Physicochemical Properties of Cellulose Separators for Lithium Ion Battery: Comparison with Celgard2325
Abstract
:1. Introduction
2. Materials and Methods
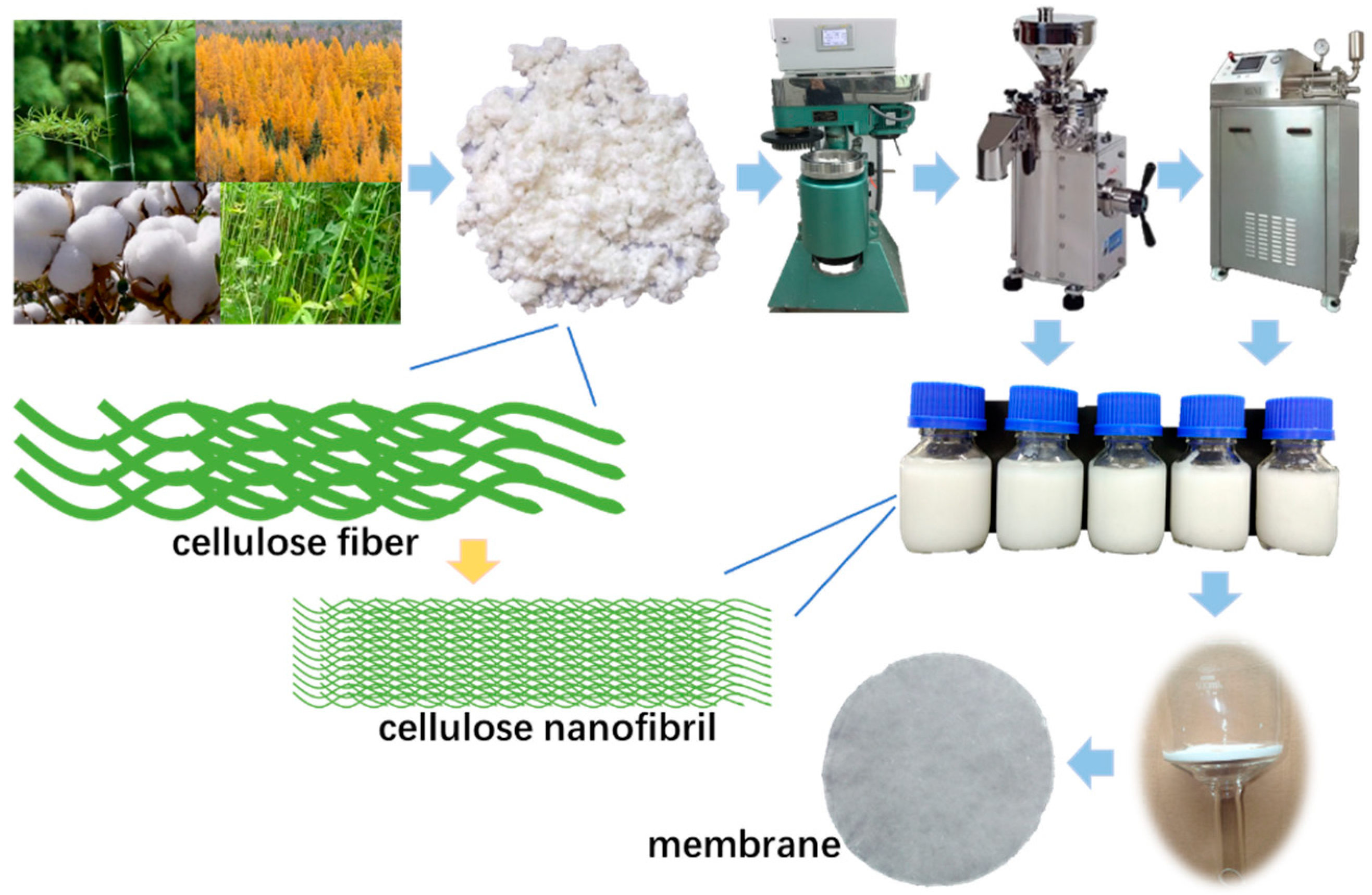
2.1. Materials
2.2. Preparation of the Cellulose Nanofibril Membrane
2.3. Characterizations
3. Results
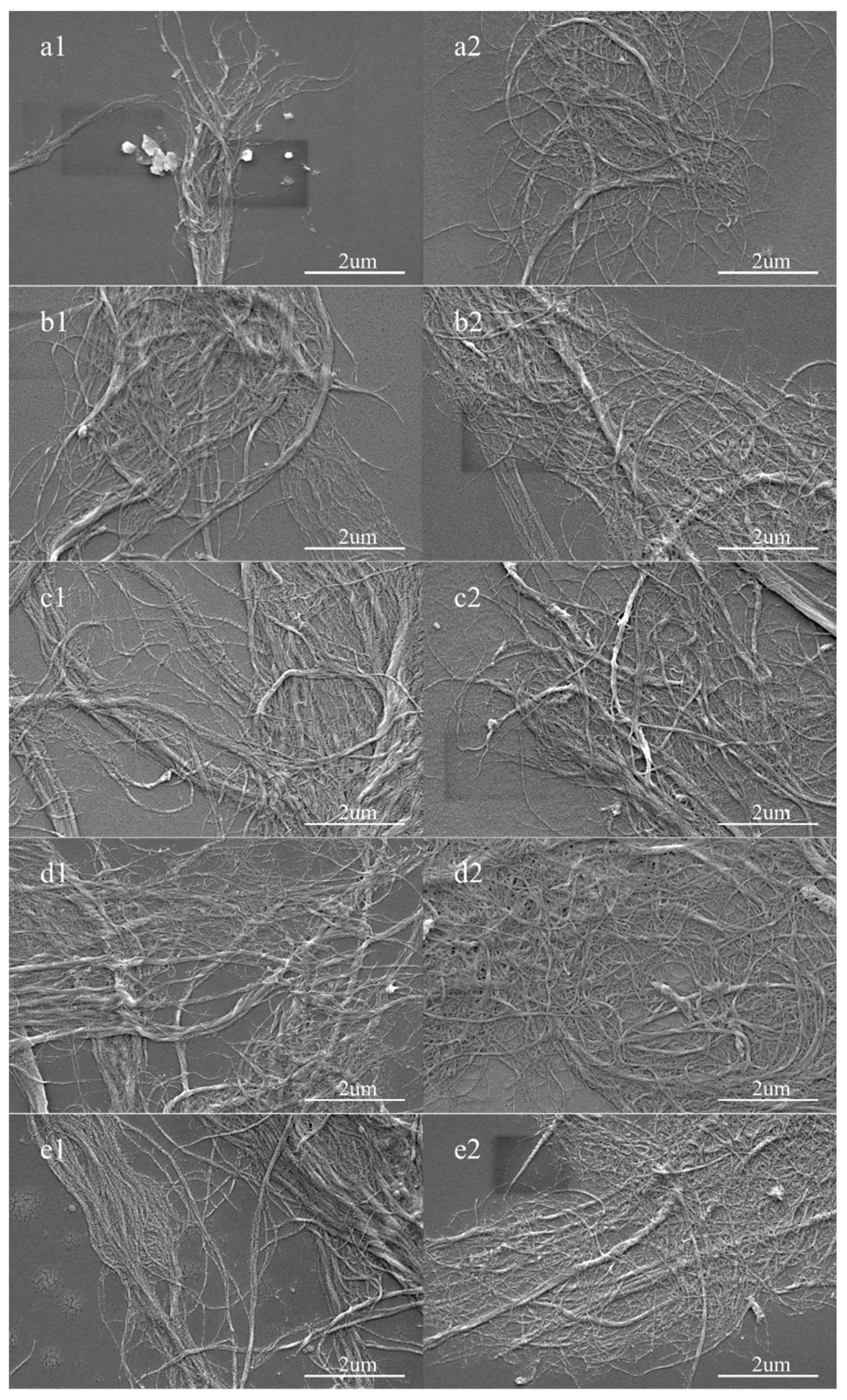
3.1. Morphology
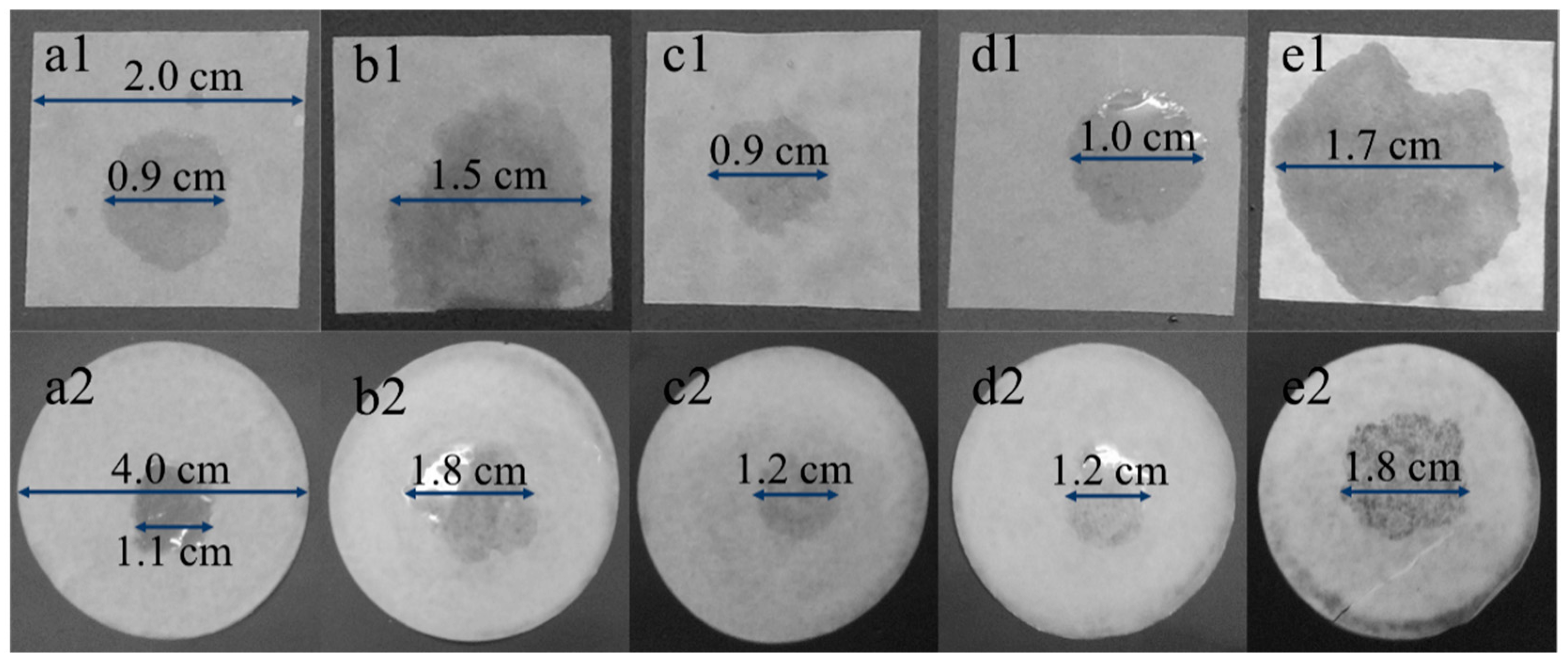
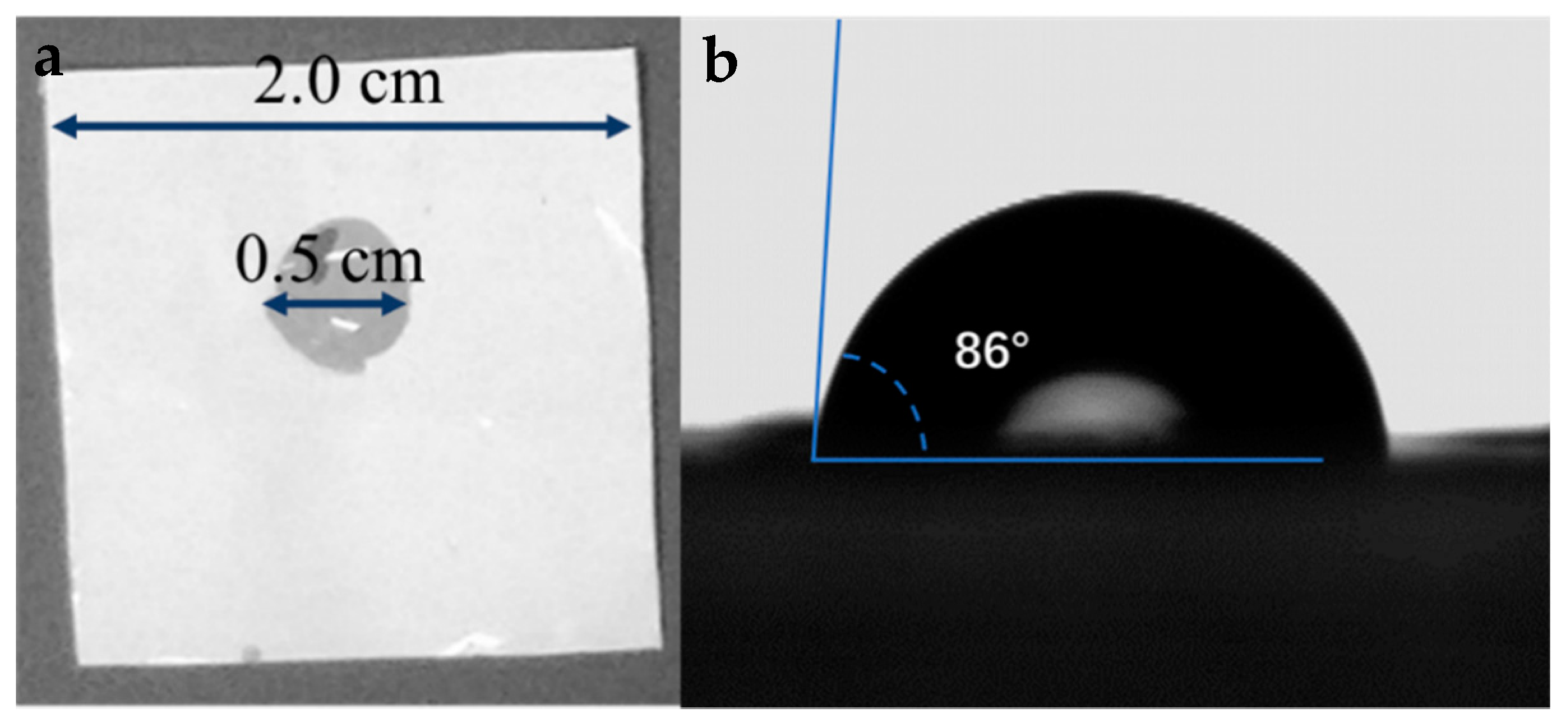
3.2. Wettability
3.3. Thermal Stability
3.4. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, C.; Tammela, P.; Huo, J.; Stromme, M.; Edstrom, K.; Gustafsson, T.; Nyholm, L. Flexible freestanding Cladophora nanocellulose paper based Si anodes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 14109–14115. [Google Scholar] [CrossRef]
- Huang, F.; Liu, W.; Li, P.; Ning, J.; Wei, Q. Electrochemical Properties of LLTO/Fluoropolymer-Shell Cellulose-Core Fibrous Membrane for Separator of High Performance Lithium-Ion Battery. Materials 2016, 9, 75. [Google Scholar] [CrossRef]
- Sheng, J.; Tong, S.; He, Z.; Yang, R. Recent developments of cellulose materials for lithium-ion battery separators. Cellulose 2017, 24, 4103–4122. [Google Scholar] [CrossRef]
- Liang, Z.; Zheng, G.; Liu, C.; Liu, N.; Li, W.; Yan, K.; Yao, H.; Hsu, P.; Chu, S.; Cui, Y. Polymer Nanofiber-Guided Uniform Lithium Deposition for Battery Electrodes. Nano Lett. 2015, 15, 2910–2916. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Kichambare, P.; Rai, A.K.; Bhattacharya, R.; Rodrigues, S.; Subramanyam, G. A high performance ceramic-polymer separator for lithium batteries. J. Power Sources 2016, 301, 194–198. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, C.; Xia, Q.; Liu, J.; Ding, Z.; Xu, M.; Chen, L.; Wei, W. Composite electrolyte membranes incorporating viscous copolymers with cellulose for high performance lithium-ion batteries. J. Membr. Sci. 2016, 497, 259–269. [Google Scholar] [CrossRef]
- Chen, W.; Shi, L.; Zhou, H.; Zhu, J.; Wang, Z.; Mao, X.; Chi, M.; Sun, L.; Yuan, S. Water-Based Organic-Inorganic Hybrid Coating for a High-Performance Separator. ACS Sustain. Chem. Eng. 2016, 4, 3794–3802. [Google Scholar] [CrossRef]
- Jabbour, L.; Bongiovanni, R.; Chaussy, D.; Gerbaldi, C.; Beneventi, D. Cellulose-based Li-ion batteries: A review. Cellulose 2013, 20, 1523–1545. [Google Scholar] [CrossRef]
- Jiang, F.; Nie, Y.; Yin, L.; Feng, Y.; Yu, Q.; Zhong, C. Core-shell-structured nanofibrous membrane as advanced separator for lithium-ion batteries. J. Membr. Sci. 2016, 510, 1–9. [Google Scholar] [CrossRef]
- Pan, R.; Cheung, O.; Wang, Z.; Tammela, P.; Huo, J.; Lindh, J.; Edström, K.; Strømme, M.; Nyholm, L. Mesoporous Cladophora cellulose separators for lithium-ion batteries. J. Power Sources 2016, 321, 185–192. [Google Scholar] [CrossRef]
- Kim, J.; Gu, M.; Lee, D.H.; Kim, J.; Oh, Y.; Min, S.H.; Kim, B.; Lee, S. Functionalized Nanocellulose-Integrated Heterolayered Nanomats toward Smart Battery Separators. Nano Lett. 2016, 16, 5533–5541. [Google Scholar] [CrossRef]
- Li, M.X.; Wang, X.W.; Yang, Y.Q.; Chang, Z.; Wu, Y.P.; Holze, R. A dense cellulose-based membrane as a renewable host for gel polymer electrolyte of lithium ion batteries. J. Membr. Sci. 2015, 476, 112–118. [Google Scholar] [CrossRef]
- Boriboon, D.; Vongsetskul, T.; Limthongkul, P.; Kobsiriphat, W.; Tammawat, P. Cellulose ultrafine fibers embedded with titania particles as a high performance and eco-friendly separator for lithium-ion batteries. Carbohydr. Polym. 2018, 189, 145–151. [Google Scholar] [CrossRef]
- Agate, S.; Joyce, M.; Lucia, L.; Pal, L. Cellulose and nanocellulose-based flexible-hybrid printed electronics and conductive composites—A review. Carbohydr. Polym. 2018, 198, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.; Choi, E.; Lee, E.; Kim, J.H.; Lee, S.; Lee, S. Eco-friendly cellulose nanofiber paper-derived separator membranes featuring tunable nanoporous network channels for lithium-ion batteries. J. Mater. Chem. 2012, 22, 16618–16626. [Google Scholar] [CrossRef]
- Xu, Q.; Kong, Q.; Liu, Z.; Zhang, J.; Wang, X.; Liu, R.; Yue, L.; Cui, G. Polydopamine-coated cellulose microfibrillated membrane as high performance lithium-ion battery separator. RSC Adv. 2014, 4, 7845–7850. [Google Scholar] [CrossRef]
- Xu, Q.; Kong, Q.; Liu, Z.; Wang, X.; Liu, R.; Zhang, J.; Yue, L.; Duan, Y.; Cui, G. Cellulose/Polysulfonamide Composite Membrane as a High Performance Lithium-Ion Battery Separator. ACS Sustain. Chem. Eng. 2014, 2, 194–199. [Google Scholar] [CrossRef]
- Sun, X.; Wu, Q.; Zhang, X.; Ren, S.; Lei, T.; Li, W.; Xu, G.; Zhang, Q. Nanocellulose films with combined cellulose nanofibers and nanocrystals: Tailored thermal, optical and mechanical properties. Cellulose 2018, 25, 1103–1115. [Google Scholar] [CrossRef]
- Bolloli, M.; Antonelli, C.; Molméret, Y.; Alloin, F.; Iojoiu, C.; Sanchez, J. Nanocomposite poly(vynilidene fluoride)/nanocrystalline cellulose porous membranes as separators for lithium-ion batteries. Electrochim. Acta 2016, 214, 38–48. [Google Scholar] [CrossRef]
- Sheng, J.; Yang, R. A facile method to concentrate cellulose nanofibril slurries. Cellulose 2018, 1–4. [Google Scholar] [CrossRef]
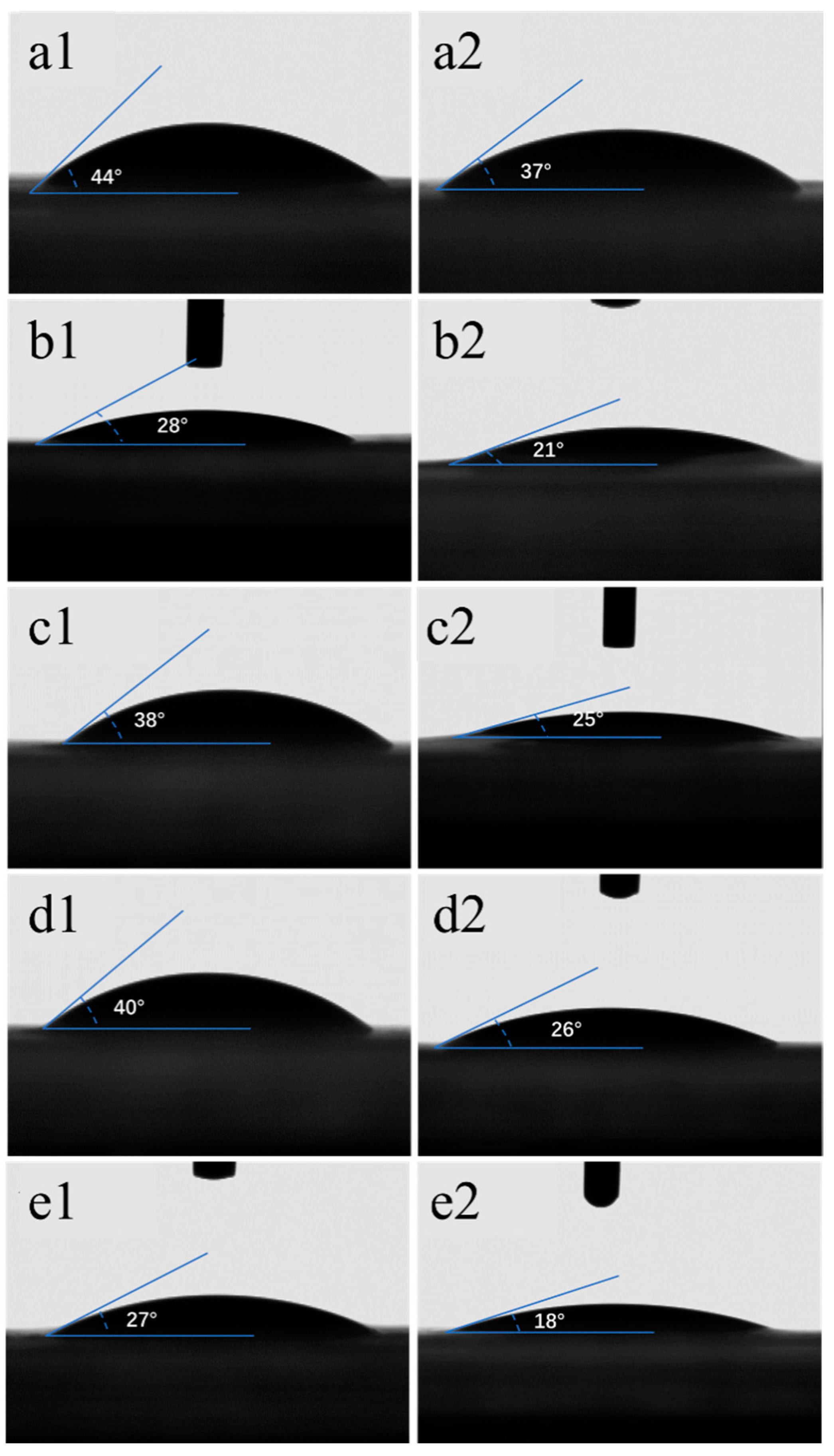
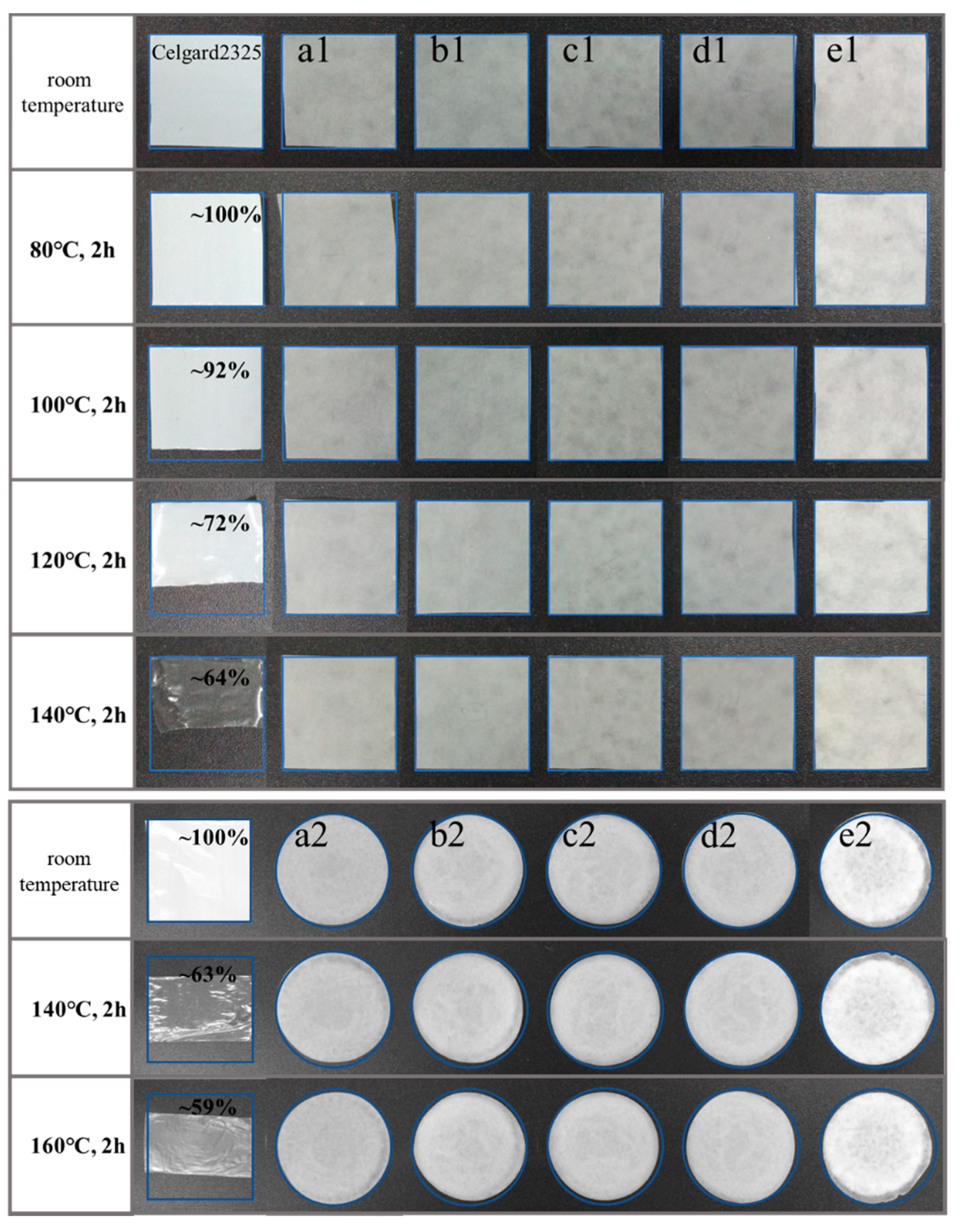
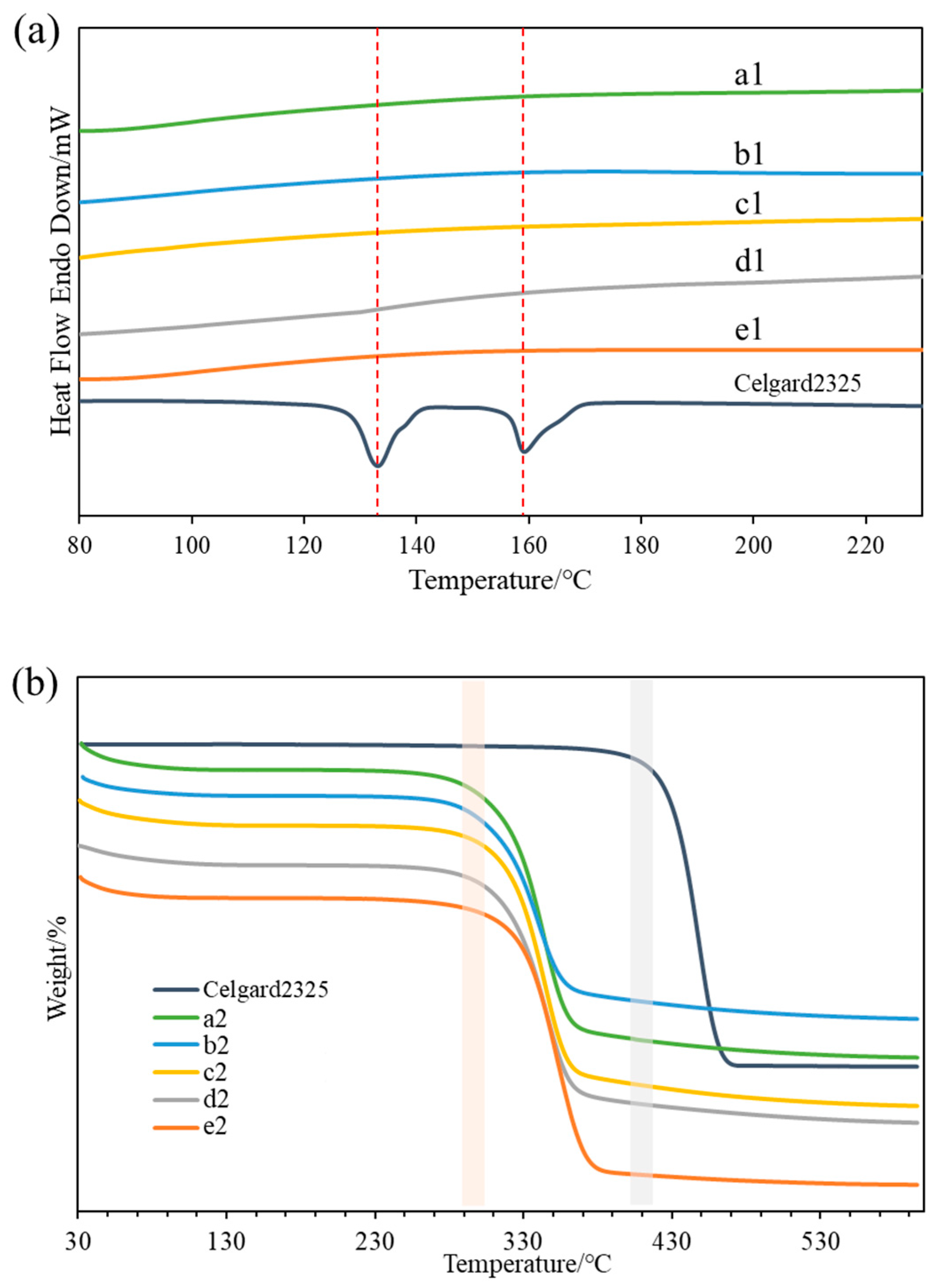
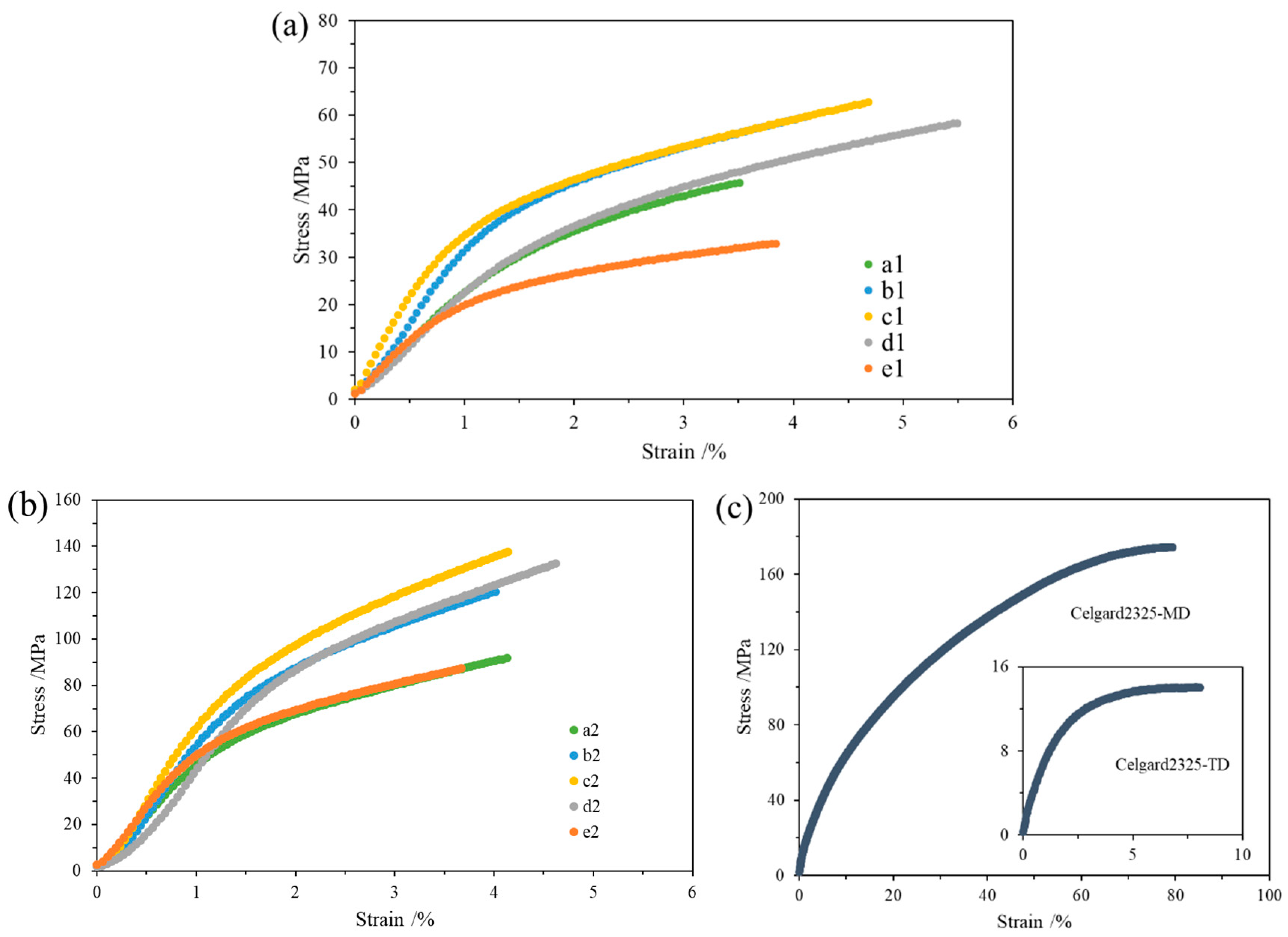
| Samples | Thickness (µm) | Samples | Thickness (µm) |
|---|---|---|---|
| a1 | 35 | a2 | 22 |
| b1 | 37 | b2 | 21 |
| c1 | 37 | c2 | 23 |
| d1 | 38 | d2 | 22 |
| e1 | 38 | e2 | 22 |
| Celgard2325 | 24 | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, J.; Wang, R.; Yang, R. Physicochemical Properties of Cellulose Separators for Lithium Ion Battery: Comparison with Celgard2325. Materials 2019, 12, 2. https://doi.org/10.3390/ma12010002
Sheng J, Wang R, Yang R. Physicochemical Properties of Cellulose Separators for Lithium Ion Battery: Comparison with Celgard2325. Materials. 2019; 12(1):2. https://doi.org/10.3390/ma12010002
Chicago/Turabian StyleSheng, Jie, Ruibin Wang, and Rendang Yang. 2019. "Physicochemical Properties of Cellulose Separators for Lithium Ion Battery: Comparison with Celgard2325" Materials 12, no. 1: 2. https://doi.org/10.3390/ma12010002
APA StyleSheng, J., Wang, R., & Yang, R. (2019). Physicochemical Properties of Cellulose Separators for Lithium Ion Battery: Comparison with Celgard2325. Materials, 12(1), 2. https://doi.org/10.3390/ma12010002





