Recycled Cobalt from Spent Li-ion Batteries as a Superhydrophobic Coating for Corrosion Protection of Plain Carbon Steel
Abstract
1. Introduction
- sulfuric acid leaching without any reductive agent;
- sulfuric acid leaching in the presence of hydrogen peroxide; and,
- sulfuric acid leaching in the presence of ascorbic acid.
2. Materials and Methods
2.1. Materials
2.2. Pretreatment of Spent LIBs
2.3. Leaching Experiments
2.4. Electrodeposition
2.5. Microstructural, Chemical and Simulation Analysis
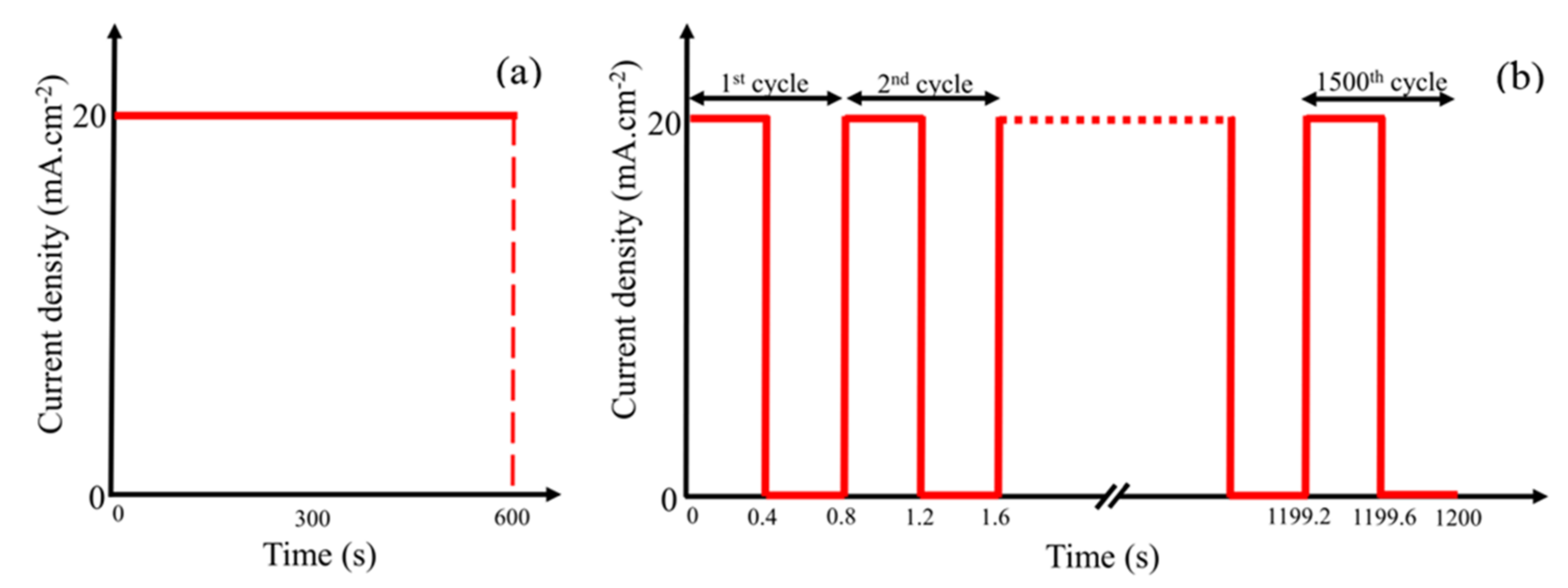
2.6. Cyclic Voltammetry and Corrosion Behavior of the Coating
3. Results
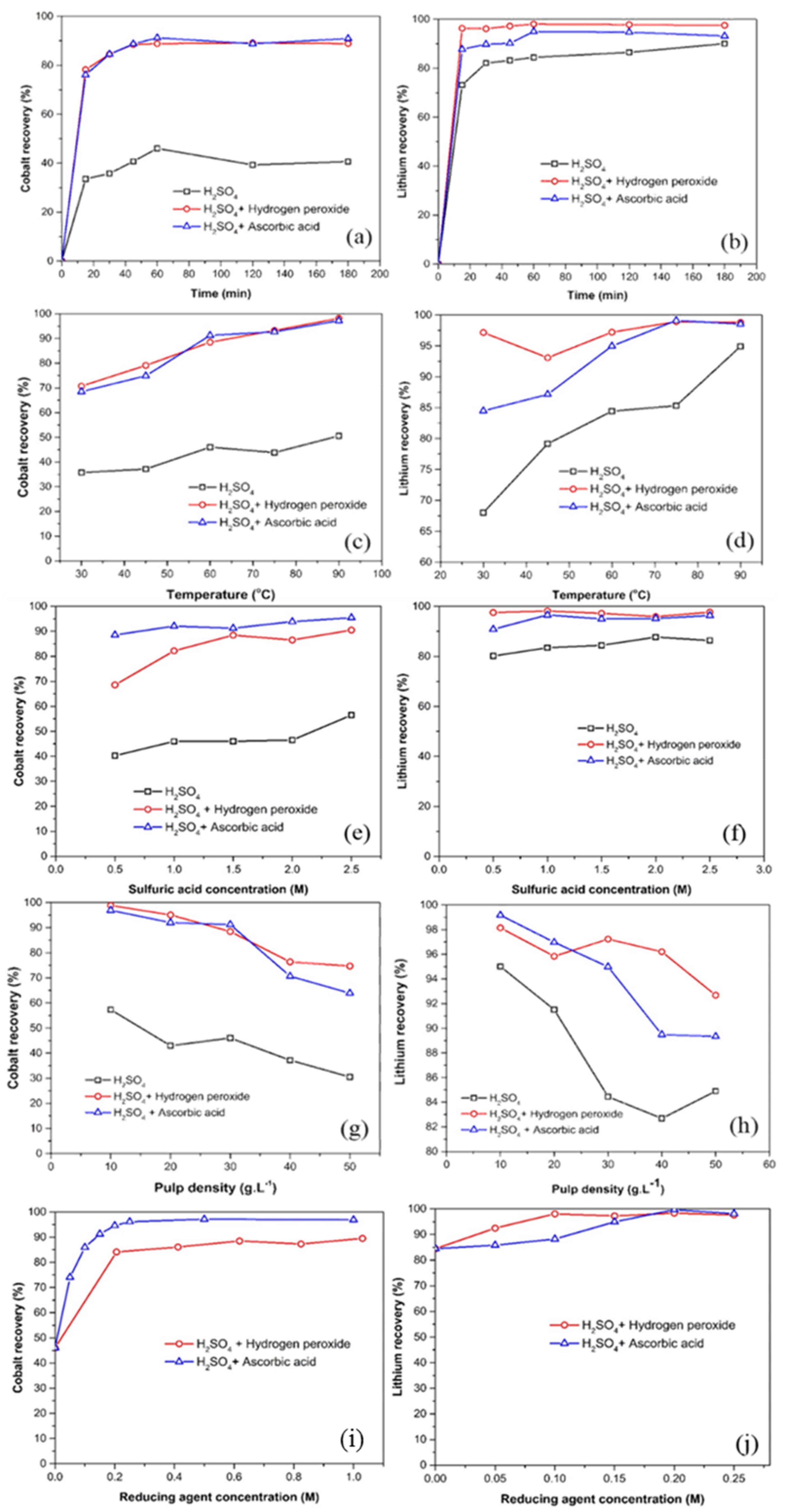
3.1. Optimization of Leaching Parameters
3.1.1. Effect of Leaching Time
3.1.2. Effect of Temperature
3.1.3. Effect of Sulfuric Acid Concentration
3.1.4. Effect of Pulp Density
3.1.5. Effect of Reducing Agent
3.2. Electrodeposition
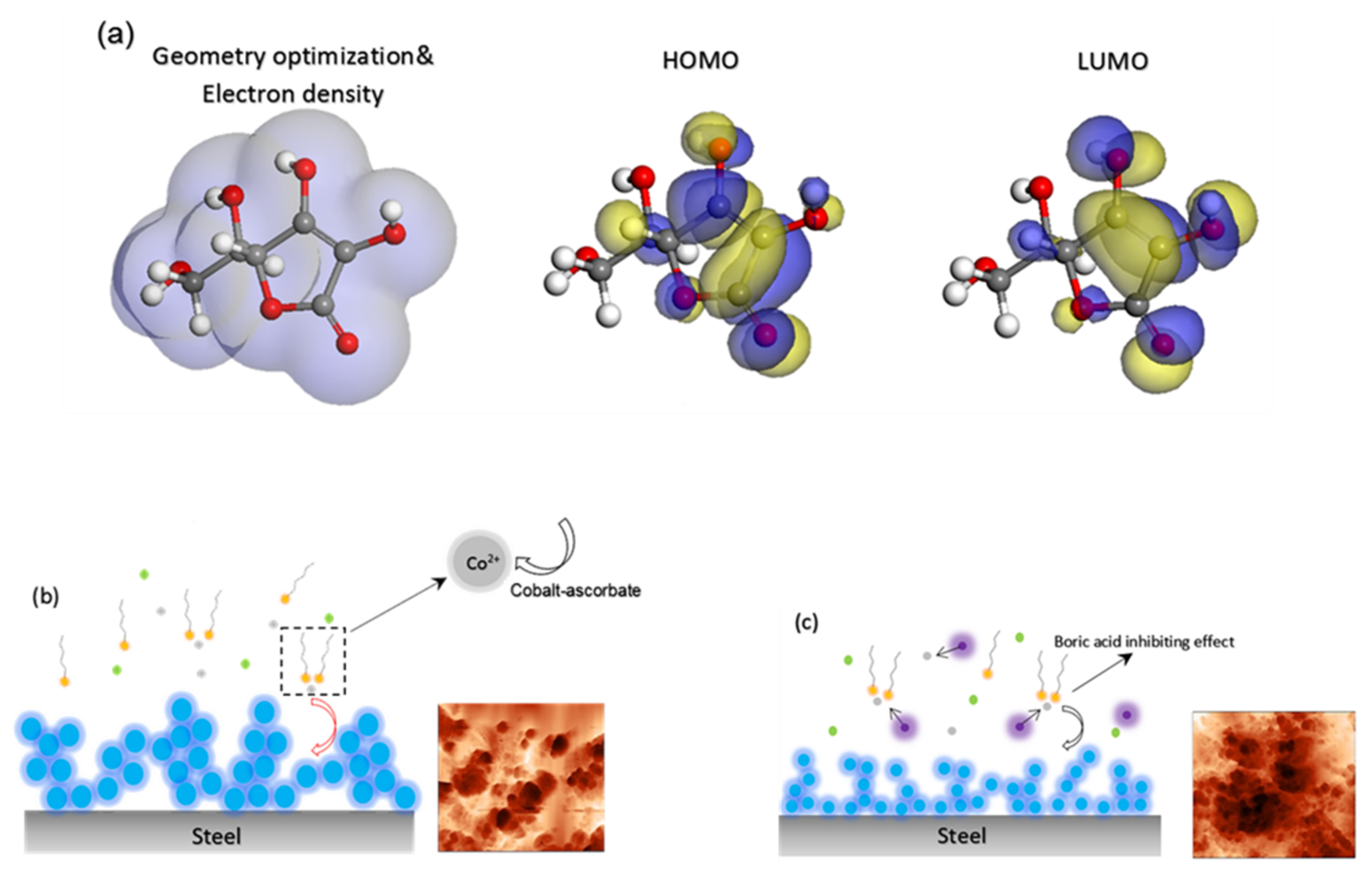
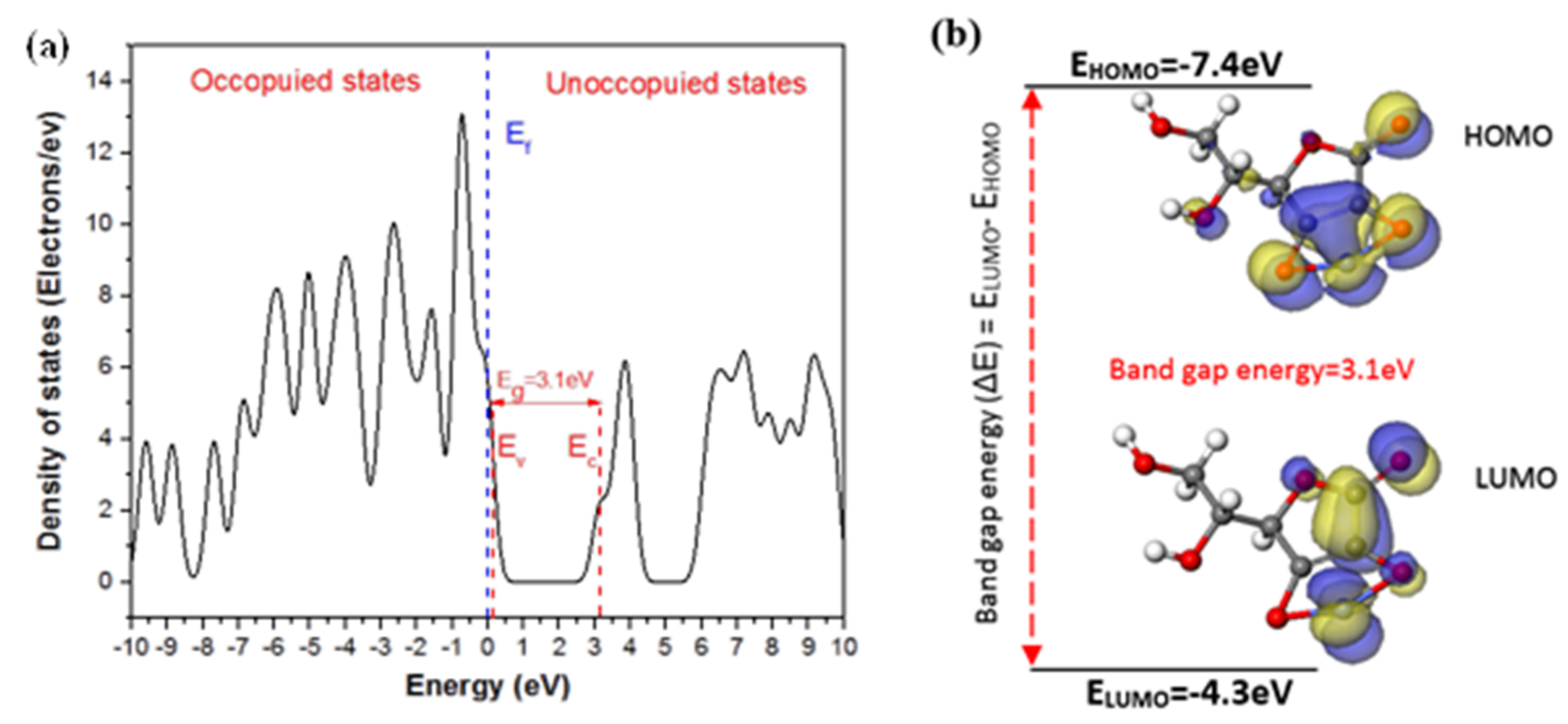
3.2.1. Cyclic Voltammetry and Simulation Studies
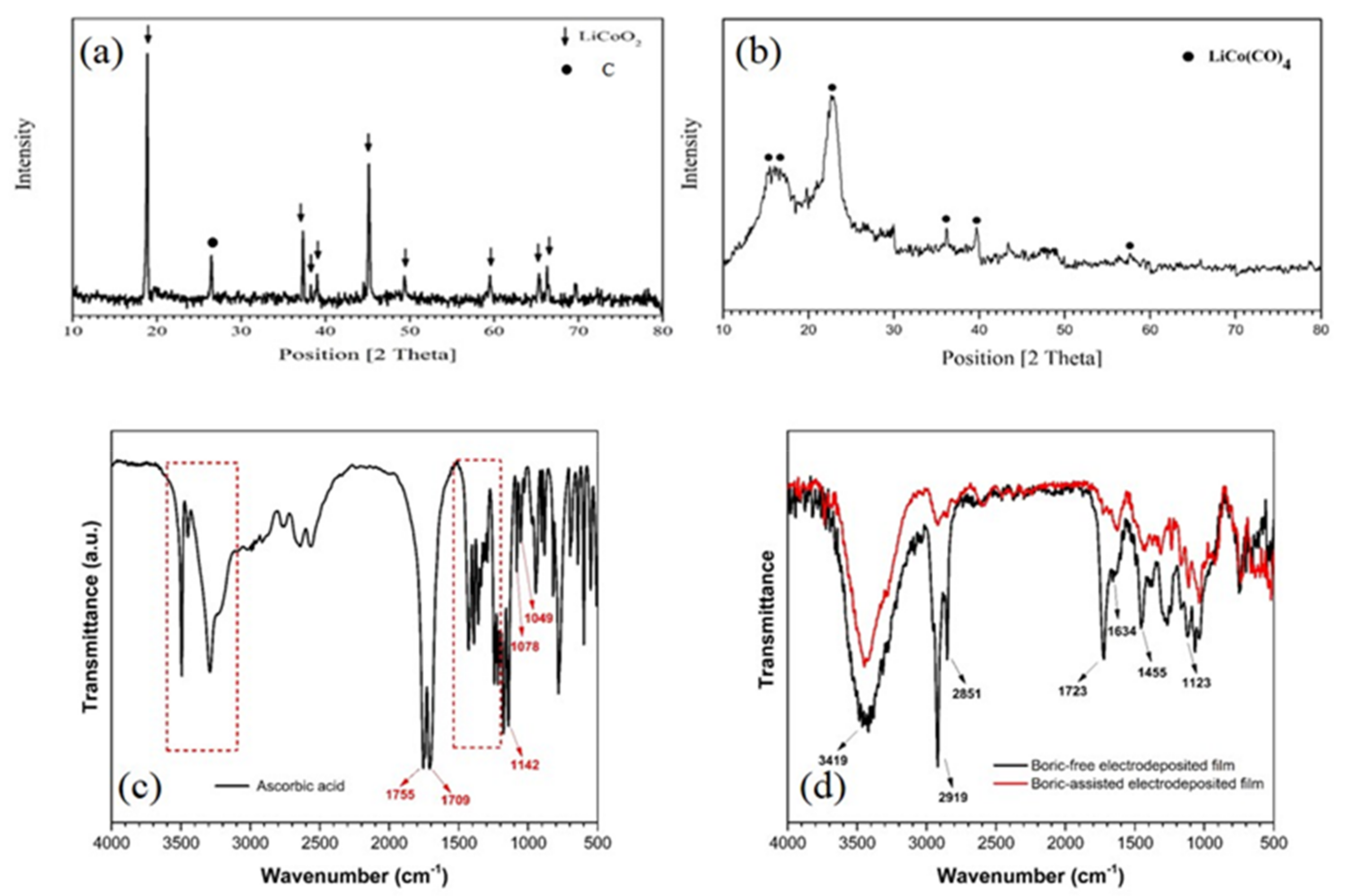
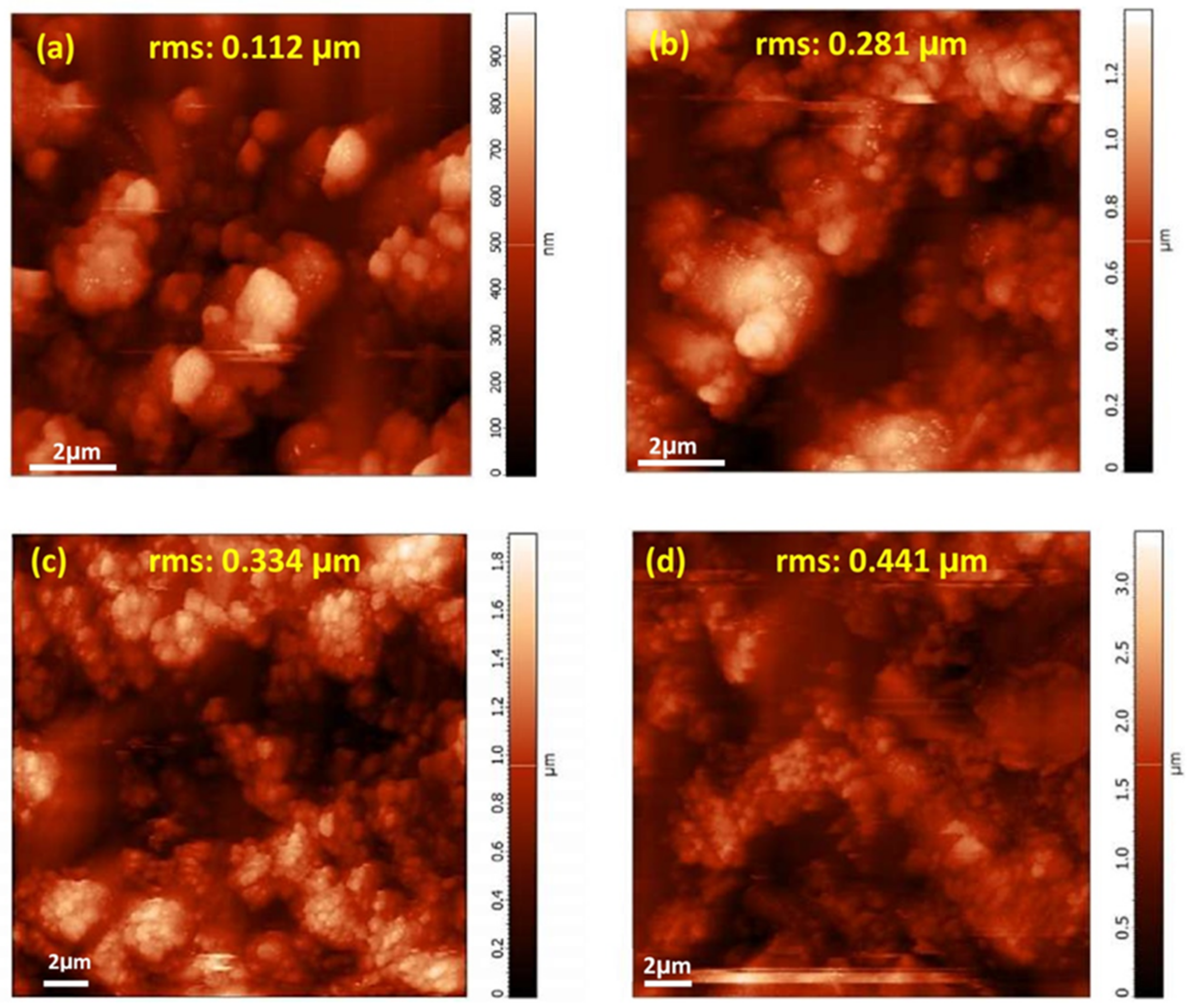
3.2.2. X-ray Diffraction and FT-IR Analysis of the Deposited Films
3.2.3. Corrosion Protection Behavior of the Electrodeposited Film
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, B.; Li, Q.; Cartmell, S.; Ferrara, S.; Deng, Z.D.; Xiao, J. Lithium and lithium ion batteries for applications in microelectronic devices: A review. J. Power Sources 2015, 286, 330–345. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, J.; Li, H.; Chen, Y.; Wang, C. A promising approach for the recovery of high value-added metals from spent lithium-ion batteries. J. Power Sources 2017, 351, 192–199. [Google Scholar] [CrossRef]
- Väyrynen, A.; Salminen, J. Lithium ion battery production. J. Chem. Thermodyn. 2012, 46, 80–85. [Google Scholar] [CrossRef]
- Kang, J.; Senanayake, G.; Sohn, J.; Shin, S.M. Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272. Hydrometallurgy 2010, 100, 168–171. [Google Scholar] [CrossRef]
- Lv, W.; Wang, Z.; Cao, H.; Sun, Y.; Zhang, Y.; Sun, Z. A critical review and analysis on the recycling of spent lithium-ion batteries. ACS Sustain. Chem. Eng. 2018, 6, 1504–1521. [Google Scholar] [CrossRef]
- Ferreira, D.A.; Prados, L.M.Z.; Majuste, D.; Mansur, M.B. Hydrometallurgical separation of aluminium, cobalt, copper and lithium from spent Li-ion batteries. J. Power Sources 2009, 187, 238–246. [Google Scholar] [CrossRef]
- Joulié, M.; Laucournet, R.; Billy, E. Hydrometallurgical process for the recovery of high value metals from spent lithium nickel cobalt aluminum oxide based lithium-ion batteries. J. Power Sources 2014, 247, 551–555. [Google Scholar] [CrossRef]
- Lee, C.K.; Rhee, K.-I. Reductive leaching of cathodic active materials from lithium ion battery wastes. Hydrometallurgy 2003, 68, 5–10. [Google Scholar] [CrossRef]
- Li, J.; Shi, P.; Wang, Z.; Chen, Y.; Chang, C.-C. A combined recovery process of metals in spent lithium-ion batteries. Chemosphere 2009, 77, 1132–1136. [Google Scholar] [CrossRef]
- Swain, B.; Jeong, J.; Lee, J.-C.; Lee, G.-H.; Sohn, J.-S. Hydrometallurgical process for recovery of cobalt from waste cathodic active material generated during manufacturing of lithium ion batteries. J. Power Sources 2007, 167, 536–544. [Google Scholar] [CrossRef]
- Li, L.; Zhai, L.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Recovery of valuable metals from spent lithium-ion batteries by ultrasonic-assisted leaching process. J. Power Sources 2014, 262, 380–385. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Zhou, T.; Liu, D.; Hu, H.; Fan, S. Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries. Waste Manag. 2015, 38, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.M.; Kim, N.H.; Sohn, J.S.; Yang, D.H.; Kim, Y.H. Development of a metal recovery process from Li-ion battery wastes. Hydrometallurgy 2005, 79, 172–181. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Ren, Y.; Zhang, X.X.; Chen, R.J.; Wu, F.; Amine, K. Ascorbic-acid-assisted recovery of cobalt and lithium from spent Li-ion batteries. J. Power Sources 2012, 218, 21–27. [Google Scholar] [CrossRef]
- Nayaka, G.; Manjanna, J.; Pai, K.; Vadavi, R.; Keny, S.; Tripathi, V. Recovery of valuable metal ions from the spent lithium-ion battery using aqueous mixture of mild organic acids as alternative to mineral acids. Hydrometallurgy 2015, 151, 73–77. [Google Scholar] [CrossRef]
- Li, L.; Bian, Y.; Zhang, X.; Guan, Y.; Fan, E.; Wu, F.; Chen, R. Process for recycling mixed-cathode materials from spent lithium-ion batteries and kinetics of leaching. Waste Manag. 2018, 71, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ge, J.; Wu, F.; Chen, R.; Chen, S.; Wu, B. Recovery of cobalt and lithium from spent lithium ion batteries using organic citric acid as leachant. J. Hazard. Mater. 2010, 176, 288–293. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Chen, R.; Wu, F.; Chen, S.; Zhang, X. Environmental friendly leaching reagent for cobalt and lithium recovery from spent lithium-ion batteries. Waste Manag. 2010, 30, 2615–2621. [Google Scholar] [CrossRef]
- Sun, L.; Qiu, K. Organic oxalate as leachant and precipitant for the recovery of valuable metals from spent lithium-ion batteries. Waste Manag. 2012, 32, 1575–1582. [Google Scholar] [CrossRef]
- Li, L.; Qu, W.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Succinic acid-based leaching system: A sustainable process for recovery of valuable metals from spent Li-ion batteries. J. Power Sources 2015, 282, 544–551. [Google Scholar] [CrossRef]
- Nayaka, G.; Pai, K.; Santhosh, G.; Manjanna, J. Recovery of cobalt as cobalt oxalate from spent lithium ion batteries by using glycine as leaching agent. J. Environ. Chem. Eng. 2016, 4, 2378–2383. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Pai, K.V.; Manjanna, J.; Keny, S.J. Use of mild organic acid reagents to recover the Co and Li from spent Li-ion batteries. Waste Manag. 2016, 51, 234–238. [Google Scholar] [CrossRef] [PubMed]
- He, L.-P.; Sun, S.-Y.; Mu, Y.-Y.; Song, X.-F.; Yu, J.-G. Recovery of lithium, nickel, cobalt, and manganese from spent lithium-ion batteries using L-tartaric acid as a leachant. ACS Sustain. Chem. Eng. 2016, 5, 714–721. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, X.; Zheng, X.; Lin, X.; Cao, H.; Zhang, Y.; Sun, Z. Lithium carbonate recovery from cathode scrap of spent lithium-ion battery: A closed-loop process. Environ. Sci. Technol. 2017, 51, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dunn, J.B.; Zhang, X.X.; Gaines, L.; Chen, R.J.; Wu, F.; Amine, K. Recovery of metals from spent lithium-ion batteries with organic acids as leaching reagents and environmental assessment. J. Power Sources 2013, 233, 180–189. [Google Scholar] [CrossRef]
- Li, L.; Fan, E.; Guan, Y.; Zhang, X.; Xue, Q.; Wei, L.; Wu, F.; Chen, R. Sustainable recovery of cathode materials from spent lithium-ion batteries using lactic acid leaching system. ACS Sustain. Chem. Eng. 2017, 5, 5224–5233. [Google Scholar] [CrossRef]
- Fernández-Merino, M.J.; Guardia, L.; Paredes, J.; Villar-Rodil, S.; Solís-Fernández, P.; Martínez-Alonso, A.; Tascon, J. Vitamin C is an ideal substitute for hydrazine in the reduction of graphene oxide suspensions. J. Phys. Chem. C 2010, 114, 6426–6432. [Google Scholar] [CrossRef]
- Rickman, R.; Sorensen, R.; Watkins, K.; Davies, G. Stoichiometry and kinetics of the cobalt (III) oxidation of L-ascorbic acid in acid perchlorate solution. Inorg. Chem. 1977, 16, 1570–1572. [Google Scholar] [CrossRef]
- Fukuyo, T.; Imai, H. Morphological evolution of silver crystals produced by reduction with ascorbic acid. J. Cryst. Growth 2002, 241, 193–199. [Google Scholar] [CrossRef]
- Miranda, O.R.; Dollahon, N.R.; Ahmadi, T.S. Critical concentrations and role of ascorbic acid (vitamin C) in the crystallization of gold nanorods within hexadecyltrimethyl ammonium bromide (CTAB)/tetraoctyl ammonium bromide (TOAB) micelles. Cryst. Growth Des. 2006, 6, 2747–2753. [Google Scholar] [CrossRef]
- Cho, S.; Jeong, H.; Park, D.-H.; Jung, S.-H.; Kim, H.-J.; Lee, K.-H. The effects of vitamin C on ZnO crystal formation. CrystEngComm 2010, 12, 968–976. [Google Scholar] [CrossRef]
- Heydarian, A.; Mousavi, S.M.; Vakilchap, F.; Baniasadi, M. Application of a mixed culture of adapted acidophilic bacteria in two-step bioleaching of spent lithium-ion laptop batteries. J. Power Sources 2018, 378, 19–30. [Google Scholar] [CrossRef]
- Ku, H.; Jung, Y.; Jo, M.; Park, S.; Kim, S.; Yang, D.; Rhee, K.; An, E.-M.; Sohn, J.; Kwon, K. Recycling of spent lithium-ion battery cathode materials by ammoniacal leaching. J. Hazard. Mater. 2016, 313, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, F.; Zhu, H.; Li, G.; Huang, J.; He, W. Leaching lithium from the anode electrode materials of spent lithium-ion batteries by hydrochloric acid (HCl). Waste Manag. 2016, 51, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, R.; Sun, F.; Wu, F.; Liu, J. Preparation of LiCoO 2 films from spent lithium-ion batteries by a combined recycling process. Hydrometallurgy 2011, 108, 220–225. [Google Scholar] [CrossRef]
- Shalchian, H.; Rafsanjani-Abbasi, A.; Vahdati-Khaki, J.; Babakhani, A. Selective Acidic Leaching of Spent Zinc-Carbon Batteries Followed by Zinc Electrowinning. Metall. Mater. Trans. B 2015, 46, 38–47. [Google Scholar] [CrossRef]
- Bian, D.; Sun, Y.; Li, S.; Tian, Y.; Yang, Z.; Fan, X.; Zhang, W. A novel process to recycle spent LiFePO4 for synthesizing LiFePO4/C hierarchical microflowers. Electrochim. Acta 2016, 190, 134–140. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, L. Definition of superhydrophobic states. Adv. Mater. 2007, 19, 3423–3424. [Google Scholar] [CrossRef]
- Celia, E.; Darmanin, T.; de Givenchy, E.T.; Amigoni, S.; Guittard, F. Recent advances in designing superhydrophobic surfaces. J. Colloid Interface Sci. 2013, 402, 1–18. [Google Scholar] [CrossRef]
- Min, W.L.; Jiang, B.; Jiang, P. Bioinspired self-cleaning antireflection coatings. Adv. Mater. 2008, 20, 3914–3918. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, X.; Zhang, J.; Yu, S.; Han, Z.; Ren, L. A electro-deposition process for fabrication of biomimetic super-hydrophobic surface and its corrosion resistance on magnesium alloy. Electrochim. Acta 2014, 125, 395–403. [Google Scholar] [CrossRef]
- Rahimi, E.; Rafsanjani-Abbasi, A.; Davoodi, A.; Kiani-Rashid, A. Shape evolution of water and saline droplets during icing/melting cycles on superhydrophobic surface. Surf. Coat. Technol. 2018, 333, 201–209. [Google Scholar] [CrossRef]
- Seo, K.; Kim, M. Candle-based process for creating a stable superhydrophobic surface. Carbon 2014, 68, 583–596. [Google Scholar] [CrossRef]
- Bormashenko, E.; Bormashenko, Y. Non-stick droplet surgery with a superhydrophobic scalpel. Langmuir 2011, 27, 3266–3270. [Google Scholar] [CrossRef]
- Notsu, H.; Kubo, W.; Shitanda, I.; Tatsuma, T. Super-hydrophobic/super-hydrophilic patterning of gold surfaces by photocatalytic lithography. J. Mater. Chem. 2005, 15, 1523–1527. [Google Scholar] [CrossRef]
- Zhu, M.; Zuo, W.; Yu, H.; Yang, W.; Chen, Y. Superhydrophobic surface directly created by electrospinning based on hydrophilic material. J. Mater. Sci. 2006, 41, 3793–3797. [Google Scholar] [CrossRef]
- Manca, M.; Cannavale, A.; de Marco, L.; Arico, A.S.; Cingolani, R.; Gigli, G. Durable superhydrophobic and antireflective surfaces by trimethylsilanized silica nanoparticles-based sol−gel processing. Langmuir 2009, 25, 6357–6362. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, M.; Lu, Q.; Shi, Z. Superhydrophobic polyimide films with a hierarchical topography: Combined replica molding and layer-by-layer assembly. Langmuir 2008, 24, 12651–12657. [Google Scholar] [CrossRef]
- Liao, R.; Zuo, Z.; Guo, C.; Yuan, Y.; Zhuang, A. Fabrication of superhydrophobic surface on aluminum by continuous chemical etching and its anti-icing property. Appl. Surf. Sci. 2014, 317, 701–709. [Google Scholar] [CrossRef]
- Ma, M.; Mao, Y.; Gupta, M.; Gleason, K.K.; Rutledge, G.C. Superhydrophobic fabrics produced by electrospinning and chemical vapor deposition. Macromolecules 2005, 38, 9742–9748. [Google Scholar] [CrossRef]
- Wang, N.; Hang, T.; Shanmugam, S.; Li, M. Preparation and characterization of nickel–cobalt alloy nanostructures array fabricated by electrodeposition. CrystEngComm 2014, 16, 6937–6943. [Google Scholar] [CrossRef]
- Xiao, H.; Hu, A.; Hang, T.; Li, M. Electrodeposited nanostructured cobalt film and its dual modulation of both superhydrophobic property and adhesiveness. Appl. Surf. Sci. 2015, 324, 319–323. [Google Scholar] [CrossRef]
- Dorella, G.; Mansur, M.B. A study of the separation of cobalt from spent Li-ion battery residues. J. Power Sources 2007, 170, 210–215. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Contact angle and wetting properties. In Surface Science Techniques; Springer: Berlin, Germany, 2013; pp. 3–34. [Google Scholar]
- Sun, L.; Qiu, K. Vacuum pyrolysis and hydrometallurgical process for the recovery of valuable metals from spent lithium-ion batteries. J. Hazard. Mater. 2011, 194, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.-B.; Mandal, M.; Jaroniec, M. Periodic Mesoporous Benzene− Silicas Prepared Using Boric Acid as Catalyst. Chem. Mater. 2011, 23, 1971–1976. [Google Scholar] [CrossRef]
- Ding, S.; Liu, N.; Li, X.; Peng, L.; Guo, X.; Ding, W. Silica nanotubes and their assembly assisted by boric acid to hierachical mesostructures. Langmuir 2010, 26, 4572–4575. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Jung, K.K.; Ko, J.S. Growth mechanism and application of nanostructures fabricated by a copper sulfate solution containing boric acid. J. Electrochem. Soc. 2016, 163, D407–D413. [Google Scholar] [CrossRef]
- Šupicová, M.; Rozik, R.; Trnkova, L.; Oriňáková, R.; Galova, M. Influence of boric acid on the electrochemical deposition of Ni. J. Solid State Electrochem. 2006, 10, 61–68. [Google Scholar] [CrossRef]
- Hu, Z.; Meng, Y.; Ma, X.; Zhu, H.; Li, J.; Li, C.; Cao, D. Experimental and theoretical studies of benzothiazole derivatives as corrosion inhibitors for carbon steel in 1 M HCl. Corros. Sci. 2016, 112, 563–575. [Google Scholar] [CrossRef]
- Nagasawa, T.; Sueoka, K. Simulation of STM Images on a Flat Si (110)-(8× 2) Surface Using Density Functional Theory. J. Electrochem. Soc. 2012, 159, H201–H207. [Google Scholar] [CrossRef]
- Kanani, N. Electroplating: Basic Principles, Processes and Practice; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Rahimi, E.; Davoodi, A.; Rashid, A.R.K. Characterization of screw dislocation-driven growth in nickel micro-nanostructure electrodeposition process by AFM. Mater. Lett. 2018, 210, 341–344. [Google Scholar] [CrossRef]
- Panicker, C.Y.; Varghese, H.T.; Philip, D. FT-IR, FT-Raman and SERS spectra of Vitamin C. Spectrochim. Acta Part A 2006, 65, 802–804. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wang, Y.; Xue, Q.; Wu, X. Synthesis of highly stable dispersions of nanosized copper particles using L-ascorbic acid. Green Chem. 2011, 13, 900–904. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, D.; Lu, Z. Advantage of super-hydrophobic surface as a barrier against atmospheric corrosion induced by salt deliquescence. Corros. Sci. 2015, 90, 23–32. [Google Scholar] [CrossRef]
- KyoungáKim, S.; BokáLee, S. Role of boric acid in nickel nanotube electrodeposition: A surface-directed growth mechanism. Chem. Commun. 2014, 50, 527–529. [Google Scholar]
- Schem, M.; Schmidt, T.; Gerwann, J.; Wittmar, M.; Veith, M.; Thompson, G.E.; Molchan, I.S.; Hashimoto, T.; Skeldon, P.; Phani, A.R.; et al. CeO2-filled sol–gel coatings for corrosion protection of AA2024-T3 aluminium alloy. Corros. Sci. 2009, 51, 2304–2315. [Google Scholar] [CrossRef]
- Khorsand, S.; Raeissi, K.; Ashrafizadeh, F.; Arenas, M.; Conde, A. Corrosion behaviour of super-hydrophobic electrodeposited nickel–cobalt alloy film. Appl. Surf. Sci. 2016, 364, 349–357. [Google Scholar] [CrossRef]
- Li, J.-H.; Liu, Q.; Wang, Y.-L.; Chen, R.-R.; Takahashi, K.; Li, R.-M.; Liu, L.-H.; Wang, J. Formation of a corrosion-resistant and anti-icing superhydrophobic surface on magnesium alloy via a single-step method. J. Electrochem. Soc. 2016, 163, 213–220. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, X.; Yan, Q. One-step electrochemical deposition to achieve superhydrophobic cobalt incorporated amorphous carbon-based film with self-cleaning and anti-corrosion. Surf. Interface Anal. 2017, 50, 290–296. [Google Scholar] [CrossRef]
- Flemming, M.; Coriand, L.; Duparré, A. Ultra-hydrophobicity through stochastic surface roughness. J. Adhes. Sci. Technol. 2009, 23, 381–400. [Google Scholar] [CrossRef]
- Rahimi, E.; Rafsanjani-Abbasi, A.; Kiani-Rashid, A.; Jafari, H.; Davoodi, A. Morphology modification of electrodeposited superhydrophobic nickel coating for enhanced corrosion performance studied by AFM, SEM-EDS and electrochemical measurements. Colloids Surf. Physicochem. Eng. Aspects 2018, 547, 81–94. [Google Scholar] [CrossRef]


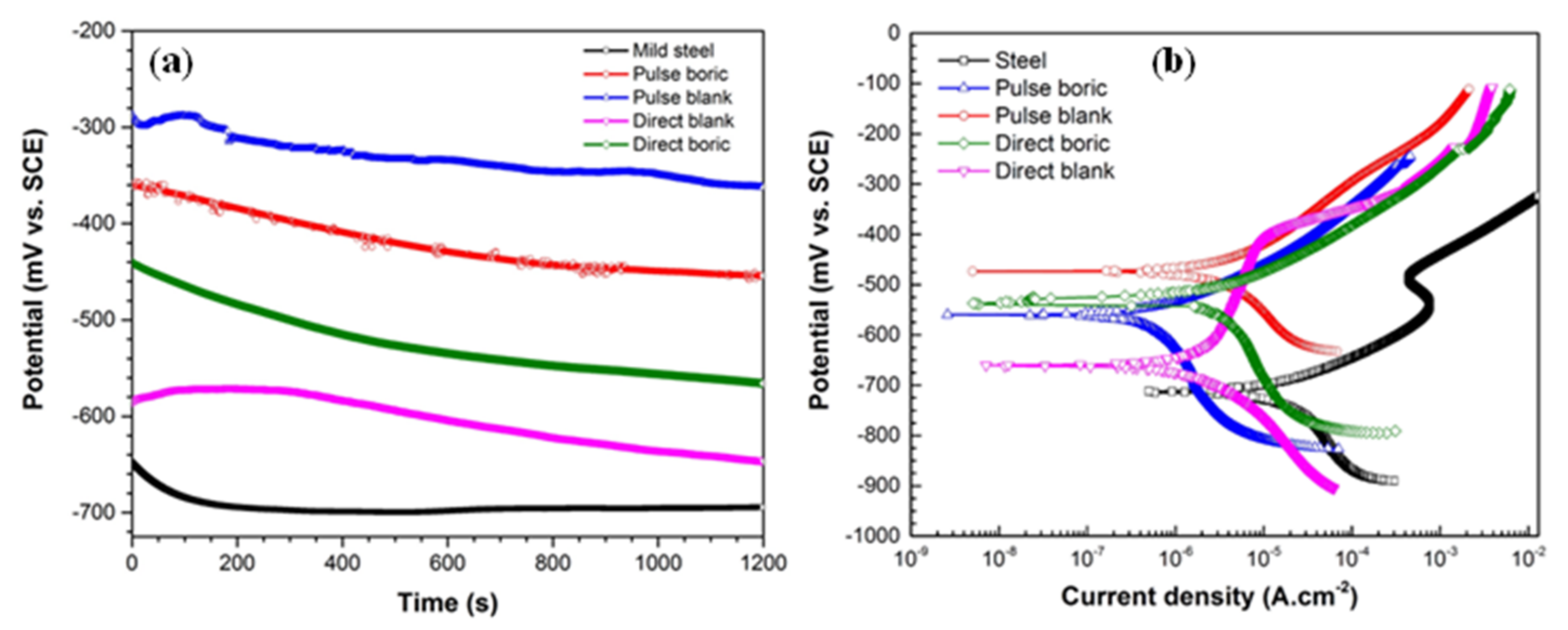


| Surface | icorr (µA·cm−2) | Ecorr (mV vs. SCE) | ba (mV/decade) | bc (mV/decade) |
|---|---|---|---|---|
| Boric-assisted pulsed electrodeposition | 0.5 ± 0.1 | −557 ± 14 | 52 ± 2 | 126 ± 2 |
| Boric-free pulsed electrodeposition | 1 ± 0.1 | −471 ± 15 | 60 ± 3 | 78 ± 4 |
| Boric-assisted direct electrodeposition | 1.4 ± 0.2 | −534 ± 10 | 75 ± 2 | 83 ± 5 |
| Boric-free direct electrodeposition | 1.6 ± 0.2 | −662 ± 12 | 210 ± 4 | 138 ± 3 |
| Plain carbon steel | 10 ± 0.5 | −712 ± 10 | 72 ± 3 | 128 ± 4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafsanjani-Abbasi, A.; Rahimi, E.; Shalchian, H.; Vahdati-Khaki, J.; Babakhani, A.; Hosseinpour, S.; Davoodi, A. Recycled Cobalt from Spent Li-ion Batteries as a Superhydrophobic Coating for Corrosion Protection of Plain Carbon Steel. Materials 2019, 12, 90. https://doi.org/10.3390/ma12010090
Rafsanjani-Abbasi A, Rahimi E, Shalchian H, Vahdati-Khaki J, Babakhani A, Hosseinpour S, Davoodi A. Recycled Cobalt from Spent Li-ion Batteries as a Superhydrophobic Coating for Corrosion Protection of Plain Carbon Steel. Materials. 2019; 12(1):90. https://doi.org/10.3390/ma12010090
Chicago/Turabian StyleRafsanjani-Abbasi, Ali, Ehsan Rahimi, Hossein Shalchian, Jalil Vahdati-Khaki, Abolfazl Babakhani, Saman Hosseinpour, and Ali Davoodi. 2019. "Recycled Cobalt from Spent Li-ion Batteries as a Superhydrophobic Coating for Corrosion Protection of Plain Carbon Steel" Materials 12, no. 1: 90. https://doi.org/10.3390/ma12010090
APA StyleRafsanjani-Abbasi, A., Rahimi, E., Shalchian, H., Vahdati-Khaki, J., Babakhani, A., Hosseinpour, S., & Davoodi, A. (2019). Recycled Cobalt from Spent Li-ion Batteries as a Superhydrophobic Coating for Corrosion Protection of Plain Carbon Steel. Materials, 12(1), 90. https://doi.org/10.3390/ma12010090







