Abstract
The new phases BaLa0.9M0.1InO3.95 (M = Ca2+, Sr2+, Ba2+) with a Ruddlesden-Popper structure were obtained. It was established that all investigated samples were capable for the water uptake from the gas phase. The ability of water incorporation was due to not only by the presence of oxygen vacancies, but also due to the presence of La-O blocks in the structure. The degree of hydration of the samples was much higher than the concentration of oxygen vacancies and the composition of the samples appear to be BaLaInO3.42(OH)1.16, BaLa0.9Ca0.1InO3.25(OH)1.4, BaLa0.9Sr0.1InO3.03(OH)1.84, BaLa0.9Ba0.1InO2.9(OH)2.1. The degree of hydration increased with an increase in the size of the dopant, i.e., with an increase in the size of the salt blocks. It was proven that doping led to the increase in the oxygen ionic conductivity. The conductivities for doped samples BaLa0.9M0.1InO3.95 were higher than for undoped composition BaLaInO4 at ~1.5 order of magnitude. The increase in the conductivity was mainly attributed to the increase of the carrier concentration as a result of the formation of oxygen vacancies during doping. The proton conductivities of doped samples increased in the order Ca2+–Sr2+–Ba2+ due to an increase in the concentration of protons. It was established that all doped samples demonstrated the dominant proton transport below 450 °C.
1. Introduction
Protonic ceramics are important materials for using as electrolytes in proton-conducting solid oxide fuel cells (H-SOFCs) [1,2,3,4,5,6]. The application of the proton-conducting complex oxides in comparison with oxygen-ion conductors (in oxygen ion-conducting solid oxide fuel cells O-SOFCs) has several advantages. First, the proton electrolytes have lower activation energies of charge carriers (~0.5 eV) than the oxygen-ion conductors (~1 eV). Consequently, they have higher conductivities. This allows us to decrease the operating temperatures from 900–1000 °C (O-SOFCs) to 500–700 °C (H-SOFCs) [7] and provides the decrease of the production cost of H-SOFCs at 27–37% [8]. Secondly, the water vapor in the H-SOFCs is produced at the air-side electrode (the cathode side) and this avoids the dilution of fuel by steam [9], and, consequently, increases the energy conversion efficiency [10,11]. Therefore, the discovery of a new structure family of proton conductors is important both in terms of the development of scientific materials and in the applied aspect.
The most studied protonic conductors are the complex oxides with perovskite or perovskite-related structures. Further development of proton conductors suggests investigations of materials with new types of structures, including Ruddlesden-Popper structure. It is common understanding that oxygen-deficient compounds are capable of manifesting not only oxygen-ionic conductivity, but also protonic conductivity in a humid atmosphere. Therefore, perovskite-related structures with oxygen-ion conductivity can be tested as potentially capable of proton transport. Recently, researchers uncovered new data about oxygen-ion transport in the complex oxides with a Ruddlesden-Popper structure [12,13,14]. The phase BaNdInO4 has a structure that is slightly different from K2NiF4 [15]. The BaNdInO4-type structure (monoclinic, P21/c) consists of alternative stacking of the [NdO] oxide unit and Nd1/4Ba3/4InO3 perovskite unit with the InO6 edge-facing Nd−O units.
The new compounds BaYInO4, BaSmInO4, BaHoInO4, BaErInO4, and BaYbInO4 were also synthesized [16,17], but the total electrical conductivity of BaNdInO4 was the highest among BaRInO4 compounds. Therefore, the investigations were mainly focused on BaNdInO4. The phase BaNdInO4 was described as a mixed oxygen ionic-electronic conductor [12,13,14] and cation doping with different metals M = Ca, Sr, Ba, Ce, Ga, Cr, Si, Mg, Zr, Nb, Ta, Ti, and Sn at the Nd-sites or In-sites were used for increasing the oxide ion conductivity [13,18]. It was shown that oxygen-ion conductivity of Ca2+ and Ti4+ doped compositions was higher than those for other dopants [13,18]. It was proven that the increase of oxygen-ion conductivity at acceptor doping is due to the formation of oxygen vacancies. At the same time, the possibility of the formation of the interstitial oxygens in the rock-salt block of BaNdIn1-xTixO4+δ due to donor doping, which leads to the increase of oxide-ion conductivity, was also considered [18]. The interstitial oxygen conduction was also discussed in the donor doped K2NiF4-type LaSrInO4 oxides [19,20]. It should also be mentioned that Kotaro Fujii et al. [17] emphasized that there is no direct evidence for the interstitial oxygen. In the BaNdInO4-type structure, there is no rock-salt block, but the A rare earth oxide unit could have an influence on the impurity phases. All this demonstrates that these phases are very interesting and further investigation is required.
Although oxygen-ion transport in the systems based on BaNdInO4 was widely discussed, no studies of proton transport have been performed. Nevertheless, the possibility of proton transport for phases with the Ruddlesden-Popper structure was described earlier for oxyfluorides Ba2InO3F and Ba3In2O5F2 [21]. K2NiF4-type materials of Pr1-xM1+xInO4 (M = Ba2+, Sr2+; x = 0, 0.1) exhibit proton conductivity and are suitable for the operation of proton-conducting solid oxide fuel cells (H-SOFCs) at targeted temperatures of 500 to 700 °C [22]. The layered perovskites Sr1+xSm1−xAlO4−δ and Sr1+xPr1−xAlO4-δ also have proton conductivity and high proton transport number at 700 to 900 °C [23]. These studies also show that, for a correct discussion, the conductivity measurements for phases with a layered structure should be carried out in atmospheres with controlled humidity.
To develop the novel proton conductors, we focused on the composition of BaLaInO4 as a parent compound, which has a Ruddlesden-Popper structure. The structural features of the phases of the composition AIILnInO4 (AA′BO4) were described earlier [24], where the relationship between transitions of K2NiF4−CaFe2O4 type structures was established. The compound BaLaInO4 crystallizes in an orthorhombic structure with space group Pbca and with lattice parameters a = 12.933 (3), b = 5.911 (1), and c = 5.905 (1) Å [24]. In the present work, the new complex oxides BaLa0.9M0.1InO3.95 (M = Ca2+, Sr2+, Ba2+) with a Ruddlesden-Popper structure were obtained via the solid-state method. The structure, water uptake, and proton transport were examined. The influence of the nature of dopant (alkaline earth metals) on the transport properties were discussed.
2. Materials and Methods
The phases BaLa0.9M0.1InO3.95 (M = Ca2+, Sr2+, Ba2+) and the parent composition BaLaInO4 were prepared by a solid-state method. The preliminary dried high-purity powders of CaCO3, SrCO3, BaCO3, In2O3, and La2O3 were used. Prior to weighing, the starting materials were dried. The stoichiometric powder mixtures were manually milling in an agate mortar and then calcined at 800, 900, 1000, 1100, 1200, and 1300 °С for 24 h. Intermediate grindings were made for every following heating step.
Phase purity was monitored by powder X-ray diffraction (XRD) on a Bruker Advance D8 diffractometer with CuKα radiation. The crystal structures of dry and hydrated samples were determined by Rietveld refinement [25]. The dry samples were prepared for XRD by heat treated at 1100 °C for 4 h and then cooled in dry Ar (pH2O = 3.5 × 10−5 atm). The hydrated samples were obtained at slow cooling from 1100 to 150 °C (1 °C/min) under a flow of wet Ar (99.999% purity, pH2O = 2 × 10−2 atm). Ar atmosphere was used to avoid any carbonization of the samples.
TG-analysis was carried out on a STA 409 PC analyzer (Netzsch, Selb, Germany) coupled with a quadrupole mass spectrometer QMS 403 C Aëolos (Netzsch, Selb, Germany) in order to determine the degree of hydration. The samples were heated at the rate of 10 °C/min in a corundum crucible under a flow of dry Ar at temperatures ranging from 40 to 1100 °C. Before TG-measurements, the powder samples were initially hydrated as described above. The number of moles of water per one formula unit was determined and the notation of the total composition as BaLa0.9M0.1InO3.95·nH2O was used for convenience of comparison. To indicate that H2O incorporation gives OH−-groups, the formulas were also written in another representation as BaLa0.9M0.1InO3.95−n(OH)2n.
The ceramics used for the electrical measurements were prepared by pressing cylindrical pellets and sintering them at 1300 °C for 24 h in dry air. The samples typically had a relative density of around ∼92% (density of the sintered samples was determined by the Archimede method). After polishing, the platinum paste electrodes were fired at 900 °C for 3 h. The ac conductivity of the samples (two-probe method) was measured over the frequency range of 1 kHz–1 MHz using a Z-1000P (Elins, RF) impedance spectrometer at an applied voltage of 150 mV. The least squares refinement program ZView (Scribner Associates Inc., Southern Pines, NC, USA) was used to fit the impedance data to an (RQ) equivalent circuit, where R is resistance and Q is the constant phase element. The bulk resistance was calculated from the impedance data and then the bulk conductivity was calculated and discussed below. From the fitted resistances and, together with the geometrical dimensions, the total conductivity can be calculated using the following equation.
where l and S are the thickness and surface area of the sample.
The measurements of the temperature dependencies of conductivities were performed in the temperature range from 200 to 1000 °C every 10 to 20 °C with a cooling rate of 1 °C/min. During the measurement, the sample was held at each temperature until the resistances became constant. The conductivity measurements were carried out under dry and wet air or Ar.
The «wet» gas was obtained by bubbling the gas at room temperature first through distilled water and then through a saturated solution of KBr (pH2O = 2 × 10−2 atm). The «dry» gas was produced by circulating the gas through P2O5 (pH2O = 3.5 × 10−5 atm). The humidity of gases was measured by the H2O-sensor (“Honeywell” HIH-3610, Charlotte, NC, USA).
3. Results and Discussions
3.1. X-ray Characterization
The X-ray diffraction (XRD) results for the parent compound BaLaInO4 and its doped analogs BaLa0.9M0.1InO3.95 (M = Ca2+, Sr2+, Ba2+) showed that the samples were single phase (orthorhombic symmetry, space group Pbca). The obtained lattice parameters for the sample BaLaInO4 were in good agreement with previously reported data [24]. The substitution of M2+ ions for La+3 ion led to changes in structural parameters (Table 1). The lattice parameters and cell volumes increased with an increasing ionic radius of the dopant [26]. It should be noted that a more significant increase in parameters and cell volume in comparison with the undoped sample was observed for the Ba2+-doped sample. At the same time, for the Ca2+-doped sample (as the closest-sized ion to lanthanum), an increase and decrease in the lattice parameters was observed. It is known that the formation of oxygen vacancy is also accompanied by decreases of the lattice parameter because of the size relationship of oxygen vacancy and the oxide ion [27]. We can assume that, for the Ca2+-doped sample, the overall effect of the influence of the size of the B-cation and oxygen vacancy occurred and no monotonic change of the parameters was observed.

Table 1.
Cell parameters and volume of anhydrous and hydrated samples.
The hydration processes led to the changes in the crystal structure for the samples compared with anhydrous samples. All hydrated samples BaLaInO4∙nH2O and BaLa0.9M0.1InO3.95∙nH2O belonged to the monoclinic symmetry (space group P2/m). The increase in the ionic radii of alkaline earth metal led to the increase in the cell volume of hydrate samples (Table 1).
It should be noted that XRD-patterns for all obtained samples were refined by the Rietveld analysis. Results for anhydrous BaLa0.9Sr0.1InO3.95 and hydrated BaLa0.9Sr0.1InO3.95∙nH2O are presented in Figure 1a,b as an example of fitting.
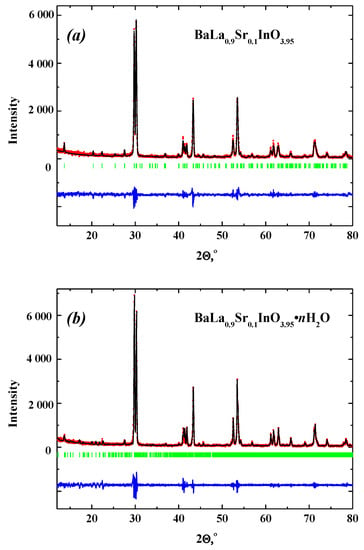
Figure 1.
XRD patterns of BaLa0.9Sr0.1InO3.95 (a) and BaLa0.9Sr0.1InO3.95∙nH2O (b). At the bottom of the figure, the pattern is the difference between the experimental (red) and the calculated one (dark) after refinement. Vertical bars show the Bragg angle positions.
3.2. Thermal Properties
The water uptake was established by TG-analysis in combination with differential scanning calorimetry (DSC) and mass-spectrometry (MS).
As is well known, the possibility of water uptake from the gas phase for perovskite-type oxide conductors is due to the presence of oxygen vacancies in the structure and can be described as follows.
where is the oxygen vacancy, is the oxygen atom in a regular position, and is the hydroxyl group in the oxygen sublattice. Based on the concentration of oxygen vacancies of obtained samples BaLaInO4[VO]0 and BaLa0.9M0.1InO3.95[VO]0.05, there should be a degree of hydration of 0 and 0.05 mol H2O per formula unit. As shown in Figure 2, for all the investigated samples, the mass loss was observed, which is attributed to a loss of water, in accordance with MS data. Water uptake of the samples was much higher than the concentration of oxygen vacancies and the composition of the samples appears to be BaLaInO4∙0.58H2O (or BaLaInO3.42(OH)1.16, which represents that H2O incorporation gives OH−-groups, BaLa0.9Ca0.1InO3.95∙0.7H2O (or BaLa0.9Ca0.1InO3.25(OH)1.4), BaLa0.9Sr0.1InO3.95∙0.92H2O (or BaLa0.9Sr0.1InO3.03(OH)1.84), and BaLa0.9Ba0.1InO3.95∙1.05H2O (or BaLa0.9Ba0.1InO2.9(OH)2.1).
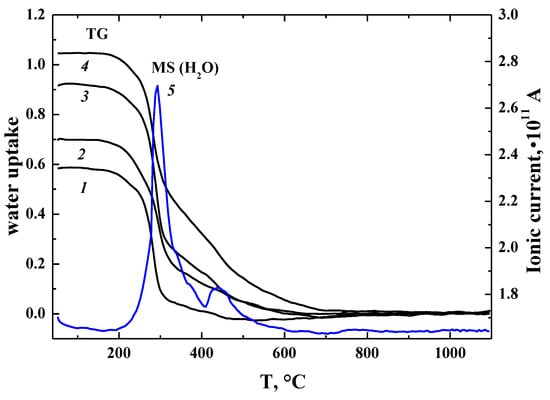
Figure 2.
TG data for hydrated samples BaLaInO4∙nH2O (1), BaLa0.9Ca0.1InO3.95∙nH2O (2), BaLa0.9Sr0.1InO3.95∙nH2O (3), BaLa0.9Ba0.1InO3.95∙nH2O (4), and MS (H2O)-data for BaLa0.9Ba0.1InO3.95∙nH2O (5).
Figure 3 represents the comparison of TG-curves, DSC-curves, and MS-curves for the hydrated sample BaLaInO4∙nH2O. As can be seen, the sample underwent a mass decrease in the temperature from 200 to 800 °C, which was accompanied by a three endothermic DSC-signals (two well pronounced and one weak signal) at ~320 °C, ~500 °C, and ~700 °C. The MS analysis indicated that the mass change can be attributed by the water loss. No other volatile components (CO2, O2) were detected. The doped samples BaLa0.9M0.1InO3.95 showed the similar TG-curves, DSC-curves, and MS-curves (Figure 2).
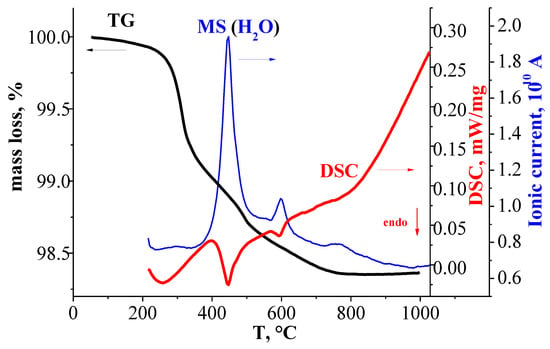
Figure 3.
TG-(dark line), DSC-(blue line), and MS (H2O)-data (red line) for hydrated sample BaLaInO4∙nH2O.
It is known that the ability of water incorporation is ensured not only by the presence of oxygen vacancies, but also by the features of the crystal structure [28]. For layered structures, it can be explained by the ability of water incorporation by the salt La−O blocks, which alternates with blocks of the perovskite matrix. For example, the formation of hydrated phases based on the Ruddlesden-Popper-type layered oxide Ba2ZrO4 [29] was proven. During hydration, this phase can incorporate up to 1.7 mol of water Ba2ZrO4∙1.7H2O. The effects of water incorporation into layered structures were summarized in the dissertation [30]. It was shown that RP phases can adopt up to two water molecules per formula unit/rock-salt block. At the same time, the large amount of water molecules in some RP-phases can be explained by the formation of hydrates and, in addition, not all RP compounds react with water under ambient conditions. This shows that the elemental composition of an RP compounds has a significant effect on the stability, and atoms with the biggest influence are the cations present in the rock-salt block. If the majority of cations in the rock-salt block are large cations, e.g., Ba, K, Rb or Cs, then the compounds have a higher tendency for the intercalation of water into the rock-salt block [30].
As can be seen from Figure 2, the degree of hydration increases in the order of BaLaInO4–BaLa0.9Ca0.1InO3.95–BaLa0.9Sr0.1InO3.95–BaLa0.9Ba0.1InO3.95. We can suggest that, with an increase in the size of the dopant, the size of the salt block increases. This contributes to the placement of a larger number of OH−-groups, which occupy interstitial sites within the rock salt layers.
Therefore, we can conclude that investigated samples BaLaInO4 and BaLa0.9M0.1InO3.95 can show the ability for water uptake not only in accordance with reaction (2), but also due to the presence in the structure of La-O blocks (rock-salt blocks).
Thus, the results show the ready ability of the RP-structure to incorporate extra water as OH−-groups, located in the sites of the rock salt layers. This ability can lead to proton transport. In addition, it is important to note that these results also show that it is possible to regulate the concentration of intercalated water by the nature of the dopant introduced into the salt layers.
3.3. Electrical Properties
For all the samples in this study, one semicircle, starting from zero coordinates of the complex Z-Z′ plane and corresponding to the bulk component, was observed. Typical impedance spectroscopy diagrams for the BaLa0.9Sr0.1InO3.95 sample, recorded at various temperatures, are given in Figure 4. The capacitance of the observed arc was estimated to be about 10−11 F∙cm−1, which is typical of a bulk response. According to the experimental data obtained for other phases with an RP structure (for example, LaSrInO4 [19,20] and NdBaInO4 [13]), two contributions can be observed in the typical impedance spectroscopy diagrams. The semicircle in the high-frequency range, corresponding to the contribution from bulk resistance, and very small low-frequency semicircle, attributed to the contribution of grain-boundary resistance. It should be noted that the grain-boundary arc was manifested in the frequency range of 100 mHz to 100 Hz. In comparison with the presented data for doped BaLaInO4 (Figure 4), the observed one semicircle is realized in the frequency range of 1 to 1000 kHz. Therefore, the arc corresponding to the grain boundary response, which appears at lower frequencies (in comparison with bulk semicircle), cannot be observed due to the instrumental limitations of the frequency range.
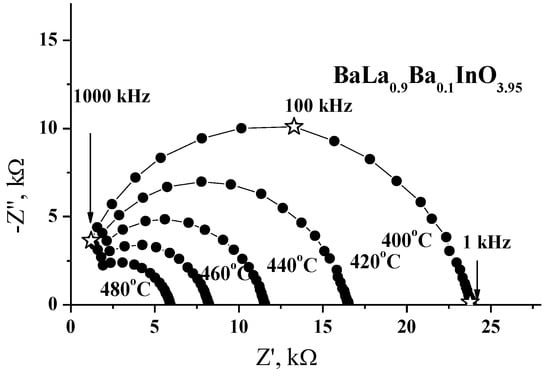
Figure 4.
Evolution of the impedance spectra with temperature for the sample BaLa0.9Ba0.1InO3.95 in dry air (pH2O = 3.5 × 10−5 atm).
3.4. Dry Conditions
The temperature dependencies of total conductivities for investigated samples BaLaInO4 and BaLa0.9M0.1InO3.95 (M = Ca2+, Sr2+, Ba2+) are shown in Figure 5. For dry air (pH2O = 3.5 × 10−5 atm) (Figure 5), the conductivities for doped samples BaLa0.9M0.1InO3.95 are higher than those for undoped composition BaLaInO4 at a ~1.5 order of magnitude. To evaluate the contribution of electronic conductivity of investigated phases, the measurements were performed in the Ar atmosphere (that is, at lower pO2 where ionic conductivity dominates). The comparison of total conductivities in air and Ar under dry conditions was shown in Figure 6. The conductivities in Ar atmosphere were lower than the conductivities, measured in air. The conductivity enhancement in air can be ascribed to the electronic conduction because of formation of holes in air, as shown by the quasi-chemical reaction.
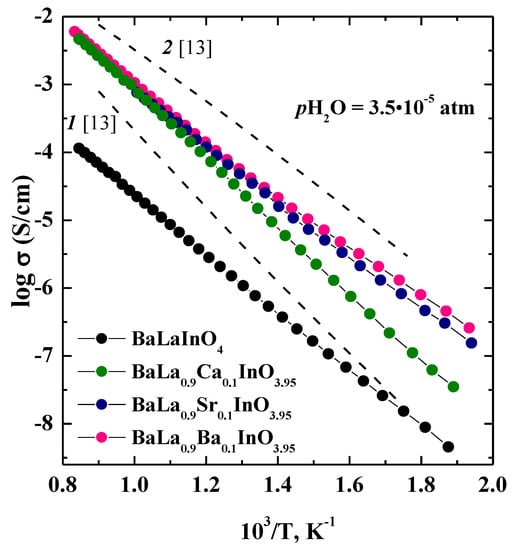
Figure 5.
The temperature dependencies of the total conductivities (pO2 = 0.21 atm) of BaLaInO4 and BaLa0.9M0.1InO3.95 (M = Ca2+, Sr2+, and Ba2+) in dry air and comparison with (1) BaNdInO4 and (2) Ba1.1Nd0.9InO3.95 in air.
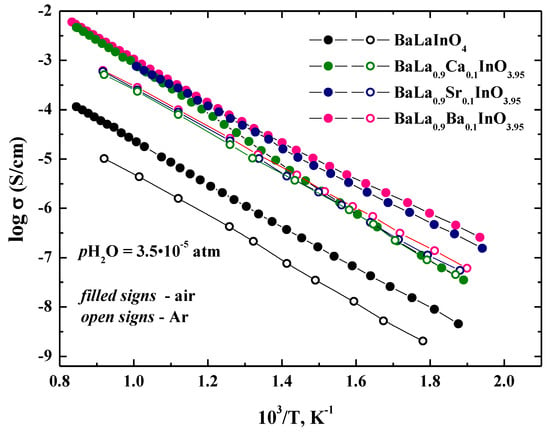
Figure 6.
The temperature dependencies of the conductivities of BaLaInO4 and BaLa0.9M0.1InO3.95 (M = Ca2+, Sr2+, and Ba2+) in dry air (filled signs) and dry Ar (open signs).
For the undoped sample, the conductivity in Ar atmosphere were lower than the conductivities, measured in air, over the whole temperature range studied. This proves the mixed oxygen ionic-electronic character of conductivity of BaLaInO4. For Sr2+, Ba2+-doped samples, the conductivity exhibited the same character, but, for the Ca2+-doped sample, the differences were more significant at higher temperatures and the conductivity values were comparable at low temperatures, which indicates that the electron contribution decreases with a falling temperature and this phase exhibited the dominant oxygen–ionic character of conductivity below 500 °C. These results also showed that for the Sr2+, Ba2+-substituted phases, the contribution of the electronic conductivity was greater when compared to the Ca2+-substituted phase.
Thus, the observed increase in the ionic conductivity (Ar atmosphere) for doped phases BaLa0.9M0.1InO3.95 in comparison with undoped composition BaLaInO4 was mainly attributed to the increase of the carrier concentration as a result of the formation of oxygen vacancies during doping. The M2+-cations are incorporated into the lattice at La3+ sites with the creation of oxygen vacancies as charge-compensating defects. This doping process can be represented by the following defect reaction.
Comparing the ionic conductivities of the doped phases, we can note the tendency of its increase in the order of BaLa0.9Ca0.1InO3.95−BaLa0.9Sr0.1InO3.95−BaLa0.9Ba0.1InO3.95, i.e., in the order of increasing the ionic radius of dopants. The concentration of the oxygen vacancies was the same for the doped samples, so the difference in the conductivity of the BaLa0.9M0.1InO3.95 might be attributed to their mobilities. In this respect, effect of the dopant nature on the conductivity should be considered.
The authors [13] have investigated NdBaInO4, doped with Ca2+, Sr2+, Ba2+. Their results show another trend in comparison with the data obtained in this work: σion of Ca2+ > Sr2+ > Ba2+. Their conductivities showed the tendency to increase with a decrease in the ionic radii of the M site ions, i.e., Ca2+ is the most favorable dopant on Nd3+ sites. The authors [13] indicate that the replacement of Nd3+ by the cations with the most comparable size may minimize local structural relaxation and this main reason may explain the enhancement of the oxygen vacancy conductivity in the order of Ca2+ > Sr2+ > Ba2+. Taking into account the differences in the radii of La3+ and Ca2+, Sr2+, and Ba2+, we could expect a similar trend in the change in conductivity of doped BaLaInO4. However, this is not the case. Clearly, other reasons should be considered.
There are not many reports in the literature on the relationships of O2−-transport in phases with the RP-structure. However, for the ABO3 perovskites, there is a lot of information on the influence of the nature of A-cations, B-cations, and dopants on the mobility of oxygen vacancies. In the general case, there are many factors that affect the mobility of oxygen vacancies. For phases with the same structure and with the same concentration of dopants, the main factors are generally identified.
(1) Geometric characteristics. It is well known that the size proportion of the A-cations and B-cations is an important factor for high ionic conductivity. The relationship of structure parameters and oxygen-ion transport properties had been studied by Wakamura [31]. The author plotted the activation energy (Ea) versus lattice volume (V) for numerous ion conductors, and found a simple inverse correlation between them, according to the expression Ea = Av/V2/3, where Av is a constant. The relationship indicates that the Ea value is decreased with a weakened long-range force due to the volume expansion. A similar tendency was observed for the ionic conductivity of mixed ionic-electronic conductors. For example, for Ln0.5M0.5FeO3-δ (M = Sr2+, Ba2+) [32], increasing the mismatch between La3+ and M2+ radii weakens the metal-oxygen bonding and increases the oxygen mobility.
Returning to the analysis of the experimental data, presented in this paper, we can make the assumption concerning the reason for the enhancement of the oxygen vacancy conductivity in the order of Ca2+–Sr2+–Ba2+ for doped BaLaInO4. The observed increase in the lattice volume and the lattice parameters weakens the metal-oxygen bonding, and, as a consequence, increases the oxygen mobility. It should be noted that the activation energy for oxygen ion conduction in doped BaLaInO4 decreased in the order of Ca2+ (0.86 eV)–Sr2+ (0.85 eV)–Ba2+ (0.82 eV).
As can be seen, the results obtained for changes in oxygen-ion conductivity are consistent with the main trends described for perovskites. At the same time, it should be emphasized that the nature of the dopant has little effect on the oxygen ionic conductivity of doped BaLaInO4. The differences reached less than 0.3 order at low temperatures. This is due to the fact that the replacement of cations is carried out in the salt block and the change in geometrical parameters also occurs mainly in the salt block. The formation of oxygen vacancies and their migration occurs in the perovskite block. Therefore, the nature of the dopant introduced into the salt block does not significantly affect the oxygen ionic conductivity.
(2) Binding energies for dopant−oxygen vacancy pair clusters. It is well-known that interactions between dopant ions and their charge-compensating defects can lead to the formation of clusters, which can trap the migrating species. This implies that the oxide ion vacancies might be strongly bound to the dopant species, and the migration of the oxygen vacancy might depend on the binding energy of the dopant-oxygen vacancy, which, consequently, leads to lower oxide ion conductivities. As for the calculations for dopant-vacancy clusters in Ca2+-, Sr2+-, and Ba2+-doped NdBaInO4 with RP-structure, it was shown [13] that the calculated binding energies are comparable to ca.–0.9 eV, which indicates that trapping for the migrating oxygen vacancies in NdBaInO4 from the Ca2+, Sr2+, and Ba2+ dopants could be comparable. If we assume that the same tendency is observed for the doped phase BaLaInO4, then it can also be said that the reason for the cluster formation does not explain the change in conductivity due to the nature of the dopant.
This result shows that the nature of the dopant, by occupying sites within the rock salt layers, does not have a very significant effect on the oxygen-ion conduction and that the key parameter governing the oxygen-ion conductivity of these materials is the concentration of oxygen vacancies. Likely, the variation in the concentration of oxygen vacancies of solid solutions, which will be studied in the future, will reveal new regularities of ionic transport in phases with the RP-structure.
The comparison of the conductivities of the investigated samples based on BaLaInO4 with Nd-structural analogues BaNdInO4 [13] showed the total and ionic conductivities were in good agreement and comparable for Sr2+ and Ba2+ doped phases at low temperatures, but Nd-structural analogues exhibited higher conductivities at higher temperatures [13]. Figure 5 shows a comparison of the temperature dependencies of conductivity for undoped phases BaLaInO4 and BaNdInO4 and Ba2+ doped phases BaLa0.9Ba0.1InO3.95 (or Ba1.1La0.9InO3.95), Ba1.1Nd0.9InO3.95 [13].
3.5. Wet Conditions
Figure 7 shows a comparison of the temperature dependencies of conductivity of investigated samples in Ar under dry and wet conditions. For wet Ar (pH2O = 2 × 10−2 atm) (Figure 7, curves with open signs), the conductivities of all samples were higher than those in dry Ar. Clearly, this behavior was a result of the insertion of water and the formation of proton charge carriers. The conductivity differences were more significant at lower temperatures as the hydration increased with decreasing temperature. We can suppose that the nature of dopant does not have significant effects on the type of dominant charge carrier, and increasing in the conductivity values in wet atmospheres is due to the appearance of proton carriers for all investigated samples.
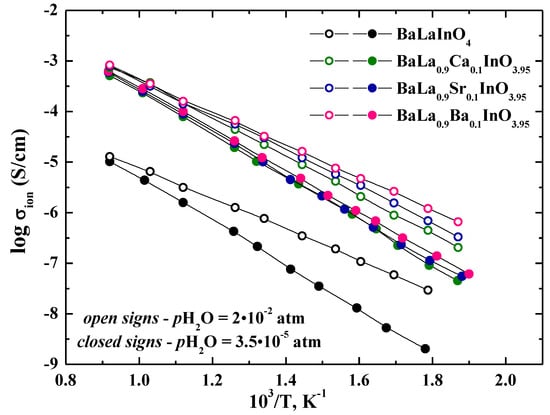
Figure 7.
The temperature dependencies of the ionic conductivities of BaLaInO4 and BaLa0.9M0.1InO3.95 (M = Ca2+, Sr2+, Ba2+) at dry Ar (filled signs) and wet Ar (closed signs).
The proton conductivity was calculated as the difference between the conductivity in wet and dry Ar, that is, σH = σ (wet Ar) − σ (dry Ar), and the temperature dependencies are shown in Figure 8.
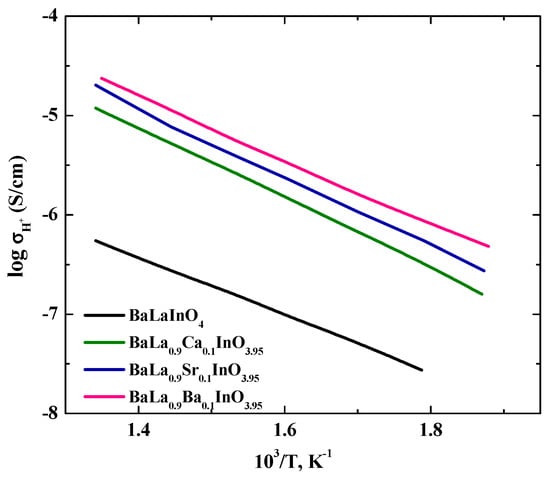
Figure 8.
The temperature dependencies of the proton conductivities of BaLaInO4 and BaLa0.9M0.1InO3.95 (M = Ca2+, Sr2+, and Ba2+).
As can be seen from Figure 8, the proton conductivities of doped samples increased in the order Ca2+–Sr2+–Ba2+. We can assume that these differences were due to an increase in the concentration of protons in the same order. The activation energies decreased in the order of Ca2+ (0.70 eV)–Sr2+ (0.68 eV)–Ba2+ (0.65 eV). These values are greater than the activation energy of proton transport in doped perovskites ~0.5 eV [33]. As was discussed above in the analysis of TG data, during hydration, the main part of the protons was in the salt block. This situation is like hydroxides. It is well known that proton mobility is dependent on the strength of hydrogen bonds with the next-nearest oxygen atoms and energy barriers are high for proton transport in non-H-bonded systems [34,35]. It is clear that proton migration in the layer of salt block is a difficult in comparison with the proton migration in the bulk of perovskite block. Moreover, from a general point of view, four key steps for hydration can be distinguished [36]: these are (1) water adsorption on the oxide surface, (2) proton migration from the surface to the bulk of the oxide, (3) proton migration in the bulk, and (4) oxide ion vacancy migration in the bulk. The proton migration from the surface to the bulk is difficult. For investigated systems, the proton migration from the salt layer is assumed to be the same as the proton migration from the surface to the bulk of the perovskite structure. Taking into account these differences in the structures of perovskites and phases with the RP-structure, as well as differences in hydration mechanisms, it can be concluded that the proton transport in the investigated phases is realized with higher activation energies compared to the substituted ordinary perovskites.
The proton transport numbers were calculated according to the formula tH = σH/σ (wet air) and were plotted as a function of temperatures in Figure 9. It can be seen that the proton transport numbers increased with decreasing temperature and the investigated samples were nearly pure proton conductors below 450 °C.
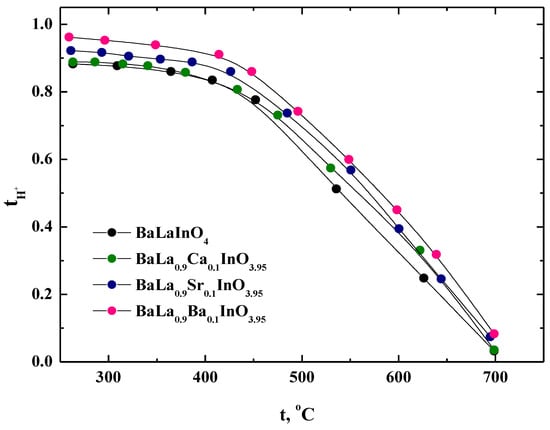
Figure 9.
The temperature dependencies of the proton transport numbers of BaLaInO4 and BaLa0.9M0.1InO3.95 (M = Ca2+, Sr2+, and Ba2+).
The results indicate that new phases BaLa0.9M0.1InO3.95 (M = Ca2+, Sr2+, Ba2+) might be a promising proton-conducting oxide in future applications. Further modification of the composition will improve the H+-conductivity.
4. Conclusions
The new phases BaLa0.9M0.1InO3.95 (M = Ca2+, Sr2+, and Ba2+) with the Ruddlesden-Popper structure were obtained. The analysis of the effect of nature of dopant (alkaline earth metals) on the hydration processes and on the transport properties of BaLaInO4 was carried out. It was established that all investigated samples were capable of water uptake from the gas phase. The ability of water incorporation was due to, not only the presence of oxygen vacancies, but also due to the presence in the structure of La-O blocks. The degree of hydration increased with a growth in the size of the dopant, i.e., with an increase in the size of the salt blocks, that contributed to the placement of a larger number of OH−-groups, which occupied interstitial sites within the rock salt layers.
It was proven that doping led to the increase of the oxygen ionic conductivity values. The conductivity values for doped samples BaLa0.9M0.1InO3.95 were higher than for undoped composition BaLaInO4 at a ~1.5 order of magnitude. The increase in the conductivity values was mainly attributed to the increase of the carrier concentration as a result of the formation of oxygen vacancies during doping. The oxygen ionic conductivity of doped samples increased in the order of BaLa0.9Ca0.1InO3.95–BaLa0.9Sr0.1InO3.95–BaLa0.9Ba0.1InO3.95, i.e., in the order of increasing the ionic radius of dopants. The increase in the lattice volume and the lattice parameters weakens the metal-oxygen bonding, and, as a consequence, increases the oxygen mobility. The activation energy for oxygen ion conduction decreased in the same order.
The conductivity increased in wet conditions as a result of the insertion of water and the formation of proton charge carriers. The proton conductivity was estimated by subtracting the conductivity data for dry Ar from the corresponding data in wet Ar. The proton conductivities of doped samples increased in the order Ca2+–Sr2+–Ba2+ due to an increase in the concentration of protons. The activation energies for the proton conductivities were 0.70–0.68–0.65 eV, respectively.
Author Contributions
Conceptualization, I.A. and N.T.; methodology, I.A.; investigation, A.G. and D.K.; data curation, N.T. and A.G.; writing—original draft preparation, N.T.; writing—review and editing, I.A.
Funding
A grant from the President of the Russian Federation (project MK-24.2019.3) supported this research.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Shim, J.H. Ceramics breakthrough. Nat. Energy 2018, 3, 168–169. [Google Scholar] [CrossRef]
- Fabbri, E.; Bi, L.; Pergolesi, D.; Traversa, E. Towards the next generation of solid oxide fuel cells operating below 600 °C with chemically stable proton-conducting electrolytes. Adv. Mater. 2012, 24, 195–208. [Google Scholar] [CrossRef]
- Marrony, M.; Dailly, J. Advanced Proton Conducting Ceramic Cell as Energy Storage Device. ECS Trans. 2017, 78, 3349–3363. [Google Scholar] [CrossRef]
- Norby, T. Advances in proton ceramic fuel cells, steam electrolyzers, and dehydrogenation reactors based on materials and process optimizations. ECS Trans. 2017, 80, 23–32. [Google Scholar] [CrossRef]
- Colomban, P.; Zaafrani, O.; Slodczyk, A. Proton Content and Nature in Perovskite Ceramic Membranes for Medium Temperature Fuel Cells and Electrolysers. Membranes 2012, 2, 493–509. [Google Scholar] [CrossRef]
- Kochetova, N.; Animitsa, I.; Medvedev, D.; Demin, A.; Tsiakaras, P. Recent activity in the development of proton-conducting oxides for high-temperature applications. RSC Adv. 2016, 6, 73222–73268. [Google Scholar] [CrossRef]
- Duan, C.; Tong, J.; Shang, M.; Nikodemski, S.; Sanders, M.; Ricote, S.; Almansoori, A.; O’Hayre, R. Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 2015, 349, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Dubois, A.; Ricote, S.; Braun, R. Benchmarking the expected stack manufacturing cost of next generation, intermediate-temperature protonic ceramic fuel cells with solid oxide fuel cell technology. J. Power Sources 2017, 369, 65–77. [Google Scholar] [CrossRef]
- De Souza, E.C.C.; Muccillo, R. Properties and applications of perovskite proton conductors. Mater. Res. 2010, 13, 385–394. [Google Scholar] [CrossRef]
- Duan, C.; Kee, R.J.; Zhu, H.; Karakaya, C.; Chen, Y.; Ricote, S.; Jarry, A.; Crumlin, E.J.; Hook, D.; Braun, R.; et al. Highly durable, coking and sulfur tolerant, fuel-flexible protonic ceramic fuel cells. Nature 2018, 557, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kucharczyk, C.J.; Liang, Y.; Zhang, X.; Takeuchi, I.; Ji, H.-I.; Haile, S.M. Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nat. Energy 2018, 3, 202–210. [Google Scholar] [CrossRef]
- Fujii, K.; Shiraiwa, M.; Esaki, Y. Improved oxide-ion conductivity of NdBaInO4 by Sr doping. J. Mater. Chem. A 2015, 3, 11985–11990. [Google Scholar] [CrossRef]
- Yang, X.; Liu, S.; Lu, F.; Xu, J.; Kuang, X. Acceptor Doping and Oxygen Vacancy Migration in Layered Perovskite NdBaInO4-Based Mixed Conductors. J. Phys. Chem. C 2016, 120, 6416–6426. [Google Scholar] [CrossRef]
- Shiraiwa, M.; Fujii, K.; Esaki, Y.; Kim, S.J.; Lee, S.; Yashima, M. Crystal Structure and Oxide-Ion Conductivity of Ba1+xNd1−xInO4−x/2. J. Electrochem. Soc. 2017, 164, F1392–F1399. [Google Scholar] [CrossRef]
- Birgeneau, R.J.; Guggenheim, H.J.; Shirane, G. Neutron scattering investigation of phase transitions and magnetic correlations in the two-dimensional antiferromagnets K2NiF4, Rb2MnF4, Rb2FeF4. Phys. Rev. B 1970, 1, 2211–2230. [Google Scholar] [CrossRef]
- Cochrane, A.K.; Telfer, M.; Dixon, C.A.L.; Zhang, W.; Halasyamani, P.S.; Bousquet, E.; Lightfoot, P. NdBaScO4: Aristotype of a new family of geometric ferroelectrics. Chem. Commun. 2016, 52, 10980–10983. [Google Scholar] [CrossRef]
- Fuji, K.; Yashima, M. Discovery and development of BaNdInO4—A brief review. J. Ceram. Soc. Jpn. 2018, 126, 852–859. [Google Scholar] [CrossRef]
- Ishihara, T.; Yan, Y.; Sakai, T.; Ida, S. Oxide ion conductivity in doped NdBaInO4. Solid State Ion. 2016, 288, 262–265. [Google Scholar] [CrossRef]
- Troncoso, L.; Alonso, J.A.; Fernández-Díaz, M.T.; Aguadero, A. Introduction of interstitial oxygen atoms in the layered perovskite LaSrIn1−xBxO4+δ system (B = Zr, Ti). Solid State Ion. 2015, 282, 82–87. [Google Scholar] [CrossRef]
- Troncoso, L.; Alonso, J.A.; Aguadero, A. Low activation energies for interstitial oxygen conduction in the layered perovskites La1+xSr1−xInO4+δ. J. Mater. Chem. A 2015, 3, 17797–17803. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I. Protonic transport in oxyfluorides Ba2InO3F and Ba3In2O5F2 with Ruddlesden-Popper structure. Solid State Ion. 2015, 275, 53–57. [Google Scholar] [CrossRef]
- Li, X.; Shimada, H.; Ihara, M. Conductivity of New Electrolyte Material Pr1−xM1+xInO4 (M = Ba, Sr) with Related Perovskite Structure for Solid Oxide Fuel Cells. ECS Trans. 2013, 50, 3–14. [Google Scholar] [CrossRef]
- Matsuhira, T.; Kurahashi, Y.; Hasegawa, K.; Ihara, M. Proton Conducting Properties of Sr1+xLn1−xAlO4−δ (Ln = Pr, Sm) with Layered Perovskite Structure for Solid Oxide Fuel Cells. In Proceedings of the 232nd ECS Meeting, National Harbor, MD, USA, 1–5 October 2017. [Google Scholar]
- Tyitov, Y.O.; Byilyavina, N.M.; Markyiv, V.Y.; Slobodyanik, M.S.; Krajevs’ka, Y.A. Synthesis and crystal structure of BaLaInO4 and SrLnInO4 (Ln−La, Pr). Dopovyidyi Natsyional'noyi Akademyiyi Nauk Ukrayini 2009, 10, 160–166. [Google Scholar]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. App. Cryst. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Act. Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Jedvik, E.; Lindman, A.; Benediktsson, M.; Wahnström, G. Size and shape of oxygen vacancies and protons in acceptor-doped barium zirconate. Solid State Ion. 2015, 275, 2–8. [Google Scholar] [CrossRef]
- Norby, T. Chapter 11. Proton Conductivity in Perovskite Oxides. In Perovskite Oxide for Solid Oxide Fuel Cells; Ishihara, T., Ed.; Springer Science+Business Media, LLC: Berlin/Heidelberg, Germany, 2009; pp. 217–241. [Google Scholar]
- Shpanchenko, R.V.; Antipov, E.V.; Kovba, L.M. Ba2ZrO4 and its crystallohydrates. Russ. J. Inorg. Chem. 1993, 38, 599. [Google Scholar]
- Lehtimäki, M. Stability, Cation Ordering and Oxygen Non-Stoichiometry of Some Perovskites and Related Layered Oxides. Ph.D. Dissertation, Aalto University, Aalto, Finland, 2013. [Google Scholar]
- Wakamura, K. Ion conduction in proton- and related defect (super) ionic conductors: Mechanical, electronic and structure parameters. Solid State Ion. 2009, 180, 1343–1349. [Google Scholar] [CrossRef]
- Kharton, V.V.; Marques, F.M.B.; Kilner, J.A.; Atkinson, A. Oxygen Ion-Conducting Materials in Solid State Electrochemistry I: Fundamentals, Materials and Their Applications; Kharton, V.V., Ed.; WILEY-VCH Verlag GmbH & Co.: Weinheim, Germany, 2009; ISBN 978-3-527-32318-0. [Google Scholar]
- Liang, K.C.; Du, Y.; Nowick, A.S. Fast high-temperature proton transport in nonstoichiometric mixed perovskites. Solid State Ion. 1994, 69, 117–120. [Google Scholar] [CrossRef]
- Wakamura, K. Empirical relationships for ion conduction based on vibration amplitude in perovskite-type proton and superionic conductors. J. Phys. Chem. Solids 2005, 66, 133–142. [Google Scholar] [CrossRef]
- Proton Conductors: Solids, Membranes and Gels—Materials and Devices (Chemistry of Solid State Materials); Colomban, P., Ed.; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar] [CrossRef]
- Jing, Y.; Matsumoto, H.; Aluru, N.R. Mechanistic Insights into Hydration of Solid Oxides. Chem. Mater. 2018, 30, 138–144. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).