Studies on Pitting Corrosion of Al-Cu-Li Alloys Part I: Effect of Li Addition by Microstructural, Electrochemical, In-situ, and Pit Depth Analysis
Abstract
1. Introduction
2. Experimental
2.1. Materials and Microstructure Characterization
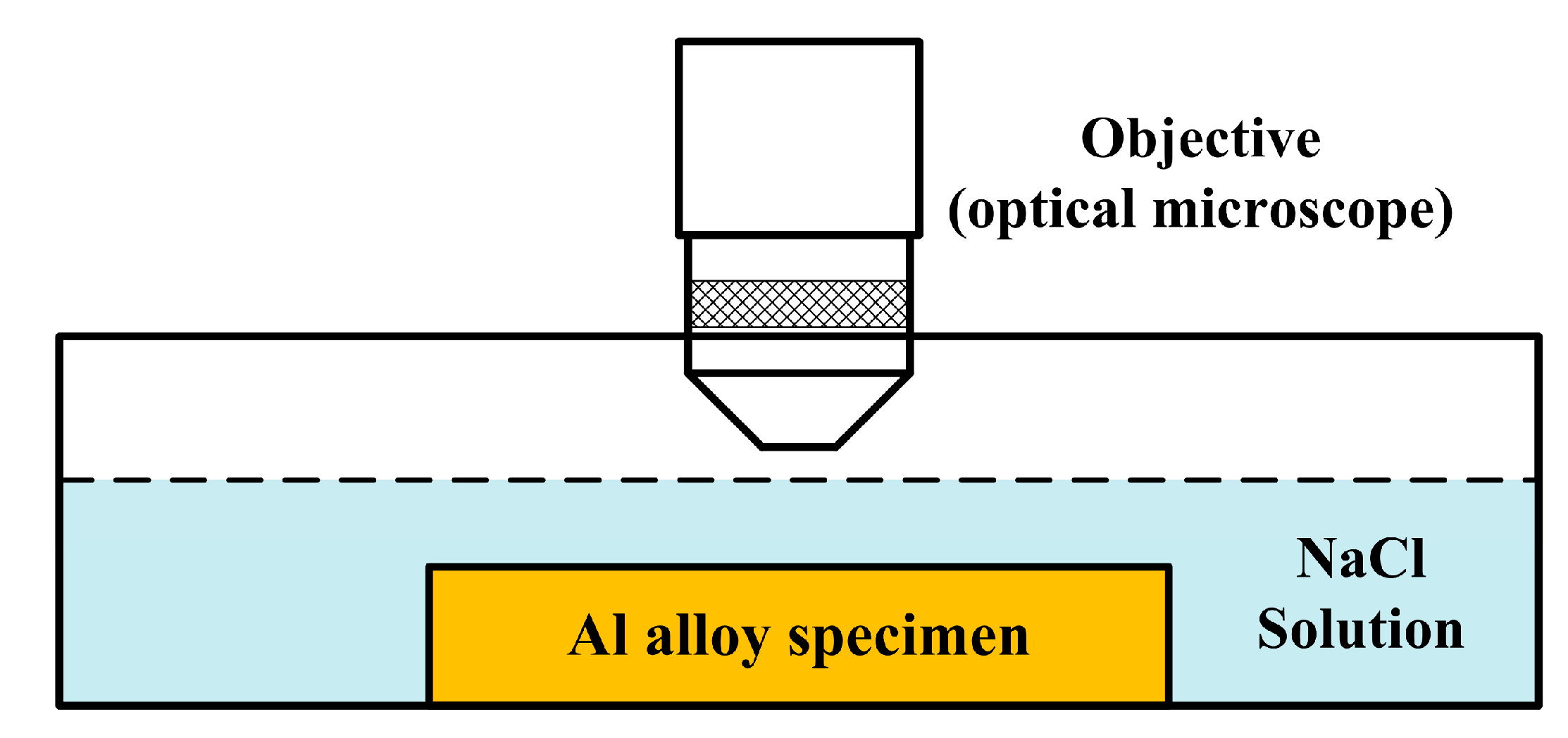
2.2. In-Situ Immersion Test
2.3. Electrochemical Measurements
3. Results
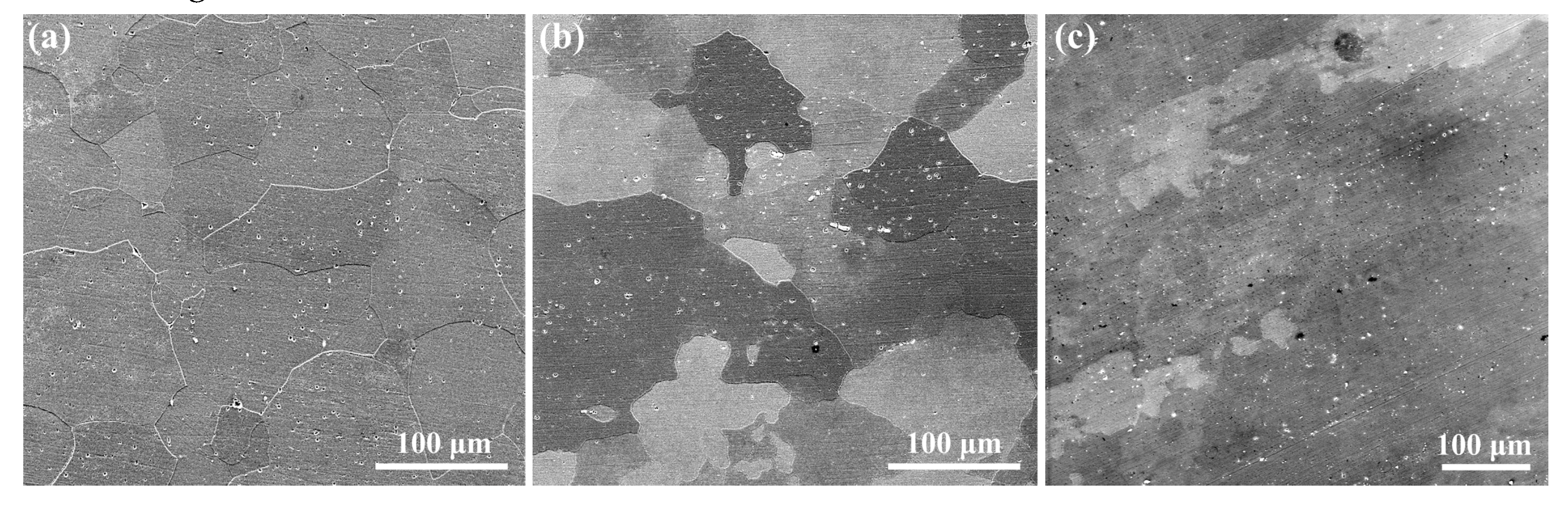
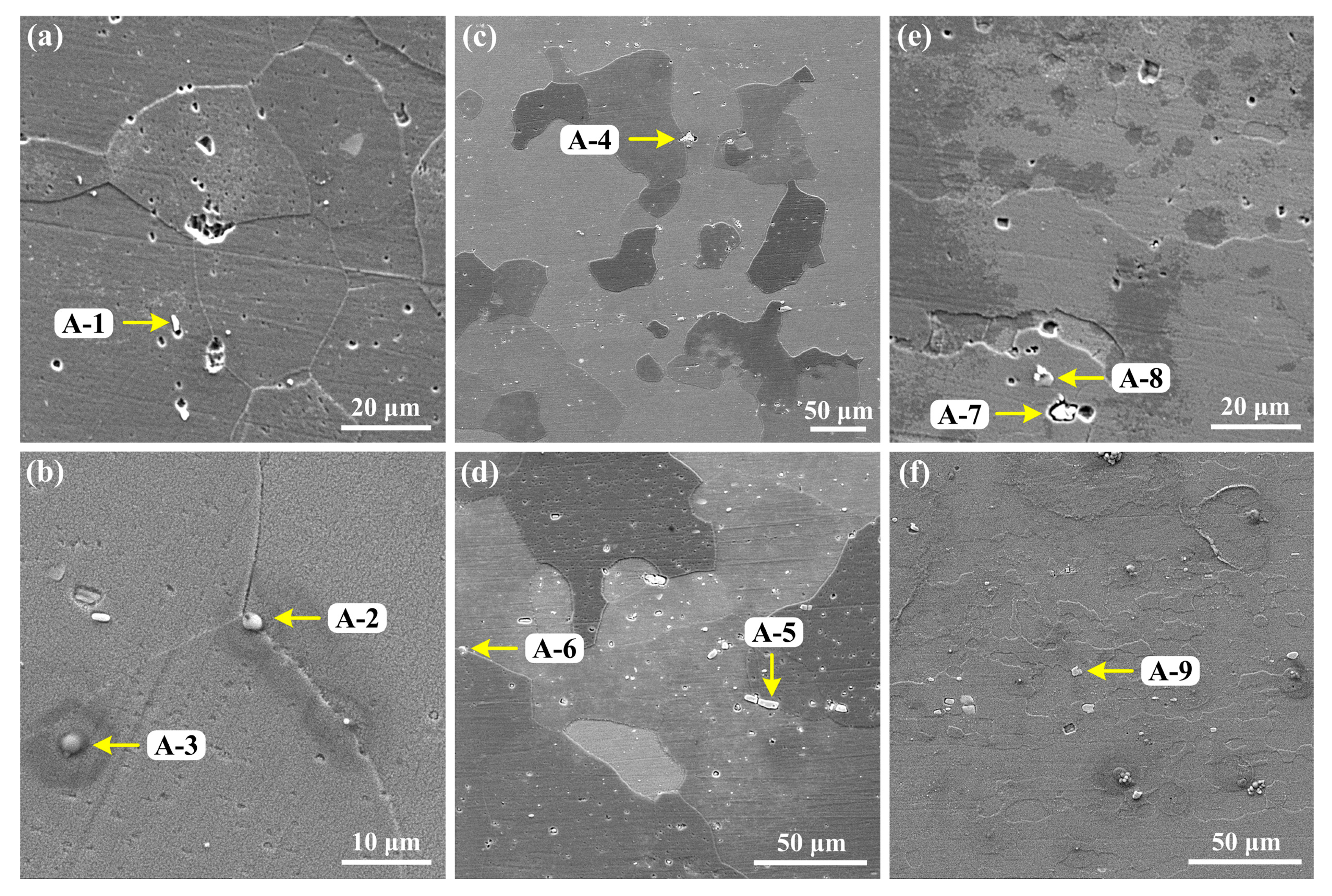
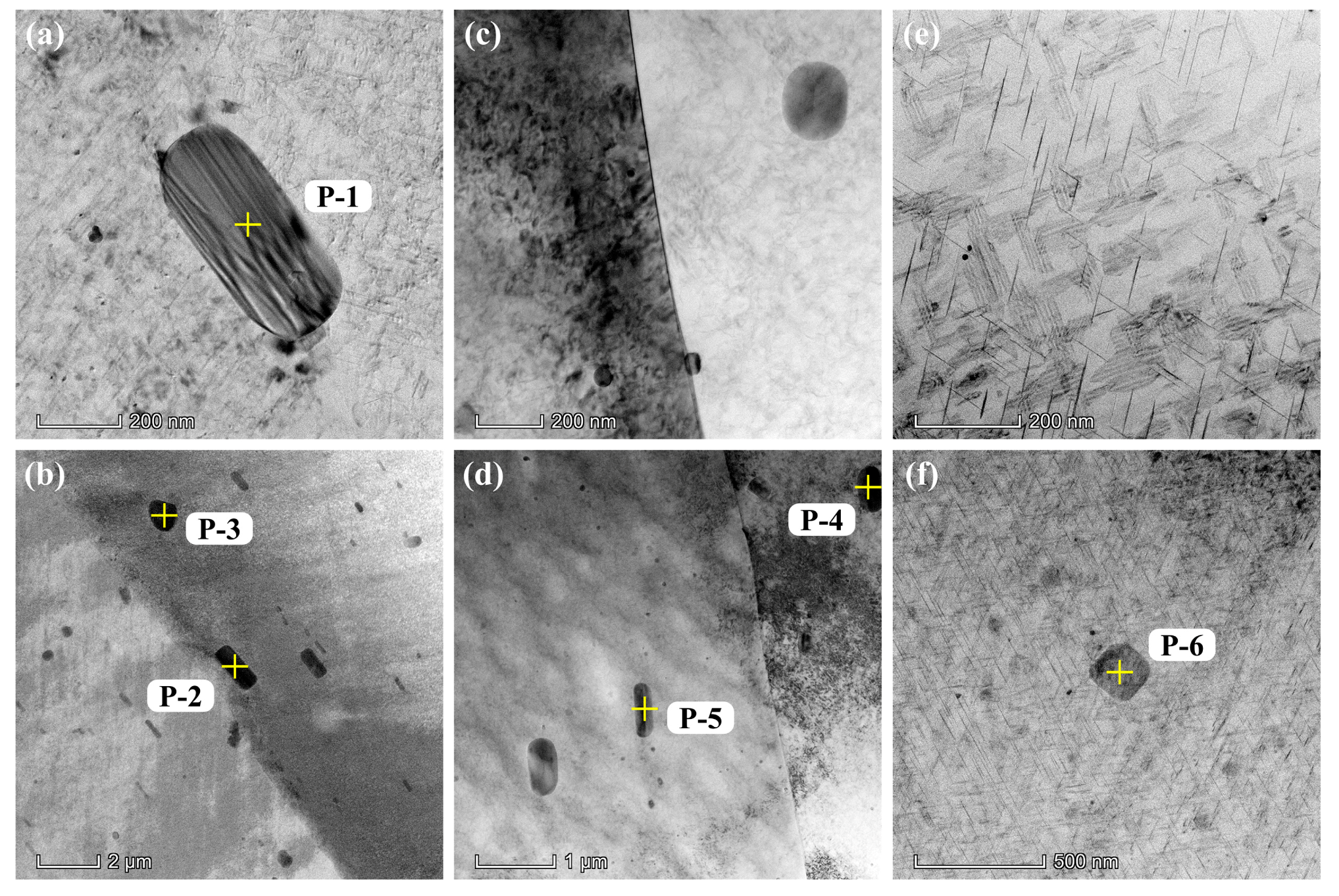
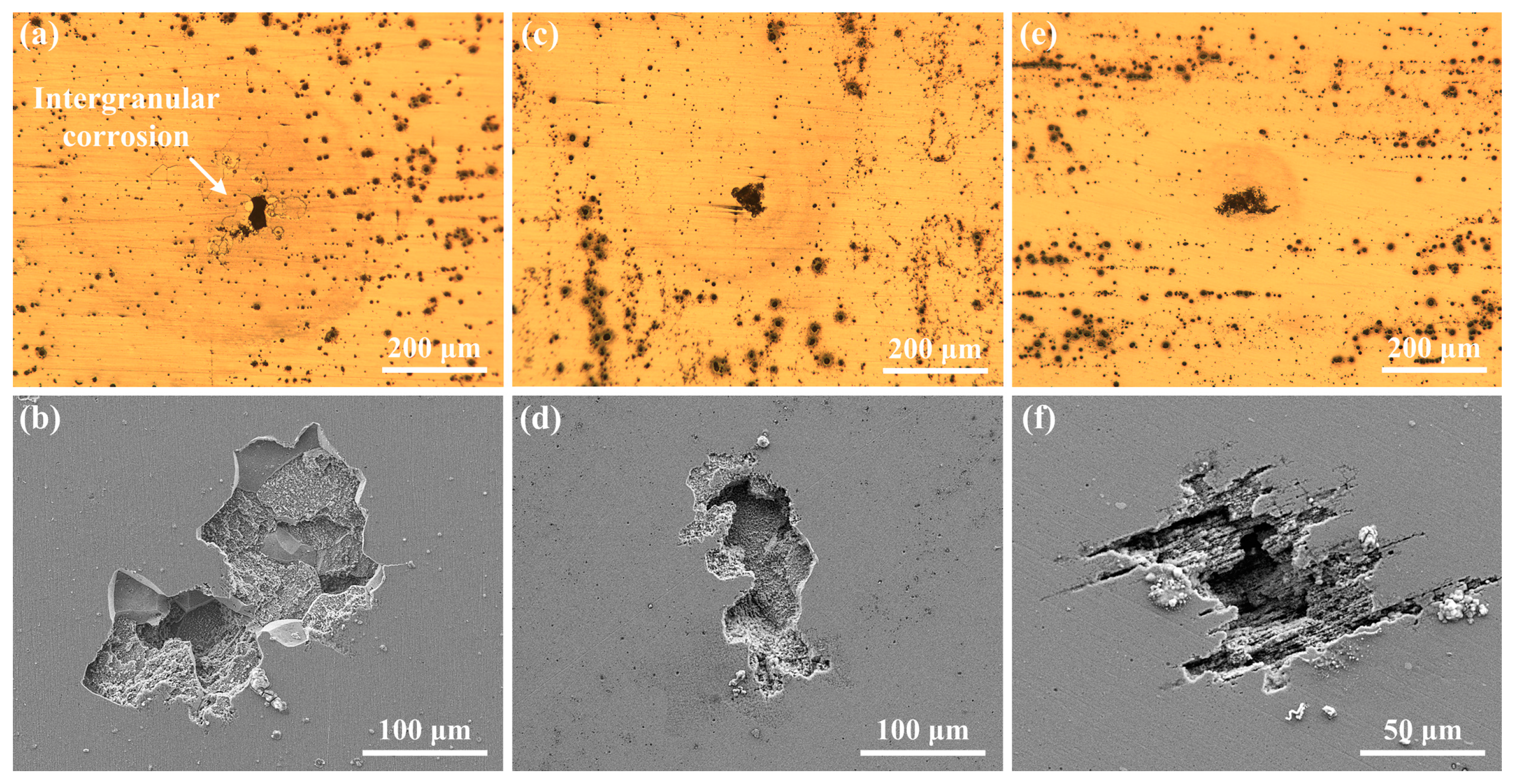
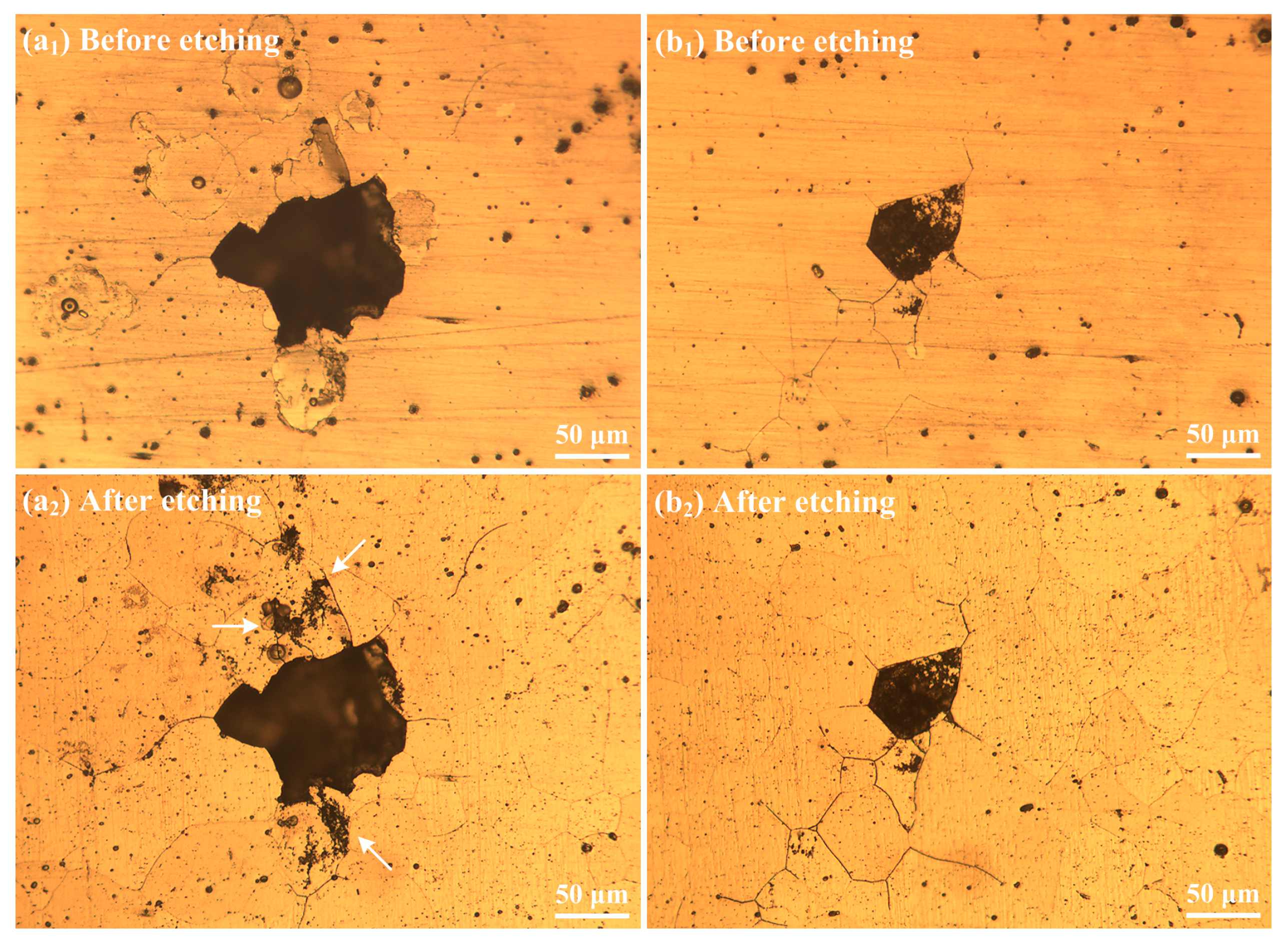
3.1. Microstructures
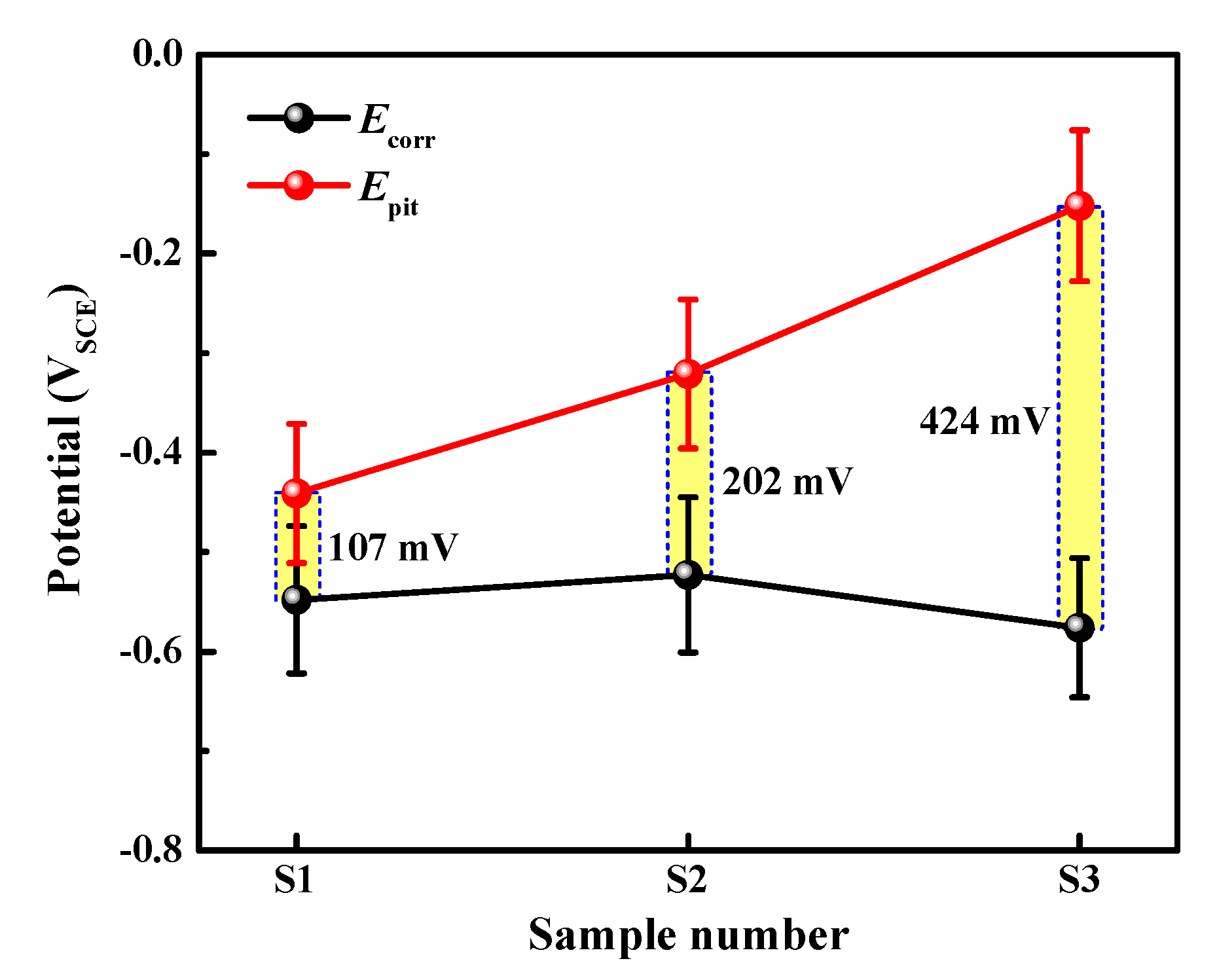
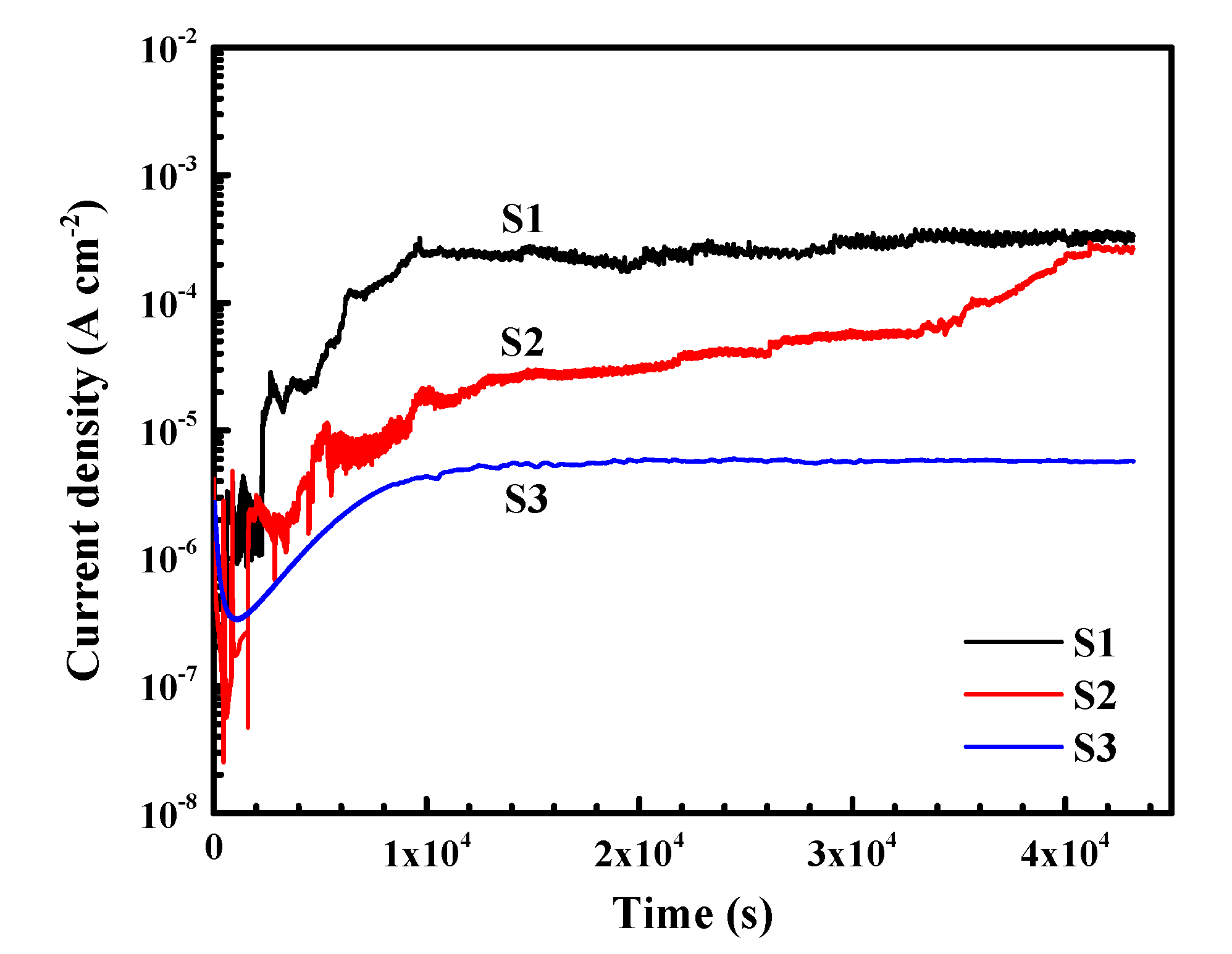
3.2. Electrochemical Measurements
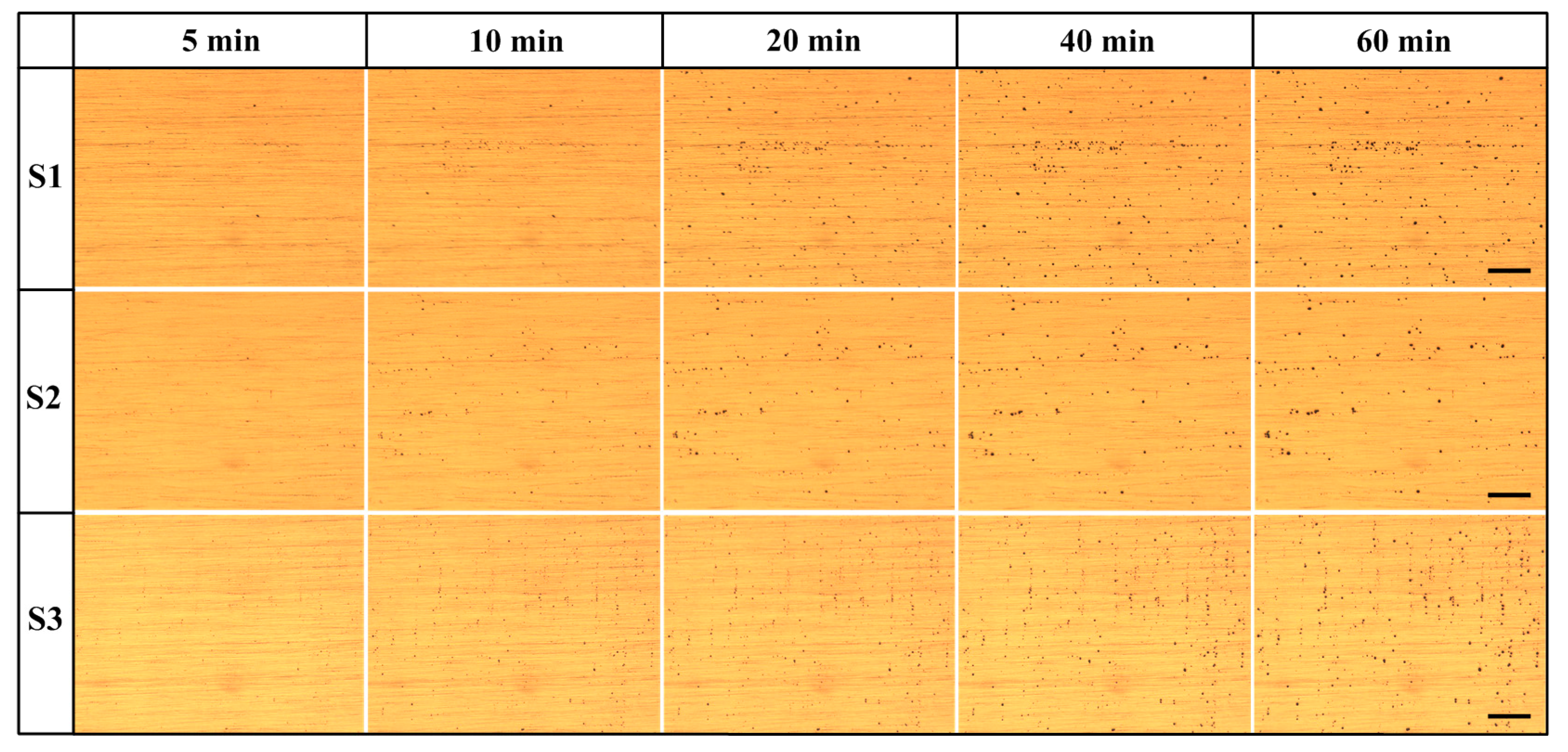
3.3. Immersion Test
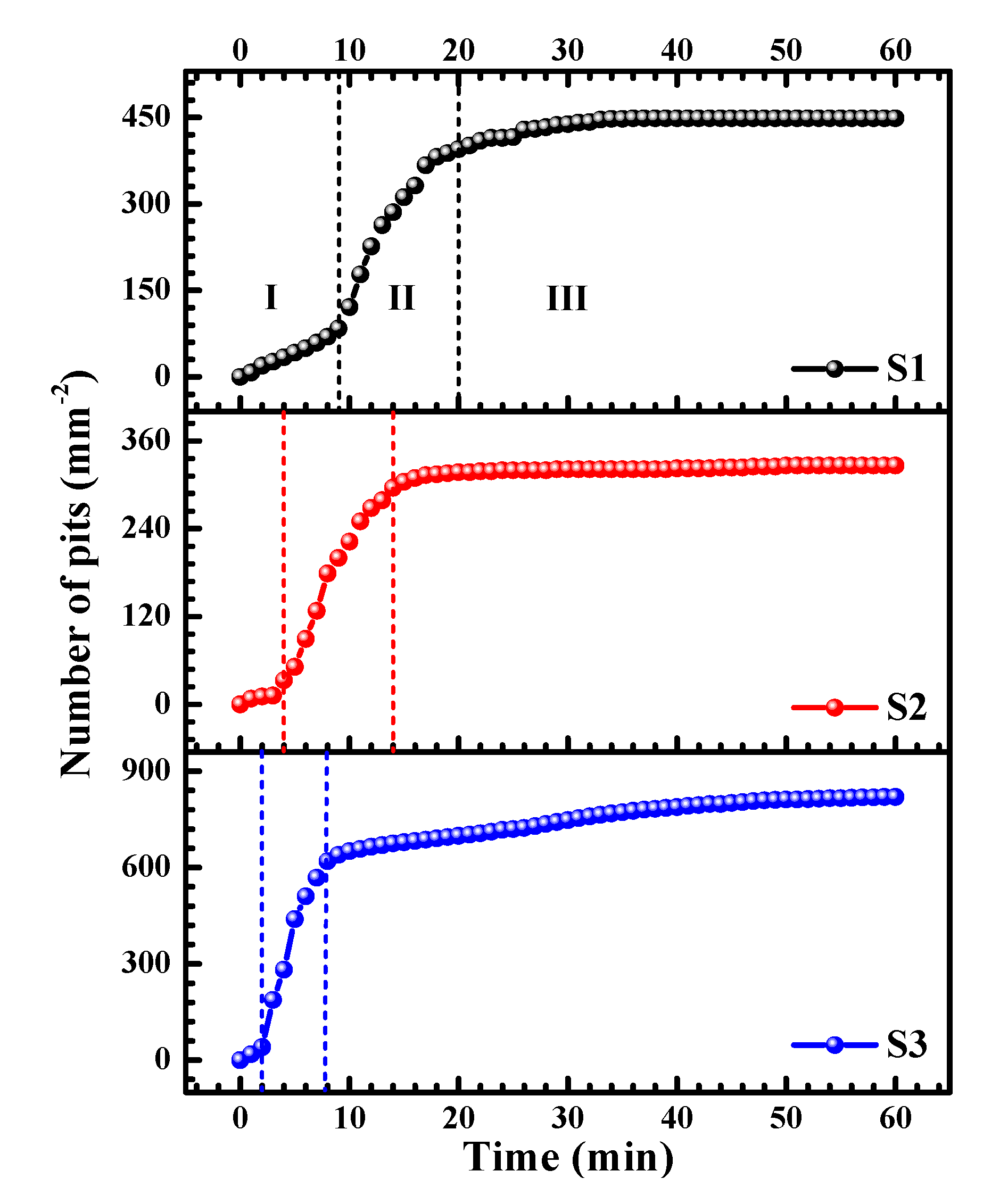
3.3.1. Number of Pits
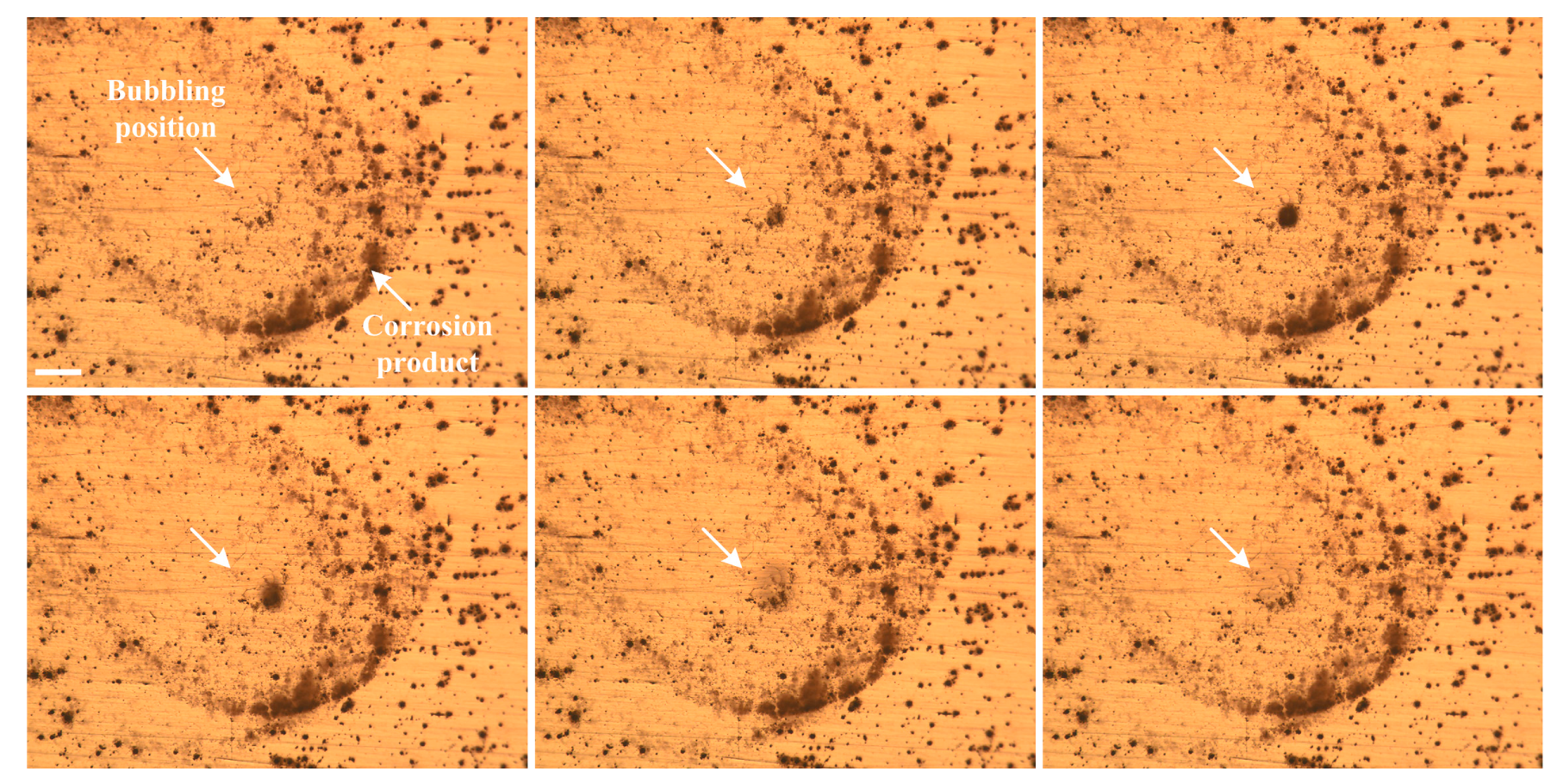
3.3.2. Morphology of Pits
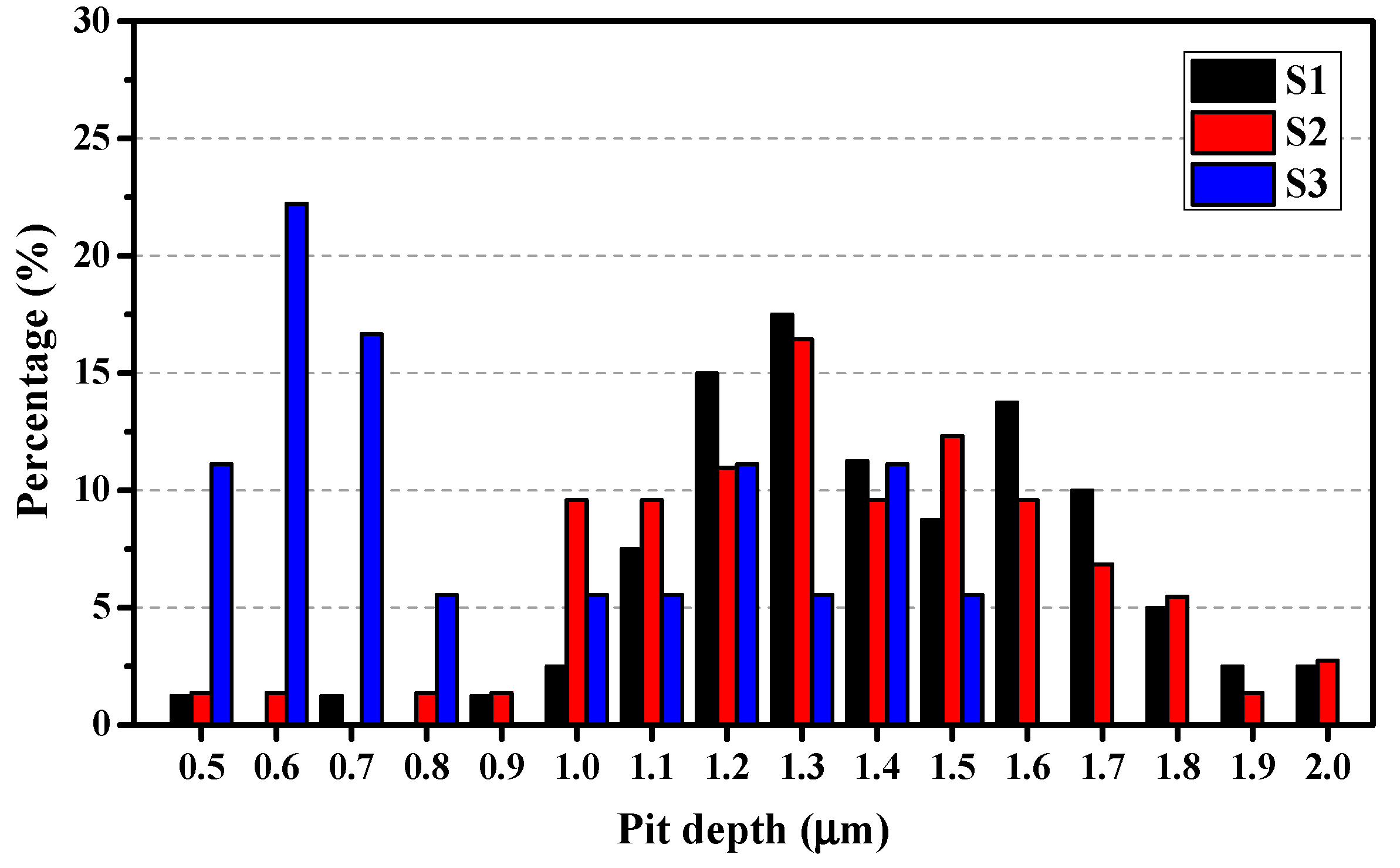
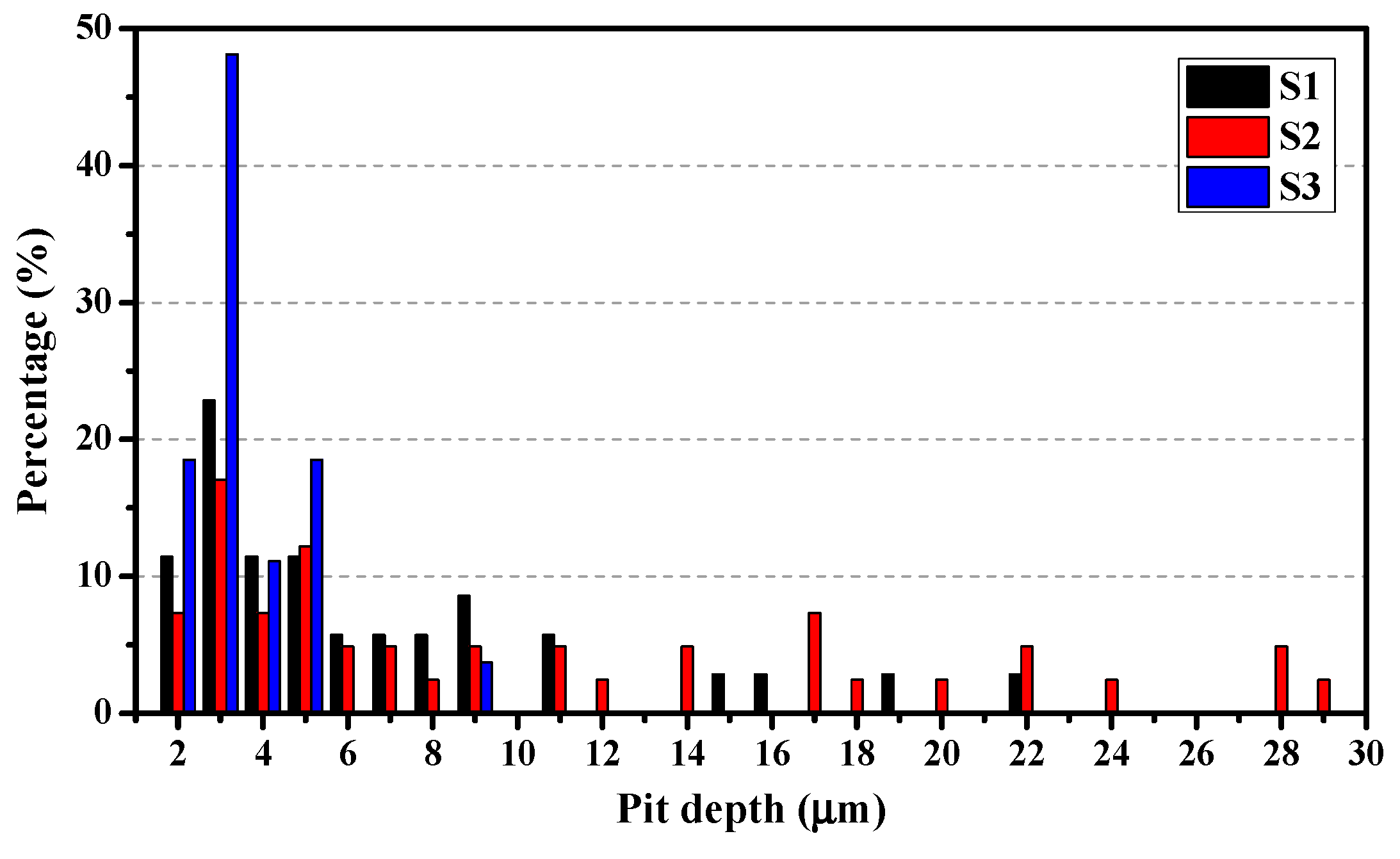
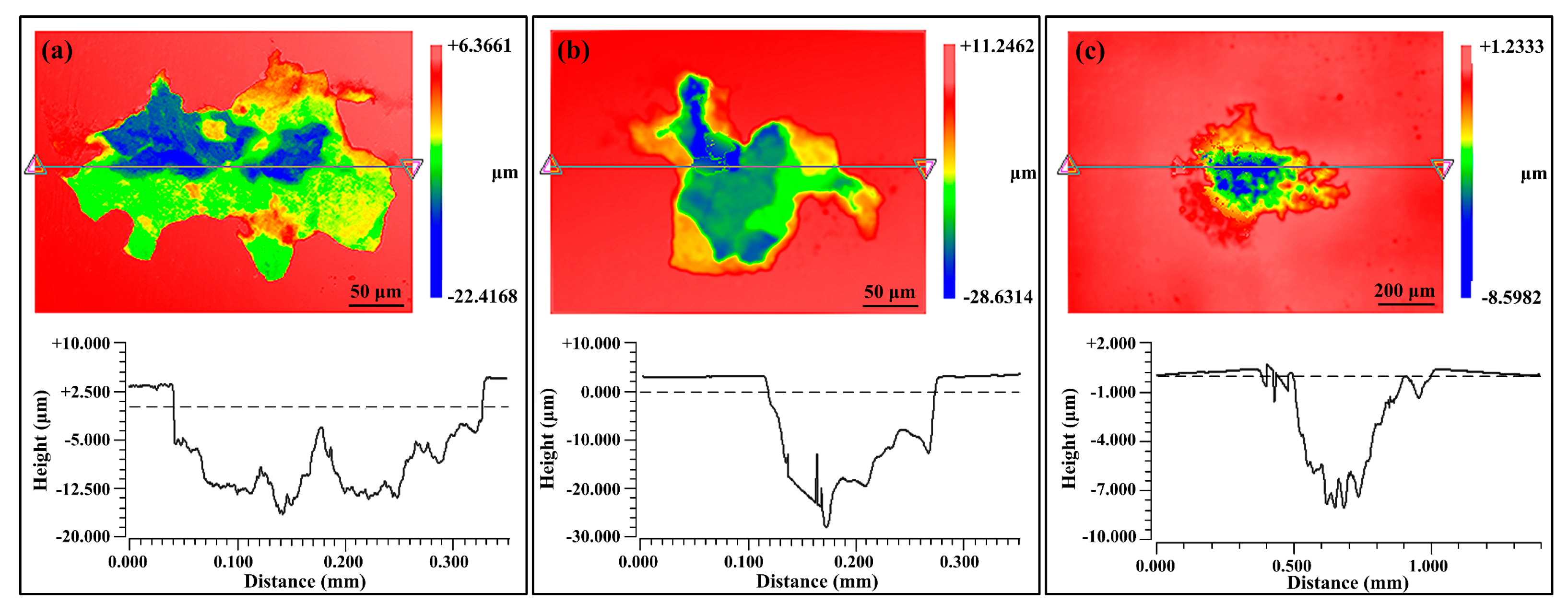
3.3.3. Statistical Analysis of Pit Depth
4. Discussions
4.1. Effect of Li on Pitting Corrosion
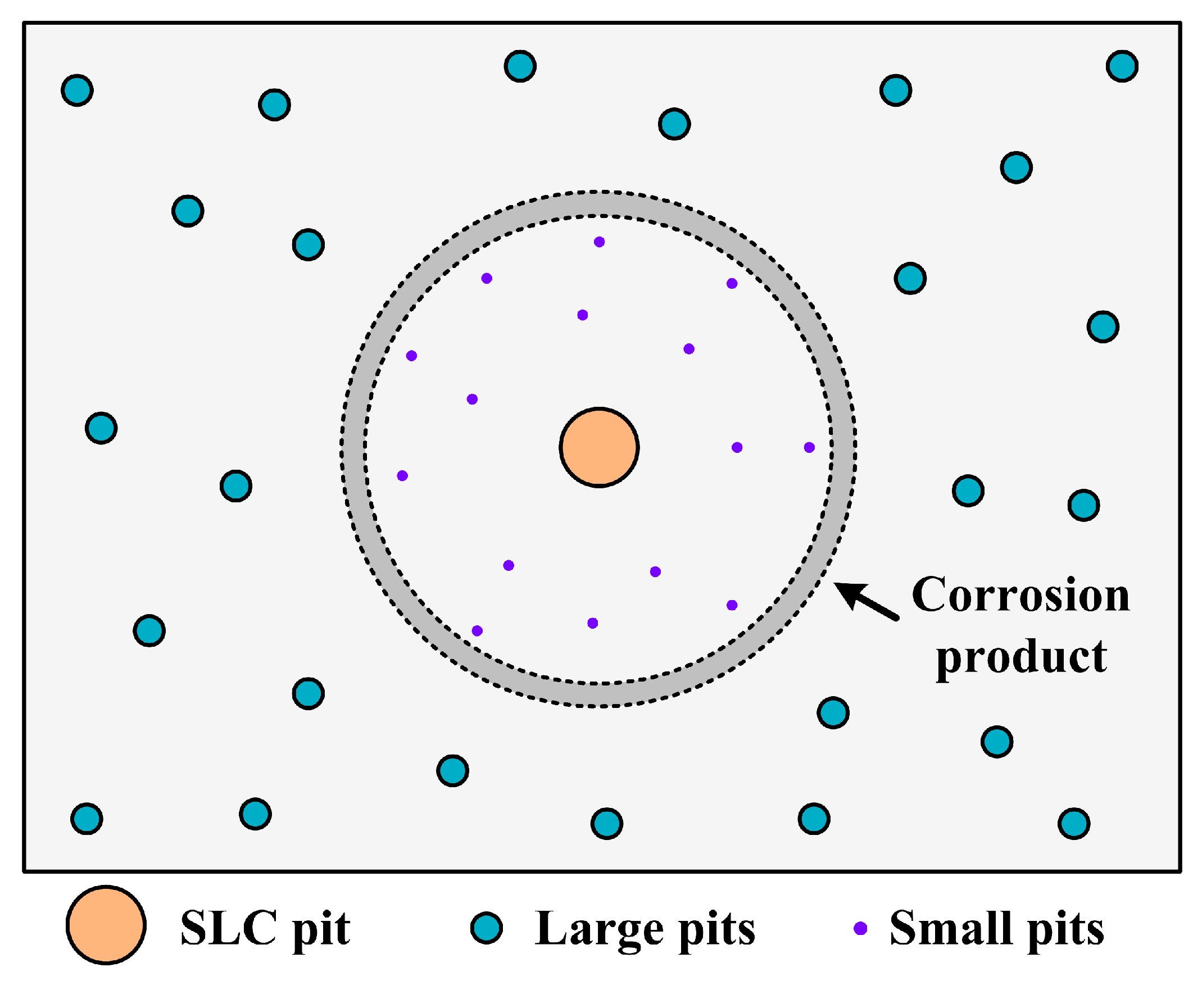
4.2. From Pitting Corrosion to SLC Pits
5. Conclusions
- (1)
- The pitting potential of the three alloys lies in the following order: S1 < S2 < S3. With the comparatively highest lithium content, S3 shows the widest passive region and lowest steady-state current density.
- (2)
- The development of the pitting process can be divided in to an initial slow nucleation stage (Stage I), a fast development stage (Stage II), and a stabilized growth stage (Stage III). The accumulative number of pitting sites rises more quickly with increasing lithium content (S1 → S2 → S3), whereas S1 and S2 possess deeper pits than does S3.
- (3)
- Circular characteristics were found near the SLC sites, which should be due to the precipitation of corrosion products on the alloy surfaces external to the pit.
- (4)
- A well-established pit partially protects the surrounding area cathodically, thereby inhibiting the nucleation and growth rates of new pits under the “hemisphere of influence” of the central pit.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, J.F.; Birbilis, N.; Liu, D.Y.; Chen, Y.L.; Zhang, X.H.; Cai, C. Intergranular corrosion of Zn-free and Zn-microalloyed Al-xCu-yLi alloys. Corros. Sci. 2016, 105, 44–57. [Google Scholar] [CrossRef]
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Prasad, N.E.; Gokhale, A.; Wanhill, R.J.H. Aluminum-Lithium Alloys: Processing, Properties, and Applications; Butterworth-Heinemann: Oxford, UK, 2014. [Google Scholar]
- Sinhmar, S.; Dwivedi, D.K. A study on corrosion behavior of friction stir welded and tungsten inert gas welded AA2014 aluminium alloy. Corros. Sci. 2018, 133, 25–35. [Google Scholar] [CrossRef]
- Gui, J.; Devine, T. Influence of Lithium on the Corrosion of Aluminum. Scr. Metall. 1987, 21, 853–857. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Liao, Y.; Yi, Y.; Wu, H.; Wang, Z.; Huang, W. Localised corrosion in AA 2099-T83 aluminium-lithium alloy: The role of grain orientation. Corros. Sci. 2016, 107, 41–48. [Google Scholar] [CrossRef]
- Ott, N.; Yan, Y.; Ramamurthy, S.; Kairy, S.; Birbilis, N. Auger electron spectroscopy analysis of grain boundary microchemistry in an Al-Cu-Li alloy. Scr. Mater. 2016, 119, 17–20. [Google Scholar] [CrossRef]
- Guérin, M.; Alexis, J.; Andrieu, E.; Laffont, L.; Lefebvre, W.; Odemer, G.; Blanc, C. Identification of the metallurgical parameters explaining the corrosion susceptibility in a 2050 aluminium alloy. Corros. Sci. 2016, 102, 291–300. [Google Scholar] [CrossRef]
- Guerin, M.; Andrieu, E.; Odemer, G.; Alexis, J.; Blanc, C. Effect of varying conditions of exposure to an aggressive medium on the corrosion behavior of the 2050 Al–Cu–Li alloy. Corros. Sci. 2014, 85, 455–470. [Google Scholar] [CrossRef]
- Rioja, R.J.; Liu, J. The Evolution of Al-Li Base Products for Aerospace and Space Applications. Metall. Mater. Trans. A 2012, 43, 3325–3337. [Google Scholar] [CrossRef]
- Carrick, D.M.; Hogg, S.C.; Wilcox, G.D. Influence of Li Additions on the Microstructure and Corrosion Response of 2XXX Series Aluminium Alloys. Mater. Sci. Forum 2014, 794–796, 193–198. [Google Scholar] [CrossRef]
- Buchheit, R.G. The Electrochemistry of θ (Al2Cu), S (Al2CuMg) and T1 (Al2CuLi) and Localized Corrosion and Environment Assisted Cracking in High Strength Al Alloys. Mater. Sci. Forum 2000, 331–337, 1641–1646. [Google Scholar] [CrossRef]
- Szklarska-Smialowska, Z. Pitting corrosion of aluminum. Corros. Sci. 1999, 41, 1743–1767. [Google Scholar] [CrossRef]
- Frankel, G.S. The effects of microstructure and composition on Al alloy corrosion. Corrosion 2015, 71, 1308–1320. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Huang, W.; Liao, Y.; Chen, X.; Zhang, X.; Thompson, G.E. Crystallographic defects induced localised corrosion in AA2099-T8 aluminium alloy. Corros. Eng. Sci. Technol. 2014, 50, 420–424. [Google Scholar] [CrossRef]
- Li, J.F.; Li, C.X.; Peng, Z.W.; Chen, W.J.; Zheng, Z.Q. Corrosion mechanism associated with T1 and T2 precipitates of Al-Cu-Li alloys in NaCl solution. J. Alloys Compd. 2008, 460, 688–693. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Wu, B.; Ma, X.L. Size-dependent role of S phase in pitting initiation of 2024Al alloy. Corros. Sci. 2016, 105, 183–189. [Google Scholar] [CrossRef]
- Wei, W.; González, S.; Hashimoto, T.; Prasath Babu, R.; Thompson, G.E.; Zhou, X. Effect of low temperature sensitization on the susceptibility to intergranular corrosion in AA5083 aluminum alloy. Mater. Corros. 2016, 67, 331–339. [Google Scholar] [CrossRef]
- Niskanen, P.; Sanders, T.; Rinker, J.; Marek, M. Corrosion of aluminum alloys containing lithium. Corros. Sci. 1982, 22, 283–304. [Google Scholar] [CrossRef]
- Zweiacker, K.W.; Liu, C.; Gordillo, M.A.; McKeown, J.T.; Campbell, G.H.; Wiezorek, J.M.K. Composition and automated crystal orientation mapping of rapid solidification products in hypoeutectic Al-4 at.%Cu alloys. Acta Mater. 2018, 145, 71–83. [Google Scholar] [CrossRef]
- Deschamps, A.; Garcia, M.; Chevy, J.; Davo, B.; De Geuser, F. Influence of Mg and Li content on the microstructure evolution of Al-Cu-Li alloys during long-term ageing. Acta Mater. 2017, 122, 32–46. [Google Scholar] [CrossRef]
- Wang, S.C.; Starink, M.J. Precipitates and intermetallic phases in precipitation hardening Al–Cu–Mg–(Li) based alloys. Int. Mater. Rev. 2005, 50, 193–215. [Google Scholar] [CrossRef]
- Huang, B.P.; Zheng, Z.Q. Effects of Li content on precipitation in Al-Cu-(Li)-Mg-Ag-Zr alloys. Scr. Mater. 1998, 38, 357–362. [Google Scholar] [CrossRef]
- Kumai, C.; Kusinski, J.; Thomas, G.; Devine, T. Influence of aging at 200 C on the corrosion resistance of Al-Li and Al-Li-Cu alloys. Corrosion 1989, 45, 294–302. [Google Scholar] [CrossRef]
- Duan, S.Y.; Wu, C.L.; Gao, Z.; Cha, L.M.; Fan, T.W.; Chen, J.H. Interfacial structure evolution of the growing composite precipitates in Al-Cu-Li alloys. Acta Mater. 2017, 129, 352–360. [Google Scholar] [CrossRef]
- Li, J.F.; Zheng, Z.Q.; Jiang, N.; Li, S.C. Study on localized corrosion mechanism of 2195 Al-Li alloy in 4.0% NaCl solution (pH 6.5) using a three-electrode coupling system. Mater. Corros. 2005, 56, 192–196. [Google Scholar] [CrossRef]
- Li, J.F.; Zheng, Z.Q.; Li, S.C.; Chen, W.J.; Ren, W.D.; Zhao, X.S. Simulation study on function mechanism of some precipitates in localized corrosion of Al alloys. Corros. Sci. 2007, 49, 2436–2449. [Google Scholar] [CrossRef]
- Yoshimura, R.; Konno, T.J.; Abe, E.; Hiraga, K. Transmission electron microscopy study of the evolution of precipitates in aged Al-Li-Cu alloys: The θ’ and T1 phases. Acta Mater. 2003, 51, 4251–4266. [Google Scholar] [CrossRef]
- Hashimoto, T.; Zhang, X.; Zhou, X.; Skeldon, P.; Haigh, S.J.; Thompson, G.E. Investigation of dealloying of S phase (Al2CuMg) in AA 2024-T3 aluminium alloy using high resolution 2D and 3D electron imaging. Corros. Sci. 2016, 103, 157–164. [Google Scholar] [CrossRef]
- Donatus, U.; Terada, M.; Ospina, C.R.; Queiroz, F.M.; Fatima Santos Bugarin, A.; Costa, I. On the AA2198-T851 alloy microstructure and its correlation with localized corrosion behaviour. Corros. Sci. 2018, 131, 300–309. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Huang, W.; Thompson, G.E.; Zhang, X.; Luo, C.; Sun, Z. Localized corrosion in AA2099-T83 aluminum–lithium alloy: The role of intermetallic particles. Mater. Chem. Phys. 2015, 161, 201–210. [Google Scholar] [CrossRef]
- Wang, H.N.; Liu, C.Z.; Lu, L.; Li, R.S.; Lin, D. Effect of Li content on electrochemical corrosion behavior for Al-Li alloy in acidic NaCl solution. Rare Met. Mater. Eng. 2018, 47, 1445–1450. [Google Scholar]
- Zhang, X.; Zhou, X.; Hashimoto, T.; Liu, B.; Luo, C.; Sun, Z.; Tang, Z.; Lu, F.; Ma, Y. Corrosion behaviour of 2A97-T6 Al-Cu-Li alloy: The influence of non-uniform precipitation. Corros. Sci. 2018, 132, 1–8. [Google Scholar] [CrossRef]
- Huang, J.-L.; Li, J.-F.; Liu, D.-Y.; Zhang, R.-F.; Chen, Y.-L.; Zhang, X.-H.; Ma, P.-C.; Gupta, R.K.; Birbilis, N. Correlation of intergranular corrosion behaviour with microstructure in Al-Cu-Li alloy. Corros. Sci. 2018, 139, 215–226. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Hashimoto, T.; Lindsay, J.; Ciuca, O.; Luo, C.; Sun, Z.; Zhang, X.; Tang, Z. The influence of grain structure on the corrosion behaviour of 2A97-T3 Al-Cu-Li alloy. Corros. Sci. 2017, 116, 14–21. [Google Scholar] [CrossRef]
- Bargeron, C.B.; Givens, R.B. Precursive blistering in the localized corrosion of aluminum. Corrosion 1980, 36, 618–625. [Google Scholar] [CrossRef]
- Bargeron, C.B.; Givens, R.B. Blister formation and pitting corrosion in aluminum. J. Electrochem. Soc. 1978, 125, C119. [Google Scholar]
- Buravova, S.N.; Gordopolov, Y.A. Cavitation erosion as a kind of dynamic damage. Int. J. Fract. 2011, 170, 83–93. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Engelhardt, G.R. Predictive Modeling of Corrosion; Elsevier Science Bv: Amsterdam, The Netherlands, 2010; pp. 1630–1679. [Google Scholar]


| Number | Material | Cu | Li | Mg | Mn | Ag | Zr | Zn | Al |
|---|---|---|---|---|---|---|---|---|---|
| S1 | 2029-T8 | 3.46 | - | 0.80 | 0.26 | 0.04 | - | 0.01 | Bal. |
| S2 | 2060-T8 | 3.63 | 0.78 | 0.67 | 0.25 | 0.04 | 0.06 | 0.29 | Bal. |
| S3 | 2098-T851 | 3.71 | 1.29 | 0.26 | 0.03 | 0.03 | 0.06 | 0.01 | Bal. |
| Element | Al | Cu | O | Al/Cu | |
|---|---|---|---|---|---|
| S1 | A-1 | 13.01 | 69.94 | 17.05 | 0.186 |
| A-2 | 43.75 | 13.28 | 42.97 | 3.294 | |
| A-3 | 35.68 | 12.09 | 52.23 | 2.951 | |
| S2 | A-4 | 18.85 | 72.84 | 8.31 | 0.259 |
| A-5 | 15.59 | 69.43 | 14.98 | 0.225 | |
| A-6 | 49.30 | 42.66 | 8.04 | 1.156 | |
| S3 | A-7 | 2.13 | 87.92 | 9.95 | 0.024 |
| A-8 | 56.74 | 17.06 | 26.20 | 3.326 | |
| A-9 | 19.74 | 71.51 | 8.75 | 0.276 | |
| Element | Al | Cu | O | Mg | Mn | C | |
|---|---|---|---|---|---|---|---|
| S1 | P-1 | 90.76 | 6.03 | 1.99 | 1.22 | - | - |
| P-2 | 92.67 | 5.00 | 0.19 | 1.18 | 0.96 | - | |
| P-3 | 85.39 | 7.59 | 0.21 | 0.99 | 5.82 | - | |
| S2 | P-4 | 75.72 | 10.98 | - | - | 11.96 | 1.34 |
| P-5 | 74.26 | 9.55 | - | 0.68 | 9.77 | 5.74 | |
| S3 | P-6 | 87.21 | 11.20 | 0.83 | - | 0.76 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, X.; Saatchi, A.; Ghanbari, E.; Dang, R.; Li, W.; Wang, N.; Macdonald, D.D. Studies on Pitting Corrosion of Al-Cu-Li Alloys Part I: Effect of Li Addition by Microstructural, Electrochemical, In-situ, and Pit Depth Analysis. Materials 2019, 12, 1600. https://doi.org/10.3390/ma12101600
Lei X, Saatchi A, Ghanbari E, Dang R, Li W, Wang N, Macdonald DD. Studies on Pitting Corrosion of Al-Cu-Li Alloys Part I: Effect of Li Addition by Microstructural, Electrochemical, In-situ, and Pit Depth Analysis. Materials. 2019; 12(10):1600. https://doi.org/10.3390/ma12101600
Chicago/Turabian StyleLei, Xiaowei, Alireza Saatchi, Elmira Ghanbari, Runze Dang, Wenzhe Li, Nan Wang, and Digby D. Macdonald. 2019. "Studies on Pitting Corrosion of Al-Cu-Li Alloys Part I: Effect of Li Addition by Microstructural, Electrochemical, In-situ, and Pit Depth Analysis" Materials 12, no. 10: 1600. https://doi.org/10.3390/ma12101600
APA StyleLei, X., Saatchi, A., Ghanbari, E., Dang, R., Li, W., Wang, N., & Macdonald, D. D. (2019). Studies on Pitting Corrosion of Al-Cu-Li Alloys Part I: Effect of Li Addition by Microstructural, Electrochemical, In-situ, and Pit Depth Analysis. Materials, 12(10), 1600. https://doi.org/10.3390/ma12101600








