Bone-Healing Pattern on the Surface of Titanium Implants at Cortical and Marrow Compartments in Two Topographic Sites: an Experimental Study in Rabbits
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Study Design and Experimental Animals
2.3. Randomization and Allocation Concealment
2.4. Implant Features
2.5. Surgical Procedures
2.6. Post-Operative Care, Housing and Husbandry
2.7. Euthanasia
2.8. Histological Preparation
2.9. Histological Examination
2.10. Data Analysis
3. Results
3.1. Clinical and Histological Outcomes
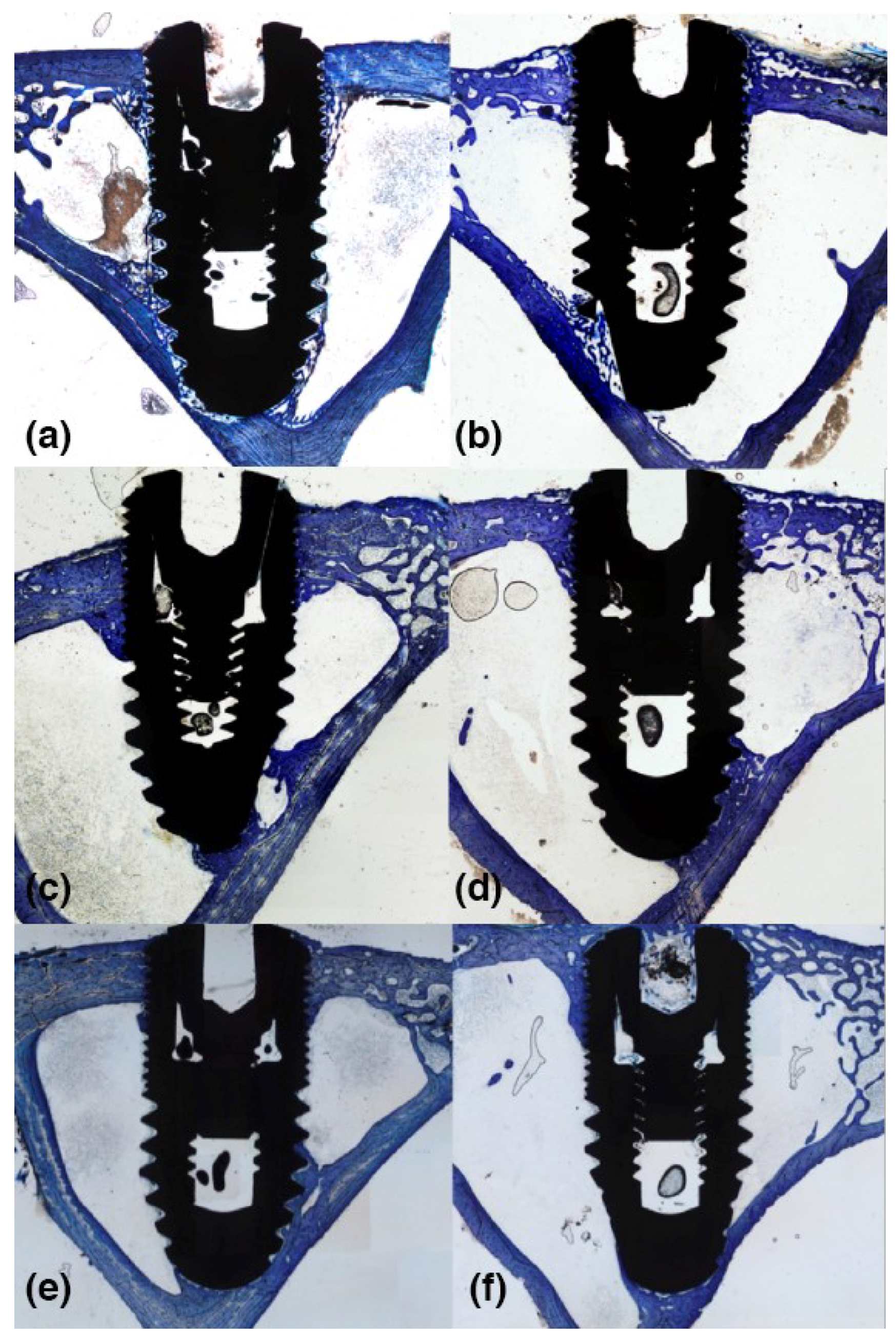
3.2. 2-Weeks of Healing
3.3. 4-Weeks of Healing
3.4. 8-Weeks of Healing
4. Discussion
5. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BIC | bone to implant contact |
| RBM | resorbable blasted media |
| CaP | calcium phosphate bioceramic |
| NB | new bone |
| ST | soft tissue |
| OB | old bone |
References
- Abrahamsson, I.; Linder, E.; Lang, N.P.; Berglundh, T.; Linder, E.; Lang, N.P.; Lindhe, J. Early bone formation adjacent to rough and tumed endosseous implant surfaces An experimental study in the dog. Clin. Oral Implants Res. 2003, 15, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, D.; Berglundh, T.; Persson, L.G.; Lindhe, J. Bone regeneration at implants with turned or rough surfaces in self-contained defects. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, D.; Lang, N.P. Dynamics of osseointegration in various human and animal models—A comparative analysis. Clin. Oral Implants Res. 2017, 28, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.B.; Campos, F.E.; Marin, C.; Teixeira, H.S.; Bonfante, E.A.; Suzuki, M.; Witek, L.; Zanetta-Barbosa, D.; Coelho, P.G. Implant Biomechanical Stability Variation at Early Implantation Times in Vivo: An Experimental Study in Dogs. Int. J. Oral Maxillofac. Implants 2013, 28, e128–e134. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Eriksson, A.R.; Friberg, B.; Lekholm, U.; Lindahl, L.; Nevins, M.; Oikarinen, V.; Roos, J.; Sennerby, L.; Astrand, P. Histologic investigations on 33 retrieved Nobelpharma implants. Clin. Mater. 1993, 12, 1–9. [Google Scholar] [CrossRef]
- Kumar, G.; Narayan, B. Osseointegrated titanium implants: Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Class Pap. Orthop. 2014, 52, 507–509. [Google Scholar]
- Lioubavina-Hack, N.; Lang, N.P.; Karring, T. Significance of primary stability for osseointegration of dental implants. Clin. Oral Implants Res. 2006, 17, 244–250. [Google Scholar] [CrossRef]
- Marco, F.; Milena, F.; Gianluca, G.; Vittoria, O. Peri-implant osteogenesis in health and osteoporosis. Micron 2005, 36, 630–644. [Google Scholar] [CrossRef]
- Falco, A.; Berardini, M.; Trisi, P. Correlation Between Implant Geometry, Implant Surface, Insertion Torque, and Primary Stability: In Vitro Biomechanical Analysis. Int. J. Oral Maxillofac. Implants 2018, 33, 824–830. [Google Scholar] [CrossRef]
- Sela, M.N.; Badihi, L.; Rosen, G.; Steinberg, D.; Kohavi, D. Adsorption of human plasma proteins to modified titanium surfaces. Clin. Oral Implants Res. 2007, 18, 630–638. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implants Res. 2009, 20, 172–184. [Google Scholar] [CrossRef]
- Park, J.Y.; Gemmell, C.H.; Davies, J.E. Platelet interactions with titanium: Modulation of platelet activity by surface topography. Biomaterials 2001, 22, 2671–2682. [Google Scholar] [CrossRef]
- Koh, J.-W.; Yang, J.-H.; Han, J.-S.; Lee, J.-B.; Kim, S.-H. Biomechanical evaluation of dental implants with different surfaces: Removal torque and resonance frequency analysis in rabbits. J. Adv. Prosthodont. 2009, 1, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Davies, J. Understanding peri-implant endosseous healing. J. Dent. Educ. 2003, 67, 932–949. [Google Scholar] [PubMed]
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A critical review of multifunctional titanium surfaces: New frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018, 79, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Barfeie, A.; Wilson, J.; Rees, J. Implant surface characteristics and their effect on osseointegration. Br. Dent. J. 2015, 218, E9. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.-S. Reality of dental implant surface modification: A short literature review. Open Biomed. Eng. J. 2014, 8, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Piattelli, M.; Scarano, A.; Paolantonio, M.; Iezzi, G.; Petrone, G.; Piattelli, A. Bone response to machined and resorbable blast material titanium implants: An experimental study in rabbits. J. Oral Implantol. 2002, 28, 2–8. [Google Scholar] [CrossRef]
- Coelho, P.G.; Bonfante, E.A.; Pessoa, R.S.; Marin, C.; Granato, R.; Giro, G.; Witek, L.; Suzuki, M. Characterization of five different implant surfaces and their effect on osseointegration: A study in dogs. J. Periodontol. 2011, 82, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.-S.; Han, J.-S.; Yang, J.-H. Biomechanical and histomorphometric study of dental implants with different surface characteristics. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 87, 303–311. [Google Scholar] [CrossRef]
- Müeller, W.-D.; Gross, U.; Fritz, T.; Voigt, C.; Fischer, P.; Berger, G.; Rogaschewski, S.; Lange, K.P. Evaluation of the interface between bone and titanium surfaces being blasted by aluminium oxide or bioceramic particles. Clin. Oral Implants Res. 2003, 14, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-G.; Jeong, Y.-S.; Huh, Y.-H.; Park, C.-J.; Cho, L.-R. Impact of Surface Chemistry Modifications on Speed and Strength of Osseointegration. Int. J. Oral Maxillofac. Implants 2018, 33, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Lang, N.P.; De Santis, E.; Morelli, F.; Favero, G.; Botticelli, D. Bone-healing pattern at the surface of titanium implants: An experimental study in the dog. Clin. Oral Implants Res. 2014, 25, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Morelli, F.; Lang, N.P.; Bengazi, F.; Baffone, D.; Dadonim Vila Morales, C.; Botticelli, D. Influence of bone marrow on osseointegration in long bones: An experimental study in sheep. Clin. Oral Implants Res. 2015, 26, 300–306. [Google Scholar] [CrossRef]
- Caneva, M.; Lang, N.P.; Calvo Guirado, J.L.; Spriano, S.; Iezzi, G.; Botticelli, D. Bone healing at bicortically installed implants with different surface configurations. An experimental study in rabbits. Clin. Oral Implants Res. 2015, 26, 293–299. [Google Scholar] [CrossRef]
- Raghavendra, S.; Wood, M.C.; Taylor, T.D. Early wound healing around endosseous implants: A review of the literature. Int. J. Oral Maxillofac. Implants 2005, 20, 425–431. [Google Scholar]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. The ARRIVE Guidelines Checklist Animal Research: Reporting In Vivo Experiments. Br. J. Pharmacol. 2010, 8, 8–9. [Google Scholar]
- Bosshardt, D.D.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Lang, N.P. The role of bone debris in early healing adjacent to hydrophilic and hydrophobic implant surfaces in man. Clin. Oral Implants Res. 2011, 22, 357–364. [Google Scholar] [CrossRef]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral Implants Res. 2011, 22, 349–356. [Google Scholar] [CrossRef]
- Berglundh, T.; Abrahamsson, I.; Lang, N.P.; Lindhe, J. De novo alveolar bone formation adjacent to endosseous implants: A model study in the dog. Clin. Oral Implants Res. 2003, 14, 251–262. [Google Scholar] [CrossRef]
- Gottlow, J.; Barkamo, S.; Sennerby, L. An Experimental Comparison of Two Different Clinically Used Implant Designs and Surfaces. Clin. Implant Dent. Relat. Res. 2012, 14, e204-12. [Google Scholar] [CrossRef] [PubMed]
- Isidor, F. Influence of forces on peri-implant bone. Clin. Oral Implants Res. 2006, 17, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.K.; Denzer, A.J.; Cochran, D.L.; Hoffmann, B.; Lussi, A.; Steinemann, S.G. Enhanced bone apposition to a chemically modified SLA titanium surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Leonard, G.; Coelho, P.; Polyzois, I.; Stassen, L.; Claffey, N. A study of the bone healing kinetics of plateau versus screw root design titanium dental implants. Clin. Oral Implants Res. 2009, 20, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.G.; Suzuki, M.; Guimaraes, M.V.M.; Marin, C.; Granato, R.; Gil, J.N.; Miller, R.J. Early bone healing around different implant bulk designs and surgical techniques: A study in dogs. Clin. Implant Dent. Relat. Res. Can. 2010, 12, 202–208. [Google Scholar] [CrossRef] [PubMed]



| Compartment | Follow-up | Topographic Site | Statistic | New Bone | Soft Tissue | Old Bone |
|---|---|---|---|---|---|---|
| Cortical compartment | 2 w | Cort-dia | Mean | 17.8 | 70.0 | 12.2 |
| SD | 10.6 | 8.9 | 6.6 | |||
| Median | 14.0 | 68.2 | 8.3 | |||
| Cort-meta | Mean | 15.1 | 76.3 | 8.5 | ||
| SD | 5.8 | 6.4 | 6.5 | |||
| Median | 14.8 | 78.9 | 6.2 | |||
| Differences | p | 0.39 | 0.09 | 0.11 | ||
| 4 w | Cort-dia | Mean | 21.4 | 74.6 | 4.0 *,# | |
| SD | 6.9 | 7.8 | 3.2 | |||
| Median | 21.6 | 74.6 | 3.0 | |||
| Cort-meta | Mean | 19.7 * | 78.6 * | 1.7 # | ||
| SD | 8.3 | 8.0 | 1.5 | |||
| Median | 17.3 | 79.3 | 1.5 | |||
| Differences | p | 0.57 | 0.26 | 0.04 | ||
| 8 w | Cort-dia | Mean | 37.0 * | 58.9 * | 4.1 | |
| SD | 5.7 | 6.8 | 2.6 | |||
| Median | 37.3 | 58.5 | 4.3 | |||
| Cort-meta | Mean | 35.5 * | 61.3 * | 3.2 * | ||
| SD | 8.7 | 9.8 | 3.4 | |||
| Median | 33.8 | 60.9 | 2.6 | |||
| Differences | p | 0.63 | 0.62 | 0.88 | ||
| Marrow compartment | 2 w | Marrow-dia | Mean | 13.8 | 78.9 | 7.3 |
| SD | 9.2 | 12.3 | 8.9 | |||
| Median | 13.2 | 82.8 | 2.9 | |||
| Marrow-meta | Mean | 10.3 | 86.1 | 3.6 | ||
| SD | 8.2 | 8.0 | 5.1 | |||
| Median | 9.3 | 89.5 | 0.5 | |||
| Differences | p | 0.18 | 0.07 | 0.23 | ||
| 4 w | Marrow-dia | Mean | 20.4 # | 77.9 # | 1.7 * | |
| SD | 6.8 | 6.9 | 2.3 | |||
| Median | 19.7 | 79.4 | 0.4 | |||
| Marrow-meta | Mean | 13.0 *,# | 86.4 *,# | 0.6 | ||
| SD | 8.2 | 8.5 | 0.8 | |||
| Median | 13.3 | 86.7 | 0.0 | |||
| Differences | p | 0.02 | 0.01 | 0.16 | ||
| 8 w | Marrow-dia | Mean | 24.6 * | 73.6 | 1.8 | |
| SD | 12.9 | 16.3 | 3.8 | |||
| Median | 21.6 | 78.5 | 0.0 | |||
| Marrow-meta | Mean | 25.1 * | 74.7 * | 0.2 * | ||
| SD | 9.6 | 9.7 | 0.4 | |||
| Median | 23.2 | 75.9 | 0.0 | |||
| Differences | p | 0.878 | 0.79 | 0.25 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Peñaloza, D.; Caneva, M.; Viña-Almunia, J.; Martín-de-Llano, J.J.; Peñarrocha-Oltra, D.; Peñarrocha-Diago, M. Bone-Healing Pattern on the Surface of Titanium Implants at Cortical and Marrow Compartments in Two Topographic Sites: an Experimental Study in Rabbits. Materials 2019, 12, 85. https://doi.org/10.3390/ma12010085
Soto-Peñaloza D, Caneva M, Viña-Almunia J, Martín-de-Llano JJ, Peñarrocha-Oltra D, Peñarrocha-Diago M. Bone-Healing Pattern on the Surface of Titanium Implants at Cortical and Marrow Compartments in Two Topographic Sites: an Experimental Study in Rabbits. Materials. 2019; 12(1):85. https://doi.org/10.3390/ma12010085
Chicago/Turabian StyleSoto-Peñaloza, David, Marco Caneva, José Viña-Almunia, José Javier Martín-de-Llano, David Peñarrocha-Oltra, and Miguel Peñarrocha-Diago. 2019. "Bone-Healing Pattern on the Surface of Titanium Implants at Cortical and Marrow Compartments in Two Topographic Sites: an Experimental Study in Rabbits" Materials 12, no. 1: 85. https://doi.org/10.3390/ma12010085
APA StyleSoto-Peñaloza, D., Caneva, M., Viña-Almunia, J., Martín-de-Llano, J. J., Peñarrocha-Oltra, D., & Peñarrocha-Diago, M. (2019). Bone-Healing Pattern on the Surface of Titanium Implants at Cortical and Marrow Compartments in Two Topographic Sites: an Experimental Study in Rabbits. Materials, 12(1), 85. https://doi.org/10.3390/ma12010085





