Microstructures and Properties of a Low-Carbon-Chromium Ferritic Stainless Steel Treated by a Quenching and Partitioning Process
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
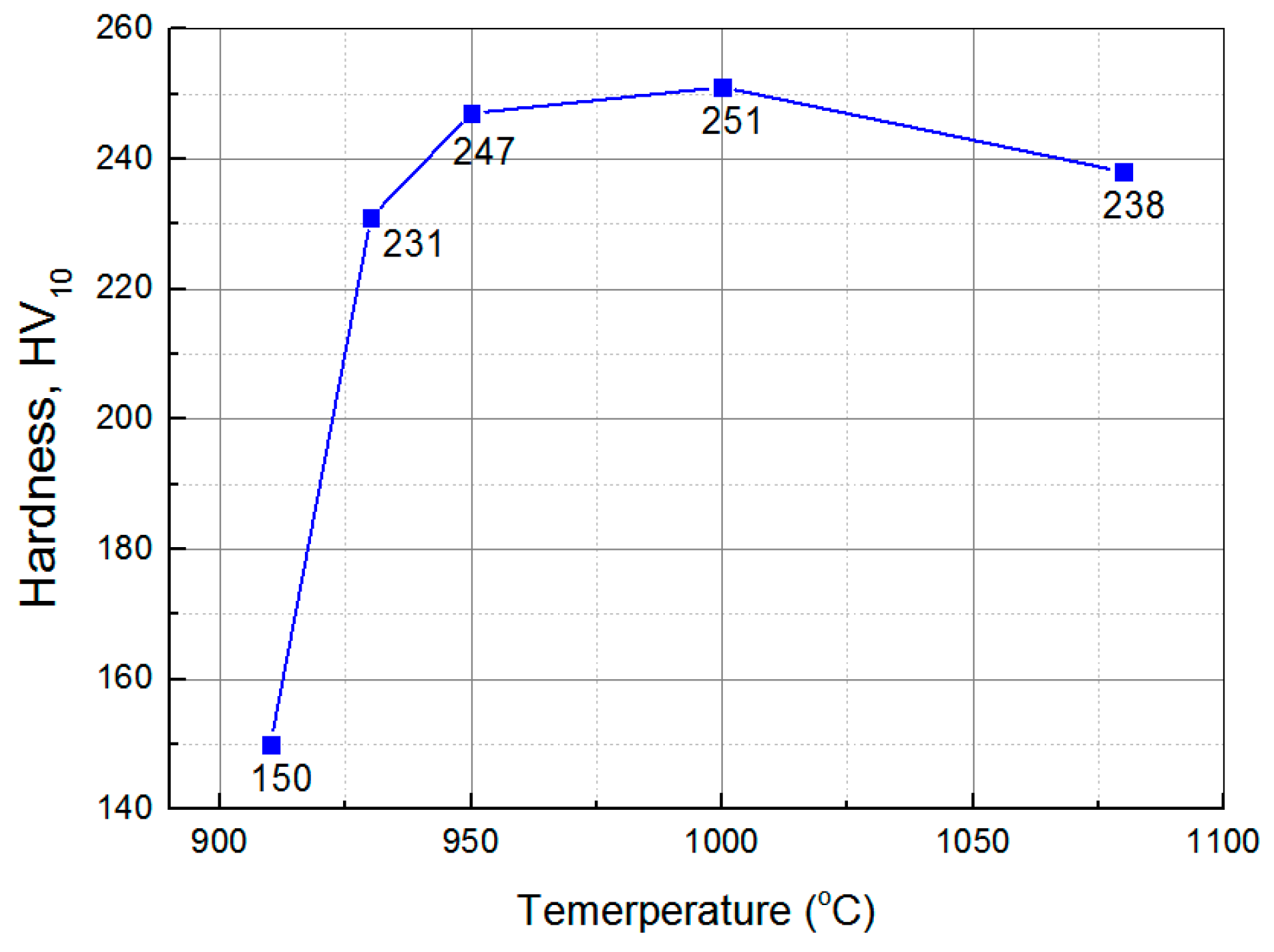
3.1. Mircostructure
3.2. Mechanical Properties
3.3. Flatness of Plate
3.4. Corrosion Resisting Property
3.5. Comparing with the AHSS
4. Conclusions
- (1)
- Combination of conventional annealing “soft state” with high elongation and traditional quenching-tempering process “hard state” with high strength. It achieves a good match between strength and plasticity, and has better impact safety (for the quenching-partitioning [Q-P] process, quenching-tempering process, and annealing process, the production of strength plasticity is about 16 GPa%, 15 GPa%, and 14 GPa%, respectively).
- (2)
- The material has low yield strength, high work hardening index (compared with Q-T), smooth tensile curve, and no yield plateau (compared with annealing), so it has better forming performance, and finally, the corrosion resistance of the material is improved by 20% in terms of pitting potential (compared with annealing).
Author Contributions
Funding
Conflicts of Interest
References
- Kuziak, R.; Kawalla, R.; Waengler, S. Advanced high strength steels for automotive industry. Arch. Civil Mech. Eng. 2008, 8, 103–117. [Google Scholar] [CrossRef]
- Matlock, D.K.; Speer, J.G.; De Moor, E.; Gibbs, P.J. Recent developments in advanced high strength sheet steels for automotive applications: an overview. Jestech 2012, 15, 1–12. [Google Scholar]
- Matlock, D.K.; Speer, J.G. Processing opportunities for new advanced high-strength sheet steels. Mater. Manuf. Process. 2010, 25, 7–13. [Google Scholar] [CrossRef]
- Yan, L.; Wang, A.; Chen, Q.; Li, J. Dynamic material flow analysis of zinc resources in China. Resour. Conserv. Recy. 2013, 75, 23–31. [Google Scholar] [CrossRef]
- Speer, J.G.; Matlock, D.K. Recent developments in low-carbon sheet steels. JOM 2002, 54, 19–24. [Google Scholar] [CrossRef]
- Bellhouse, E.M.; Mertens, A.; McDermid, J.R. Development of the surface structure of TRIP steels prior to hot-dip galvanizing. Mater. Sci. Eng. A 2007, 463, 147–156. [Google Scholar]
- Khondker, R.; Mertens, A.; McDermid, J.R. Effect of annealing atmosphere on the galvanizing behavior of a dual-phase steel. Mater. Sci. Eng. A 2007, 463, 157–165. [Google Scholar] [CrossRef]
- Kim, M.; Kwak, J.; Kim, J.; Liu, Y.; Gao, N.; Tang, N. Galvanizability of advanced high-strength steels 1180TRIP and 1180CP. Metall. Mater. Trans. A 2009, 40, 1903–1910. [Google Scholar] [CrossRef]
- Mahieu, J.; Claessens, S.; De Cooman, B. Galvanizability of high-strength steels for automotive applications. Metall. Mater. Trans. A 2001, 32, 2901–2905. [Google Scholar] [CrossRef]
- Shibli, S.; Meena, B.; Remya, R. A review on recent approaches in the field of hot dip zinc galvanizing process. Surf. Coat. Tech. 2015, 262, 210–215. [Google Scholar] [CrossRef]
- Daigo, I.; Osako, S.; Adachi, Y.; Matsuno, Y. Time-series analysis of global zinc demand associated with steel. Resour. Conserv. Recy. 2014, 82, 35–40. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Li, Y.; Li, H.; Wang, W.; Ye, B. Impacts of lead/zinc mining and smelting on the environment and human health in China. Environ. Monit. Assess. 2012, 184, 2261–2273. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Pub. Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Li, Y.; Li, H.; Wang, W.; Ge, Q. Estimation of lead and zinc emissions from mineral exploitation based on characteristics of lead/zinc deposits in China. Trans. Nonferr. Metal. Soc. China 2011, 21, 2513–2519. [Google Scholar] [CrossRef]
- Johnson, J.; Reck, B.; Wang, T.; Graedel, T. The energy benefit of stainless steel recycling. Energ. Policy 2008, 36, 181–192. [Google Scholar] [CrossRef]
- Baddoo, N. Stainless steel in construction: A review of research, applications, challenges and opportunities. J. Constr. Steel Res. 2008, 64, 1199–1206. [Google Scholar] [CrossRef]
- Eskandari, M.; Najafizadeh, A.; Kermanpur, A.; Karimi, M. Potential application of nanocrystalline 301 austenitic stainless steel in lightweight vehicle structures. Mater. Des. 2009, 30, 3869–3872. [Google Scholar] [CrossRef]
- Hyun, P.J.; Kwon, H.S. Development of high Mn–N duplex stainless steel for automobile structural components. Corros. Sci. 2008, 50, 404–410. [Google Scholar]
- Kyröläinen, A.; Vilpas, M.; Hänninen, H. Use of stainless steels in bus coach structures. J. Mater. Eng. Perform. 2000, 9, 669–677. [Google Scholar] [CrossRef]
- Schuberth, S.; Schedin, E.; Ratte, T.F.E. Next generation vehicle–engineering guidelines for stainless steel in automotive application. In Proceedings of the 6th Stainless Steel Science and Market Conference, Lusignan, France, 10–13 June 2018; pp. 637–644. [Google Scholar]
- Charenton, J.; Rombeaux, P.; Hurtaud, B.; Hauser, J. Stainless Steel, with 11 per cent Chromium and High Yield Strength, for Welded Constructions Resistant to Corrosion and Abrasion. In Proceedings of the 1st International Chromiu Sleel and Alloys Congress, Cape Town, South Africa, 1992; pp. 229–234. Available online: https://www.pyrometallurgy.co.za/InfaconVI/2229-Charenton.pdf (accessed on 26 May 2019).
- Bracke, L.; Mertens, G.; Penning, J.; De Cooman, B.C.; Liebeherr, M.; Akdut, N. Influence of phase transformations on the mechanical properties of high-strength austenitic Fe-Mn-Cr steel. Metall. Mater. Trans. A 2006, 37, 307–317. [Google Scholar] [CrossRef]
- Taban, E.; Deleu, E.; Dhooge, A.; Kaluc, E. Laser welding of modified 12% Cr stainless steel: Strength, fatigue, toughness, microstructure and corrosion properties. Mater. Design 2009, 30, 1193–1200. [Google Scholar] [CrossRef]
- Rumeng, W.; Xiaoning, Y.; Laizhu, J. Development of high strength ferrite-martensite stainless steels (FMSSs) for railway cargo transportation. Baosteel Tech. Res. 2013, 7, 57. [Google Scholar]
- Speer, J.; Matlock, D.K.; De Cooman, B.C.; Schroth, J.G. Carbon partitioning into austenite after martensite transformation. Acta Mater. 2003, 51, 2611–2622. [Google Scholar] [CrossRef]
- Speer, J.; Assunção, F.; Matlock, D.; Edmonds, D. The “quenching and partitioning” process: background and recent progress. Mater. Res. 2005, 8, 417–423. [Google Scholar] [CrossRef]
- Wang, L.; Speer, J.G. Quenching and partitioning steel heat treatment. Metallogr. Microstruct. Anal. 2013, 2, 268–281. [Google Scholar] [CrossRef]
- Yan, S.; Liu, X.; Liu, W.J.; Liang, T.; Zhang, B.; Liu, L.; Zhao, Y. Comparative study on microstructure and mechanical properties of a C-Mn-Si steel treated by quenching and partitioning (Q&P) processes after a full and intercritical austenitization. Mater. Sci. Eng. A 2017, 684, 261–269. [Google Scholar]
- Mola, J.; De Cooman, B.C. Quenching and partitioning processing of transformable ferritic stainless steels. Scripta Mater. 2011, 65, 834–837. [Google Scholar] [CrossRef]
- Tsuchiyama, T.; Tobata, J.; Tao, T.; Nakada, N.; Takaki, S. Quenching and partitioning treatment of a low-carbon martensitic stainless steel. Mater. Sci. Eng. A 2012, 532, 585–592. [Google Scholar] [CrossRef]
- Mola, J.; De Cooman, B.C. Quenching and partitioning (Q&P) processing of martensitic stainless steels. Metall. Mater. Trans. A 2013, 44, 946–967. [Google Scholar]
- Tobata, J.; Ngo-Huynh, K.-L.; Nakada, N.; Tsuchiyama, T.; Takaki, S. Role of silicon in quenching and partitioning treatment of low-carbon martensitic stainless steel. ISIJ Int. 2012, 52, 1377–1382. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Yao, K.-F.; Chen, Y.-B.; Wang, M.-H.; Chen, N.; Ge, X.-Y.J.C.S. Effect of quenching and partitioning on the microstructure evolution and electrochemical properties of a martensitic stainless steel. Corros. Sci. 2016, 100, 95–104. [Google Scholar] [CrossRef]
- Koıstinen, D.P.; Marbürger, R.E. A General Equation Prescribing Extent of Austenite-Martensite Transformation in Pure Fe-C Alloy and Plain Carbon Steels. Acta Metall. 1959, 7, 59–60. [Google Scholar] [CrossRef]
- Mola, J.; Jung, I.; Park, J.; Chae, D.; De Cooman, B.C. Ridging control in transformable ferritic stainless steels. Metall. Mater. Trans. A 2012, 43, 228–244. [Google Scholar] [CrossRef]
- Dong, H.; Sun, X.; Cao, W.; Liu, Z.; Wang, M.; Weng, Y. On the performance improvement of steels through M 3 structure control. In Advanced Steels; Springer: Berlin, Germany, 2011; pp. 35–37. [Google Scholar]
- Hsu, T.; Jin, X. Ultra-high Strength Steel Treated by Using Quenching-Partitioning-Tempering Process. In Advanced Steels; Springer: Berlin, Germany, 2011; pp. 67–73. [Google Scholar]
- Lesch, C.; Kwiaton, N.; Klose, F.B. Advanced high strength steels (AHSS) for automotive applications− tailored properties by smart microstructural adjustments. Steel Res. Int. 2017, 88, 1700210. [Google Scholar] [CrossRef]
- Qu, H.; Michal, G.M.; Heuer, A.H. A 3rd generation advanced high-strength steel (AHSS) produced by dual stabilization heat treatment (DSHT). Metall. Mater. Trans. A 2013, 44, 4450–4453. [Google Scholar] [CrossRef]
- Zackay, V.F.; Parker, E.R.; Fahr, D.; Busch, R. The enhancement of ductility in high-strength steels. ASM Trans. Quart. 1967, 60, 252–259. [Google Scholar]









| C | Si | Mn | P | S | Cr | Ni | N |
|---|---|---|---|---|---|---|---|
| 0.030 | 0.65 | 0.24 | 0.023 | 0.001 | 12.97 | 0.08 | 0.018 |
| Heat Treatment | Yield Stress Rp0.2/MPa | Tensile Strength Rm/MPa | Elongation A50/% | Work Hardening Index | Strength-Elongation Production Rm × A50/GPa % |
|---|---|---|---|---|---|
| Q-P | 544 | 833 | 18.7 | 0.16 | 15.6 |
| Q-T1 | 670 | 872 | 15.3 | 0.11 | 13.3 |
| Q-T2 | 635 | 848 | 16.5 | 0.11 | 14.0 |
| Annealing | 304 | 474 | 30.5 | 0.29 | 14.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, G.; Li, H.; Li, Y.; Mo, J. Microstructures and Properties of a Low-Carbon-Chromium Ferritic Stainless Steel Treated by a Quenching and Partitioning Process. Materials 2019, 12, 1704. https://doi.org/10.3390/ma12101704
Luo G, Li H, Li Y, Mo J. Microstructures and Properties of a Low-Carbon-Chromium Ferritic Stainless Steel Treated by a Quenching and Partitioning Process. Materials. 2019; 12(10):1704. https://doi.org/10.3390/ma12101704
Chicago/Turabian StyleLuo, Gang, Huaying Li, Yugui Li, and Jinqiang Mo. 2019. "Microstructures and Properties of a Low-Carbon-Chromium Ferritic Stainless Steel Treated by a Quenching and Partitioning Process" Materials 12, no. 10: 1704. https://doi.org/10.3390/ma12101704
APA StyleLuo, G., Li, H., Li, Y., & Mo, J. (2019). Microstructures and Properties of a Low-Carbon-Chromium Ferritic Stainless Steel Treated by a Quenching and Partitioning Process. Materials, 12(10), 1704. https://doi.org/10.3390/ma12101704




