Effect of the MgO/Silica Fume Ratio on the Reaction Process of the MgO–SiO2–H2O System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Sample Preparation
2.2.1. Preparation of MgO/SF Pastes
2.2.2. Preparation of M–S–H Gel
2.3. Testing Methods
2.3.1. Measurement of Mechanical Strength
2.3.2. Measurement of Heat Evolution
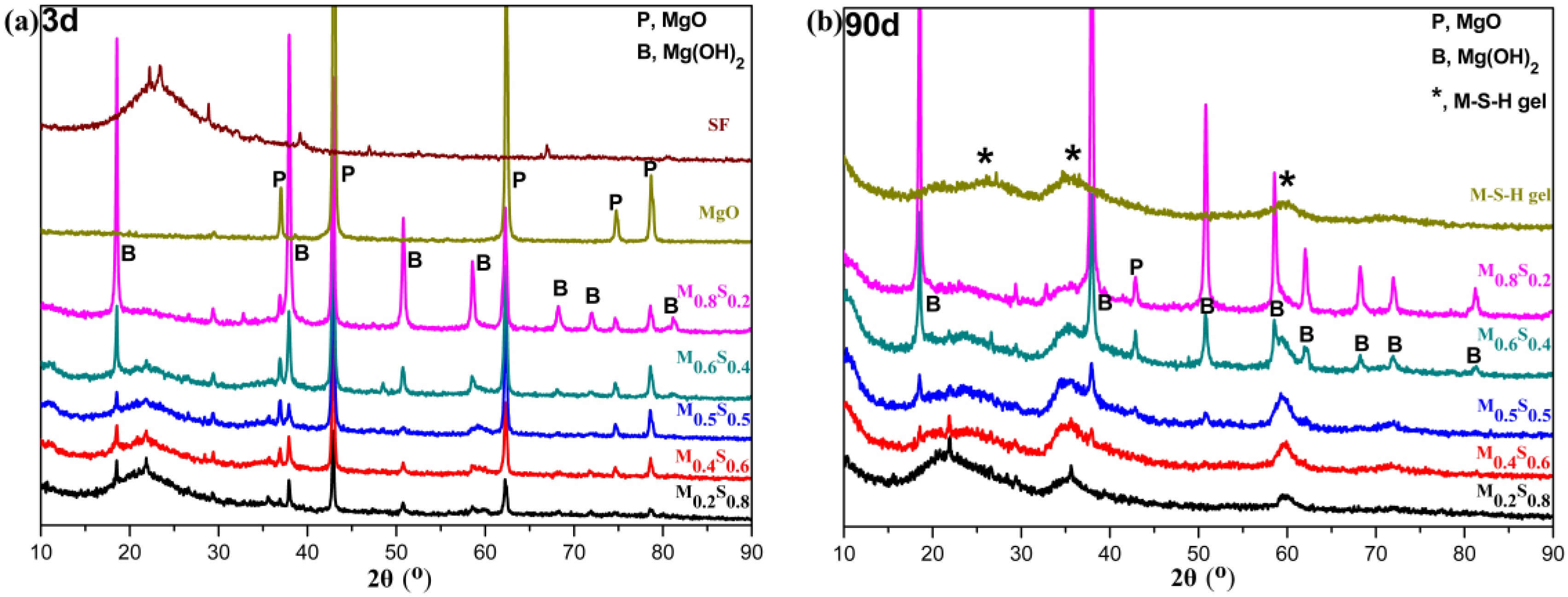
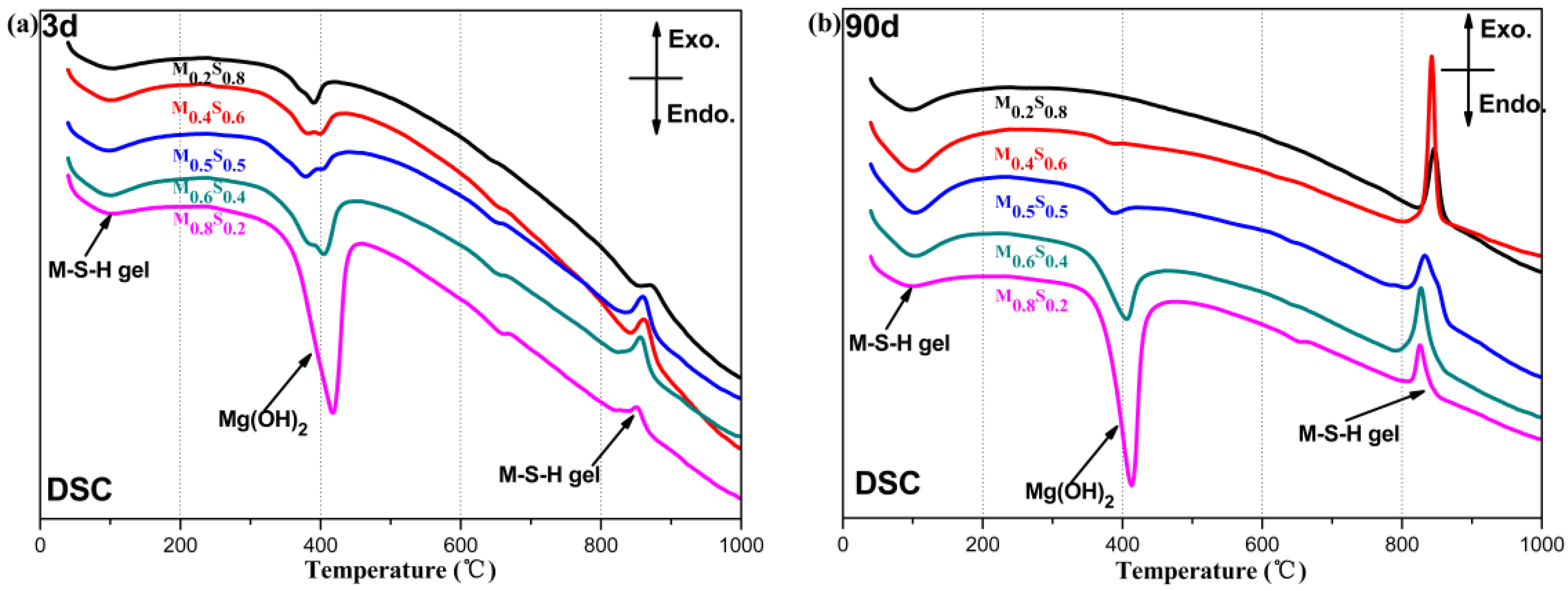
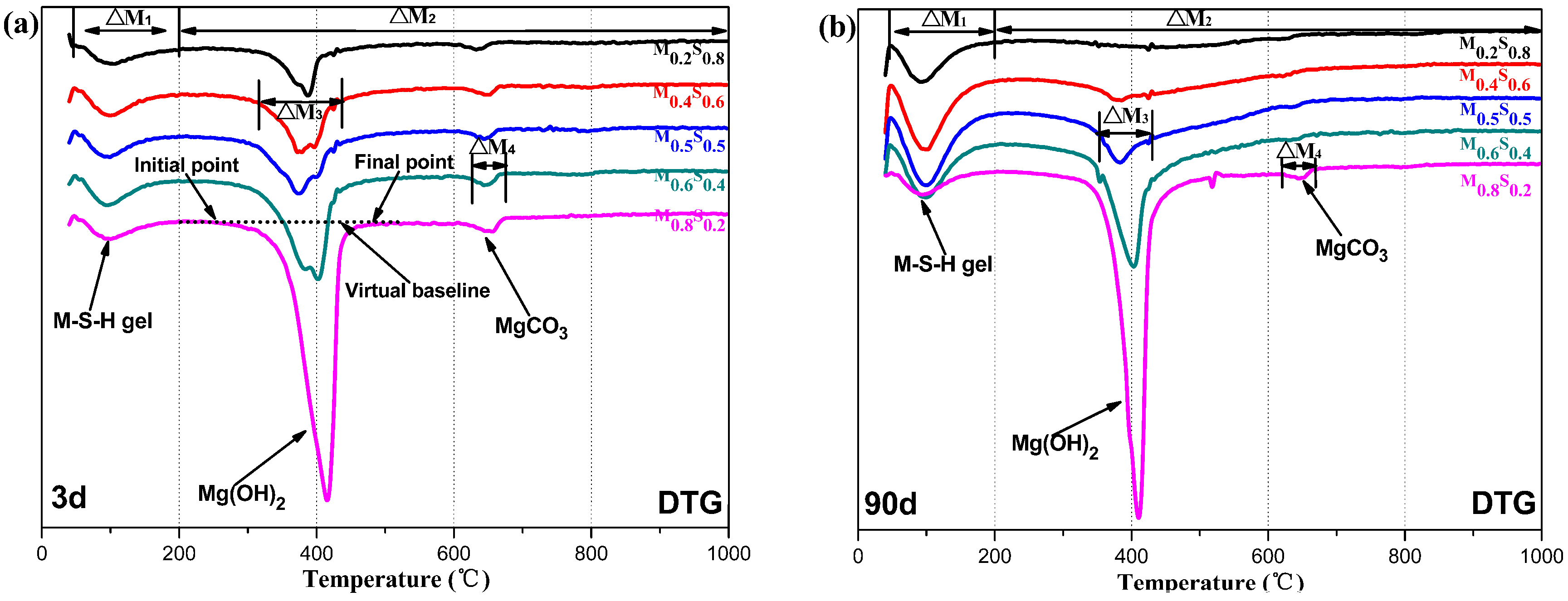
2.3.3. Characterization of the Reaction Products
2.3.4. Measurement of MgO Content
2.3.5. Calculation of Mg(OH)2 and M–S–H Gel Contents
2.3.6. Degree of Reaction
3. Results and Discussion
3.1. Reaction Process
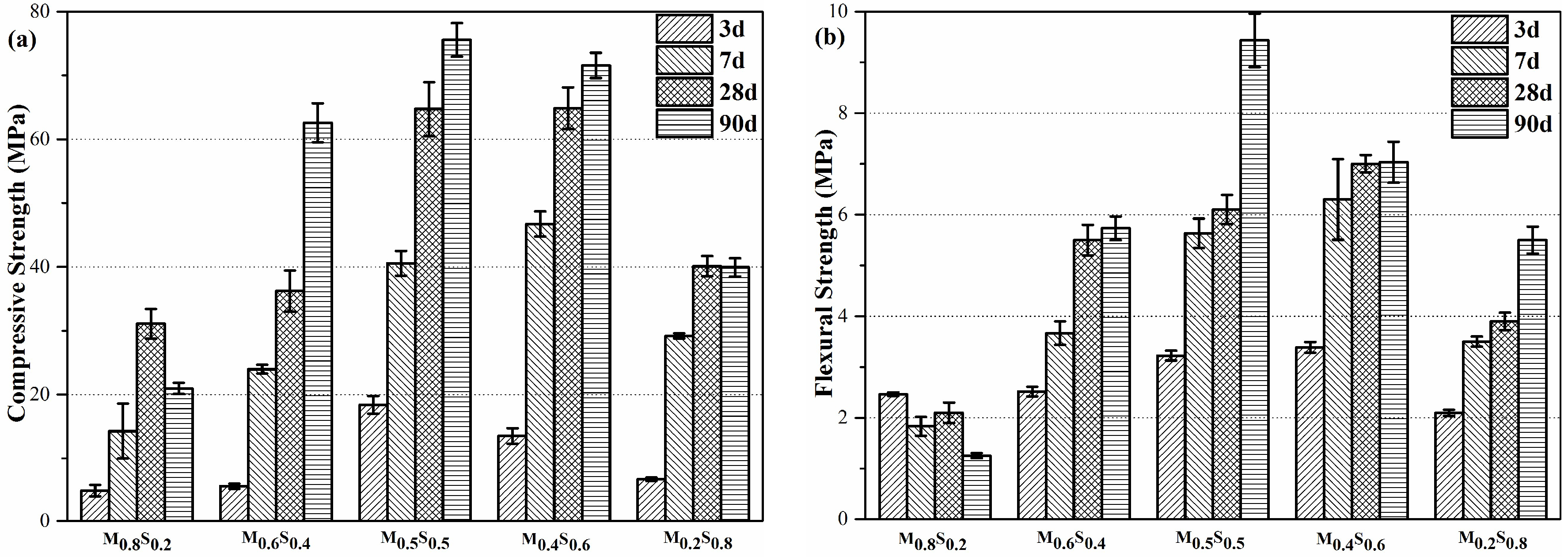
3.1.1. Compressive Strength and Flexural Strength
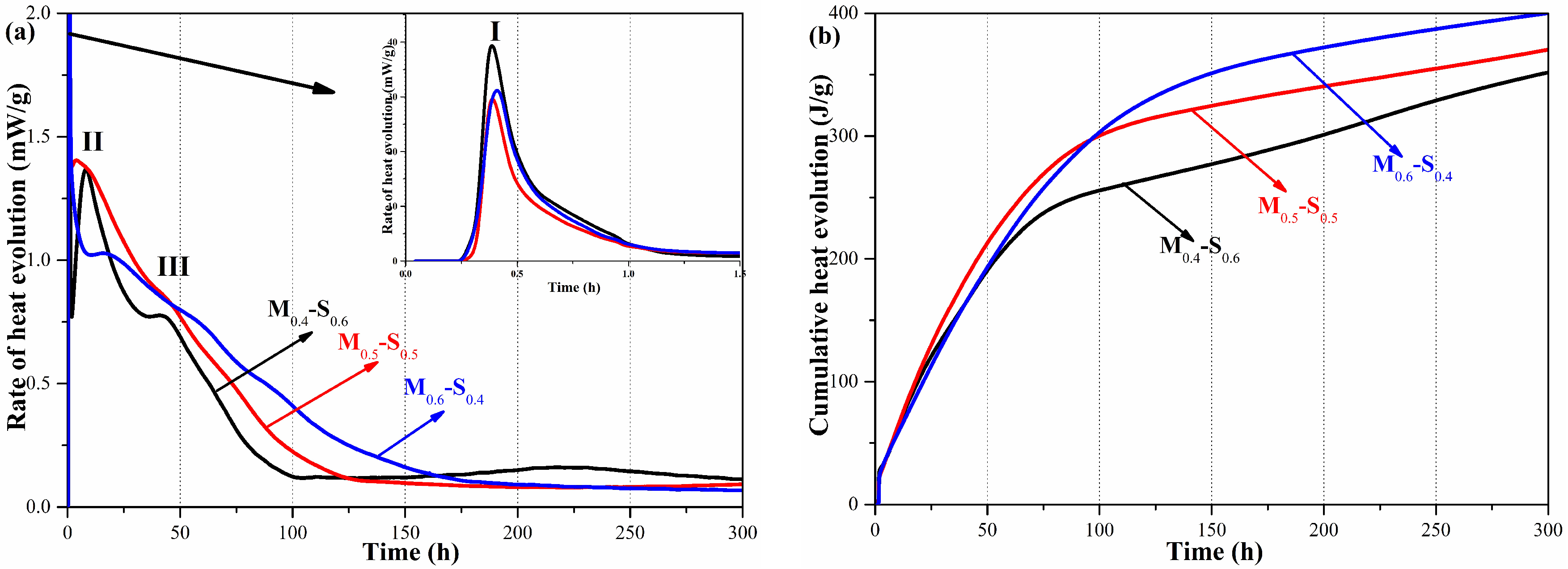
3.1.2. Heat Evolution
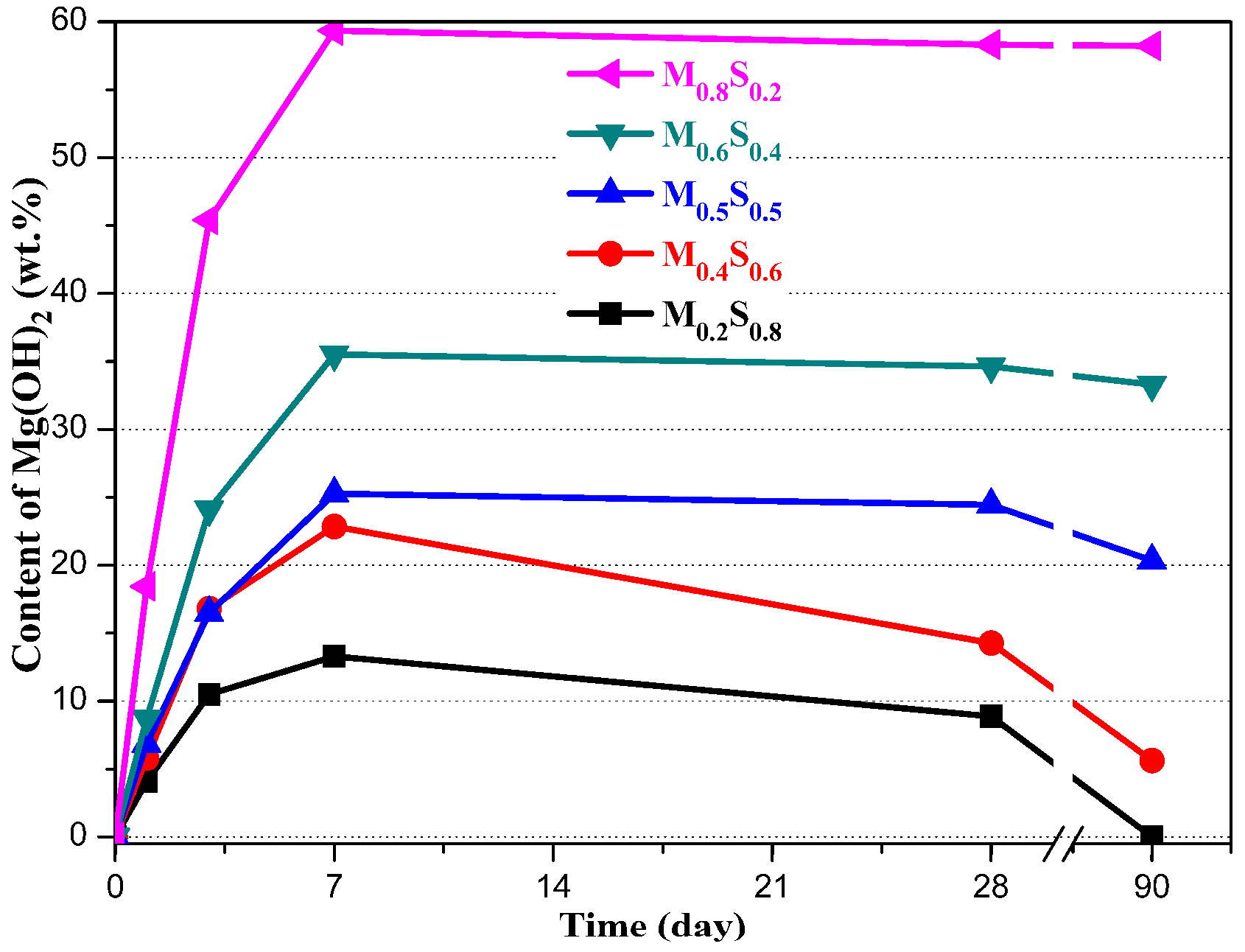
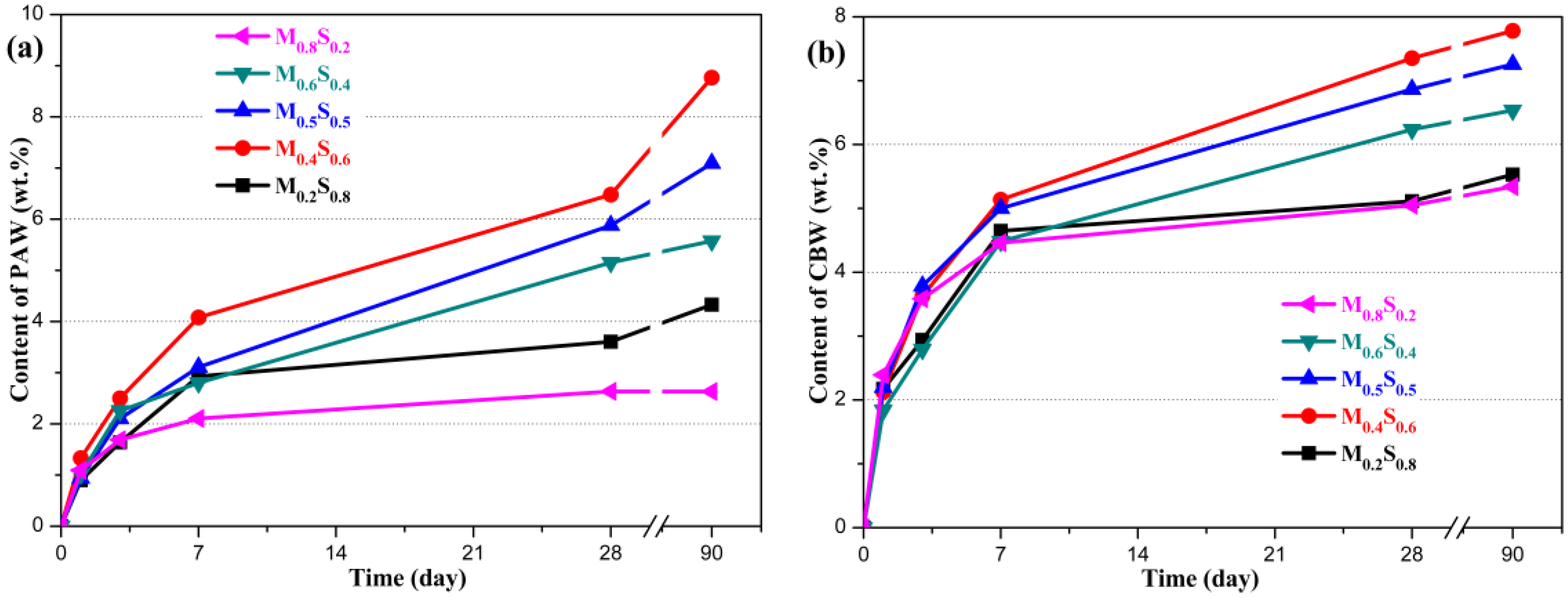
3.1.3. Variation in the Composition of MGO/SF Pastes
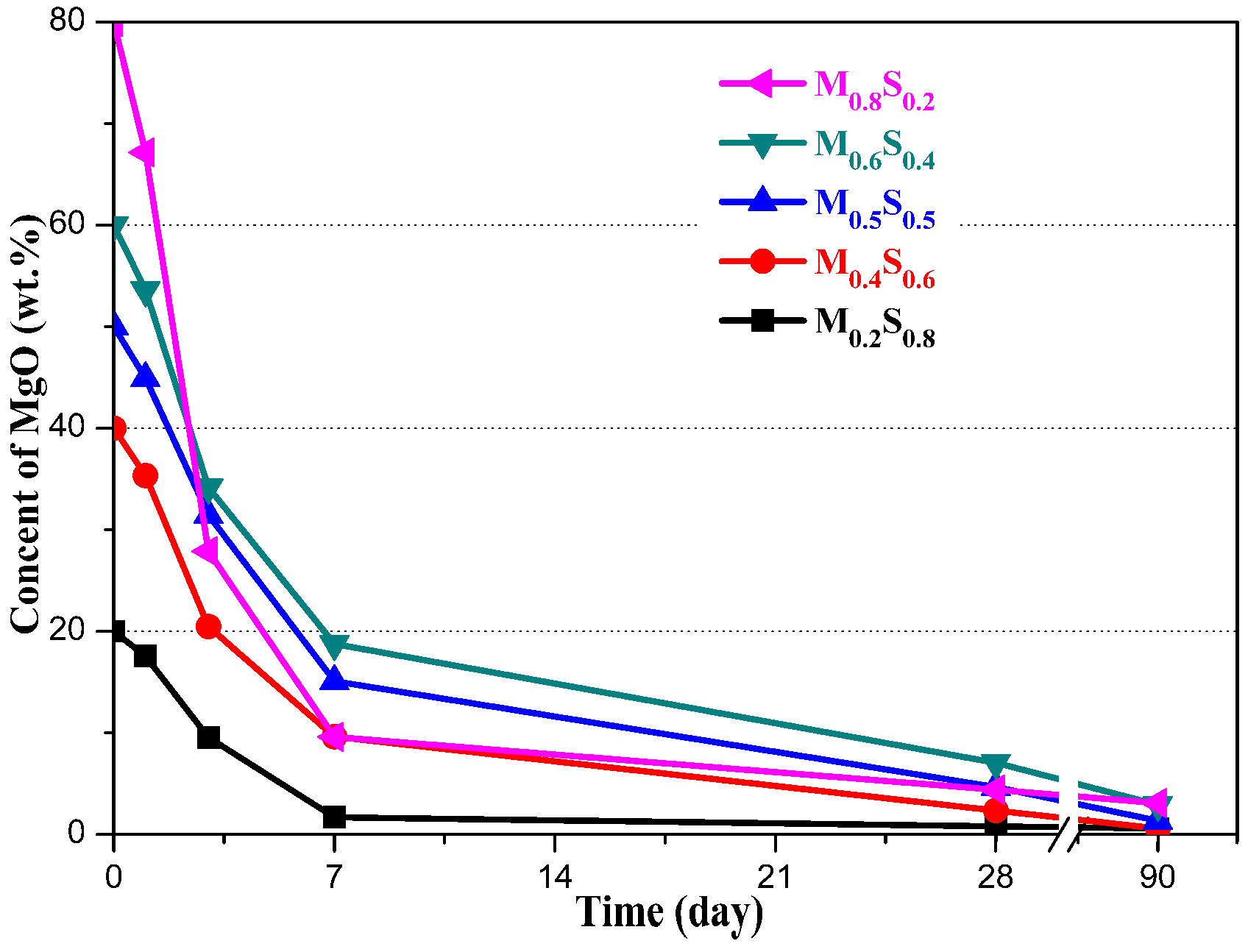
MgO Content
Mg(OH)2 Content
PAW and CBW Contents
3.2. Reaction Thermodynamics
3.3. Reaction Kinetics
3.3.1. Theoretical Deduction of the Reaction Kinetics Equation
3.3.2. Verification of the Reaction Kinetics Equation
4. Conclusions
- Mg(OH)2 results from the dissolution of MgO. M–S–H gels generates from the reaction between Mg2+ and hydrated silica, and consequently the dissolution of Mg(OH)2 and SiO2 is promoted.
- The formation reaction for the M–S–H gel is the main reaction in the MgO–SiO2–H2O system when the dosage of MgO is lower than 50%, while the formation reaction of Mg(OH)2 is the main reaction when the dosage of MgO is higher than 50%.
- Based on the thermodynamic calculations, M–S–H gels are more stable than Mg(OH)2. Furthermore, the formation reactions for the M–S–H gels occurred more completely than those for Mg(OH)2.
- The reaction kinetics of MgO in the MgO–SiO2–H2O system conforms to α = 1 − e−kt (R2 > 0.97). Because of the decrease in the SF dosage, the rate constant decreased with decreasing SF content when the dosage of MgO was lower than 50%. As a result of the formation rate of the M–S–H gels being lower than Mg(OH)2, the rate constant increased with increasing MgO content when the dosage of MgO was higher than 50%.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jang, J.K.; Kim, H.G.; Kim, J.H.; Ryou, J.S. The evaluation of damage effects on MgO added concrete with slag cement exposed to calcium chloride deicing salt. Materials 2018, 11, 793. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Chen, Y.; Li, Y. The reaction mechanism between MgO and microsilica at room temperature. J. Wuhan Univ. Technol. 2006, 21, 88–91. [Google Scholar]
- Li, Z.; Zhang, T.; Hu, J.; Tang, Y.; Niu, Y.; Wei, J.; Yu, Q. Characterization of reaction products and reaction process of MgO–SiO2–H2O system at room temperature. Constr. Build. Mater. 2014, 61, 252–259. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Q.; Chen, X.; Liu, H.; Zhang, J.; Yang, Y.; Wei, J. The role of MgO in the thermal behavior of MgO–silica fume pastes. J. Therm. Anal. Calorim. 2017, 127, 1897–1909. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Y.; Zhang, T.; Hu, J.; Wei, J.; Yu, Q. Effect of MgO calcination temperature on the reaction products and kinetics of MgO–SiO2–H2O system. J. Am. Ceram. Soc. 2018, in press. [Google Scholar] [CrossRef]
- Mo, L.; Deng, M.; Tang, M. Effects of calcination condition on expansion property of MgO-type expansive agent used in cement-based materials. Cem. Concr. Res. 2010, 40, 437–446. [Google Scholar] [CrossRef]
- Zhang, T.; Cheeseman, C.R.; Vandeperre, L.J. Development of low pH cement systems forming magnesium silicate hydrate (M–S–H). Cem. Concr. Res. 2011, 41, 439–442. [Google Scholar] [CrossRef]
- Szczerba, J.; Prorok, R.; Sniezek, E.; Madej, D.; Maślona, K. Influence of time and temperature on ageing and phases synthesis in the MgO–SiO2–H2O system. Thermochim. Acta 2013, 567, 57–64. [Google Scholar] [CrossRef]
- Behnood, A. Soil and clay stabilization with calcium-and non-calcium-based additives: A state-of-the-art review of challenges, approaches and techniques. Transp. Geotech. 2018, 17, 14–32. [Google Scholar] [CrossRef]
- Wunder, B.; Schreyer, W. Antigorite: High-pressure stability in the system MgO–SiO2–H2O (MSH). Lithos 1997, 41, 213–227. [Google Scholar] [CrossRef]
- Nadezda, S.; Jozef, J.; Vojtech, V. Effect of silica fume as a component of alternative binder on the selected technically important characteristics of bio-aggregate-based composites. Materials 2018, 11, 2153. [Google Scholar]
- Wei, J. Research on Magnesium Silicate Hydrate Cementitious Materials. Ph.D. Thesis, Building Materials Academy, Beijing, China, September 2004. (In Chinese). [Google Scholar]
- Wei, J.; Yu, Q.; Zhang, W.; Zhang, H. Reaction products of MgO and microsilica cementitious materials at different temperatures. J. Wuhan Univ. Technol. 2011, 26, 745–748. [Google Scholar] [CrossRef]
- Zhang, T.; Jing, Z.; Wang, B.; Wu, Z.; Jia, Y.; Cheeseman, C.R. Characterization of magnesium silicate hydrate (MSH) gel formed by reacting MgO and silica fume. Materials 2018, 11, 909. [Google Scholar] [CrossRef]
- Cole, W.F. A crystalline hydrated magnesium silicate formed in the breakdown of a concrete sea-wall. Nature 1953, 171, 354–355. [Google Scholar] [CrossRef]
- Gollop, R.S.; Taylor, H.F.W. Microstructural and microanalytical studies of sulfate attack. IV. Reactions of a slag cement paste with sodium and magnesium sulfate solutions. Cem. Concr. Res. 1996, 26, 1013–1028. [Google Scholar] [CrossRef]
- Brew, D.R.M.; Glasser, F.P. Synthesis and characterisation of magnesium silicate hydrate gels. Cem. Concr. Res. 2005, 35, 85–98. [Google Scholar] [CrossRef]
- Brew, D.R.M.; Glasser, F.P. The magnesia-silica gels phase in slag cements, alkali (K, Cs) sorption potential of synthetic gels. Cem. Concr. Res. 2005, 35, 77–83. [Google Scholar] [CrossRef]
- Vandeperre, L.J.; Liska, M.; Al-Tabbaa, A. Microstructures of reactive magnesia cement blends. Cem. Concr. Compos. 2008, 30, 706–714. [Google Scholar] [CrossRef]
- Jin, F.; Gu, K.; Al-Tabbaa, A. Strength and drying shrinkage of reactive MgO modified alkali-activated slag paste. Constr. Build. Mater. 2014, 51, 395–404. [Google Scholar] [CrossRef]
- Zhang, X.; Glasser, F.P.; Scrivener, K.L. Reaction kinetics of dolomite and portlandite. Cem. Concr. Res. 2014, 66, 11–18. [Google Scholar] [CrossRef]
- ASTM C 349 Standard Test Method for Compressive Strength of Hydraulic-Cement Mortars (Using Portions of Prisms Broken in Flexure); ASTM International: West Conshohocken, PA, USA, 2002.
- ASTM C 1702-09 Standard Test Method for Measurement of Heat of Hydration of Hydraulic Cementitious Materials Using Isothermal Conduction Calorimetry; ASTM International: West Conshohocken, PA, USA, 2009.
- Gallé, C. Effect of drying on cement-based materials pore structure as identified by mercury intrusion porosimetry: A comparative study between oven-, vacuum-, and freeze-drying. Cem. Concr. Res. 2001, 31, 1467–1477. [Google Scholar] [CrossRef]
- Li, J.; Yu, Q.; Wei, J.; Zhang, T. Structural characteristics and hydration kinetics of modified steel slag. Cem. Concr. Res. 2011, 41, 324–329. [Google Scholar] [CrossRef]
- Iller, R.K. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties and Biochemistry; John Wiley & Sons: New York, NY, USA, 1979. [Google Scholar]
- Xing, Z.; Bai, L.; Ma, Y.; Wang, D.; Li, M. Mechanism of magnesium oxide hydration based on the multi-rate model. Materials 2018, 11, 1835. [Google Scholar] [CrossRef] [PubMed]
- Damidot, D.; Lothenbach, B.; Herfort, D.; Glasser, F.P. Thermodynamics and cement science. Cem. Concr. Res. 2011, 41, 679–695. [Google Scholar] [CrossRef]
- Sun, L.; Zhu, Y. A serial two-stage viscoelastic–viscoplastic constitutive model with thermodynamical consistency for characterizing time-dependent deformation behavior of asphalt concrete mixtures. Constr. Build. Mater. 2013, 40, 584–595. [Google Scholar] [CrossRef]
- Lin, C.; Bai, Z.; Zhang, Z. Minerals and Related Compound Thermodynamic Data Manual, 1st ed.; Science Press: Beijing, China, 1985. (In Chinese) [Google Scholar]


| Material | Composition (wt.%) | Specific Density (g/cm3) | BET Specific Surface Area (m2/g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | MgO | CaO | K2O | Na2O | Al2O3 | P2O5 | SO3 | |||
| MgO | 0.29 | 97.22 | 1.45 | – | – | 0.21 | 0.06 | 0.20 | 2.96 | 51.60 |
| SF | 95.74 | 0.63 | 1.25 | 1.07 | 0.33 | 0.50 | 0.20 | 0.15 | 2.03 | 16.04 |
| Sample ID | MgO (wt.%) | SF (wt.%) | W/B Ratio |
|---|---|---|---|
| M0.2S0.8 | 20 | 80 | 1.0 |
| M0.4S0.6 | 40 | 60 | 1.0 |
| M0.5S0.5 | 50 | 50 | 1.0 |
| M0.6S0.4 | 60 | 40 | 1.0 |
| M0.8S0.2 | 80 | 20 | 1.0 |
| Minerals or Species | Smθ (kJ·mol−1) | ∆fHmθ (kJ·mol−1) | ∆fGmθ (kJ·mol−1) |
|---|---|---|---|
| Mg2+ (aq) | −138.00 | −466.85 | −454.89 |
| OH− (aq) | −10.71 | −230.02 | −157.34 |
| H+ (aq) | 0.00 | 0.00 | 0.00 |
| H2O (aq) | 69.95 | −285.83 | −237.19 |
| MgO (s) | 26.95 | −601.50 | −569.23 |
| SiO2 (Amorphous) | 47.40 | −903.20 | −850.59 |
| H3SiO4− (aq) | 112.55 | −1426.16 | −1253.98 |
| H2SiO42− (aq) | −12.97 | −1396.62 | −1187.02 |
| Mg(OH)2 (s) | 63.14 | −924.54 | −833.56 |
| M3S2H2 (Chrysotile) | 221.30 | −4361.66 | −4034.02 |
| M3S4H (Talc) | 260.80 | −5915.90 | −5536.27 |
| ID | Minerals or Species | Reaction Equation | ∆rSθ (kJ·mol−1) | ∆rHθ (kJ·mol−1) | ∆rGθ (kJ·mol−1) | logK |
|---|---|---|---|---|---|---|
| ① | MgO | MgO + 2H+ → Mg2+ + H2O | −95.00 | −151.18 | −122.85 | 21.52 |
| ② | H2SiO42− | SiO2 + 2OH− → H2SiO42− | −38.95 | −33.38 | −21.75 | 3.81 |
| ③ | H3SiO4− | SiO2 + OH− + H2O → H3SiO4− | 5.91 | −7.11 | −8.86 | 1.55 |
| ④ | Mg(OH)2 | Mg2+ + 2OH− ↔ Mg(OH)2 | 222.56 | 2.35 | −63.99 | 11.21 |
| ⑤ | Chrysotile | 3Mg2+ + 6OH− + 2SiO2 ↔ 3MgO·2SiO2·2H2O + H2O | 674.71 | −60.42 | −261.32 | 45.78 |
| ⑥ | Talc | 3Mg2+ + 6OH− + 4SiO2 ↔ 3MgO·4SiO2·H2O + 2H2O | 689.36 | −94.09 | −299.58 | 52.48 |
| Sample ID | K (10−6 s−1) | R2 |
|---|---|---|
| M0.2S0.8 | 2.748 | 0.973 |
| M0.4S0.6 | 2.040 | 0.988 |
| M0.5S0.5 | 1.542 | 0.984 |
| M0.6S0.4 | 1.577 | 0.971 |
| M0.8S0.2 | 3.018 | 0.977 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Xu, Y.; Liu, H.; Zhang, J.; Wei, J.; Yu, Q. Effect of the MgO/Silica Fume Ratio on the Reaction Process of the MgO–SiO2–H2O System. Materials 2019, 12, 80. https://doi.org/10.3390/ma12010080
Li Z, Xu Y, Liu H, Zhang J, Wei J, Yu Q. Effect of the MgO/Silica Fume Ratio on the Reaction Process of the MgO–SiO2–H2O System. Materials. 2019; 12(1):80. https://doi.org/10.3390/ma12010080
Chicago/Turabian StyleLi, Zhaoheng, Yudong Xu, Hao Liu, Jianwei Zhang, Jiangxiong Wei, and Qijun Yu. 2019. "Effect of the MgO/Silica Fume Ratio on the Reaction Process of the MgO–SiO2–H2O System" Materials 12, no. 1: 80. https://doi.org/10.3390/ma12010080
APA StyleLi, Z., Xu, Y., Liu, H., Zhang, J., Wei, J., & Yu, Q. (2019). Effect of the MgO/Silica Fume Ratio on the Reaction Process of the MgO–SiO2–H2O System. Materials, 12(1), 80. https://doi.org/10.3390/ma12010080






