Waste Brick Dust as Potential Sorbent of Lead and Cesium from Contaminated Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Waste Brick Dust
2.2. Model Solutions
2.3. Sorption Experiments
2.4. Leaching Test
2.5. Analytical Methods
3. Results and Discussion
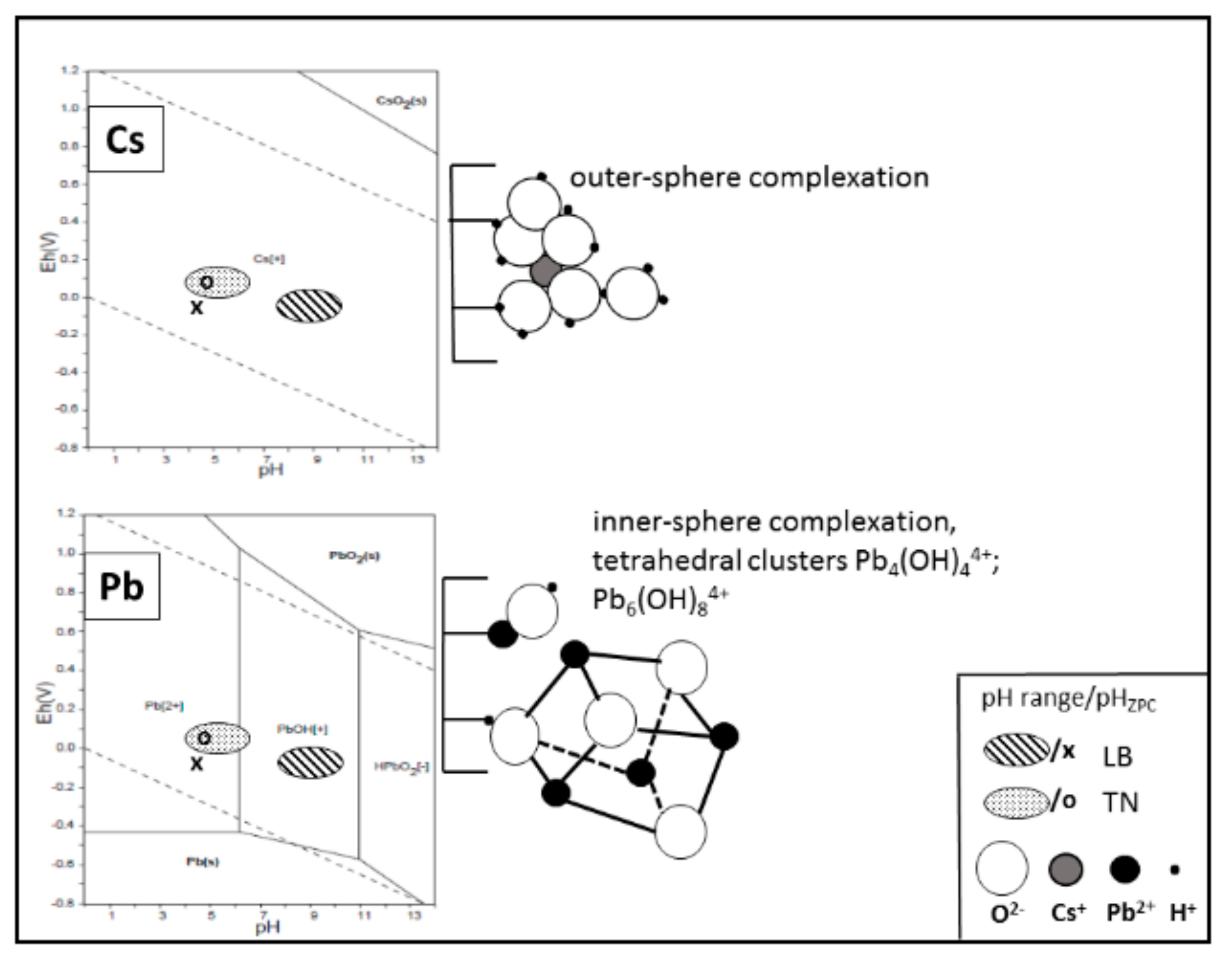
3.1. Specification of WBD Adsorbents in Relation to Pb/Cs Chemistry and pH Value
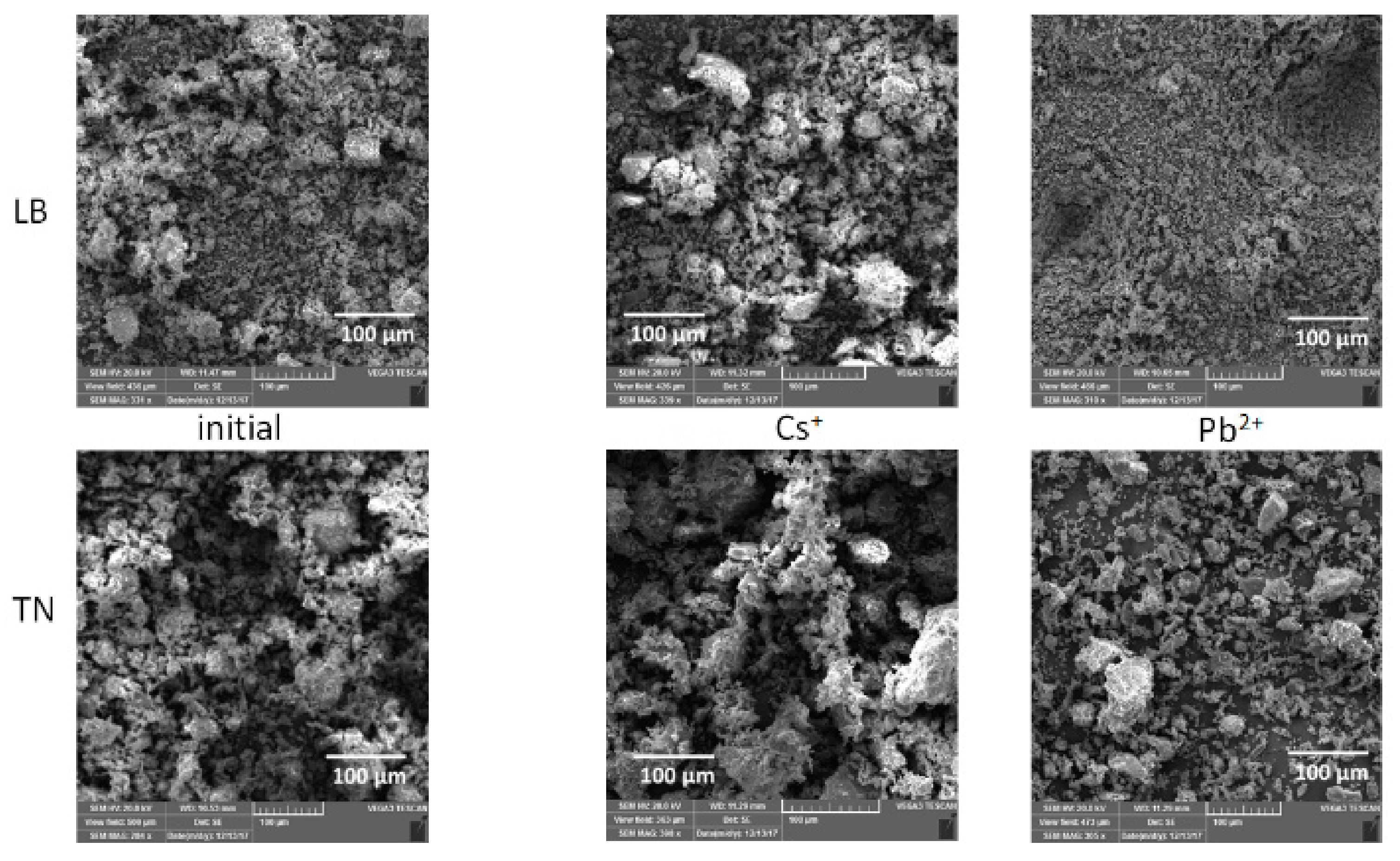
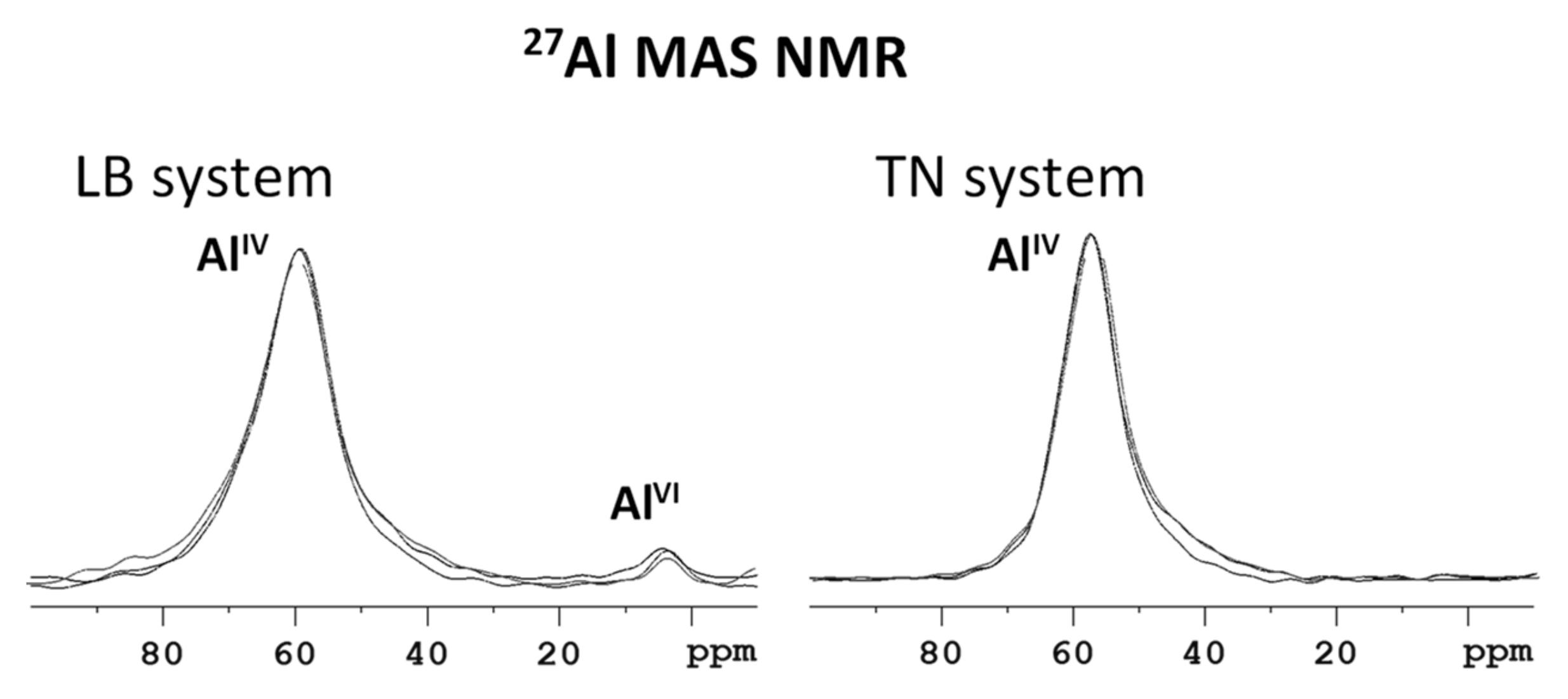
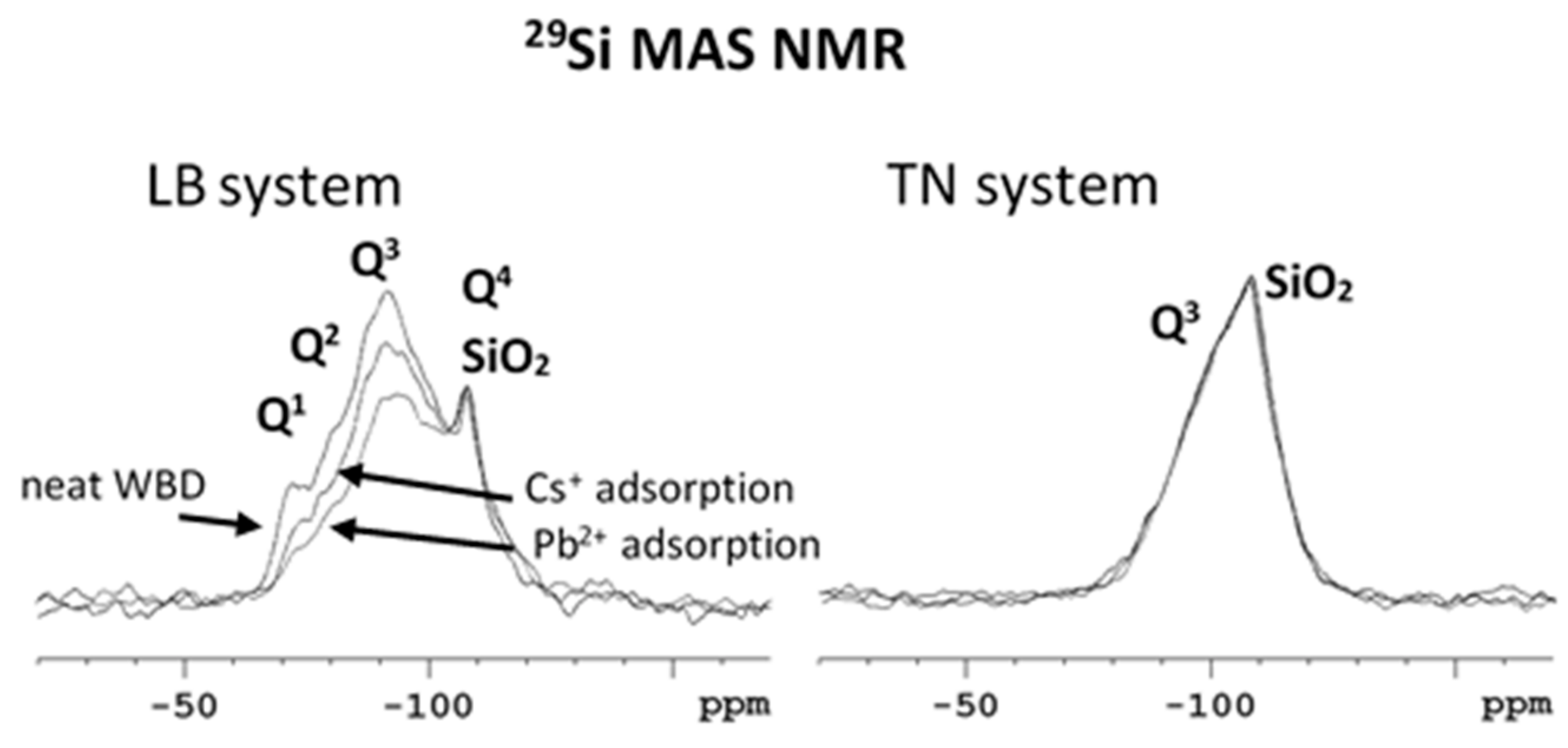
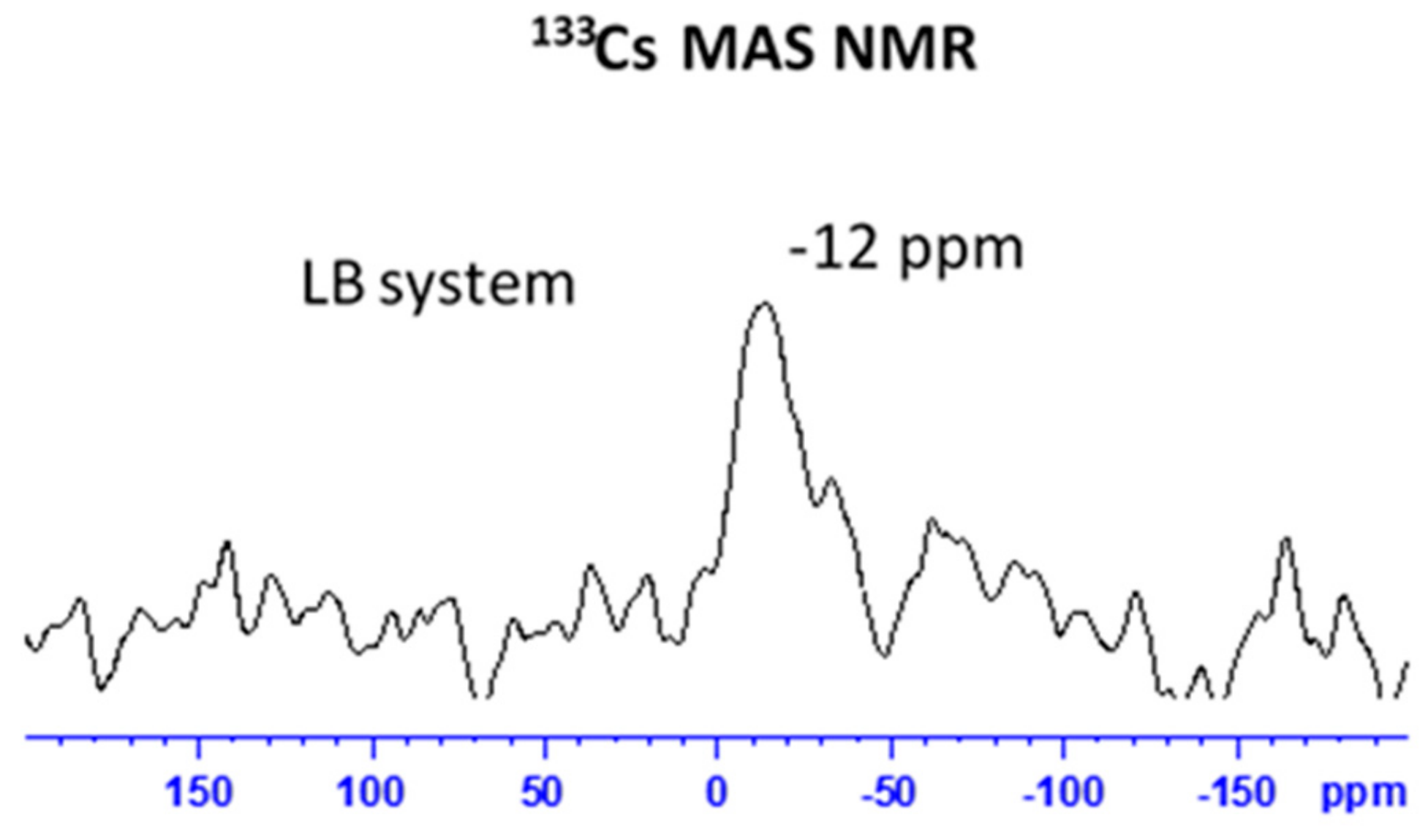
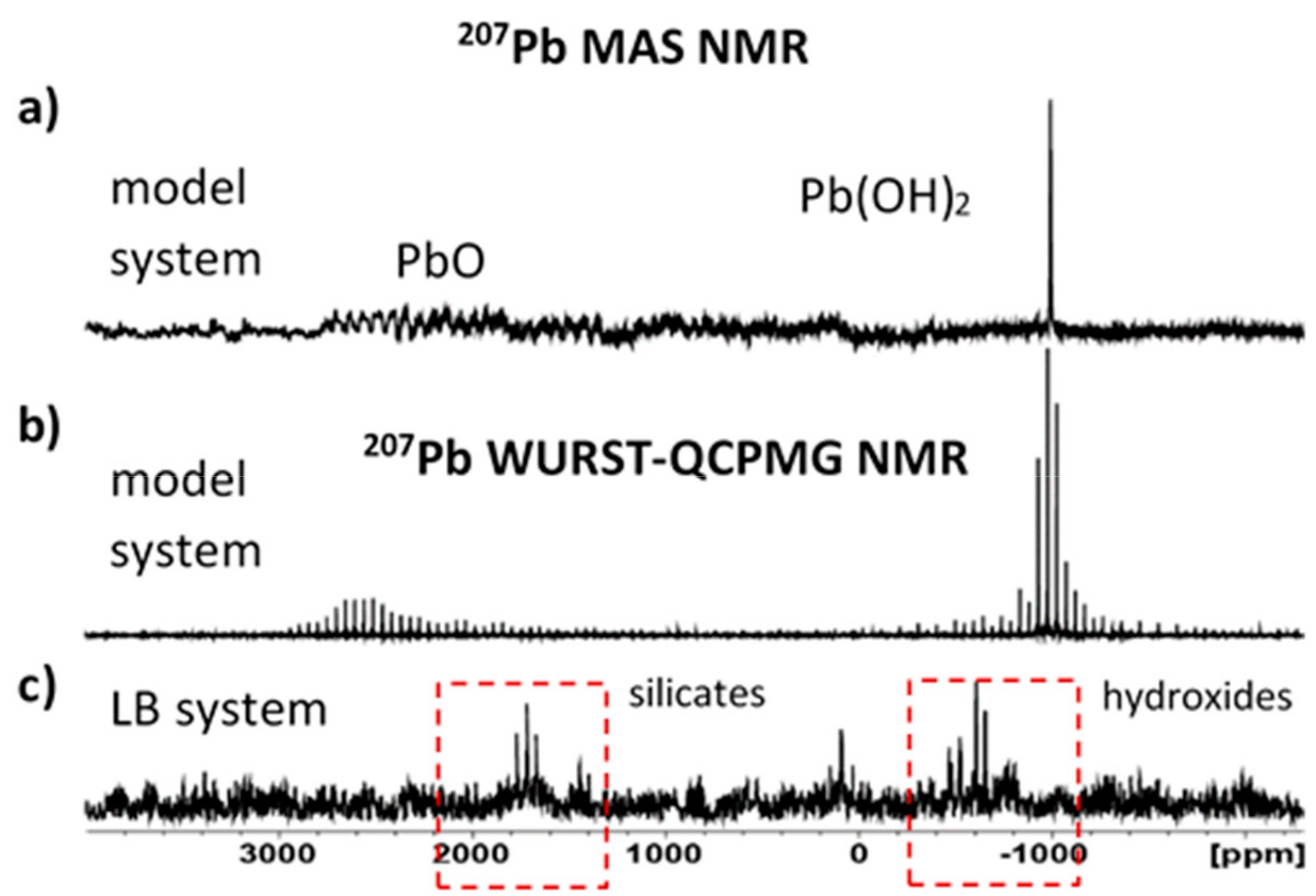
3.2. Structural Changes Following Adsorption
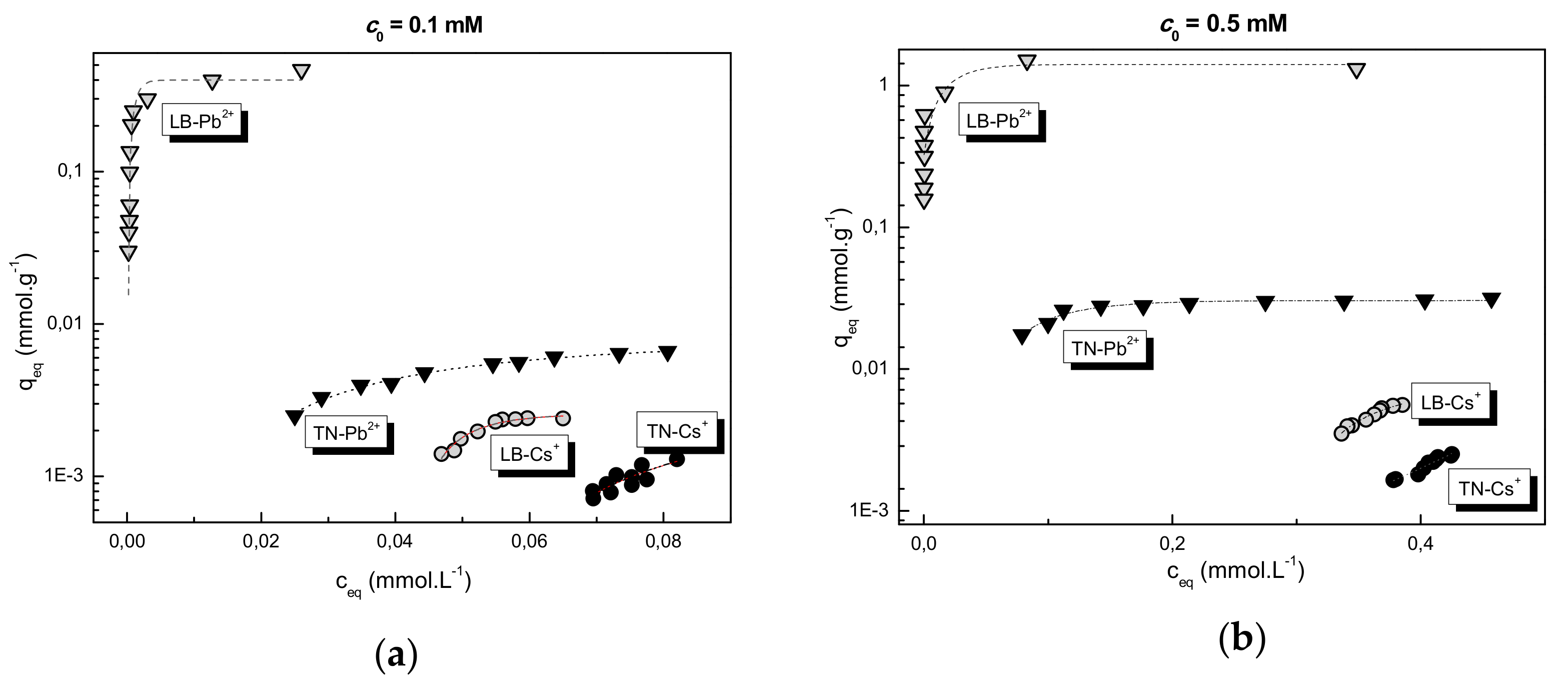
3.3. Adsorption of Pb2+/Cs+ on WBD
3.4. Leachability of Pb2+/Cs+ from Saturated Sorbents
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wong, C.S.C.; Li, X.D. Pb contamination and isotopic composition of urban soils in Hong Kong. Sci. Total Environ. 2004, 319, 185–195. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, Q.; Jin, X.; Chen, Z. Removal of Pb(II) from aqueous solution using modified and unmodified kaolinite clay. J. Hazard. Mater. 2009, 170, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Benedicto, A.; Missana, T.; Fernández, A.M. Interlayer Collapse Affects on Cesium Adsorption onto Illite. Environ. Sci. Technol. 2014, 48, 4909–4915. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M. Adsorption of cesium on minerals: A review. J. Radioanal. Nuclear Chem. 1993, 171, 483–500. [Google Scholar] [CrossRef]
- Dahiya, S.; Tripathi, R.M.; Hegde, A.G. Biosorption of heavy metals and radionuclide from aqueous solutions by pre-treated arcashell biomass. J. Hazard. Mater. 2008, 150, 376–386. [Google Scholar] [CrossRef]
- Honeyman, B.D.; Santsch, P.H. Metals in aquatic environment. Environ. Sci. Technol. 1988, 22, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Song, K.C.; Lee, H.K.; Moon, H.; Lee, K.J. Simultaneous removal of the radiotoxic nuclides 137Cs & 129I from aqueous solution. Sep. Purif. Technol. 1997, 12, 215–217. [Google Scholar]
- Jalali, R.R.; Ghafourian, H.; Asef, Y.; Dalir, S.T.; Sahafipour, M.H.; Gharanjik, B.M. Biosorption of cesium by native and chemically modified biomass of marine algae: Introduce the new biosorbent for biotechnology applications. J. Hazard. Mater. 2004, B116, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Lu, Z.; Zou, W.; Daotong, W.; Shi, J.; Jiujun, Y. Removal of copper(II) and lead(II) from aqueous solution by manganese oxide coated sand. II. Equilibrium study and competitive adsorption. J. Hazard. Mater. 2006, B137, 480–488. [Google Scholar] [CrossRef]
- Mousavi, H.Z.; Seyedi, S.R. Kinetic and equilibrium studies on the removal of Pb(II) from aqueous solution using nettle ash. J. Chil. Chem. Soc. 2010, 55, 307–311. [Google Scholar] [CrossRef]
- Ding, D.; Lei, Z.; Yang, Y.; Feng, C.; Zhang, Z. Selective removal of cesium from aqueous solutions with nickel (II) hexacyanoferrate (III) functionalized agricultural residue–walnut shell. J. Hazard. Mater. 2014, 270, 187–195. [Google Scholar] [CrossRef]
- Badescu, I.S.; Bulgariu, D.; Ahmad, I.; Bulgariu, L. Valorisation possibilities of exhausted biosorbents loaded with metal ions—A review. J. Environ. Manag. 2018, 224, 288–297. [Google Scholar] [CrossRef]
- Silva, R.V.; de Brito, J.; Dhir, R.K. Properties and composition of recycled aggregates from construction and demolition waste suitable for concrete production. Con. Build. Mat. 2014, 65, 201–217. [Google Scholar] [CrossRef]
- Vejmelková, E.; Keppert, M.; Rovnaníková, P.; Ondráček, M.; Keršner, Z.; Černý, R. Properties of high performance concrete containing fine-ground ceramics as supplementary cementitious material. Cement Concrete Compos. 2012, 34, 55–61. [Google Scholar] [CrossRef]
- Dousova, B.; Kolousek, D.; Keppert, M.; Machovic, V.; Lhotka, M.; Urbanova, M.; Brus, J.; Holcova, L. Use of waste ceramics in adsorption technologies. Appl. Clay Sci. 2016, 134, 145–152. [Google Scholar] [CrossRef]
- Doušová, B.; Grygar, T.; Martaus, A.; Fuitová, L.; Koloušek, D.; Machovič, V. Sorption of AsV on aluminosilicates treated with FeII nanoparticles. J. Colloid Interface Sci. 2006, 302, 424–431. [Google Scholar] [CrossRef]
- Jeong, Y.; Fan, M.; Singh, S.; Chuang, C.L.; Saha, B.; van Leeuwen, J.H. Evaluation of iron oxide and aluminium oxide as potential arsenic(V) adsorbents. Chem. Eng. Process. 2007, 46, 1030–1039. [Google Scholar] [CrossRef]
- Misak, N.Z. Langmuir isotherm and its application in ion-exchange reactions. React. Polym. 1993, 21, 53–64. [Google Scholar] [CrossRef]
- CEN 2002. CEN 2002. Characterization of Waste-Leaching-Compliance Test for Leaching of Granular Waste Material and Sludge Part 2. Comite Europeen de Normalisation 2002, EN 12457-2. Available online: https://www.sis.se/en/produkter/environment-health-protection-safety/wastes/solid-wastes/ssen124574/ (accessed on 1 October 2003).
- Massiot, D.; Farnan, I.; Gautier, N.; Trumeau, D.; Trokiner, A.; Coutures, J.P. Ga-71 and Ga-69 Nuclear-Magnetic-Resonance Study of Beta-Ga2O3-Resolution of 4-Fold and 6-Fold Coordinated Ga Sites in Static Conditions. Solid State Nucl. Magn. Reson. 1995, 4, 241–248. [Google Scholar] [CrossRef]
- Brus, J. Heating of samples induced by fast magic-angle spinning. Solid State Nucl. Magn. Reson. 2000, 16, 151–160. [Google Scholar] [CrossRef]
- Walther, J.V. Essentials of Geochemistry, 2nd ed.; Jones and Bartlett Publishers Inc.: Sudbury, MA, USA, 2009; 486p. [Google Scholar]
- Bohn, H.L.; Mc Neal, B.L.; O’Conner, G.A. Soil Chemistry, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1985; 341p. [Google Scholar]
- Um, W.; Papelis, C. Sorption mechanisms of Sr(II) and Pb(II) on zeolitized tuffs from the Nevada Test Site as a function of pH and ionic strength. Am. Mineral. 2003, 88, 2028–2039. [Google Scholar] [CrossRef]
- Marcus, Y. Thermodynamics of Solvation of Ions. J. Chem. Soc. Faraday Trans. 1991, 87, 2995–2999. [Google Scholar] [CrossRef]
- Ding, M.; de Jong, B.H.W.S.; Roosendaal, S.J.; Vredenberg, A. XPS studies on the electronic structure of bonding between solid and solutes: Adsorption of arsenate, chromate, phosphate, Pb2+, and Zn2+ ions on amorphous black ferric oxyhydroxide. Geochim. Cosmochim. Acta 2000, 64, 1209–1219. [Google Scholar] [CrossRef]
- Wen, X.; Du, Q.; Tang, H. Surface complexation model for the heavy metal adsorption on natural sediment. Environ. Sci. Technol. 1998, 32, 870–875. [Google Scholar] [CrossRef]
- Fayon, F.; Farnan, I.; Bessada, C.; Coutures, J.; Massiot, D.; Coutures, J.P. Empirical Correlations between 207Pb NMR Chemical Shifts and Structure in Solids. J. Am. Chem. Soc. 1997, 119, 6837–6843. [Google Scholar] [CrossRef]
- Rajec, P.; Macasek, F.; Feder, M.; Misaelides, P.; Samajova, E. Sorption of caesium and strontium on clinoptilolite- and mordenite-containing sedimentary rocks. J. Radioanal. Nucl. Chem. 1998, 229, 49–56. [Google Scholar] [CrossRef]
- Faghihian, H.; Marageh, M.G.; Kazemian, H. The use of clinoptilolite and its sodium form for removal of radioactive cesium and strontium from nuclear wastewater and Pb2+, Ni2+, Cd2+, Ba2+ from municipal wastewater. Appl. Radiat. Isotopes 1999, 50, 655–660. [Google Scholar] [CrossRef]
- Bergaoui, L.; Lambert, J.F.; Prost, R. Caesium adsorption on soil clay: Macroscopic and spectroscopic measurements. Appl. Clay Sci. 2005, 29, 23–29. [Google Scholar] [CrossRef]

| Composition (wt%) | LB | TN |
|---|---|---|
| Na2O/K2O | 1.1/4.3 | 2.5/2.8 |
| MgO/CaO | 1.5/14.4 | 1.3/1.6 |
| Al2O3 | 19.7 | 32.6 |
| SiO2 | 46.4 | 60.3 |
| P2O5 | 0.4 | 0.3 |
| SO3 | 1.5 | 0.6 |
| Fe2O3 | 3.7 | 5.4 |
| TiO2 | 0.4 | 0.8 |
| MnO2 | 0.03 | 0.04 |
| SBET (m2 g−1) | 4.1 | 2.5 |
| Vtotal (cm3 g−1)*) | 0.01 | 0.006 |
| pH **) | ~9–10 | ~5–6 |
| pHZPC | 4.3 | 5.5 |
| Cation | r(Å) | r′(Å) | n | ΔGhydr (kJ mol−1) |
|---|---|---|---|---|
| Cs+ | 1.69 | 3.28 | 2.1 | −250 |
| Pb2+ | 1.32 | 4.03 | 6.1 | −1425 |
| Sorbent/c0 (mM) | Pb2+ | Cs+ | ||||||
|---|---|---|---|---|---|---|---|---|
| qmax (mmol g−1) | Qtheor (mmol g−1) | KL (L mmol−1) | R2 | qmax (mmol g−1) | Qtheor (mmol g−1) | KL (L mmol−1) | R2 | |
| LB/0.5 | 1.32 | 1.6 | 776.4 | 0.973 | 5.5 × 10−3 | - | - | - |
| LB/0.1 | 0.85 | 1.1 | 171.5 | 0.930 | 2.4 × 10−3 | - | - | - |
| TN/0.5 | 3.4 × 10−2 | 4.5 × 10−2 | 16.7 | 0.827 | 2.9 × 10−3 | - | - | - |
| TN/0.1 | 5.5 × 10−3 | 6.8 × 10−3 | 39.8 | 0.838 | 1.1 × 10−3 | - | - | - |
| Element/Initial Concentration | Initial Amount in Saturated WBD (mg·g−1) | Leached in H2O (%) | Class of Leachability* |
|---|---|---|---|
| Libochovice (LB) | |||
| Cs+/0.1 mM | 0.32 | 3.7 | not limited |
| Cs+/0.5 mM | 0.74 | 7.4 | not limited |
| Pb2+/0.1 mM | 146.3 | 1.2 × 10−3 | IIa—other waste |
| Pb2+/0.5 mM | 212.8 | 6.6 × 10−4 | I—inert waste |
| Tyn nad Vltavou (TN) | |||
| Cs+/0.1 mM | 0.15 | 12.3 | not limited |
| Cs+/0.5 mM | 0.33 | 11.4 | not limited |
| Pb2+/0.1 mM | 0.93 | 0.25 | IIa—other waste |
| Pb2+/0.5 mM | 5.98 | 0.18 | IIa—other waste |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doušová, B.; Koloušek, D.; Lhotka, M.; Keppert, M.; Urbanová, M.; Kobera, L.; Brus, J. Waste Brick Dust as Potential Sorbent of Lead and Cesium from Contaminated Water. Materials 2019, 12, 1647. https://doi.org/10.3390/ma12101647
Doušová B, Koloušek D, Lhotka M, Keppert M, Urbanová M, Kobera L, Brus J. Waste Brick Dust as Potential Sorbent of Lead and Cesium from Contaminated Water. Materials. 2019; 12(10):1647. https://doi.org/10.3390/ma12101647
Chicago/Turabian StyleDoušová, Barbora, David Koloušek, Miloslav Lhotka, Martin Keppert, Martina Urbanová, Libor Kobera, and Jiří Brus. 2019. "Waste Brick Dust as Potential Sorbent of Lead and Cesium from Contaminated Water" Materials 12, no. 10: 1647. https://doi.org/10.3390/ma12101647
APA StyleDoušová, B., Koloušek, D., Lhotka, M., Keppert, M., Urbanová, M., Kobera, L., & Brus, J. (2019). Waste Brick Dust as Potential Sorbent of Lead and Cesium from Contaminated Water. Materials, 12(10), 1647. https://doi.org/10.3390/ma12101647






