Thermal Analysis and SEM Microscopy Applied to Studying the Efficiency of Ionic Liquid Immobilization on Solid Supports
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
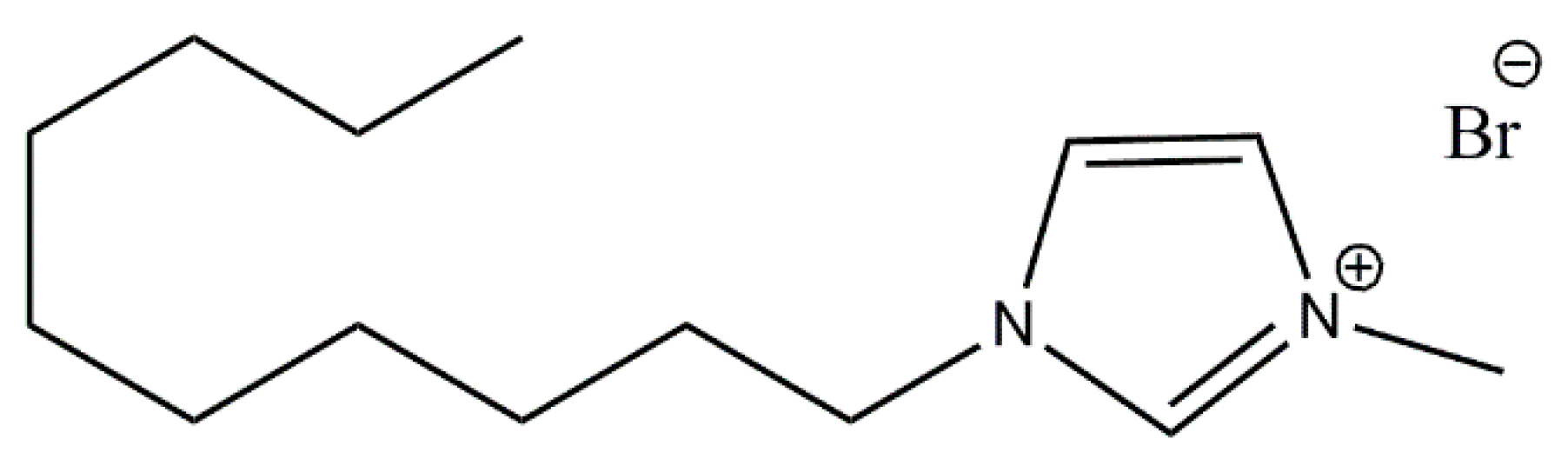
2.2. Modification of Fillers Surface with DmiBr
2.3. Characterization of Pure and Modified Fillers
3. Results and Discussion
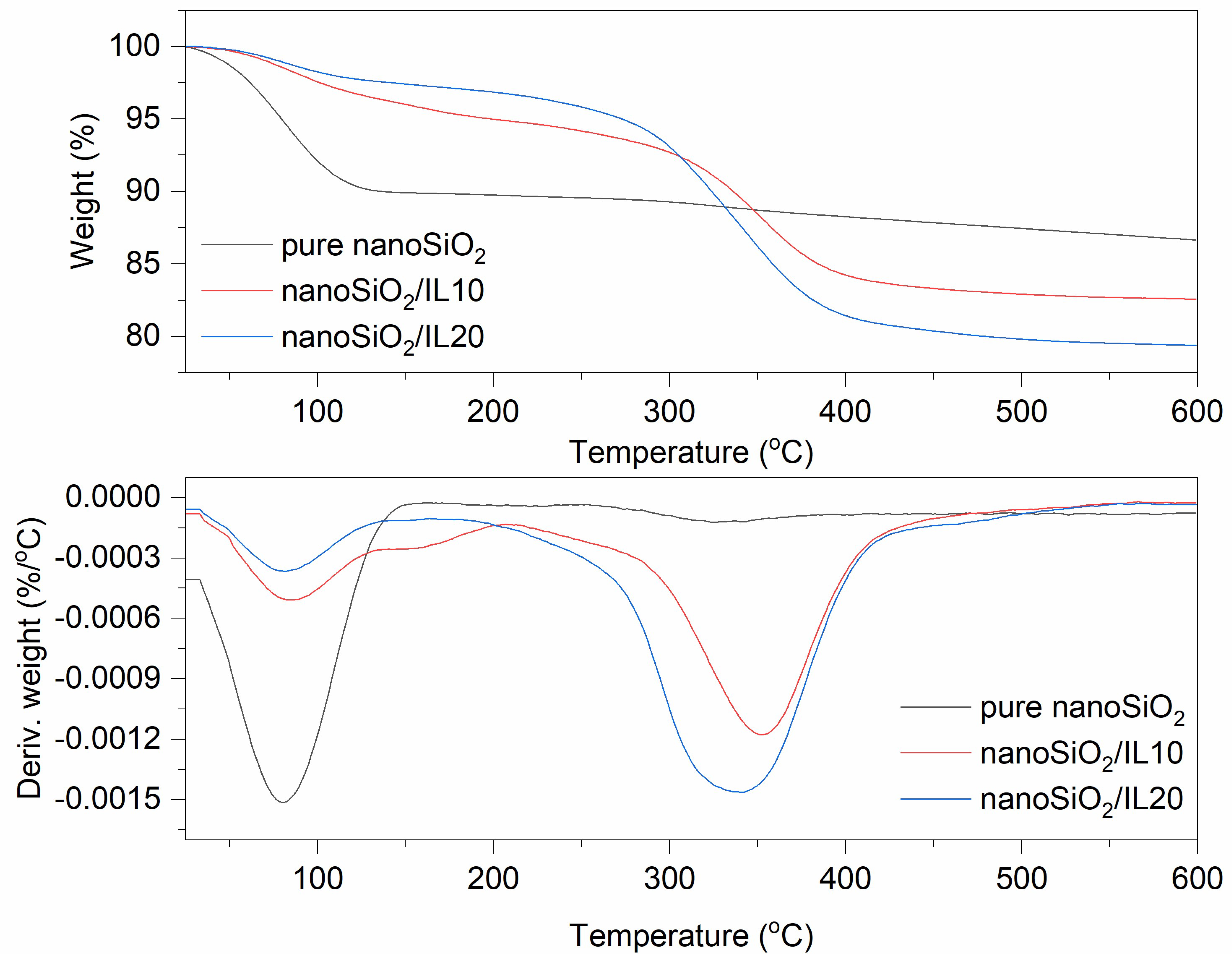
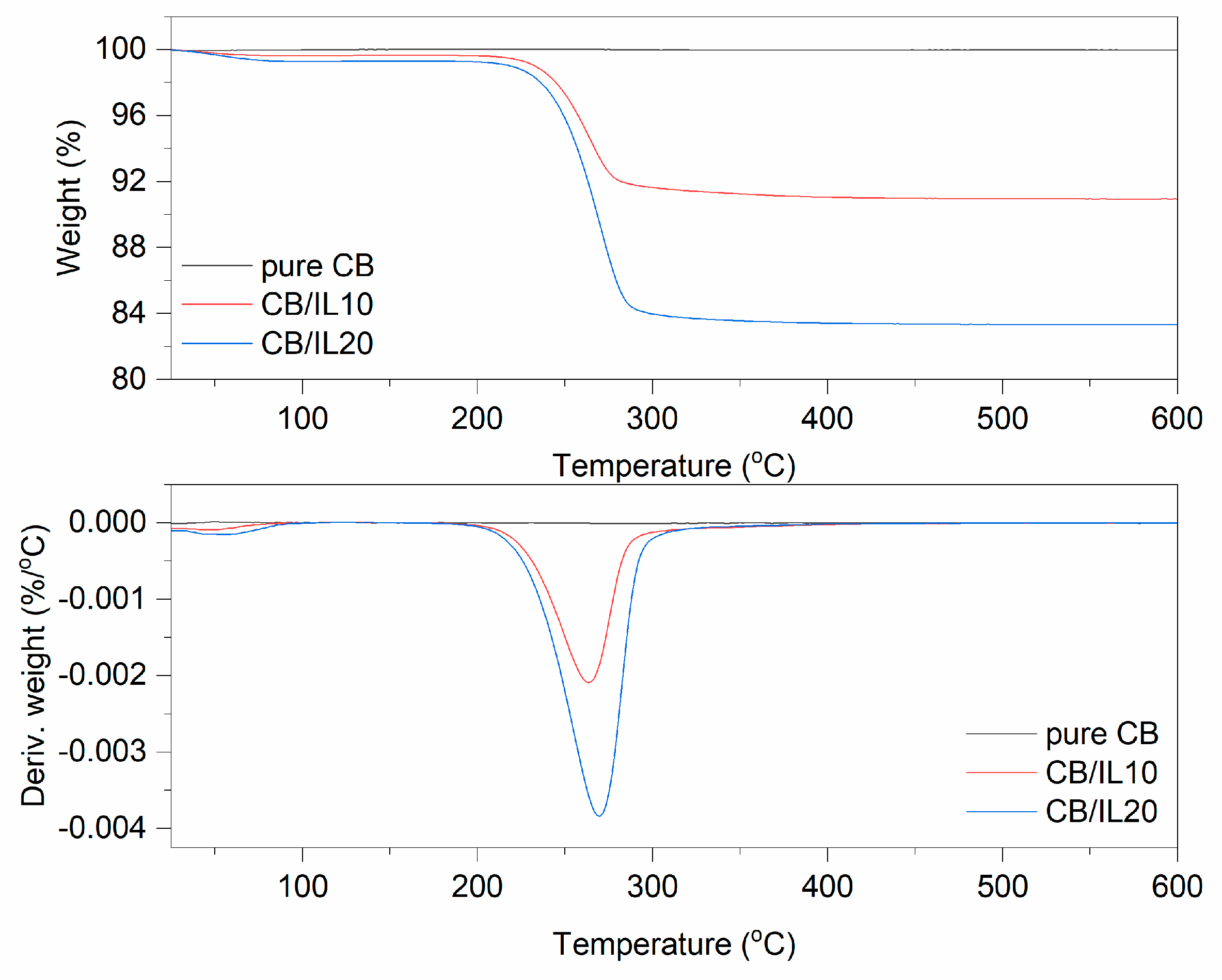
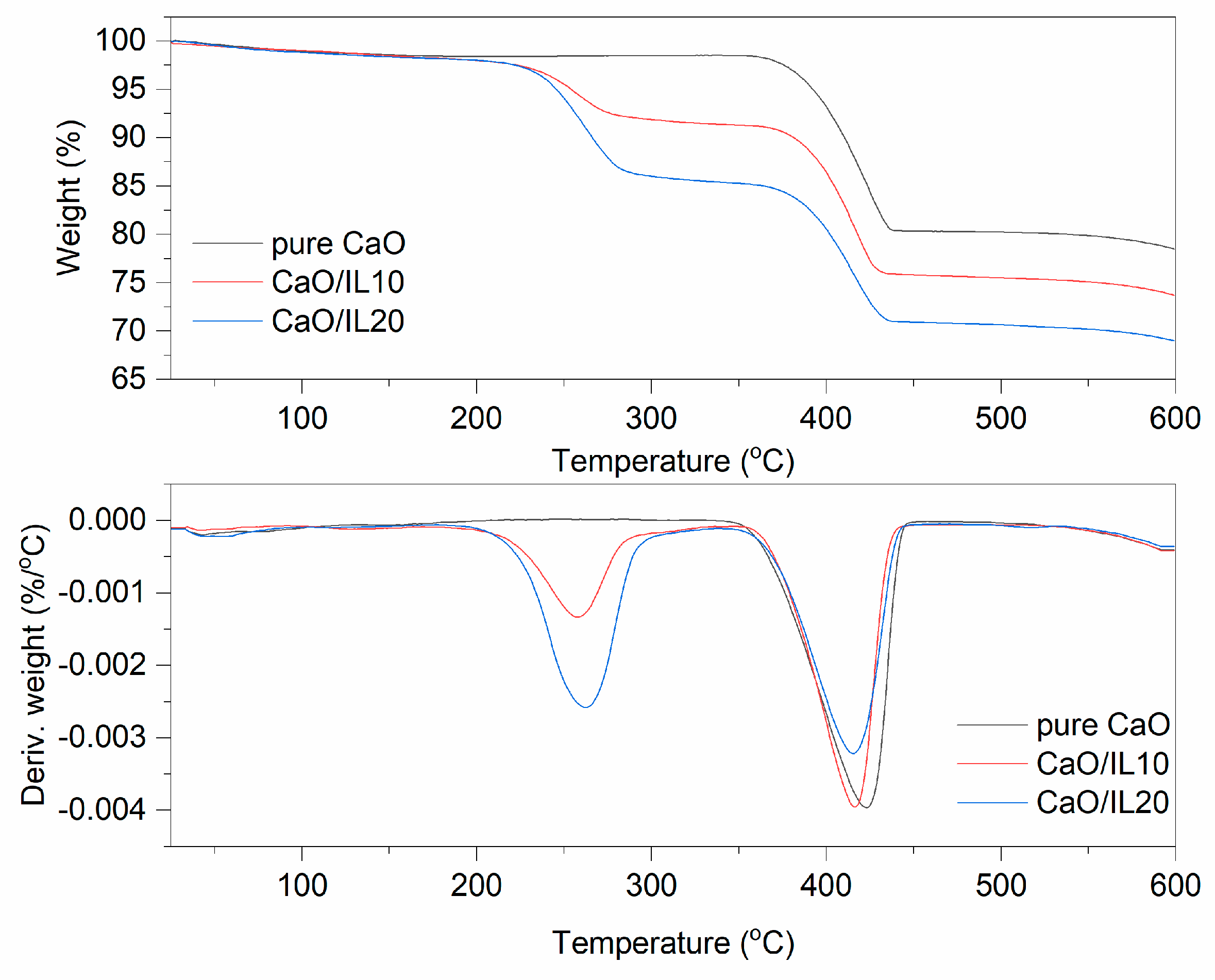
3.1. Thermogravimetric Analysis (TG)
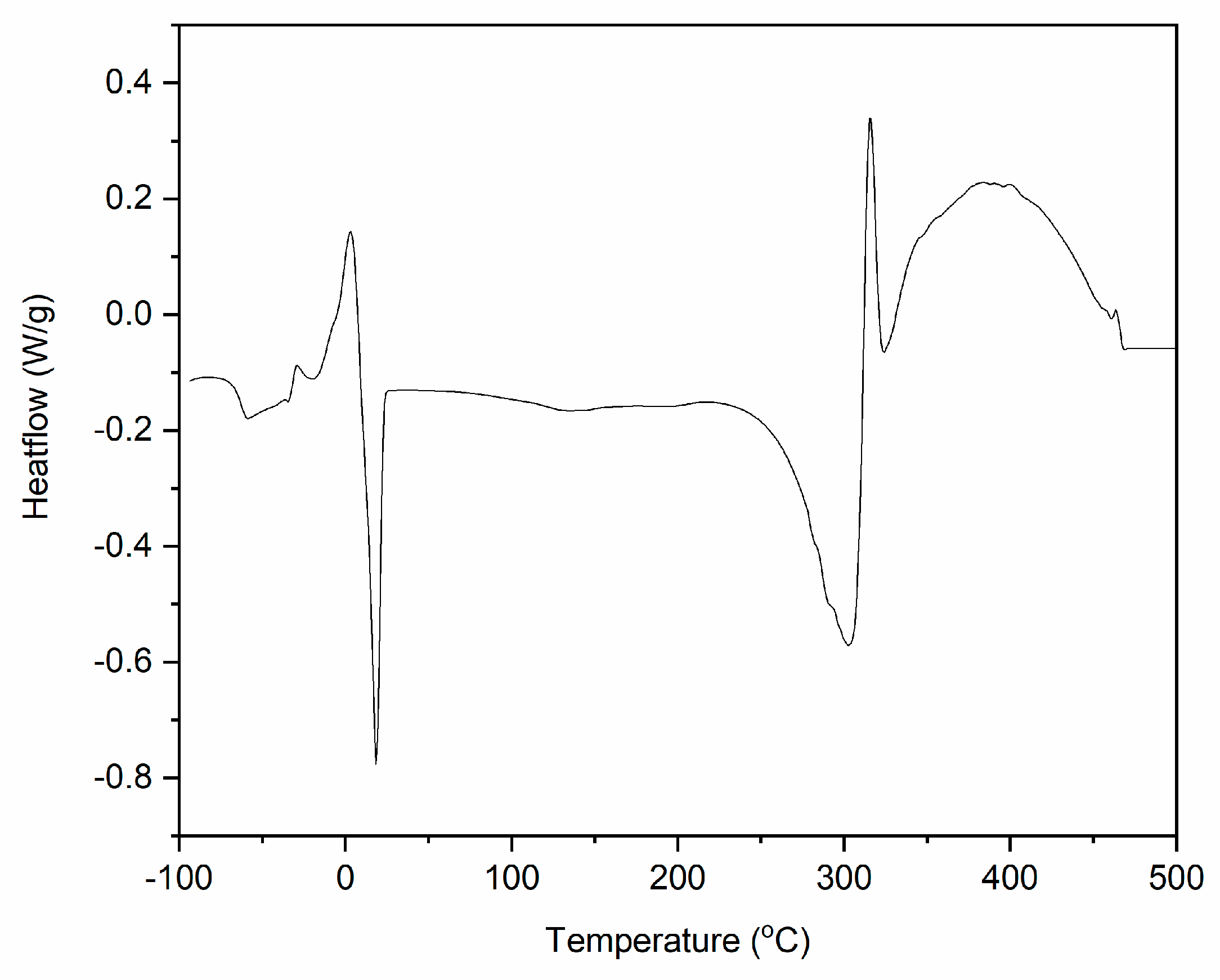
3.2. Differential Scanning Calorimetry (DSC)
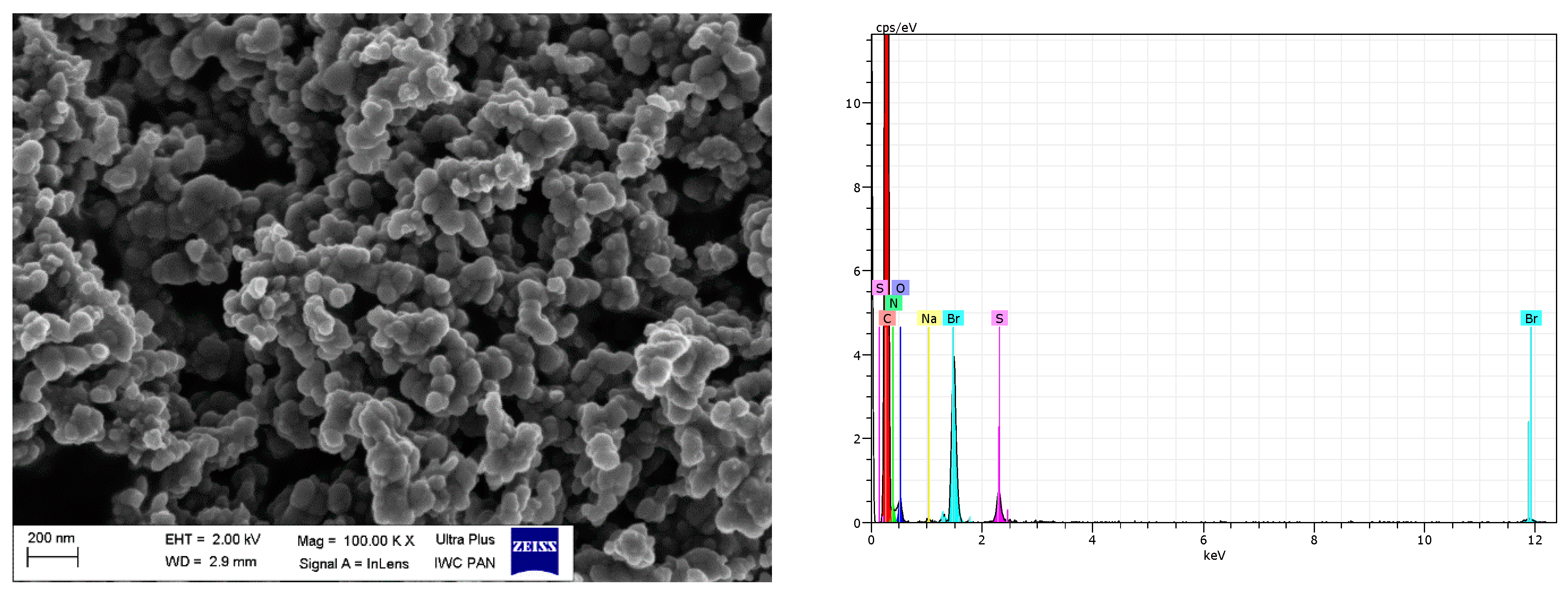
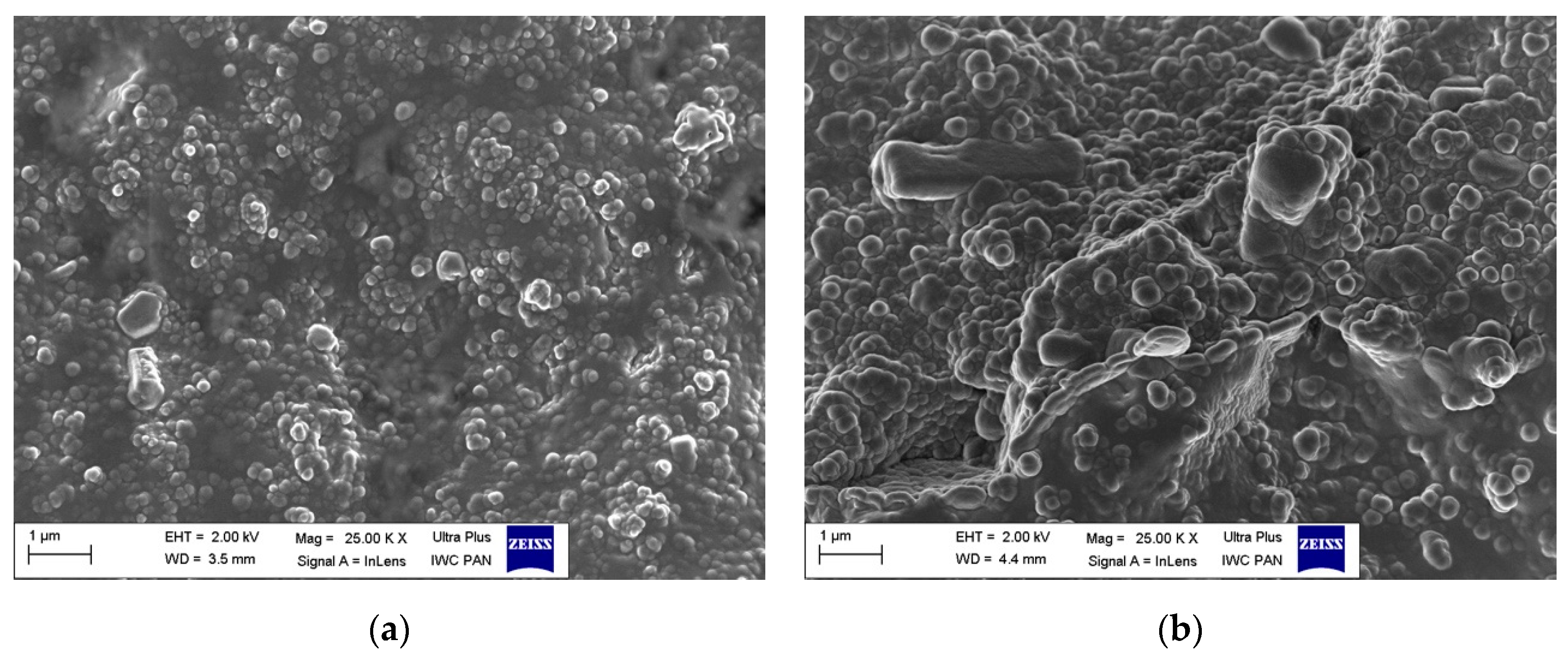
3.3. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Subramaniam, K.; Das, A.; Simon, F.; Heinrich, G. Networking of ionic liquid modified CNTs in SSBR. Eur. Polym. J. 2013, 49, 345–352. [Google Scholar] [CrossRef]
- Zaborski, M.; Donnet, J.B. Activity of fillers in elastomer networks of different structure. Macromol. Symp. 2003, 194, 87–99. [Google Scholar] [CrossRef]
- Khan, I.; Bhat, A.H. Micro and nano calcium carbonate filled natural rubber composites and nanocomposites. In Natural Rubber Materials; Thomas, S., Chan, C.H., Pothen, L., Joy, J., Maria, H., Eds.; RSC Publishing: Cambridge, UK, 2014; Volume 2, pp. 467–487. [Google Scholar]
- Wypych, G. The effect of fillers on the mechanical properties of filled materials. In Handbook of Fillers; ChemTec Publishing: Toronto, ON, Canada, 2016; pp. 467–531. [Google Scholar]
- Wang, S.; Chester, S.A. Experimental characterization and continuum modeling of inelasticity in filled rubber-like materials. Int. J. Solids Struct. 2018, 136, 125–136. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Chow, L.C.; Yang, M.; Mitchell, J.W. Effects of inorganic fillers on the thermal and mechanical properties of poly(lactic acid). Int. J. Polym. Sci. 2014, 2014, 827028. [Google Scholar] [CrossRef]
- Dorfmann, A.; Ogden, R.W. A pseudo-elastic model for loading, partial unloading and reloading of particle-reinforced rubber. Int. J. Solids Struct. 1981, 40, 2699–2714. [Google Scholar] [CrossRef]
- Donnet, J.-B.; Custodero, E. Reinforcement of elastomers by particulate fillers. In Science and Technology of Rubber, 3rd ed.; Matk, J.E., Erman, B., Eirich, F.R., Eds.; Academic Press: Cambridge, UK, 2005; pp. 383–415. [Google Scholar]
- Wolf, S.; Wang, M.J. Filler elastomer interactions. Part IV, The effect of the surface energies of fillers on elastomer reinforcement. Rubber Chem. Technol. 1992, 65, 329–342. [Google Scholar] [CrossRef]
- Ismail, H.; Omar, N.F.; Othman, N. Effect of carbon black loading on curing characteristics and mechanical properties of waste tyre dust/carbon black hybrid filler filled natural rubber compounds. J. Appl. Polym. Sci. 2011, 121, 1143–1150. [Google Scholar] [CrossRef]
- Tinker, A.J.; Jones, K.P. Blends of Natural Rubber. 3 Solutions to the basic problems of poor physical properties of NR/EPDM blends. In Novel Techniques for Blending with Speciality Polymers; Chapman and Hall: London, UK, 1998; pp. 169–183. [Google Scholar]
- Choi, S.-S.; Nah, C.; Jo, B.-W. Properties of natural rubber composites reinforced with silica or carbon black: Influence of cure accelerator content and filler dispersion. Polym. Int. 2003, 52, 1382–1389. [Google Scholar] [CrossRef]
- Brochard Wyart, F.; de Gennes, P.G. Viscosity at small scales in polymer melts. Eur. Phys. J. E 2000, 1, 93–97. [Google Scholar] [CrossRef]
- Gerspacher, M.; Nikiel, L.; Yang, H.H.; O’Farrell, C.P.; Schwartz, G.A. Flocculation in carbon black filled compound. Kautsch. Gummi Kunstst. 2002, 55, 596–604. [Google Scholar]
- Ulfah, I.M.; Fidyaningsih, R.; Rahayu, S.; Fitriani, D.A.; Saputra, D.A.; Winarto, D.A.; Wisojodharmo, L.A. Infulence of carbon black and silica filler on the rheological and mechanical properties of natural rubber compound. Procedia Chem. 2015, 16, 258–264. [Google Scholar] [CrossRef]
- Fröhlich, J.; Niedermeier, W.; Luginsland, H.-D. The effect of filler–filler and filler–elastomer interaction on rubber reinforcement. Compos. Part A 2005, 36, 449–460. [Google Scholar] [CrossRef]
- Sarkawia, S.S.; Dierkes, W.K.; Noordermeer, J.W.M. Elucidation of filler-to-filler and filler-to-rubber interactions in silica-reinforced natural rubber by TEM network visualization. Eur. Polym. J. 2014, 54, 118–127. [Google Scholar] [CrossRef]
- Klüppel, M. The role of disorder in filler reinforcement of elastomers on various length scales. Adv. Polym. Sci. 2003, 164, 1–86. [Google Scholar]
- Heinrich, G.; Klüppel, M. Recent advances in the theory of filler networking in elastomers. In Filled Elastomers Drug Delivery Systems, Advances in Polymer Science; Springer: Berlin, Germany, 2002; Volume 160, pp. 1–44. [Google Scholar]
- Schröeder, A.; Klüppel, M.; Schuster, R.H. Characterisation of surface activity of carbon black and its relation to polymer-filler interaction. Macromol. Mater. Eng. 2007, 292, 885–916. [Google Scholar] [CrossRef]
- Bieliński, D.M.; Dobrowolski, O.; Ślusarski, L. Dispersion of a filler in the rubber blend. Polimery 2007, 52, 546–555. [Google Scholar] [CrossRef]
- Ou, Y.C.; Yu, Z.Z.; Vidal, A.; Donnet, J.B. Effect of alkylation of silica filler on rubber reinforcement. Rubber Chem. Technol. 1994, 67, 834–844. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.J.; Zhang, T.; Zhang, F.; Fu, X. Study on dispersion morphology of silica in rubber. Rubber Chem. Technol. 1994, 67, 693–699. [Google Scholar] [CrossRef]
- Katueangngan, K.; Tulyapitak, T.; Saetung, A.; Soontaranon, S.; Nithi-uthai, N. Renewable interfacial modifier for silica filled natural rubber compound. Procedia Chem. 2016, 19, 447–454. [Google Scholar] [CrossRef]
- Ciesielski, A. An Introduction to Rubber Technology, 2nd ed.; Rapra Technology Limited: Shawbury, UK, 2000; pp. 37–40. [Google Scholar]
- Al-Hartomy, O.A.; Al-Ghamdi, A.A.; Al-Said, S.F.; Dishovsky, N.; Mihaylov, M.; Ivanov, M. Influence of carbon black/silica ratio on the physical and mechanical properties of composites based on epoxized natural rubber. J. Compos. Mater. 2015, 50, 377–386. [Google Scholar] [CrossRef]
- Ten Brinke, J.W.; Debnath, S.C.; Reuvekamp, L.A.E.M.; Noordermeer, J.W.M. Mechanistic aspects of the role of coupling agents in silica–rubber composites. Compos. Sci. Technol. 2003, 63, 1165–1174. [Google Scholar] [CrossRef]
- Li, Y.; Han, B.; Wen, S.; Lu, Y.; Yang, H.; Zhang, L.; Liu, L. Effect of the temperature on surface modification of silica and properties of modified silica filled rubber composites. Compos. Part A 2014, 62, 52–59. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, J.S. Modifications produced by electrochemical treatments on carbon blacks: Microstructures and mechanical interfacial properties. Carbon 2001, 39, 2011–2016. [Google Scholar] [CrossRef]
- Meissner, B.; Karásek, L. Gel-like behaviour of polybutadiene-carbon black compounds. Polymer 1998, 39, 3083–3086. [Google Scholar] [CrossRef]
- Busca, G. The surface acidity and basicity of solid oxides and zeolites. In Metal Oxides, Chemistry and Applications; Fierro, J.L.G., Ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 247–318. [Google Scholar]
- Maciejewska, M.; Zaborski, M.; Krzywania-Kaliszewska, A. Nanosized mineral oxides modified with unsaturated acids as coagents for peroxide vulcanization. Soft Mater. 2013, 11, 22–31. [Google Scholar] [CrossRef]
- Rogers, R.D.; Seddon, K.R. Ionic liquids-solvents of the future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef]
- Kubisa, P. Ionic liquids in the synthesis and modification of polymers. J. Polym. Sci. Pol. Chem. 2005, 43, 4675–4683. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.C.; Chen, F.; Lei, Y.D.; Liu, X.L.; Wan, J.J.; Jia, D.M. Styrene-butadiene rubber/halloysite nanotubes nanocomposites modified by sorbid acid. Appl. Surf. Sci. 2009, 255, 7329–7336. [Google Scholar] [CrossRef]
- Kreyenschulte, H.; Richter, S.; Götze, T.; Fischer, D.; Steinhauser, D.; Klüppel, M.; Heinrich, G. Interaction of 1-allyl-3-methylimidazolium chloride and carbon black and its influence on carbon black filled rubbers. Carbon 2012, 50, 3649–3658. [Google Scholar] [CrossRef]
- Fontana, J.P.; Camilo, F.C.; Bizeto, M.A.; Faez, R. Evaluation of the role of an ionic liquid as organophilization agent into montmorillonite for NBR rubber composite production. Appl. Clay Sci. 2013, 83, 203–209. [Google Scholar] [CrossRef]
- Likozar, B. The effect of ionic liquid type on the properties of hydrogenated nitrile elastomer/hydroxyl-functionalized multi-walled carbon nanotube/ionic liquid composites. Soft Mater. 2011, 7, 970–977. [Google Scholar] [CrossRef]
- Xu, P.; Wang, Z.; Hu, Y.; Ding, Y. Piezoresistive properties of nanocomposites based on silicone rubber and ionic liquid-functionalized carbon black. Mater. Lett. 2016, 182, 218–222. [Google Scholar] [CrossRef]
- Lei, Y.D.; Tang, Z.H.; Guo, B.C.; Zhu, L.X.; Wan, J.J.; Jia, D.M. Synthesis of novel functional liquid and its application as a modifier in SBR/silica composites. eXPRESS Polym. Lett. 2010, 4, 692–703. [Google Scholar] [CrossRef] [Green Version]
- Lemus, J.; Palomar, J.; Gilarranz, M.A.; Rodriguez, J.J. Characterization of supported ionic liquid phase (SILP) materials prepared from different supports. Adsorption 2011, 17, 561–571. [Google Scholar] [CrossRef]
- Gadenne, B.; Hesemann, P.; Moreau, J.J.E. Supported ionic liquids: Ordered mesoporous silicas containing covalently linked ionic species. Chem. Commun. 2004, 1768–1769. [Google Scholar] [CrossRef]
- Haumann, M.; Dentler, K.; Joni, J.; Riisager, A.; Wasserscheid, P. Continuous gas-phase hydroformylation of 1-butene using supported ionic liquid phase (SILP) catalysts. Adv. Synth. Catal. 2007, 349, 425–431. [Google Scholar] [CrossRef]
- Riisager, A.; Jørgensen, B.; Wasserscheid, P.; Fehrmann, R. First application of supported ionic liquid phase (SILP) catalysis for continuous methanol carbonylation. Chem. Commun. 2006, 994–996. [Google Scholar] [CrossRef]
- Fehér, C.; Kriván, E.; Hancsók, J.; Skoda-Földes, R. Oligomerisation of isobutene with silica supported ionic liquid catalysts. Green Chem. 2012, 14, 403–409. [Google Scholar] [CrossRef]
- Urbán, B.; Srankó, D.; Sáfrán, G.; Ürged, L.; Darvas, F.; Bakos, J.; Skoda-Földes, R. Evaluation of SILP-Pd catalysts for Heck reactions in a microfluidics-based high throughput flow reactor. J. Mol. Catal. A Chem. 2014, 395, 364–372. [Google Scholar] [CrossRef]
- Pavia, C.; Ballerini, E.; Bivona, L.A.; Giacalone, F.; Aprile, C.; Vaccaro, L.; Gruttadauria, M. Palladium supported on cross-linked imidazolium network on silica as highly sustainable catalysts for the Suzuki reaction under flow conditions. Adv. Synth. Catal. 2013, 355, 2007–2018. [Google Scholar] [CrossRef]
- Selvam, T.; Machoke, A.; Schwieger, W. Supported ionic liquids on non-porous and porous inorganic materials-a topical review. Appl. Catal. A Gen. 2012, 445–446, 92–101. [Google Scholar] [CrossRef]
- Jiang, T.; Zhou, Y.; Liang, S.; Liu, H.; Han, B. Hydrogenolysis of glycerol catalyzed by Ru-Cu bimetallic catalysts supported on clay with the aid of ionic liquids. Green Chem. 2009, 11, 1000–1006. [Google Scholar] [CrossRef]
- Zhu, A.; Jiang, T.; Han, B.; Zhang, J.; Xie, Y.; Ma, X. Supported choline chloride/urea as a heterogeneous catalyst for chemical fixation of carbon dioxide to cyclic carbonates. Green Chem. 2007, 9, 169–172. [Google Scholar] [CrossRef]
- Taher, A.; Kim, J.-B.; Jung, J.-Y.; Ahn, W.-S.; Jin, M.-J. Highly active and magnetically recoverable Pd-NHC catalyst immobilized on Fe3O4 nanoparticle-ionic liquid matrix for Suzuki reaction in water. Synlett 2009, 2009, 2477–2482. [Google Scholar]
- Maciejewska, M.; Zaborski, M. Thermal analysis and mechanical methods applied to studying properties of SBR compounds containing ionic liquids. Polym. Test. 2017, 61, 349–363. [Google Scholar] [CrossRef]
- Maciejewska, M.; Zaborski, M. Ionic liquids as coagents for sulfur vulcanization of butadiene–styrene elastomer filled with carbon black. Polym. Bull. 2018, 75, 4499–4514. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, E.; Ferro, V.R.; Palomar, J.; Ortega, J.; Rodriquez, J.J. Interactions of ionic liquids and acetone: Thermodynamic properties, quantum-chemical calculations, and NMR analysis. J. Phys. Chem. 2013, 117, 7388–7398. [Google Scholar] [CrossRef]
- McLafferty, F.; Tureček, F. Interpretation of Mass Spectra, 4th ed.; University Science Book: Sausalito, CA, USA, 1993; pp. 226–278. [Google Scholar]
- D’Souza, A.S.; Pantano, C.G. Hydroxylation and dehydroxylation behavior of silica glass fracture surfaces. J. Am. Ceram. Soc. 2004, 85, 1499–1504. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, M.; Florin, N.H.; Harris, A.T. Synthesis and characterization of CaO nanopods for high temperature CO2 capture. Ind. Eng. Chem. Res. 2009, 48, 10765–10770. [Google Scholar] [CrossRef]
- Li, W.; Du, Y.; Li, J.; Chen, X.; Guo, S.; Zhang, T. Isobaric vapor-liquid equilibrium for acetone + methanol system containing different ionic liquids at 101.3 kPa. Fluid Phase Equilibria 2018, 459, 10–17. [Google Scholar] [CrossRef]
- Ngo, H.L.; LeCompte, K.; Hargens, L.; McEven, A.B. Thermal properties of imidazolium ionic liquids. Thermochim. Acta 2000, 357–358, 97–102. [Google Scholar] [CrossRef]
- Fredlake, C.P.; Crosthwaite, J.M.; Hert, D.G.; Aki, S.N.V.K.; Brennecke, J.F. Thermophysical properties of imidazolium-based ionic liquids. J. Chem. Eng. Data 2004, 49, 954–964. [Google Scholar] [CrossRef]
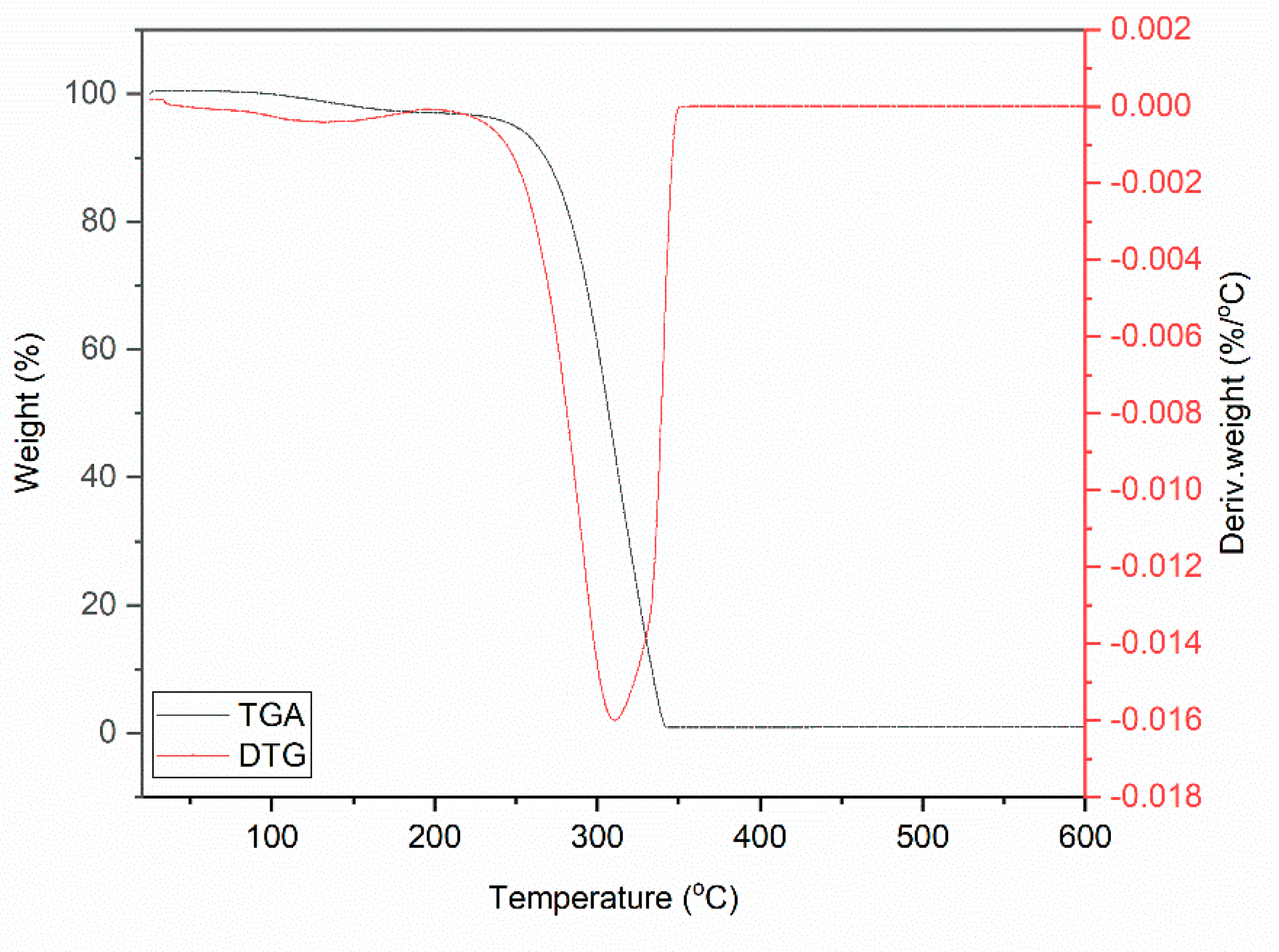
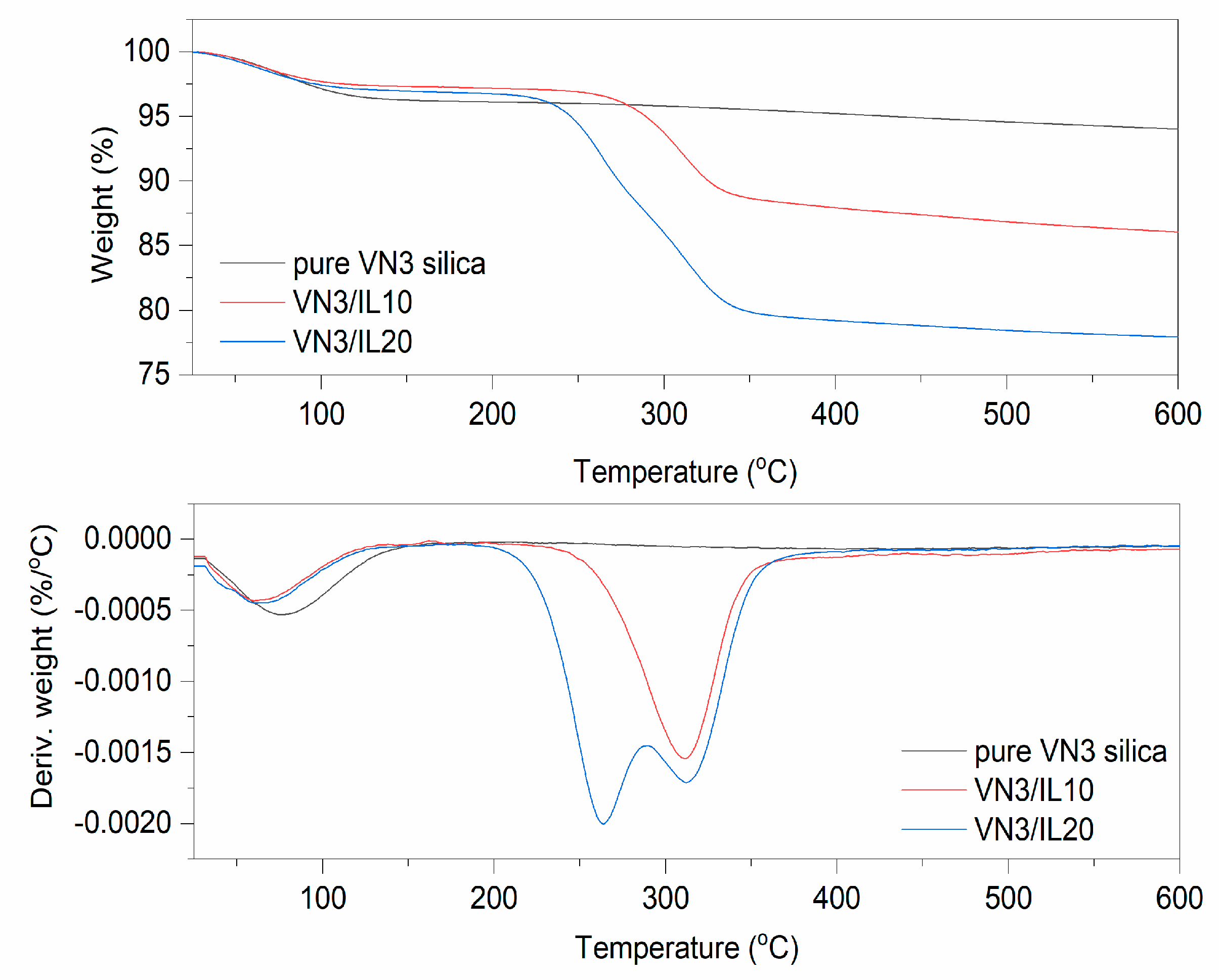
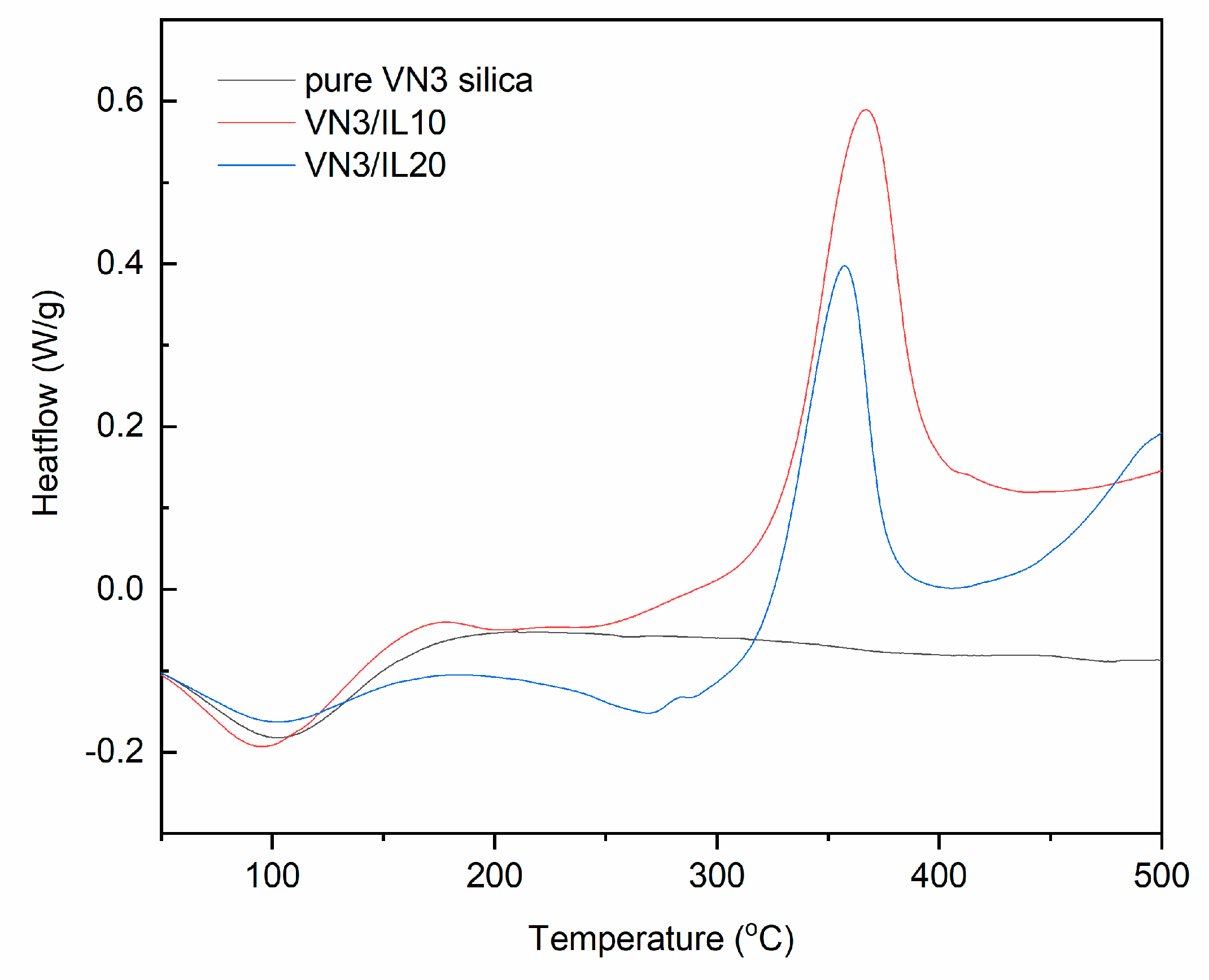
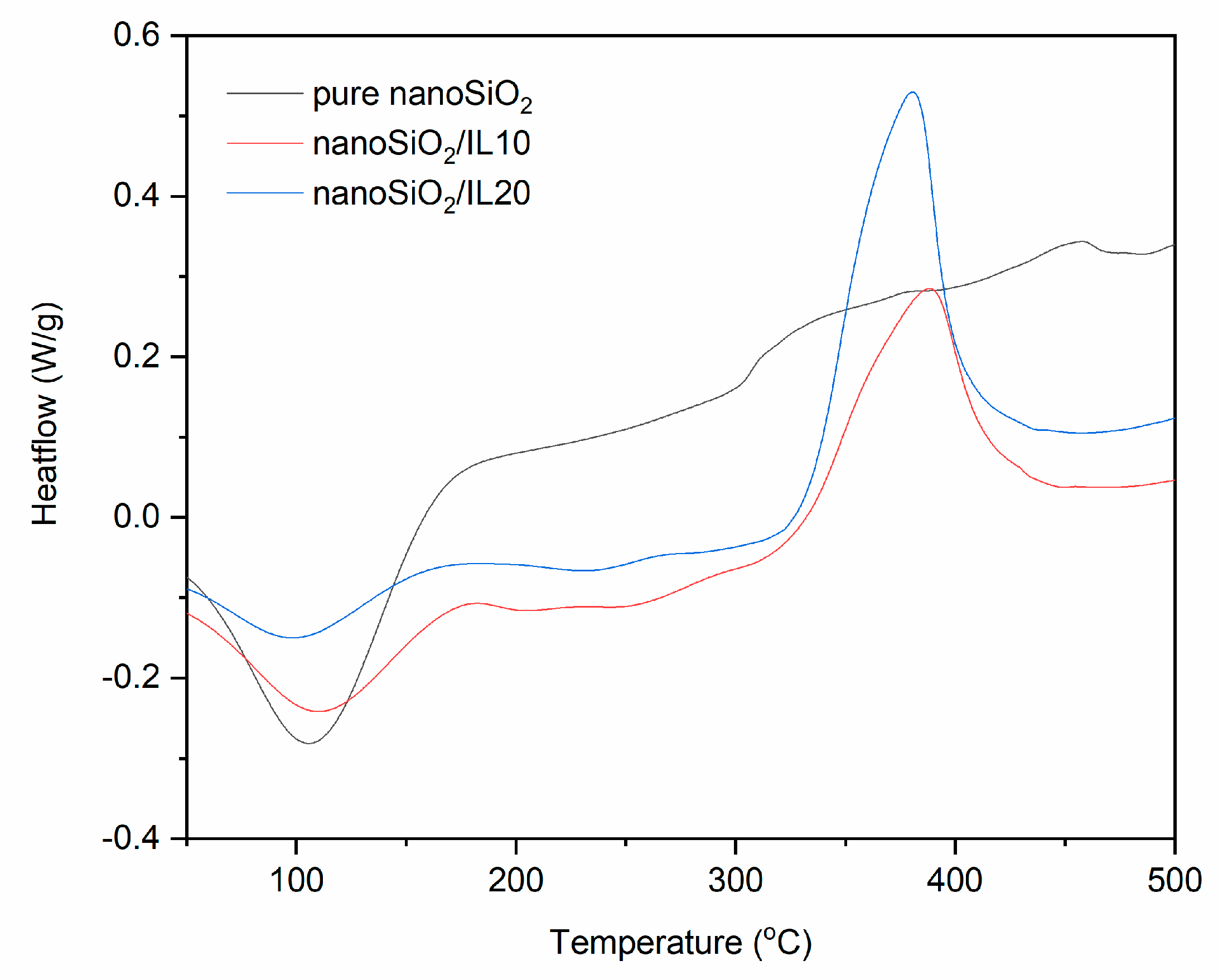
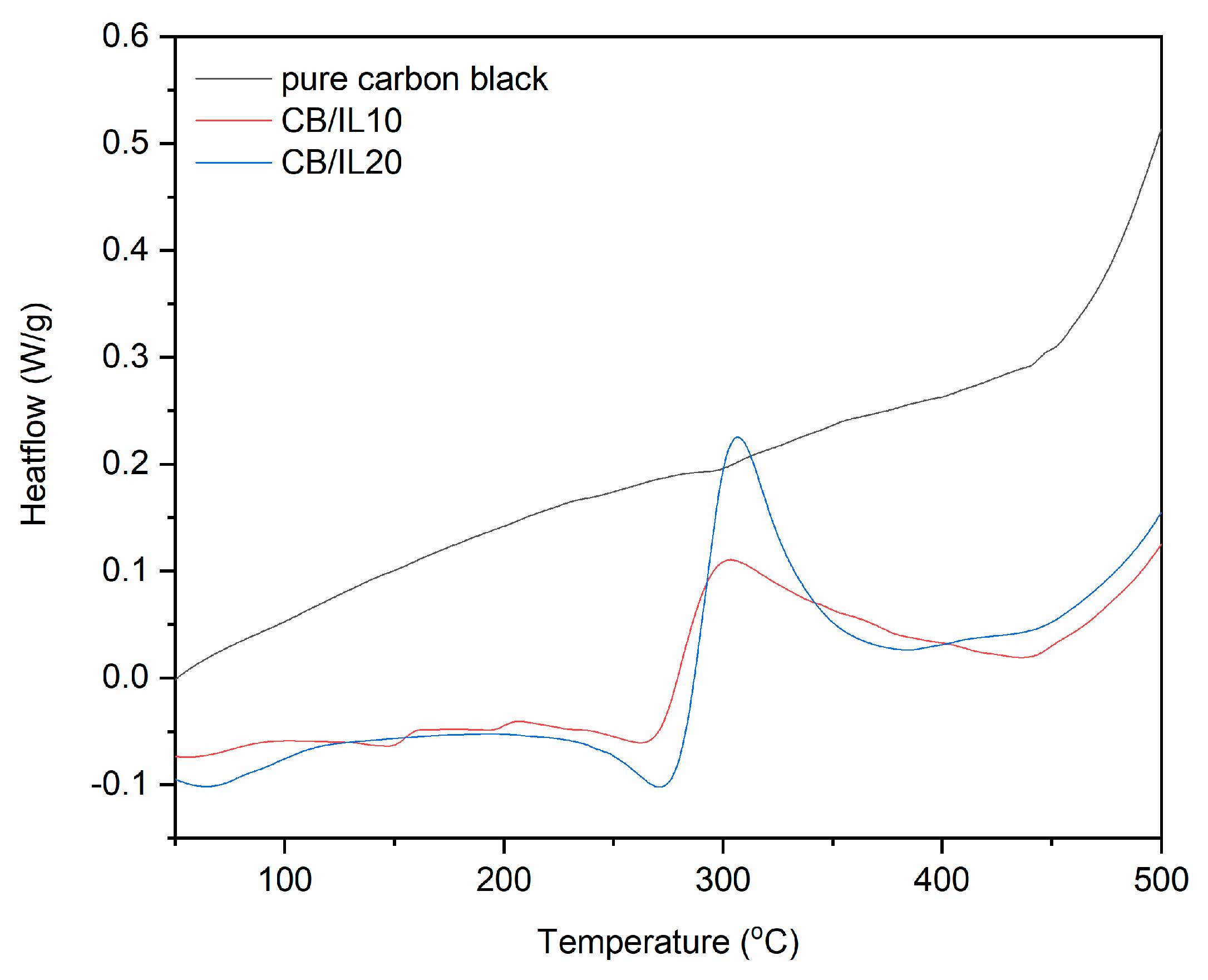
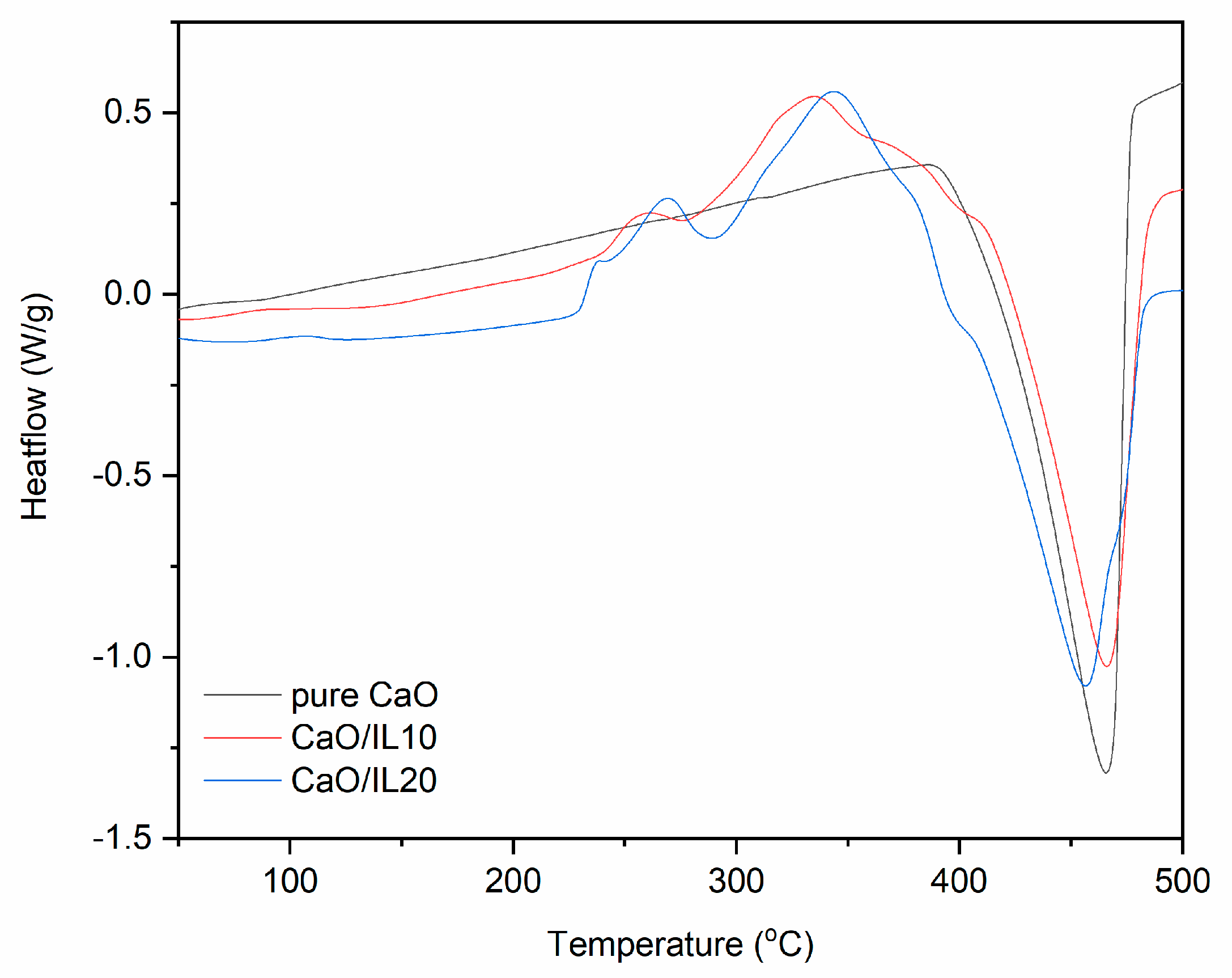

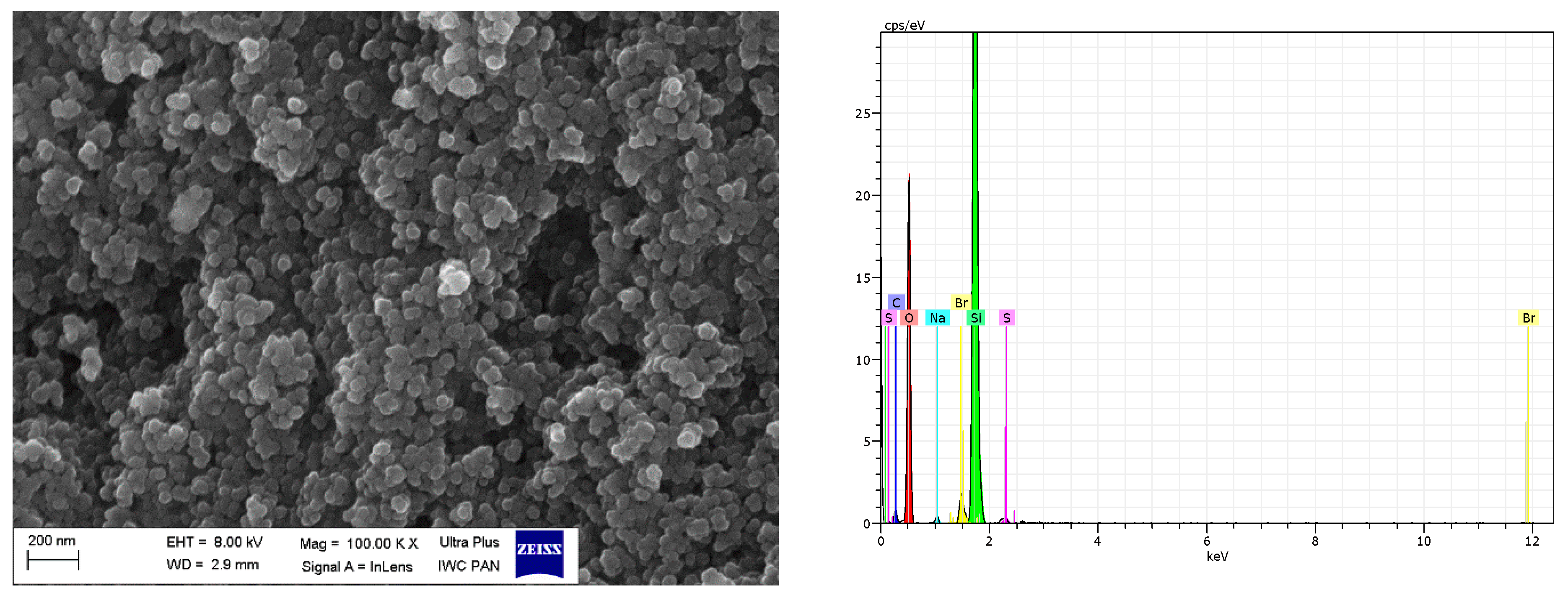
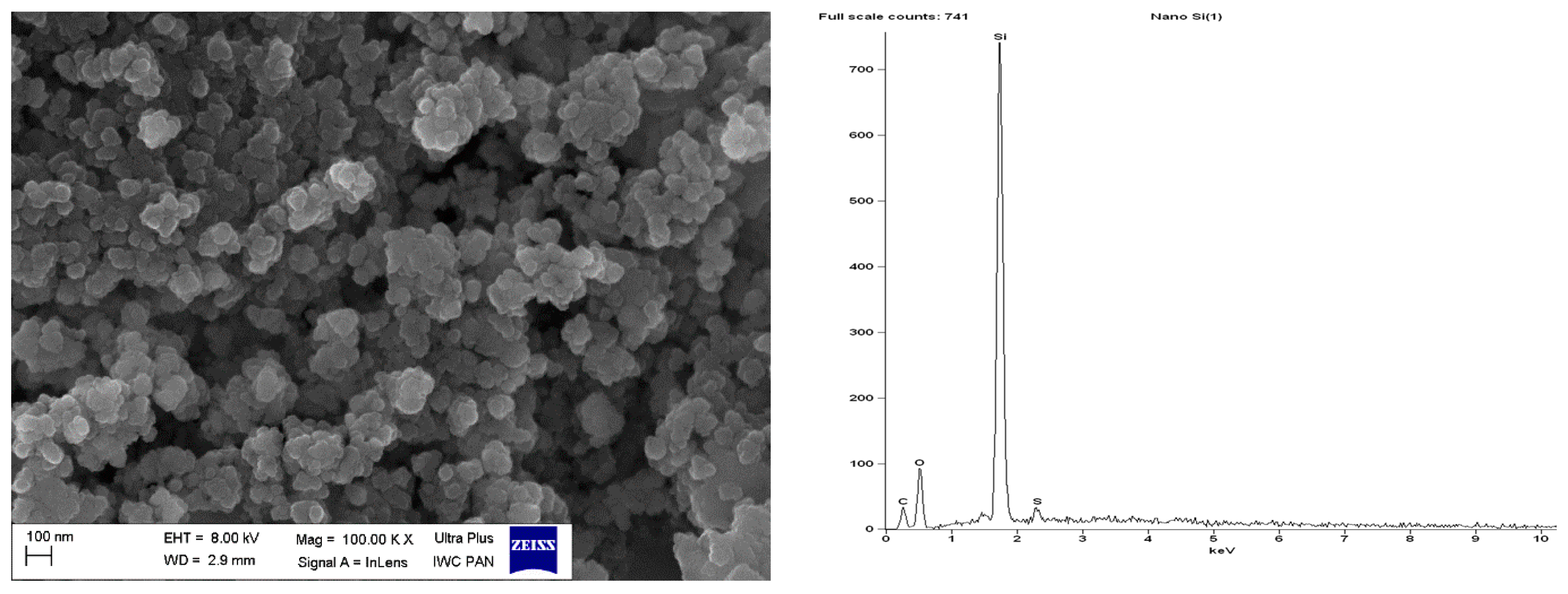
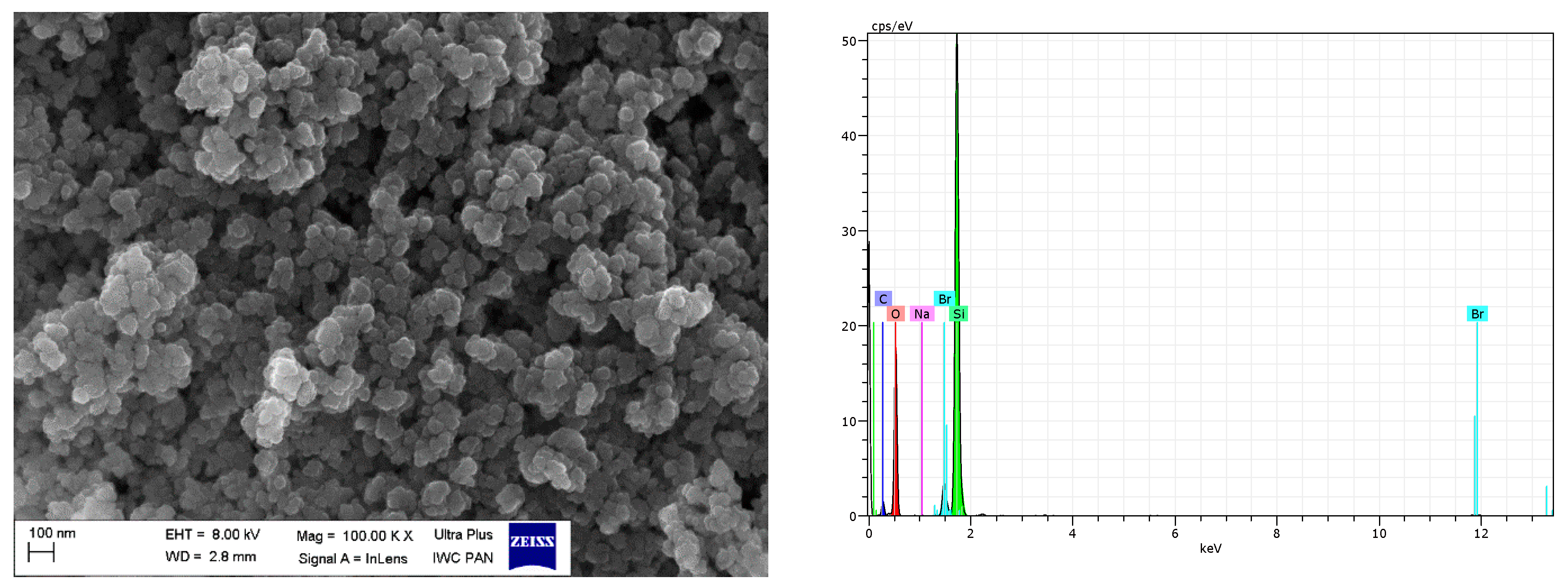
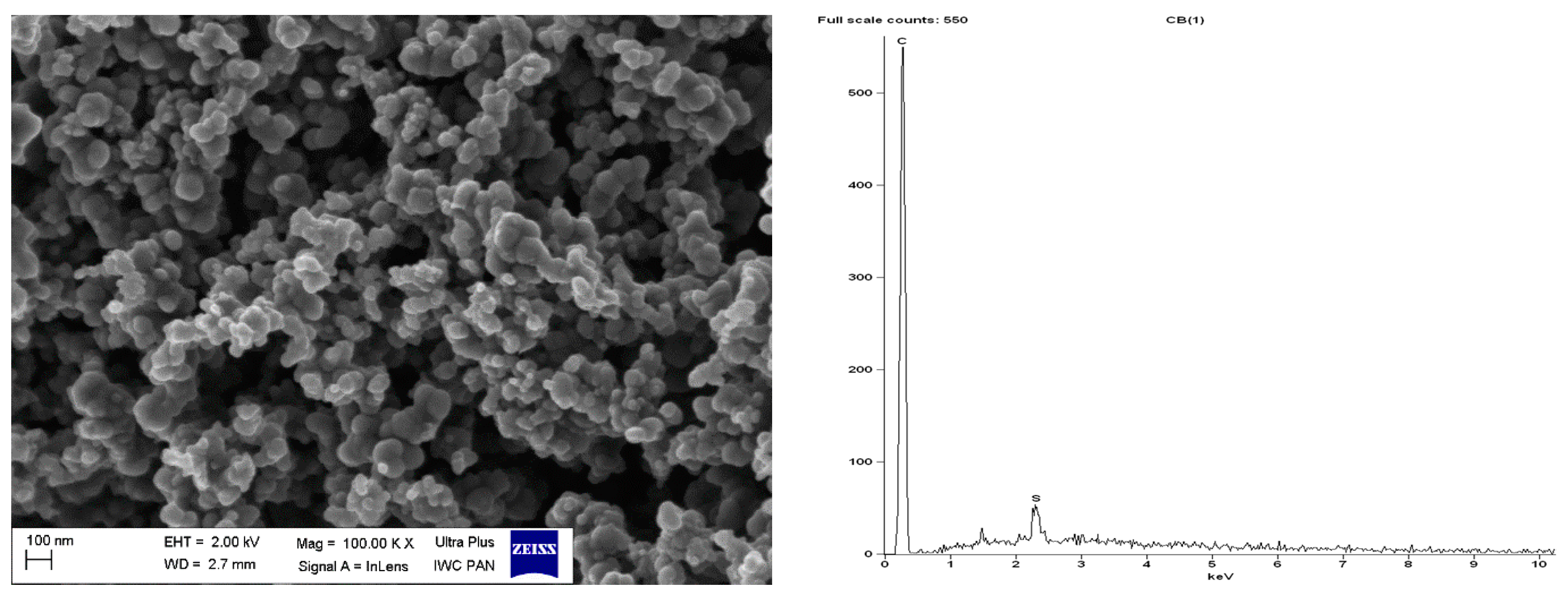
| Sample | Δm < 150 °C (%) | Δm > 150 °C (%) | Residue at 600 °C (%) | |
| DmiBr | 3.5 | 96.1 | 0.4 | |
| VN3 | 3.7 | 2.2 | 94.1 | |
| VN3/IL10 | 5.3 | 10.7 | 84.0 | |
| VN3/IL20 | 2.5 | 17.9 | 79.6 | |
| nanoSiO2 | 10.0 | 3.2 | 86.8 | |
| nanoSiO2/IL10 | 4.0 | 12.4 | 83.6 | |
| nanoSiO2/IL20 | 2.6 | 17.9 | 79.5 | |
| CB | - | 0.2 | 99.8 | |
| CB/IL10 | 0.3 | 8.8 | 90.9 | |
| CB/IL20 | 0.7 | 16.1 | 83.2 | |
| Sample | Δm < 200 °C (%) | Δm 200–350 °C (%) | Δm > 350 °C (%) | Residue at 600 °C (%) |
| CaO | 1.5 | - | 20.0 | 78.5 |
| CaO/IL10 | 1.6 | 6.7 | 17.7 | 74.0 |
| CaO/IL20 | 1.9 | 12.7 | 16.4 | 69.0 |
| Mass/Charge Ratios of Secondary Ions for DmiBr | ||
|---|---|---|
| >180 °C | >240 °C | >360 °C |
| 12, 13-C+; 15-CH3+; 39-C3H3+, 42-C3H6+; 51-C4H3+, C3NH+; 52-C4H4+, C3NH2+; 79, 80, 81-Br | 14 – CH2+; 27 – C2H3+; 30 – C2H6+; 42 – C3H6+; 43 – C3H7+; 55 – C4H7+, C3NH5+; 56 – C4H8+, C3NH6+ | 12 – C+; 26 – C2H2+; 27– C2H3+; 41– C3H5+; 42 – C3H6+; 43 – C3H7+ |
| Sample | Amount of used DmiBr (mmole/g of filler) | Amount of DmiBr in Modified Filler (mmole/g of filler) | Efficiency of Modification (%) |
|---|---|---|---|
| VN3/IL10 | 0.33 | 0.28 | 85 |
| VN3/IL20 | 0.66 | 0.52 | 79 |
| nanoSiO2/IL10 | 0.33 | 0.30 | 92 |
| nanoSiO2/IL20 | 0.66 | 0.49 | 74 |
| CB/IL10 | 0.33 | 0.28 | 86 |
| CB/IL20 | 0.66 | 0.53 | 80 |
| CaO/IL10 | 0.33 | 0.22 | 67 |
| CaO/IL20 | 0.66 | 0.42 | 64 |
| m/z of Secondary Ions for DmiBr-Modified VN3 | |||
| Sample | 120–200 °C | >200 °C | >400 °C |
| VN3/IL10 | 26, 48, 64 | 12, 13, 15, 18, 26, 27, 35, 79, 80 | 12, 18, 26, 44, 46, 48 |
| VN3/IL20 | 12, 29, 31, 52 | 12, 15, 29, 31, 35, 47, 50, 79, 80, 81 | 12, 29, 50, 52 |
| m/z of Secondary Ions for DmiBr-Modified NanoSiO2 | |||
| nanoSiO2/IL10 | 12, 17, 18, 24, 25, 26, 27, 60, 62 | 63, 64, 72, 78, 79, 80 | 12, 15, 17, 18, 24, 25, 26, 27, 30, 48, 60, 63, 64, 72, 78, 79, 80 |
| nanoSiO2/IL20 | 12, 15, 29, 26, 30 | 12, 15, 26, 29, 30 | 12, 26, 29, 30 |
| m/z of Secondary Ions for DmiBr-Modified CB | |||
| Sample | 160–200 °C | >200 °C | |
| CB/IL10 | 78 | 12, 13, 14, 15, 47, 79, 80 | |
| CB/IL20 | 18, 47 | 12, 13, 15, 18, 47, 79, 80 | |
| m/z of Secondary Ions for DmiBr-Modified CaO | |||
| Sample | 160–200 °C | >200 °C | >300 °C |
| CaO/IL10 | 12, 14, 15, 44, 45 | 15, 31, 35, 44, 47, 50, 52, 79, 80 | 12, 15, 30, 44 |
| CaO/IL20 | - | 15, 31, 35, 39, 47, 48, 50, 52, 79, 80 | 12, 15, 44 |
| Tg (°C) | Tcc (°C) | ΔHcc (kJ/mole IL) | Tm (°C) | ΔHm (kJ/mole IL) | Tdec (endo) (°C) | ΔHdec (endo) (kJ/mole IL) | Tdec (exo) (°C) | ΔHdec (exo) (kJ/mole IL) |
|---|---|---|---|---|---|---|---|---|
| −63.0 | 18.2 | −16.3 | 3.2 | 16.5 | 299.6 | −44.0 | 317.1 | 125.5 |
| Sample | Desorption of Moisture/Solvent (°C) | Desorption/Decomposition of IL Immobilized on the Filler’s Surface | Decomposition of IL | ||
|---|---|---|---|---|---|
| Temperature (°C) | Enthalpy (kJ/mole IL) | Temperature (°C) | Enthalpy (kJ/mole IL) | ||
| pure VN3 silica | 60–113 | - | - | - | - |
| VN3/IL10 | 33–123 | 231–309 | −5.5 | 330–392 | 80.1 |
| VN3/IL20 | 39–124 | 229–287 | −11.5 | 322–377 | 71.2 |
| pure nanoSiO2 | 52–162 | - | - | - | - |
| nanoSiO2/IL10 | 56–111 | 187–284 | −6.0 | 332–434 | 57.0 |
| nanoSiO2/IL20 | 49–155 | 196–321 | −7.1 | 334–403 | 84.4 |
| pure CB | - | - | - | - | - |
| CB/IL10 | 29–87 | 223–275 | −4.0 | 276–384 | 33.9 |
| CB/IL20 | 30–106 | 237–284 | −10.9 | 286–344 | 38.9 |
| pure CaO | - | - | - | - | - |
| CaO/IL10 | - | 35–260 | –22.0 | 290–402 | 54.9 |
| CaO/IL20 | - | 33–259 | –36.7 | 296–387 | 58.6 |
| Filler | Element (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| O K | Si K | C K | Br K | N K | Na K | S K | Total | |
| pure VN3 | 37.69 | 18.80 | 40.81 | - | - | 0.96 | 1.75 | 100 |
| VN3/IL10 | 50.74 | 38.72 | 8.11 | 1.50 | - | 0.53 | 0.41 | 100 |
| VN3/IL20 | 52.13 | 28.55 | 15.20 | 3.02 | - | 0.58 | 0.53 | 100 |
| pure nanoSiO2 | 37.43 | 30.22 | 31.08 | - | - | - | 1.27 | 100 |
| nanoSiO2/IL10 | 51.59 | 34.89 | 11.19 | 2.12 | - | - | 0.20 | 100 |
| nanoSiO2/IL20 | 47.91 | 34.35 | 13.16 | 4.50 | - | 0.08 | - | 100 |
| pure CB | 2.38 | - | 95.78 | - | - | - | 4.22 | 100 |
| CB/IL10 | 4.63 | - | 84.88 | 1.26 | 8.42 | 0.12 | 0.69 | 100 |
| CB/IL20 | 3.59 | - | 85.48 | 3.43 | 6.77 | 0.09 | 0.64 | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sowińska, A.; Maciejewska, M.; Guo, L.; Delebecq, E. Thermal Analysis and SEM Microscopy Applied to Studying the Efficiency of Ionic Liquid Immobilization on Solid Supports. Materials 2019, 12, 1579. https://doi.org/10.3390/ma12101579
Sowińska A, Maciejewska M, Guo L, Delebecq E. Thermal Analysis and SEM Microscopy Applied to Studying the Efficiency of Ionic Liquid Immobilization on Solid Supports. Materials. 2019; 12(10):1579. https://doi.org/10.3390/ma12101579
Chicago/Turabian StyleSowińska, Anna, Magdalena Maciejewska, Laina Guo, and Etienne Delebecq. 2019. "Thermal Analysis and SEM Microscopy Applied to Studying the Efficiency of Ionic Liquid Immobilization on Solid Supports" Materials 12, no. 10: 1579. https://doi.org/10.3390/ma12101579
APA StyleSowińska, A., Maciejewska, M., Guo, L., & Delebecq, E. (2019). Thermal Analysis and SEM Microscopy Applied to Studying the Efficiency of Ionic Liquid Immobilization on Solid Supports. Materials, 12(10), 1579. https://doi.org/10.3390/ma12101579