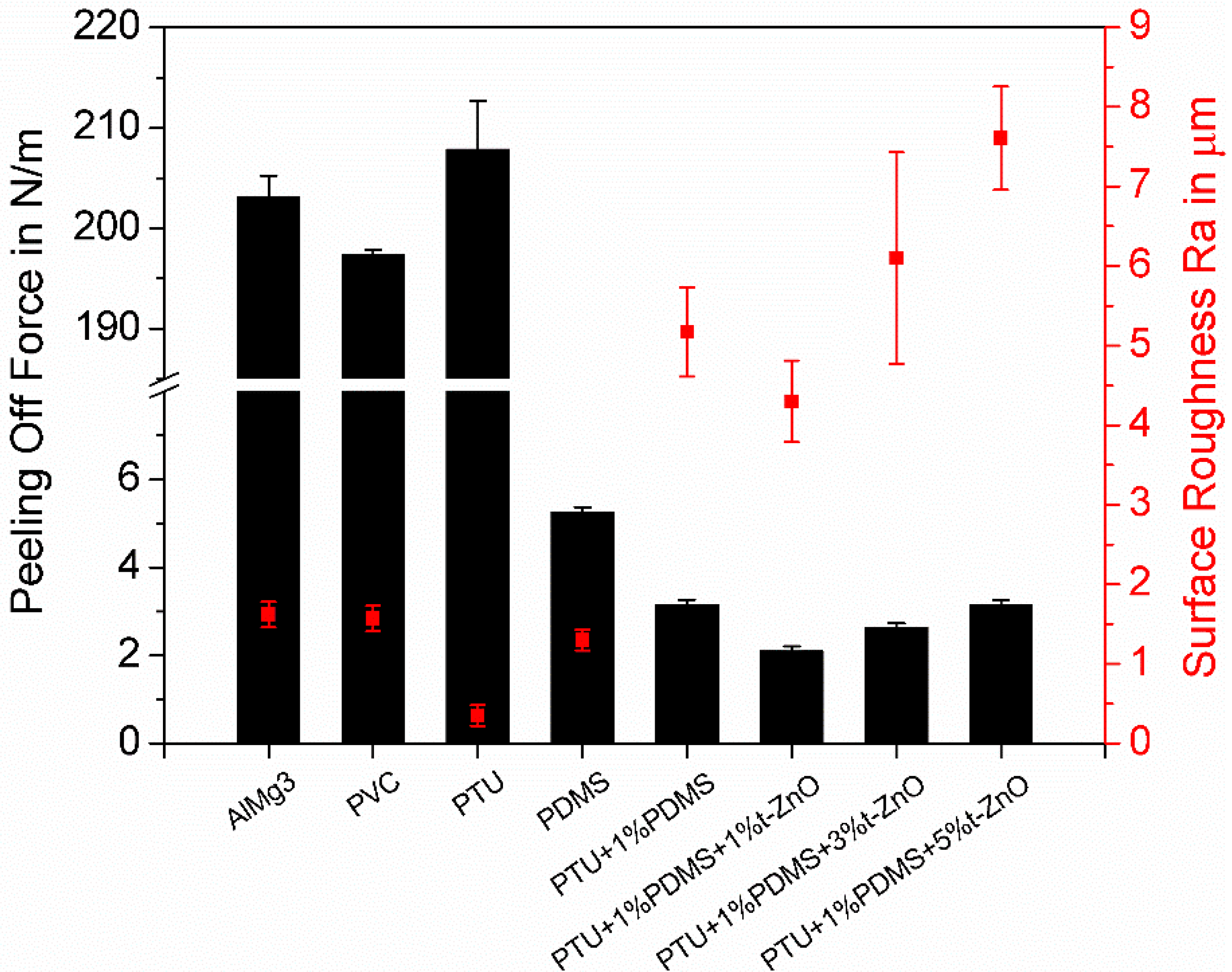
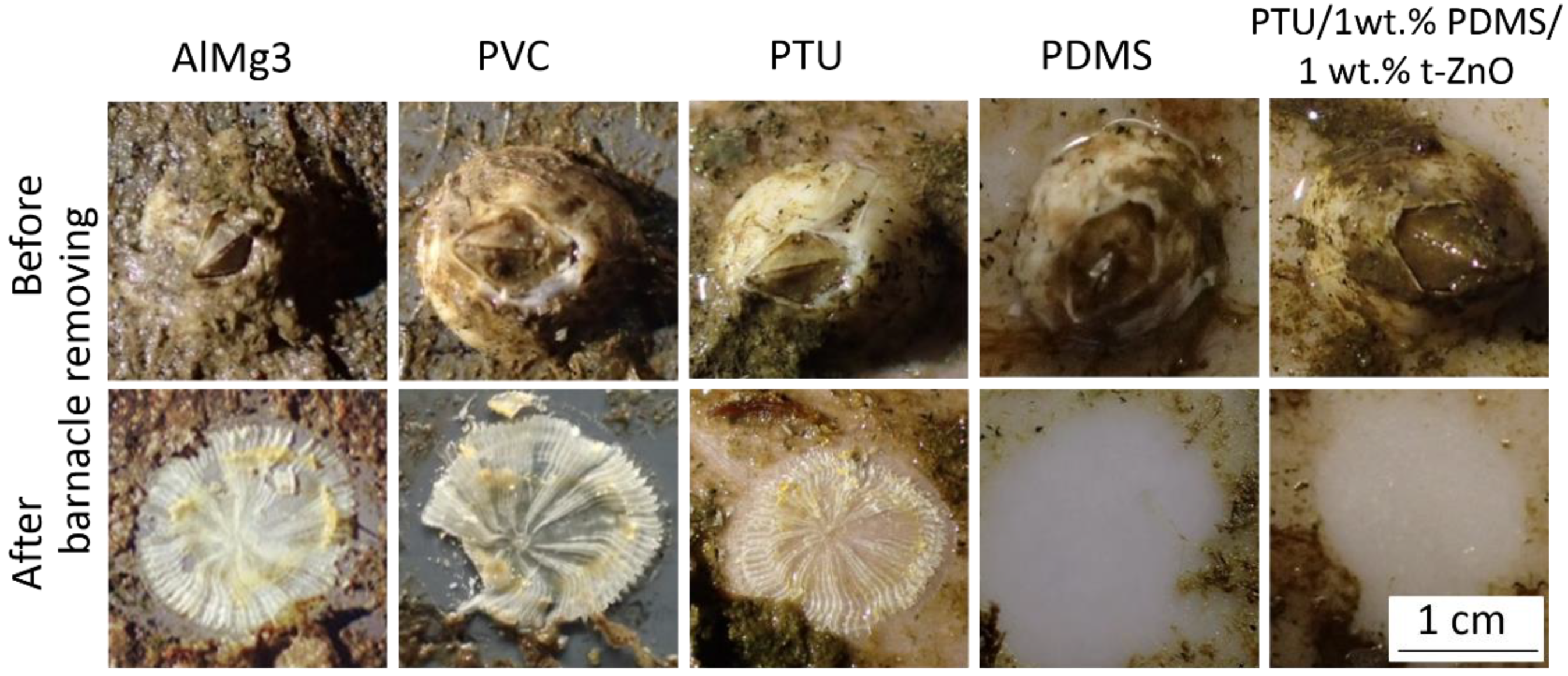
| Polymer Variations | Water Contact Angle (°) | SFE (mN/m) | γp (mN/m) | γd (mN/m) |
|---|---|---|---|---|
| PTU [37] | 67.4 ± 3.4 | 40.7 ± 0.5 | 12.4 ± 1.7 | 28.4 ± 1.2 |
| PDMS | 111.6 ± 1.1 | 20.9 ± 0.4 | 0.0 ± 0.0 | 20.9 ± 0.4 |
| PTU/PDMS | 115.0 ± 1.2 | 20.9 ± 0.8 | 0.0 ± 0.1 | 20.9 ± 0.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, H.; Hölken, I.; Gapeeva, A.; Filiz, V.; Adelung, R.; Baum, M. Development and Characterization of Mechanically Durable Silicone-Polythiourethane Composites Modified with Tetrapodal Shaped ZnO Particles for the Potential Application as Fouling-Release Coating in the Marine Sector. Materials 2018, 11, 2413. https://doi.org/10.3390/ma11122413
Qiu H, Hölken I, Gapeeva A, Filiz V, Adelung R, Baum M. Development and Characterization of Mechanically Durable Silicone-Polythiourethane Composites Modified with Tetrapodal Shaped ZnO Particles for the Potential Application as Fouling-Release Coating in the Marine Sector. Materials. 2018; 11(12):2413. https://doi.org/10.3390/ma11122413
Chicago/Turabian StyleQiu, Haoyi, Iris Hölken, Anna Gapeeva, Volkan Filiz, Rainer Adelung, and Martina Baum. 2018. "Development and Characterization of Mechanically Durable Silicone-Polythiourethane Composites Modified with Tetrapodal Shaped ZnO Particles for the Potential Application as Fouling-Release Coating in the Marine Sector" Materials 11, no. 12: 2413. https://doi.org/10.3390/ma11122413
APA StyleQiu, H., Hölken, I., Gapeeva, A., Filiz, V., Adelung, R., & Baum, M. (2018). Development and Characterization of Mechanically Durable Silicone-Polythiourethane Composites Modified with Tetrapodal Shaped ZnO Particles for the Potential Application as Fouling-Release Coating in the Marine Sector. Materials, 11(12), 2413. https://doi.org/10.3390/ma11122413






