Wear Performance Analysis of Ni–Al2O3 Nanocomposite Coatings under Nonconventional Lubrication
Abstract
:1. Introduction
2. Materials and Methods
3. Experimentation
3.1. Sample Preparation
3.1.1. Sample Preparation of Uncoated Samples
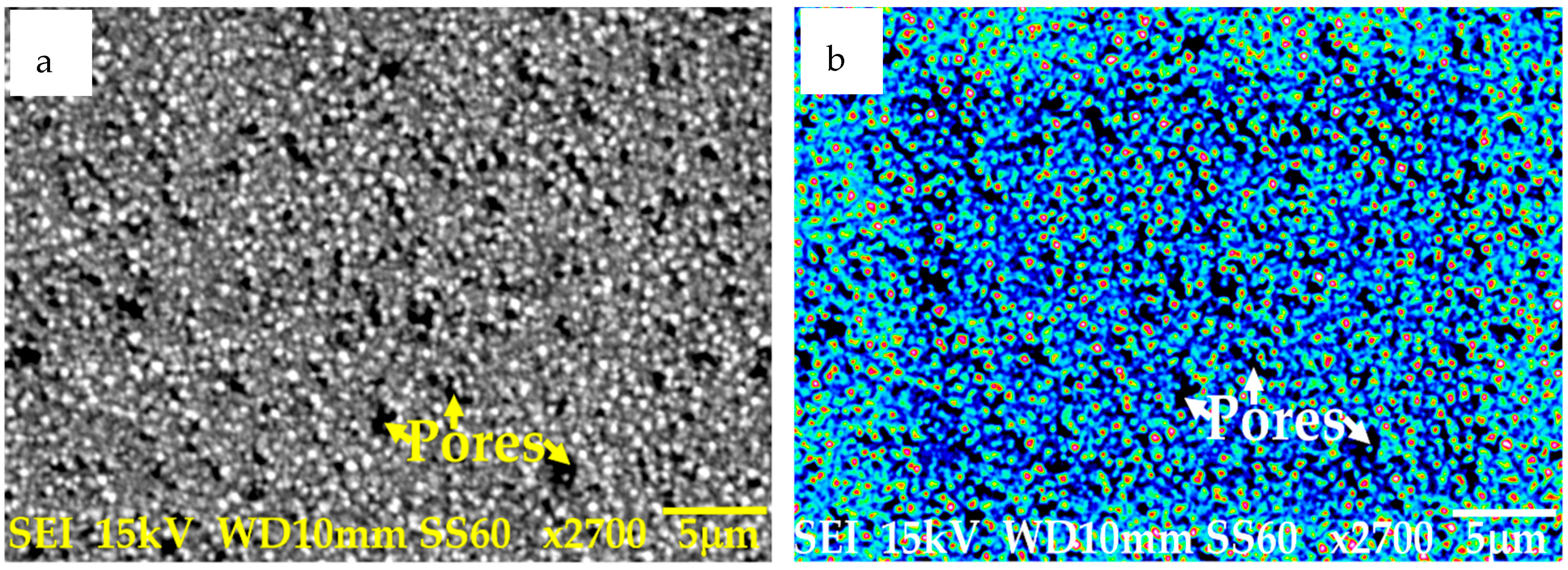
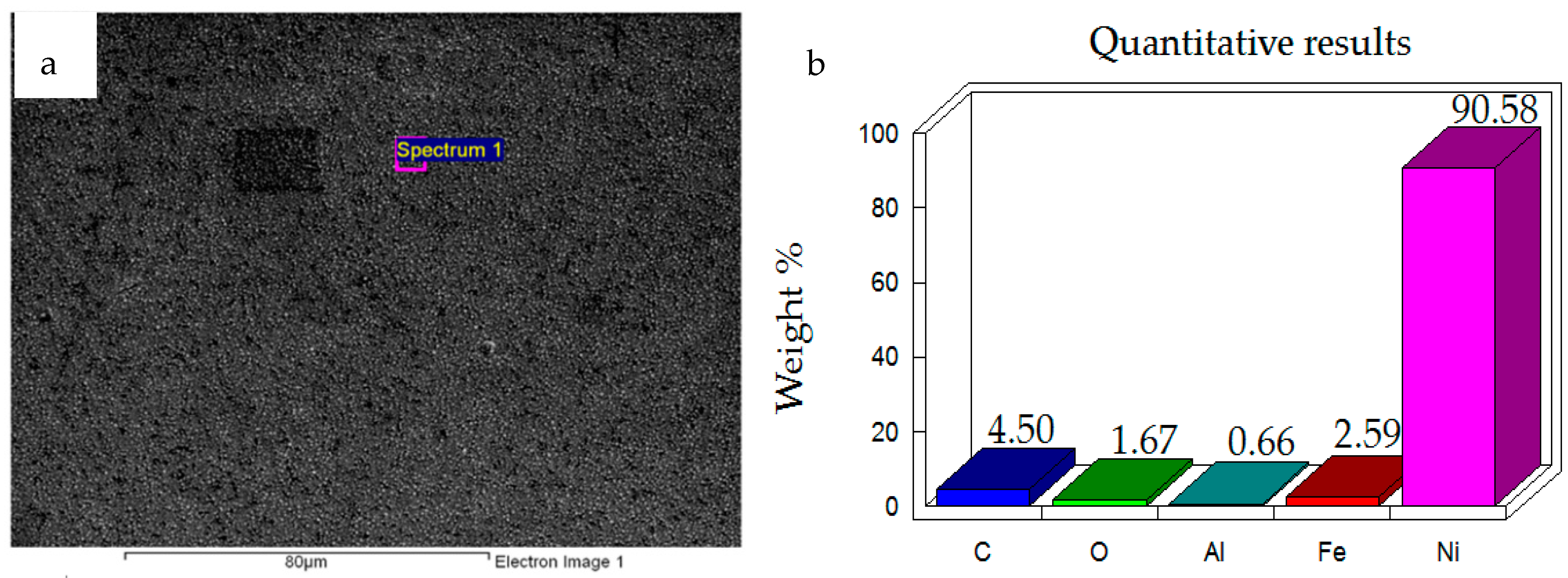
3.1.2. Sample Preparation of Coated Samples
3.2. Testing
4. Results and Discussion
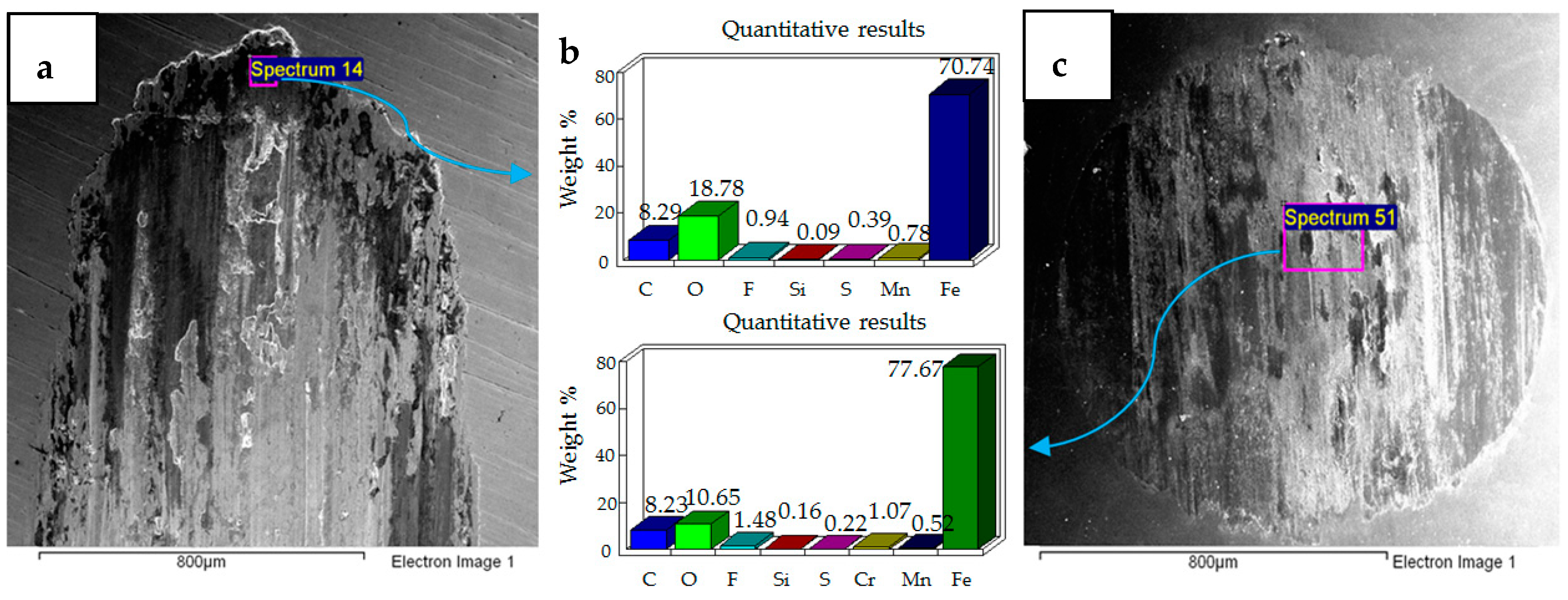
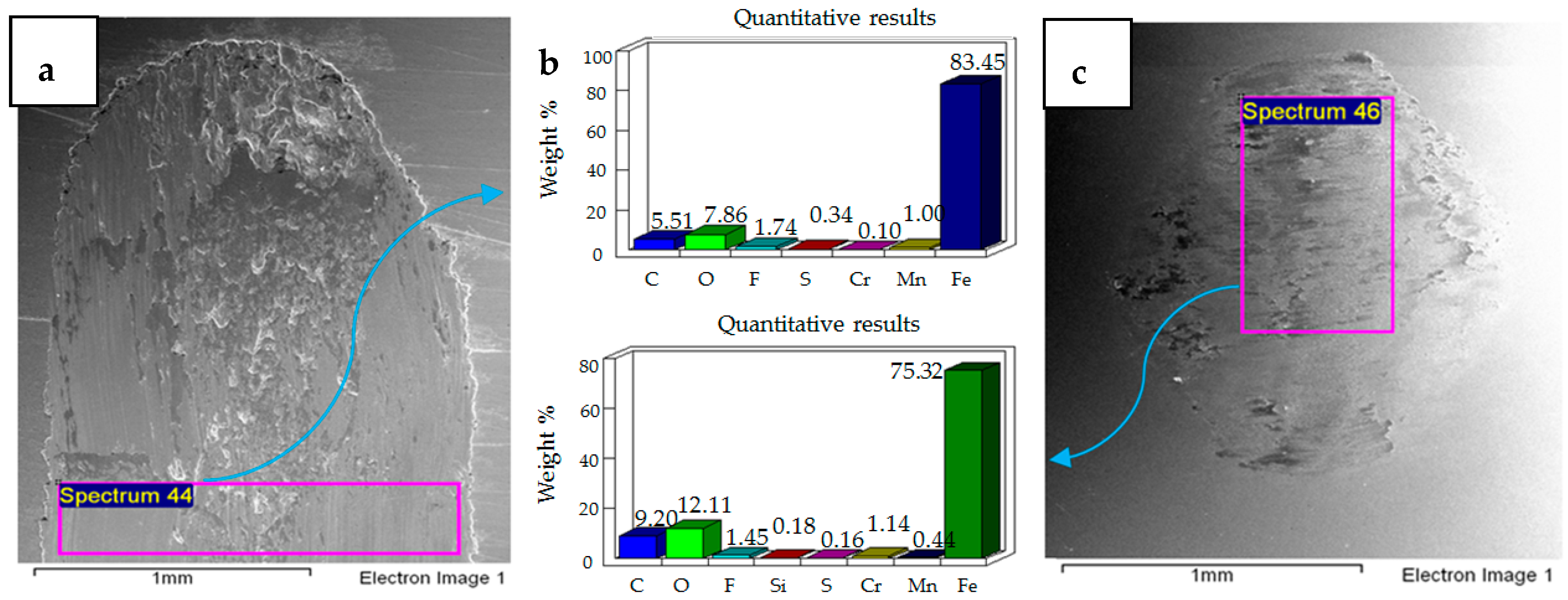
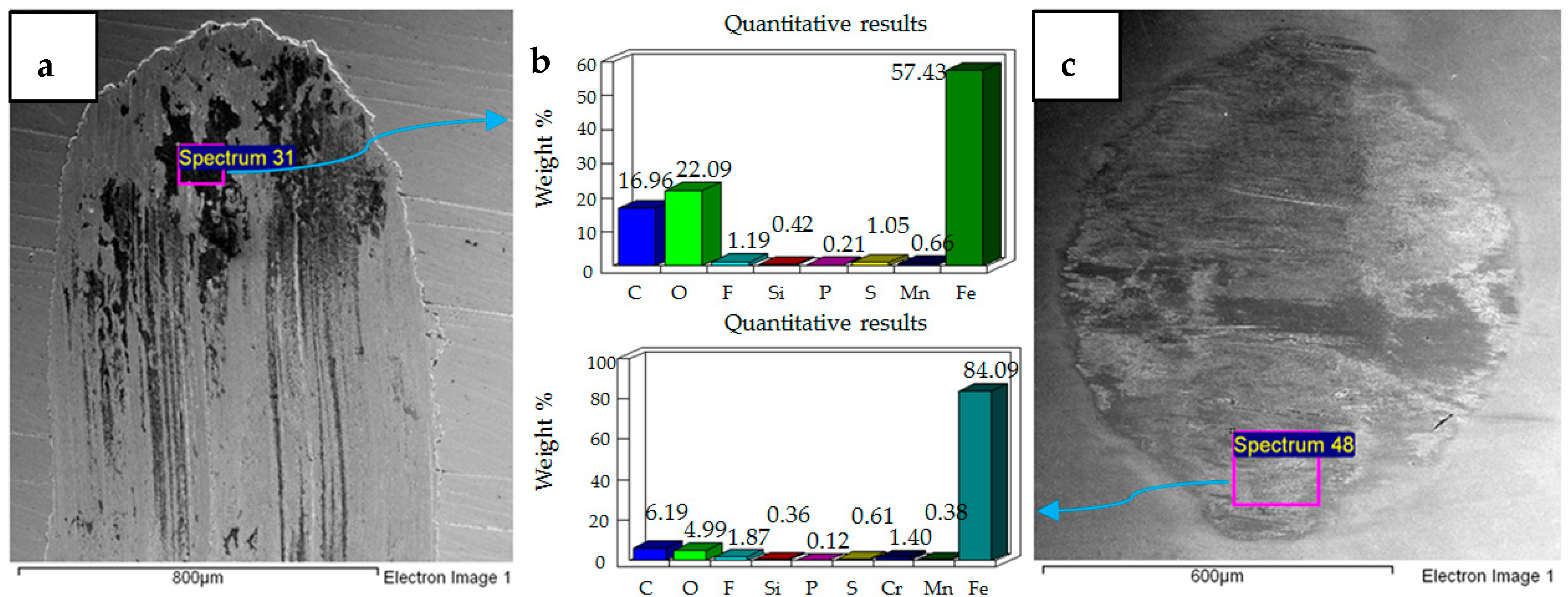
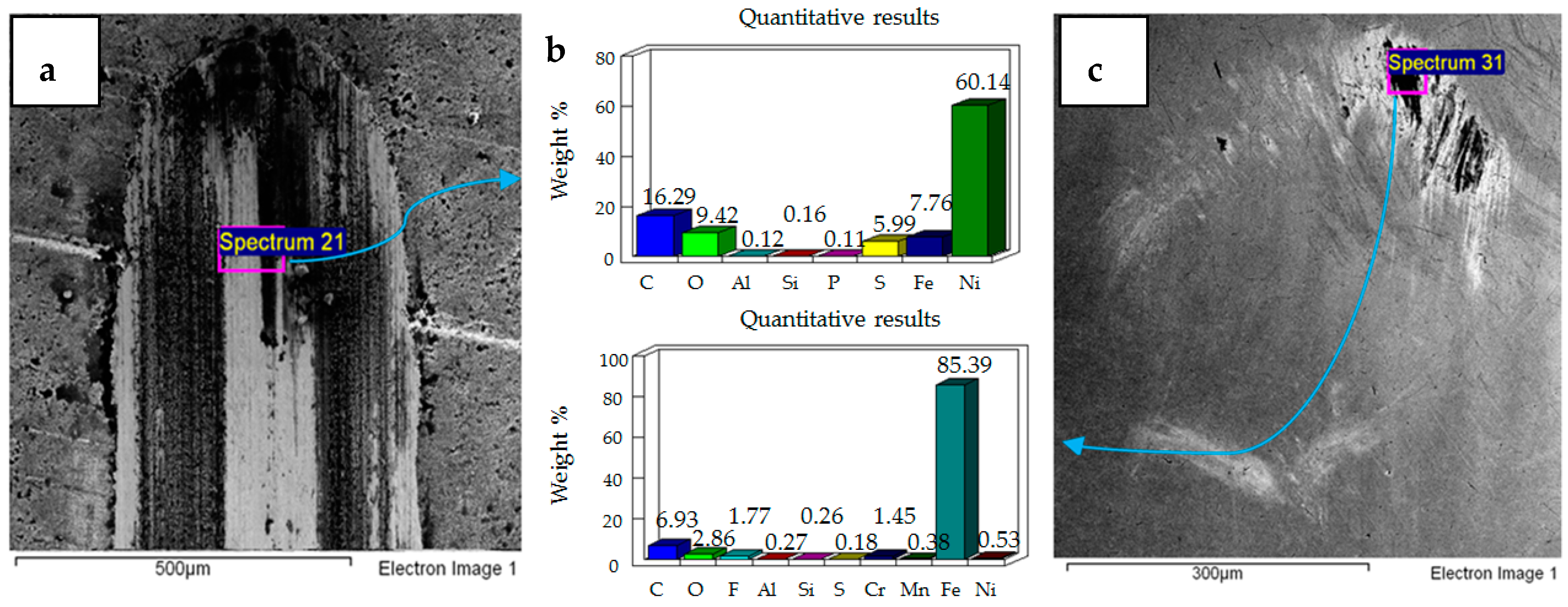
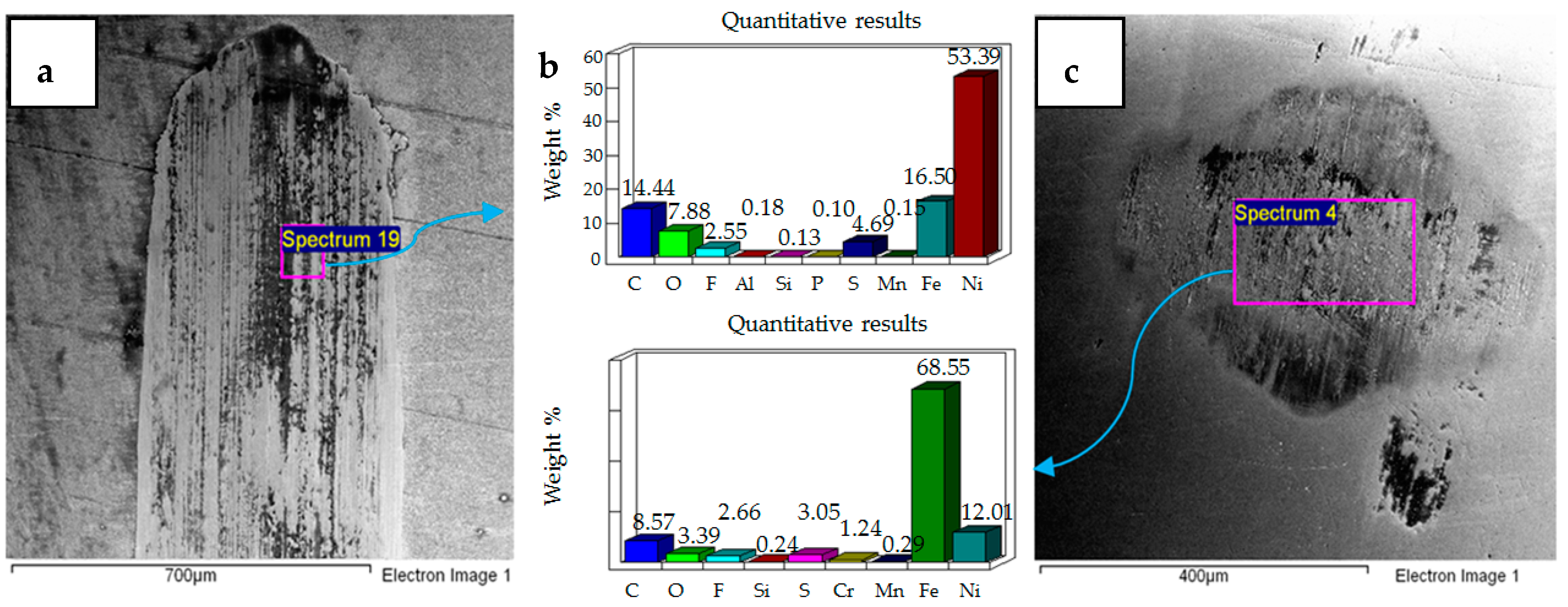
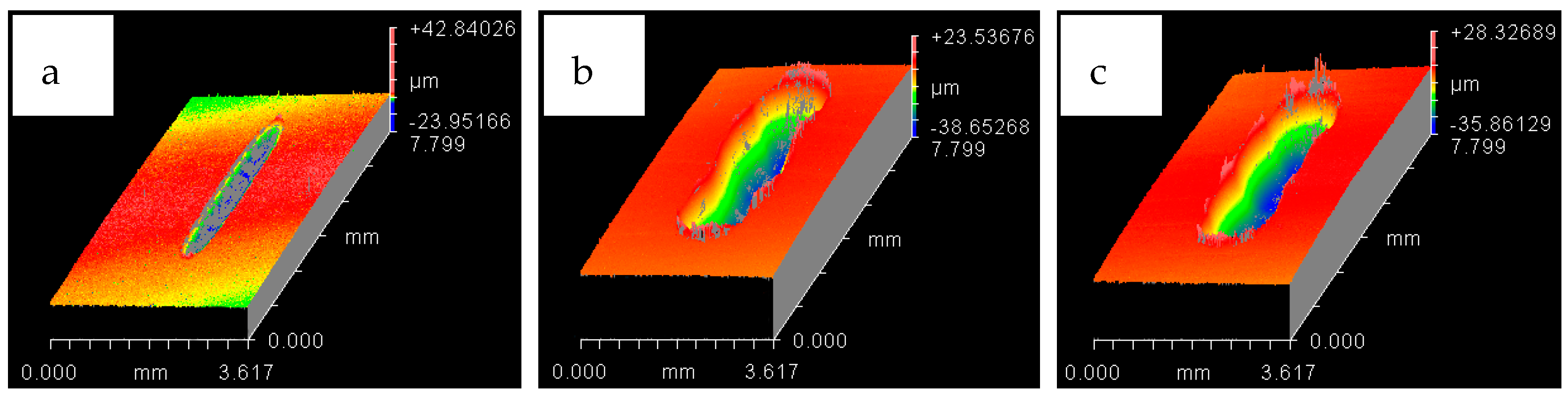
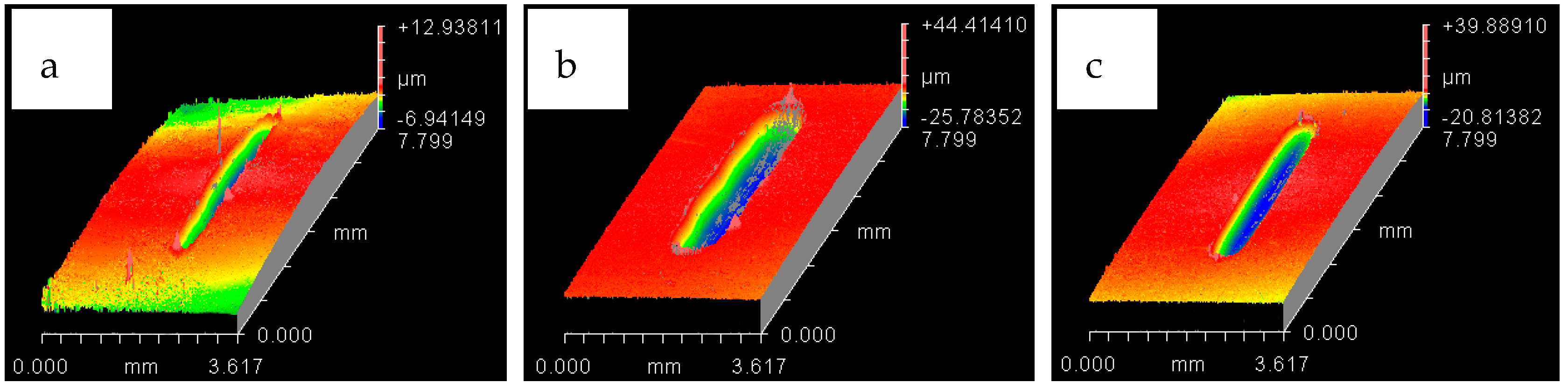
4.1. Wear of Uncoated Specimens
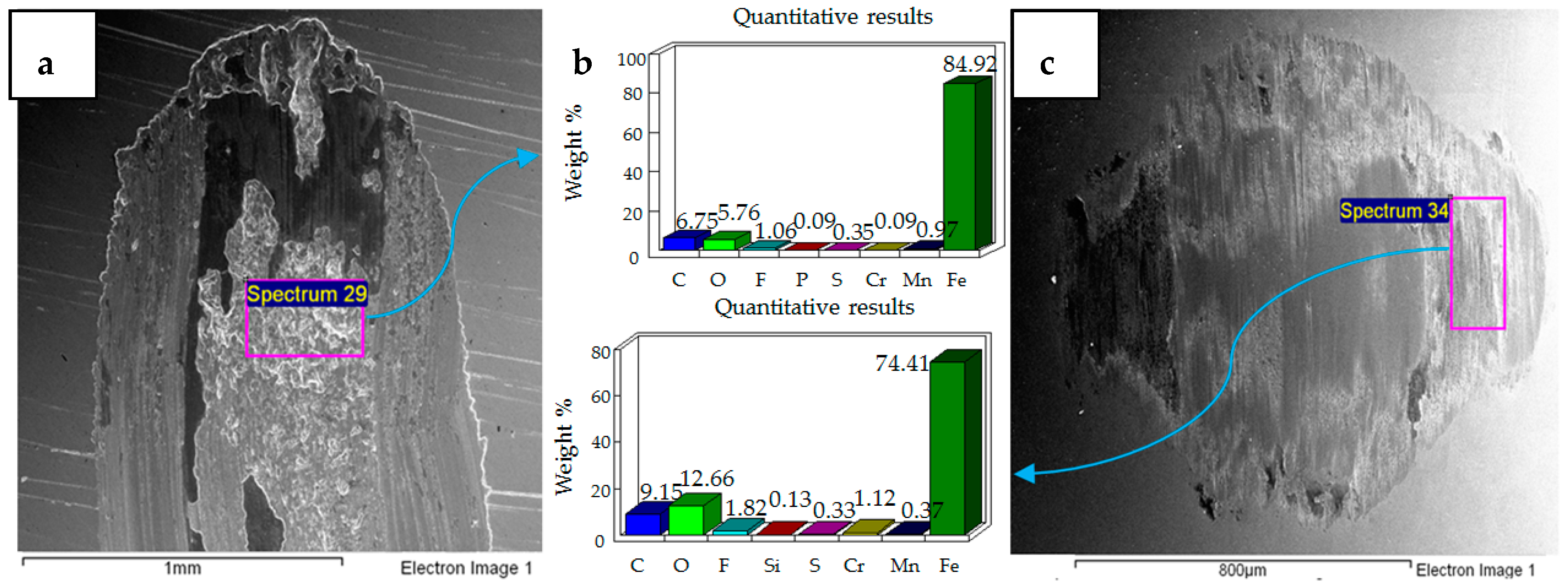
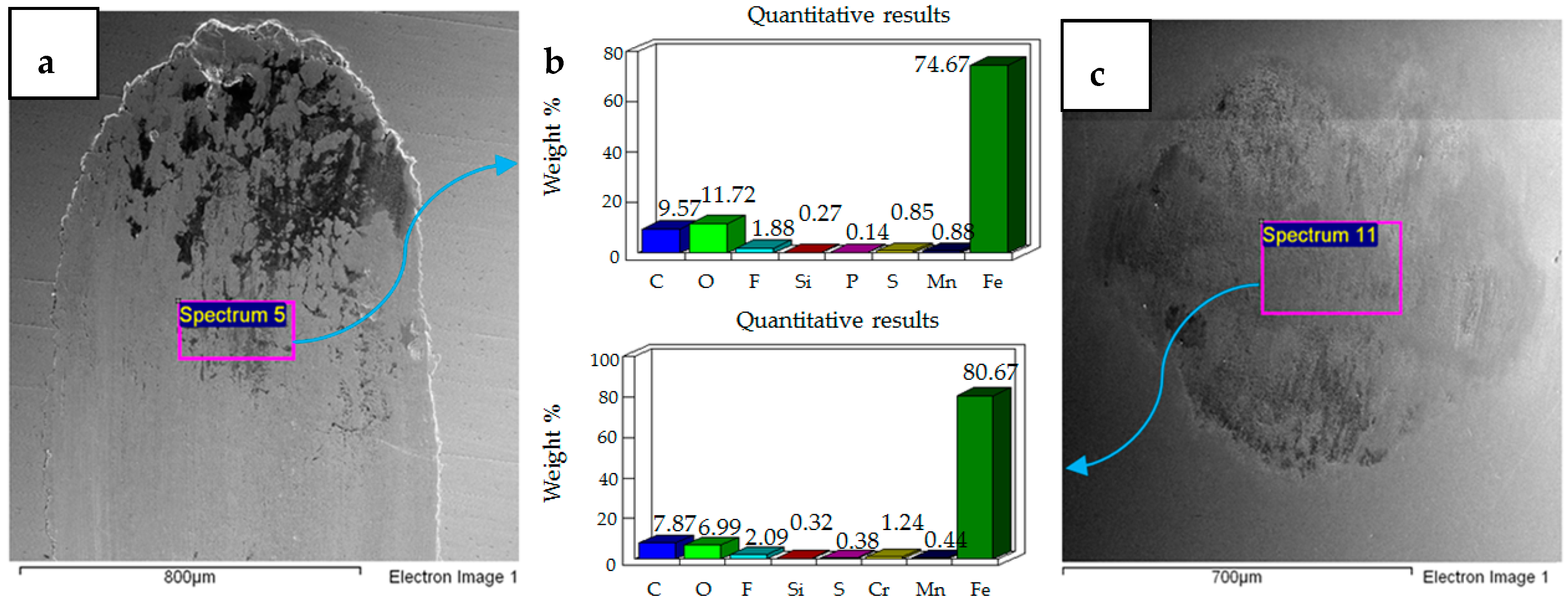
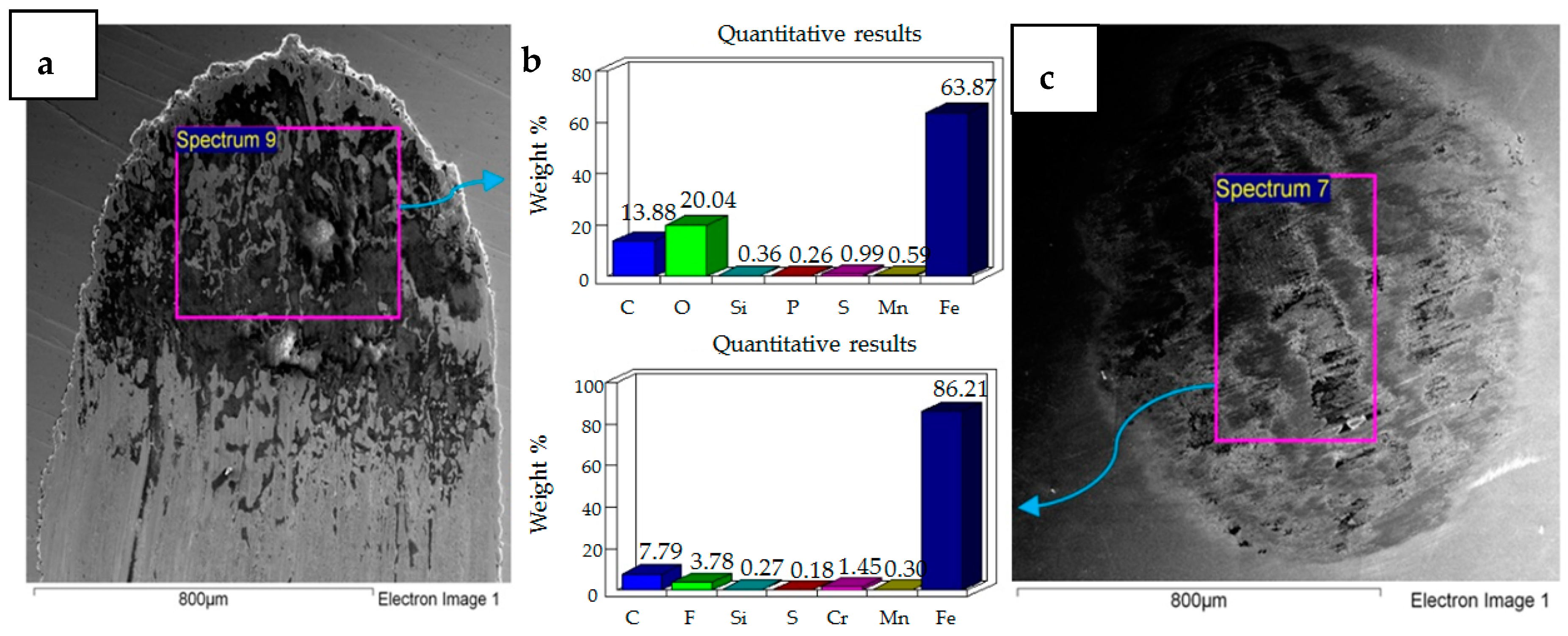
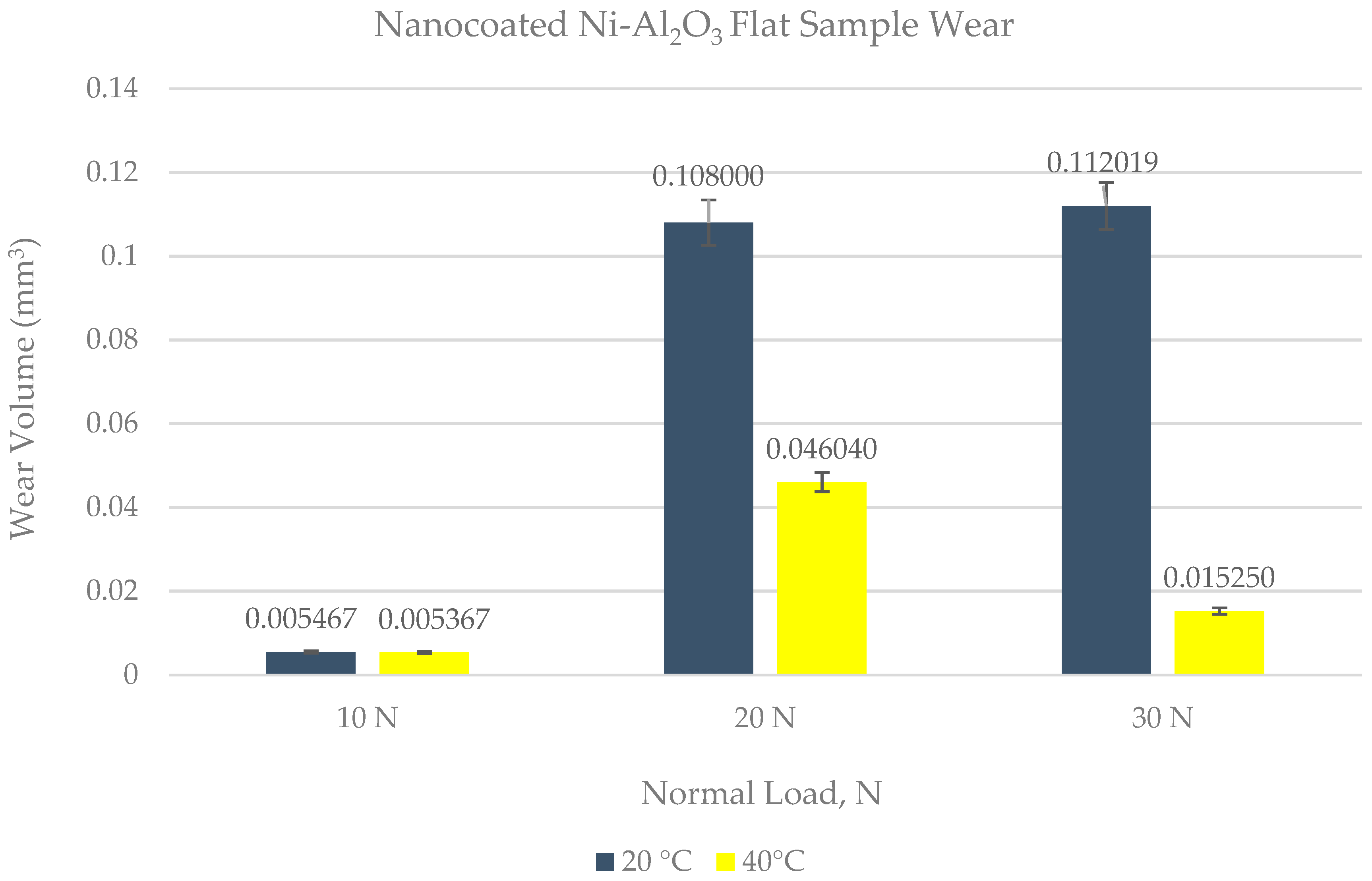
4.2. Wear of Coated Specimens
5. Conclusions
- A modified tribo-meter was used to study the wear performance of Ni–Al2O3 nanocomposite coating under HFE-7000 refrigerant lubrication under varying operating conditions.
- Ni–Al2O3 nanocomposite coatings were successfully developed using the pulse coating technique. The wear mechanism of the coatings developed has been studied and has been compared to uncoated contacts under the same operating conditions.
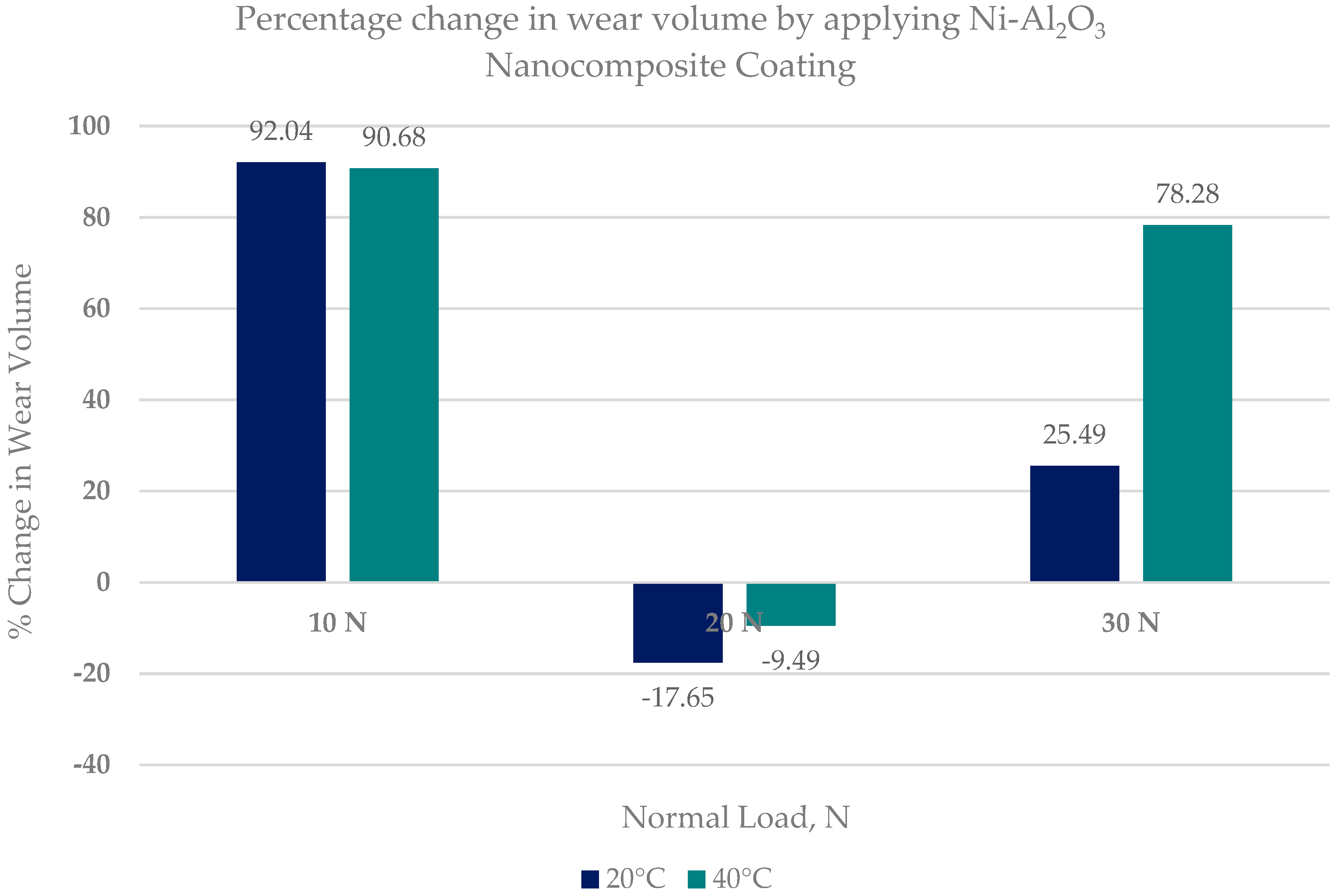
- Ni–Al2O3 nanocomposite coating shows good wear performance at low loads and can reduce wear by more than 90% compared to uncoated parts. At intermediate loads, the wear performance of these coatings showed adverse effects and increased the wear by 18% at low operating temperatures of 20 °C. Further increase in load to 30 N reduced wear by 25% at the low refrigerant temperature of 20 °C and by 78% at the higher refrigerant temperature of 40 °C.
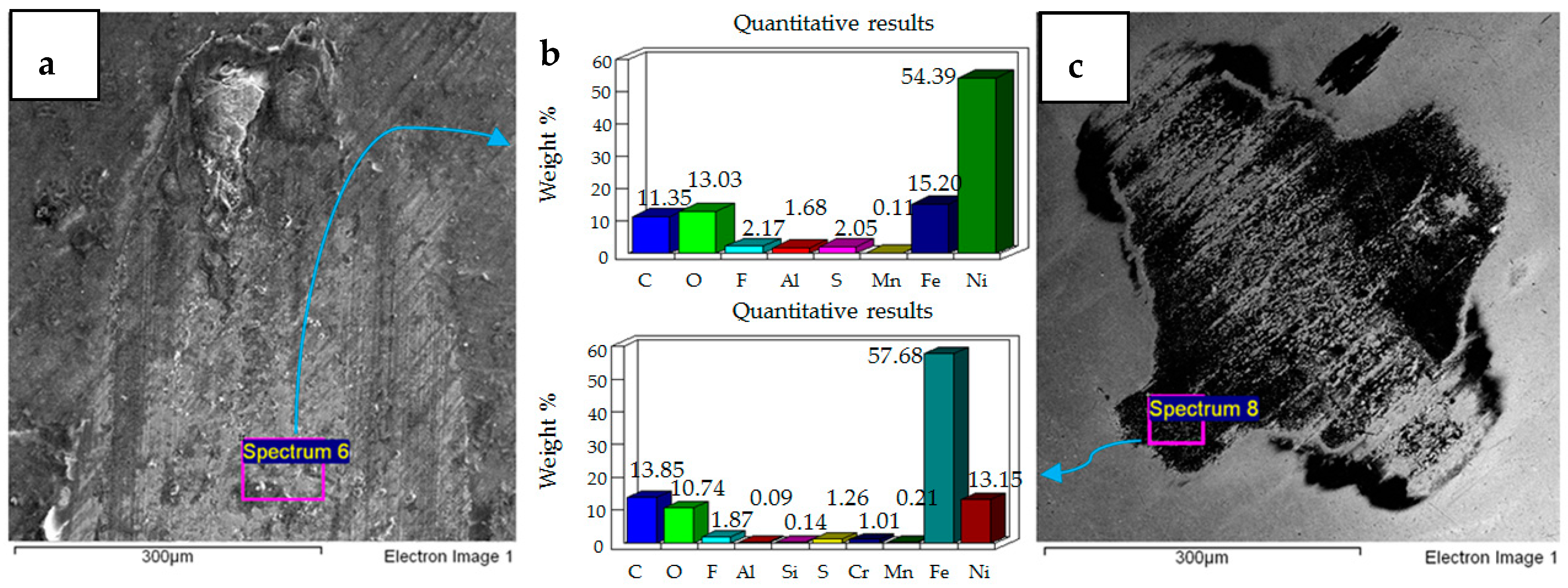
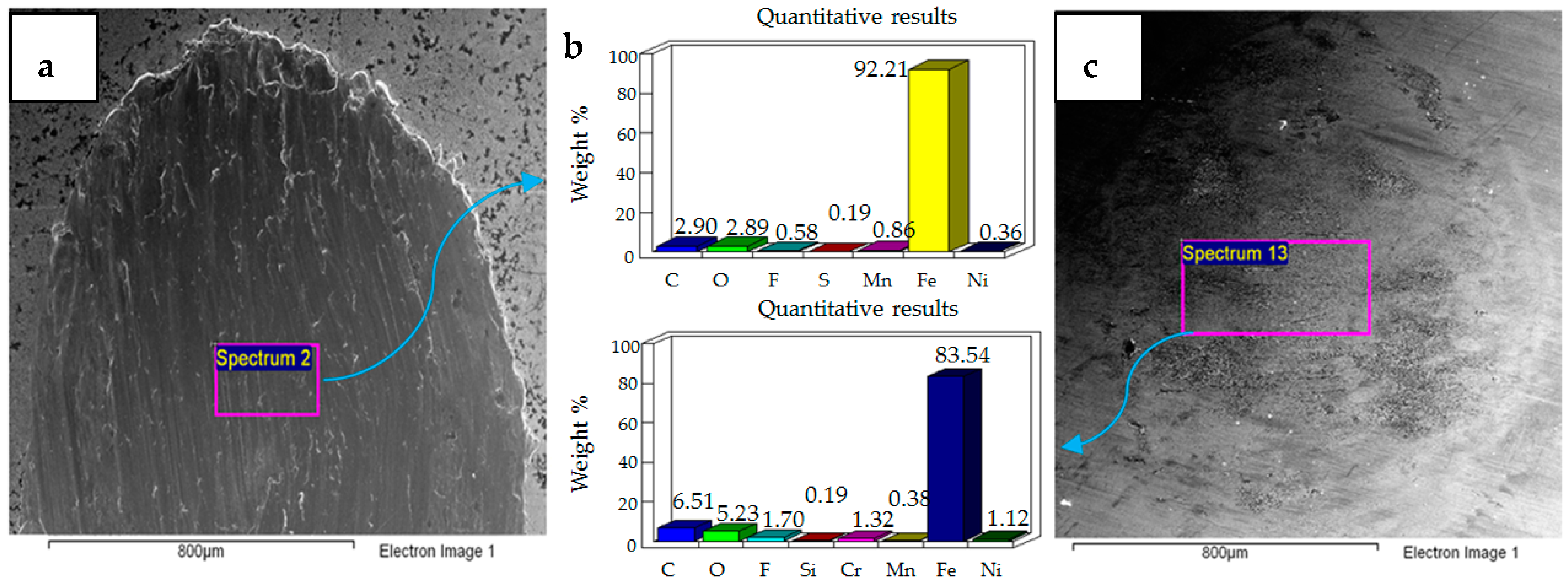
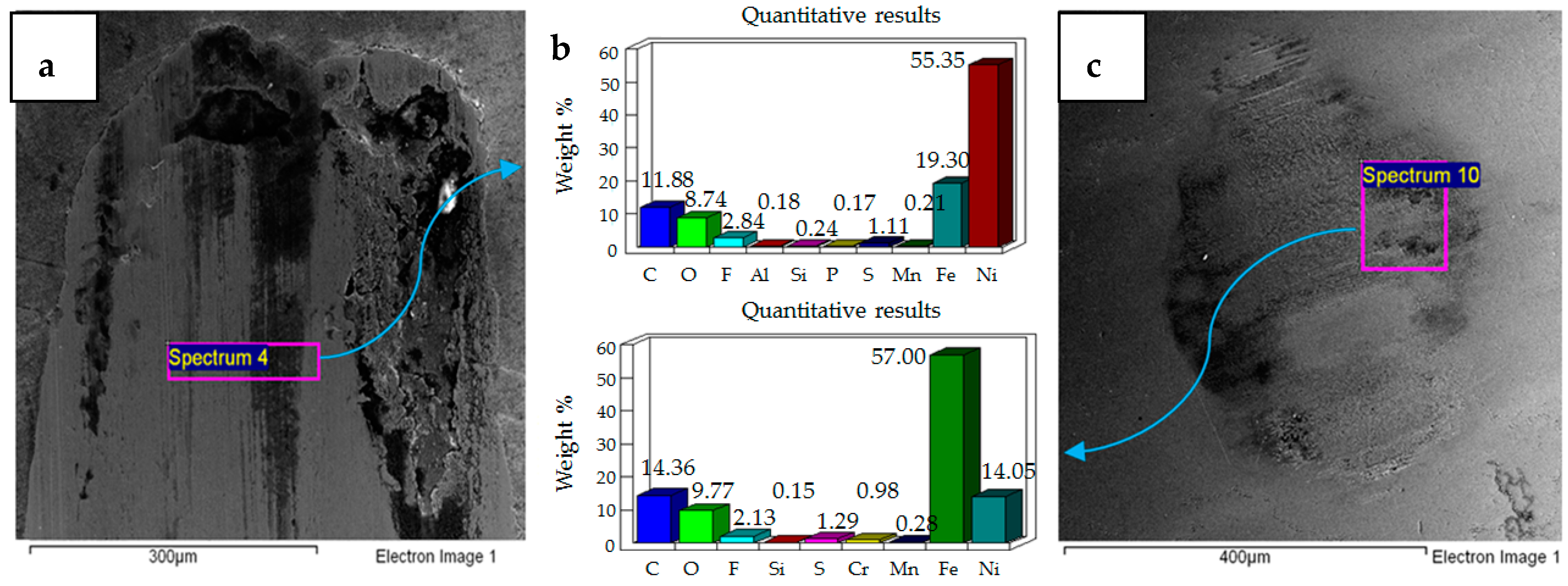
- Post-test elemental analysis of the surfaces revealed oxygen and fluorine on the interacting parts by using coated as well as uncoated steel samples. This indicates that HFE-7000 formed new compounds on the metallic surfaces, resulting in the formation of protective tribo-films which help reduce wear.
- Increase in temperature has a very positive impact on reducing wear of both the coated and uncoated samples. The ability of HFE-7000 to from protective tribo-films on the rubbing surfaces increases with increase in temperature, resulting in the accelerated formation of protective surface films on the rubbing metals.
- HFE-7000, which is an environmentally friendly future generation thermo-fluid, having good thermodynamic properties, has shown good wear performance as well.
- Wear can be reduced by using Ni–Al2O3 nanocomposite coating on EN1A-steel substrate instead of using specialized expensive metal alloys.
6. Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Energy Agency (iea). Available online: https://www.iea.org/newsroom/news/2016/august/air-conditioning-demand-set-to-grow-rapidly-over-the-coming-decades.html (accessed on 19 December 2018).
- Bhutta, M.U.; Khan, Z.A.; Garland, N.P.; Ghafoor, A. A historical review on the tribological performance of refrigerants used in compressors. Tribol. Ind. 2018, 40, 19–51. [Google Scholar] [CrossRef]
- Jollev, S. New and unique lubricants for use in compressors utilizing R-134a refrigerant. In Proceedings of the International Refrigeration and Air Conditioning Conference, Purdue University, West Lafayette, IN, USA, 17–20 July 1990; p. 96. [Google Scholar]
- Davis, B.; Sheiretov, T.K.; Cusano, C. Tribological evaluation of contacts lubricated by oil-refrigerant mixtures. Am. J. Hematol. 1992, 71, 66. [Google Scholar]
- Komatsuzaki, S.; Homma, Y. Lubricants for HFC refrigerant compressors. J. Japn. Petrol. Inst. 1994, 37, 226–235. [Google Scholar] [CrossRef]
- Mizuhara, K.; Akei, M.; Matsuzaki, T. The friction and wear behavior in controlled alternative refrigerant atmosphere. Tribol. Trans. 1994, 37, 120–128. [Google Scholar] [CrossRef]
- Sheiretov, T.; Glabbeek, W.V.; Cusano, C. Tribological evaluation of various surface treatments for M2 tool steel in a refrigerant environment. In Proceedings of the International Compressor Engineering Conference, Purdue University, West Lafayette, IN, USA, 19–22 July 1994; p. 964. [Google Scholar]
- Akei, M.; Mizuhara, K.; Taki, T.; Yamamoto, T. Evaluation of film-forming capability of refrigeration lubricants in pressurized refrigerant atmosphere. Wear 1996, 196, 180–187. [Google Scholar] [CrossRef]
- Kawahara, K.; Mishina, S.; Kamino, A.; Ochiai, K.; Okawa, T.; Fujimoto, S. Tribological evaluation of rotary compressor with HFC refrigerants. In Proceedings of the International Compressor Engineering Conference, Purdue University, West Lafayette, IN, USA, 23–26 July 1996; p. 1141. [Google Scholar]
- Fujimoto, S.; Sakitani, K.; Watada, M. Tribology analysis in rolling piston type compressor. In Proceedings of the International Compressor Engineering Conference, Purdue University, West Lafayette, IN, USA; 1984; p. 477. [Google Scholar]
- Muraki, M.; Tagawa, K.; Dong, D. Refrigeration lubricant based on polyolester for use with HFCs and prospect of its application with R-22 (Part 1) tribological characteristics. In Proceedings of the International Refrigeration and Air Conditioning Conference, Purdue University, West Lafayette, IN, USA, 23–26 July 1996; p. 336. [Google Scholar]
- Tuomas, R.; Isaksson, O. Measurement of lubrication conditions in a rolling element bearing in a refrigerant environment. Ind. Lubr. Tribol. 2009, 61, 91–99. [Google Scholar] [CrossRef]
- Molina, M.J.; Rowland, F.S. Stratospheric sink for chlorofluoromethanes: Chlorine atom-catalysed destruction of ozone. Nature 1974, 249, 810–812. [Google Scholar] [CrossRef]
- Nations, U. Montreal Protocol on Substances that Deplete the Ozone Layer Final Act 1987. J. Environ. Law 1989, 1, 128–136. [Google Scholar] [Green Version]
- Wilson, D.P.; Basu, R.S. Thermodynamic properties of a new stratospherically safe working fluid-refrigerant 134a. ASHRAE Trans. 1988, 94, 2095–2118. [Google Scholar]
- Shankland, I.R.; Basu, R.S.; Wilson, D.P. Thermal conductivity and viscosity of a new stratospherically safe refrigerant-1,1,1,2-tetrafluoroethane (R-134a). In Proceedings of the International Refrigeration and Air Conditioning Conference, Purdue University, West Lafayette, IN, USA; 1988; p. 41. [Google Scholar]
- Spauschus, H. HFC 134a as a substitute refrigerant for CFC 12. Int. J. Refrig. 1988, 11, 389–392. [Google Scholar] [CrossRef]
- Eckels, S.J.; Pate, M.B. An experimental comparison of evaporation and condensation heat transfer coefficients for HFC-134a and CFC-12. Int. J. Refrig. 1991, 14, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.H.; Zubair, S.M. Thermodynamic analyses of the CFC-12 and HFC-134a refrigeration cycles. Energy 1993, 18, 717–726. [Google Scholar] [CrossRef]
- Na, B.C.; Chun, K.J.; Han, D.-C. A tribological study of refrigeration oils under HFC-134a environment. Tribol. Int. 1997, 30, 707–716. [Google Scholar] [CrossRef]
- Wardle, F.; Jacobson, B.; Dolfsma, H.; Hoglund, E.; Jonsson, U. The effect of refrigerants on the lubrication of rolling element bearings used in screw compressors. In Proceedings of the International Compressor Engineering Conference, Purdue University, West Lafayette, IN, USA, 14–17 July 1992; p. 843. [Google Scholar]
- Jacobson, B. Lubrication of Screw Compressor Bearings in the Presence of Refrigerants. In Proceedings of the International Compressor Engineering Conference, Purdue University, West Lafayette, IN, USA, 19–22 July 1994; p. 966. [Google Scholar]
- Hamada, T.; Nishiura, N. Refrigeration lubricant based on polyolester for use with HFCs and prospect of its application with R-22 (Part 2) Hydrolytic stability and compressor endurance test results. In Proceedings of the International Refrigeration and Air Conditioning Conference, Purdue University, West Lafayette, IN, USA, 23–26 July 1996; p. 337. [Google Scholar]
- Jacobson, B. Ball Bearing Lubrication in Refrigetation Compressors. In Proceedings of the International Compressor Engineering Conference, Purdue University, West Lafayette, IN, USA, 23–26 July 1996; p. 1090. [Google Scholar]
- Akei, M.; Mizuhara, K. The Elastohydrodynamic Properties of Lubricants in Refrigerant Environments©. Tribol. Trans. 1997, 40, 1–10. [Google Scholar] [CrossRef]
- Jacobson, B.O.; Espejel, G.E.M. High pressure investigation of refrigerants HFC245fa, R134a and R123. In Proceedings of the International Compressor Engineering Conference, Purdue University, West Lafayette, IN, USA, 17–20 July 2006; p. 1789. [Google Scholar]
- Tuomas, R.; Isaksson, O. Compressibility of Oil/Refrigerant Lubricants in Elasto-Hydrodynamic Contacts. J. Tribol. 2005, 128, 218–220. [Google Scholar] [CrossRef]
- Breidenich, C.; Magraw, D.; Rowley, A.; Rubin, J.W. The Kyoto protocol to the United Nations framework convention on climate change. Am. J. Int. Law 1998, 92, 315–331. [Google Scholar] [CrossRef]
- Garland, N.; Hadfield, M. Environmental implications of hydrocarbon refrigerants applied to the hermetic compressor. Mater. Des. 2005, 26, 578–586. [Google Scholar] [CrossRef]
- Khan, Z.A.; Hadfield, M.; Wang, Y. Design of a novel pressurized chamber to assess in-use durability performance of rolling contact elements using refrigerant lubrication. In Proceedings of the 4th International Conference of Advanced Engineering Design, Glasgow, Scotland, UK, 5–8 September 2004. [Google Scholar]
- Khan, Z.A.; Hadfield, M.; Wang, Y. Pressurised chamber design for conducting rolling contact experiments with liquid refrigerant lubrication. Mater. Des. 2005, 26, 680–689. [Google Scholar] [CrossRef]
- Khan, Z.A.; Hadfield, M.; Tobe, S.; Wang, Y. Ceramic rolling elements with ring crack defects—A residual stress approach. Mater. Sci. Eng A 2005, 404, 221–226. [Google Scholar] [CrossRef]
- Khan, Z.A.; Hadfield, M.; Tobe, S.; Wang, Y. Residual stress variations during rolling contact fatigue of refrigerant lubricated silicon nitride bearing elements. Ceram. Int. 2006, 32, 751–754. [Google Scholar] [CrossRef]
- Khan, Z.A.; Hadfield, M. Manufacturing induced residual stress influence on the rolling contact fatigue life performance of lubricated silicon nitride bearing materials. Mater. Des. 2007, 28, 2688–2693. [Google Scholar] [CrossRef]
- Solzak, T.A.; Polycarpou, A.A. Tribology of protective hard coatings for use in oil-less, piston-type compressors. In Proceedings of the International Compressor Engineering Conference, Purdue University, West Lafayette, IN, USA, 17–20 July 2006; p. 1790. [Google Scholar]
- Sariibrahimoglu, K.; Kizil, H.; Aksit, M.F.; Efeoglu, I.; Kerpicci, H. Effect of R600a on tribological behavior of sintered steel under starved lubrication. Tribol. Int. 2010, 43, 1054–1058. [Google Scholar] [CrossRef] [Green Version]
- De Mello, J.; Binder, R.; Demas, N.; Polycarpou, A. Effect of the actual environment present in hermetic compressors on the tribological behaviour of a Si-rich multifunctional DLC coating. Wear 2009, 267, 907–915. [Google Scholar] [CrossRef]
- Solzak, T.A.; Polycarpou, A.A. Tribology of hard protective coatings under realistic operating conditions for use in oilless piston-type and swash-plate compressors. Tribol. Trans. 2010, 53, 319–328. [Google Scholar] [CrossRef]
- Silverio, M.; Binder, R.; Hulse, E.R.; De Mello, J.D.B. Effect of refrigerant gases (HFC134a and R600a) on the tribological behaviour of a multifunctional DLC coating. In Proceedings of the International Compressor Engineering Conference, Purdue University, West Lafayette, IN, USA, 11–14 July 2016; p. 2413. [Google Scholar]
- Górny, K.; Stachowiak, A.; Tyczewski, P.; Zwierzycki, W. Lubricity evaluation of oil–refrigerant mixtures with R134a and R290. Int. J. Refrig. 2016, 69, 261–271. [Google Scholar] [CrossRef]
- Ikeda, H.; Yagi, J.I.; Kawaguchi, Y. The development of PAG refrigeration lubricants for hermetic compressors with CO2. In Proceedings of the International Refrigeration and Air Conditioning Conference, Purdue University, West Lafayette, IN, USA, 12–15 July 2004; p. 680. [Google Scholar]
- Wu, X.; Cong, P.; Nanao, H.; Minami, I.; Mori, S. Tribological behaviors of 52100 steel in carbon dioxide atmosphere. Tribol. Lett. 2004, 17, 925–930. [Google Scholar] [CrossRef]
- Lee, K.; Suh, A.; Demas, N.; Polycarpou, A. Surface and sub-micron sub-surface evolution of Al390-T6 undergoing tribological testing under submerged lubrication conditions in the presence of CO2 refrigerant. Tribol. Lett. 2005, 18, 1–12. [Google Scholar] [CrossRef]
- Demas, N.G.; Polycarpou, A.A.; Conry, T.F. Tribological Studies on Scuffing Due to the Influence of Carbon Dioxide Used as a Refrigerant in Compressors. Tribol. Trans. 2005, 48, 336–342. [Google Scholar] [CrossRef] [Green Version]
- Demas, N.G.; Polycarpou, A.A. Ultra high pressure tribometer for testing CO2 refrigerant at chamber pressures up to 2000 psi to simulate compressor conditions. Tribol. Trans. 2006, 49, 291–296. [Google Scholar] [CrossRef]
- Cannaday, M.; Polycarpou, A. Advantages of CO2 compared to R410a refrigerant of tribologically tested Aluminum 390-T6 surfaces. Tribol. Lett. 2006, 21, 185–192. [Google Scholar] [CrossRef]
- Jeon, H.-G.; Oh, S.-D.; Lee, Y.-Z. Friction and wear of the lubricated vane and roller materials in a carbon dioxide refrigerant. Wear 2009, 267, 1252–1256. [Google Scholar] [CrossRef]
- Demas, N.G.; Polycarpou, A.A. Tribological investigation of cast iron air-conditioning compressor surfaces in CO2 refrigerant. Tribol. Lett. 2006, 22, 271–278. [Google Scholar] [CrossRef]
- Demas, N.G.; Polycarpou, A.A. Tribological performance of PTFE-based coatings for air-conditioning compressors. Surf. Coat. Tech. 2008, 203, 307–316. [Google Scholar] [CrossRef]
- Nunez, E.E.; Demas, N.G.; Polychronopoulou, K.; Polycarpou, A.A. Tribological study comparing PAG and POE lubricants used in air-conditioning compressors under the presence of CO2. Tribol. Trans. 2008, 51, 790–797. [Google Scholar] [CrossRef]
- Nunez, E.E.; Demas, N.G.; Polychronopoulou, K.; Polycarpou, A.A. Comparative scuffing performance and chemical analysis of metallic surfaces for air-conditioning compressors in the presence of environmentally friendly CO2 refrigerant. Wear 2010, 268, 668–676. [Google Scholar] [CrossRef]
- Dascalescu, D.; Polychronopoulou, K.; Polycarpou, A. The significance of tribochemistry on the performance of PTFE-based coatings in CO2 refrigerant environment. Surf. Coat. Tech. 2009, 204, 319–329. [Google Scholar] [CrossRef]
- Nunez, E.E.; Polychronopoulou, K.; Polycarpou, A.A. Lubricity effect of carbon dioxide used as an environmentally friendly refrigerant in air-conditioning and refrigeration compressors. Wear 2010, 270, 46–56. [Google Scholar] [CrossRef]
- Nunez, E.E.; Polycarpou, A.A. Wear study of metallic interfaces for air-conditioning compressors under submerged lubrication in the presence of carbon dioxide. Wear 2015, 326, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Hagita, T.; Makino, T.; Horaguchi, N.; Ukai, T. Tribology in CO2 Scroll Compressors. Available online: https://www.mhi.co.jp/technology/review/pdf/e391/e391031.pdf (accessed on 19 December 2018).
- Sasaki, T.; Nakao, H.; Maeyama, H.; Mizuno, K. Tribology characteristics of HFO and HC refrigerants with immiscible oils—Effect of refrigerant with unsaturated bond. In Proceedings of the International Compressor Engineering Conference, Purdue University, West Lafayette, IN, USA, 12–15 July 2010; p. 1946. [Google Scholar]
- Mishra, S.P.; Polycarpou, A.A. Tribological studies of unpolished laser surface textures under starved lubrication conditions for use in air-conditioning and refrigeration compressors. Tribol. Inter. 2011, 44, 1890–1901. [Google Scholar] [CrossRef]
- Akram, M.W.; Polychronopoulou, K.; Polycarpou, A.A. Lubricity of environmentally friendly HFO-1234yf refrigerant. Tribol. Int. 2013, 57, 92–100. [Google Scholar] [CrossRef]
- Akram, M.W.; Polychronopoulou, K.; Seeton, C.; Polycarpou, A.A. Tribological performance of environmentally friendly refrigerant HFO-1234yf under starved lubricated conditions. Wear 2013, 304, 191–201. [Google Scholar] [CrossRef]
- Akram, M.W.; Polychronopoulou, K.; Polycarpou, A.A. Tribological performance comparing different refrigerant–lubricant systems: The case of environmentally friendly HFO-1234yf refrigerant. Tribol. Int. 2014, 78, 176–186. [Google Scholar] [CrossRef]
- Akram, M.W.; Meyer, J.L.; Polycarpou, A.A. Tribological interactions of advanced polymeric coatings with polyalkylene glycol lubricant and r1234yf refrigerant. Tribol. Int. 2016, 97, 200–211. [Google Scholar] [CrossRef] [Green Version]
- 3M™ Novec™ 7000 Engineered Fluid. Available online: https://multimedia.3m.com/mws/media/121372O/3m-novec-7000-engineered-fluid-tds.pdf (accessed on 19 December 2018).
- Helvaci, H.; Khan, Z.A. Experimental study of thermodynamic assessment of a small scale solar thermal system. Energy Convers. Manag. 2016, 117, 567–576. [Google Scholar] [CrossRef]
- Helvaci, H.; Khan, Z. Thermodynamic modelling and analysis of a solar organic Rankine cycle employing thermofluids. Energy Convers. Manag. 2017, 138, 493–510. [Google Scholar] [CrossRef] [Green Version]
- Helvaci, H.U.; Khan, Z.A. Heat transfer and entropy generation analysis of HFE 7000 based nanorefrigerants. Int. J. Heat. Mass. Trans. 2017, 104, 318–327. [Google Scholar] [CrossRef] [Green Version]
- Meyers, M.A.; Mishra, A.; Benson, D.J. Mechanical properties of nanocrystalline materials. Prog. Mater. Sci. 2006, 51, 427–556. [Google Scholar] [CrossRef]
- Kumar, K.S.; Van Swygenhoven, H.; Suresh, S. Mechanical behavior of nanocrystalline metals and alloys. Acta Mater. 2003, 51, 5743–5774. [Google Scholar] [CrossRef]
- Zong, Y.; Zuo, L. Materials design of microstructure in grain boundary and second phase particles. J. Mater. Sci. Tech. 2003, 19, 97–101. [Google Scholar]
- Chen, L.; Wang, L.; Zeng, Z.; Xu, T. Influence of pulse frequency on the microstructure and wear resistance of electrodeposited Ni–Al2O3 composite coatings. Surf. Coat. Tech. 2006, 201, 599–605. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Zeng, Z.; Zhang, J. Effect of surfactant on the electrodeposition and wear resistance of Ni–Al2O3 composite coatings. Mater. Sci. Eng A 2006, 434, 319–325. [Google Scholar] [CrossRef]
- Zhou, Q.; He, C.L.; Cai, Q.K. Effect of Al2O3 Powders on Properties of Electrodeposited Ni Matrix. Adv. Mater. Res. 2009, 79, 631–634. [Google Scholar] [CrossRef]
- Saha, R.K.; Khan, T.I. Effect of applied current on the electrodeposited Ni–Al2O3 composite coatings. Surf. Coat. Tech. 2010, 205, 890–895. [Google Scholar] [CrossRef]
- Borkar, T.; Harimkar, S.P. Effect of electrodeposition conditions and reinforcement content on microstructure and tribological properties of nickel composite coatings. Surf. Coat. Tech. 2011, 205, 4124–4134. [Google Scholar] [CrossRef]
- Bajwa, R.S.; Khan, Z.; Bakolas, V.; Braun, W. Water-lubricated Ni-based composite (Ni–Al2O3, Ni–SiC and Ni–ZrO2) thin film coatings for industrial applications. Acta Metall. Sin. (Eng. Lett.) 2016, 29, 8–16. [Google Scholar] [CrossRef]
- Bajwa, R.S.; Khan, Z.; Bakolas, V.; Braun, W. Effect of bath ionic strength on adhesion and tribological properties of pure nickel and Ni-based nanocomposite coatings. J. Adhes. Sci. Tech. 2016, 30, 653–665. [Google Scholar] [CrossRef]
- Nazir, M.H.; Khan, Z.A.; Saeed, A.; Bakolas, V.; Braun, W.; Bajwa, R. Experimental analysis and modelling for reciprocating wear behaviour of nanocomposite coatings. Wear 2018, 416–417, 89–102. [Google Scholar] [CrossRef]
- Nazir, M.H.; Khan, Z.A.; Saeed, A.; Bakolas, V.; Braun, W.; Bajwa, R.; Rafique, S. Analyzing and Modelling the Corrosion Behavior of Ni/Al2O3, Ni/SiC, Ni/ZrO2 and Ni/Graphene Nanocomposite Coatings. Materials 2017, 10, 1225. [Google Scholar] [CrossRef]
- Yoon, H.; Sheiretov, T.; Cusano, C. Scuffing behavior of 390 aluminum against steel under starved lubrication conditions. Wear 2000, 237, 163–175. [Google Scholar] [CrossRef]
- Suh, A.Y.; Polycarpou, A.A.; Conry, T.F. Detailed surface roughness characterization of engineering surfaces undergoing tribological testing leading to scuffing. Wear 2003, 255, 556–568. [Google Scholar] [CrossRef]
- Nunez, E.E.; Yeo, S.M.; Polycarpou, A.A. Tribological behavior of PTFE, PEEK, and fluorocarbon-based polymeric coatings used in air-conditioning and refrigeration compressors. In Proceedings of the International Compressor Engineering Conference, Purdue University, West Lafayette, IN, USA, 12–15 July 2010; p. 2031. [Google Scholar]
- Bajwa, R.; Khan, Z.; Nazir, H.; Chacko, V.; Saeed, A. Wear and Friction Properties of Electrodeposited Ni-Based Coatings Subject to Nano-enhanced Lubricant and Composite Coating. Acta Metall. Sin. (Eng. Lett.) 2016, 29, 902–910. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Meng, Y.; Khan, Z.A.; Zhang, V. The Synergetic Effects of Surface Texturing and MoDDP Additive Applied to Ball-on-Disk Friction Subject to Both Flooded and Starved Lubrication Conditions. Tribol. Lett. 2017, 65, 163. [Google Scholar] [CrossRef]
- Abdullah, M.U.; Shah, S.R.; Bhutta, M.U.; Mufti, R.A.; Khurram, M.; Najeeb, M.H.; Arshad, W.; Ogawa, K. Benefits of wonder process craft on engine valve train performance. Proc. Inst. Mech. Eng. Part D J. Automb. Eng. 2018. [Google Scholar] [CrossRef]

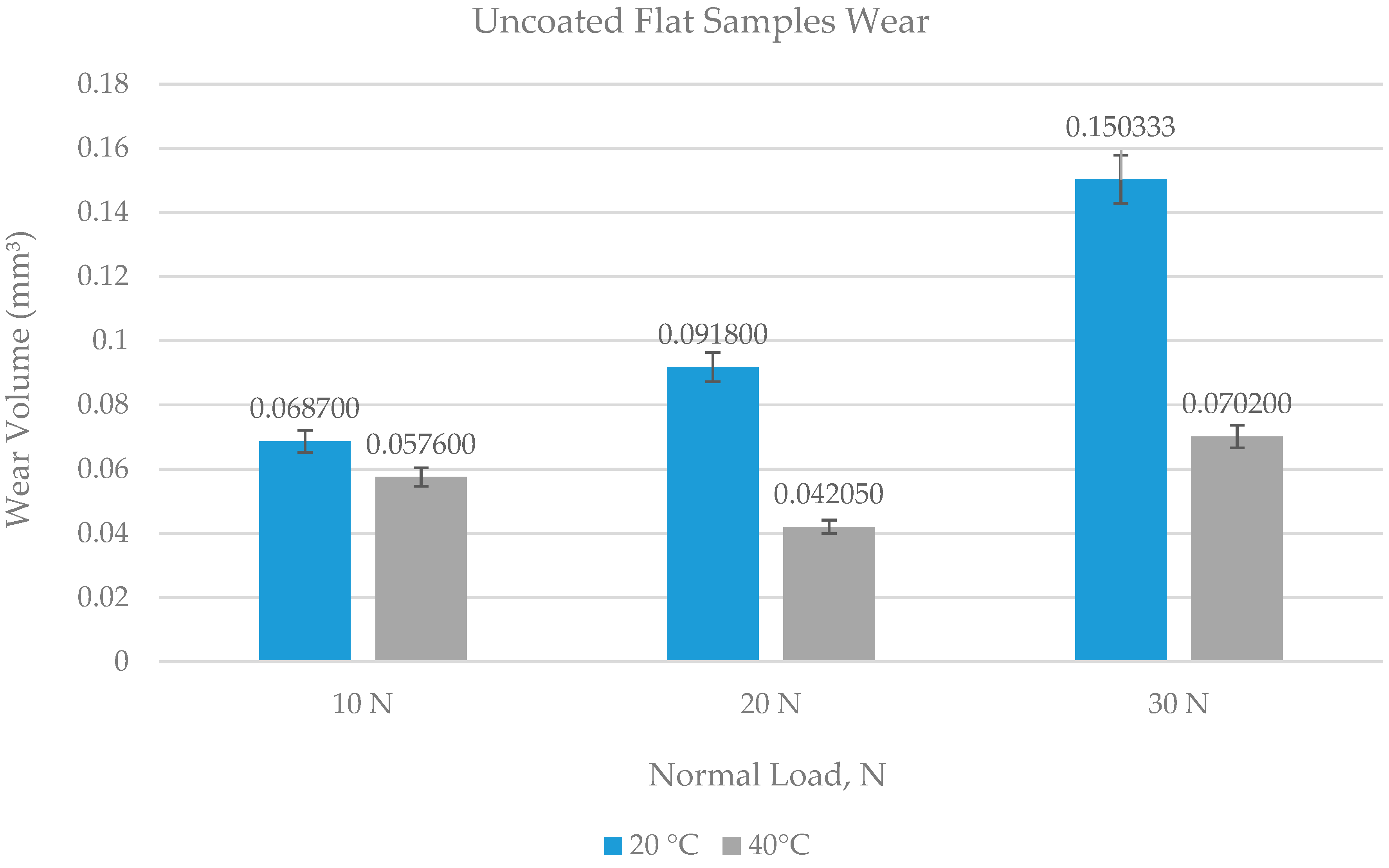

| HFE-7000 | |
|---|---|
| Structure | C3F7OCH3 |
| Molecular Weight (g/mol) | 200 |
| Freeze Point (°C) | −122.5 |
| Boiling Point @ 1 atmosphere (°C) | 34 |
| Critical Temperature (°C) | 165 |
| Critical Pressure (MPa) | 2.48 |
| Flash Point (°C) | None |
| Kinematic Viscosity @−120°C (cSt) | 17 |
| Kinematic Viscosity @20°C (cSt) | 0.32 |
| Kinematic Viscosity @40°C (cSt) | 0.27 |
| Flammability | Non-flammable |
| Ozone Depletion Potential (ODP) | Zero |
| Global Warming Potential (GWP) | 530 * |
| Specimen | Hardness (HV) | Elastic Modulus (GPa) | Average Surface Roughness (μm) |
|---|---|---|---|
| Steel ball | 810 | 210 | 0.010 |
| Uncoated steel specimen | 180 | 200 | 0.1 |
| Coated steel specimen | 450 ± 25 | 280 | 0.045 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhutta, M.U.; Khan, Z.A.; Garland, N. Wear Performance Analysis of Ni–Al2O3 Nanocomposite Coatings under Nonconventional Lubrication. Materials 2019, 12, 36. https://doi.org/10.3390/ma12010036
Bhutta MU, Khan ZA, Garland N. Wear Performance Analysis of Ni–Al2O3 Nanocomposite Coatings under Nonconventional Lubrication. Materials. 2019; 12(1):36. https://doi.org/10.3390/ma12010036
Chicago/Turabian StyleBhutta, Muhammad Usman, Zulfiqar Ahmad Khan, and Nigel Garland. 2019. "Wear Performance Analysis of Ni–Al2O3 Nanocomposite Coatings under Nonconventional Lubrication" Materials 12, no. 1: 36. https://doi.org/10.3390/ma12010036
APA StyleBhutta, M. U., Khan, Z. A., & Garland, N. (2019). Wear Performance Analysis of Ni–Al2O3 Nanocomposite Coatings under Nonconventional Lubrication. Materials, 12(1), 36. https://doi.org/10.3390/ma12010036






