Candida auris Dry Surface Biofilm (DSB) for Disinfectant Efficacy Testing
Abstract
1. Introduction
2. Materials and Methods
C. auris Growth and Maintenance
C. auris Organic Load (OL) Dry-Biofilm Model
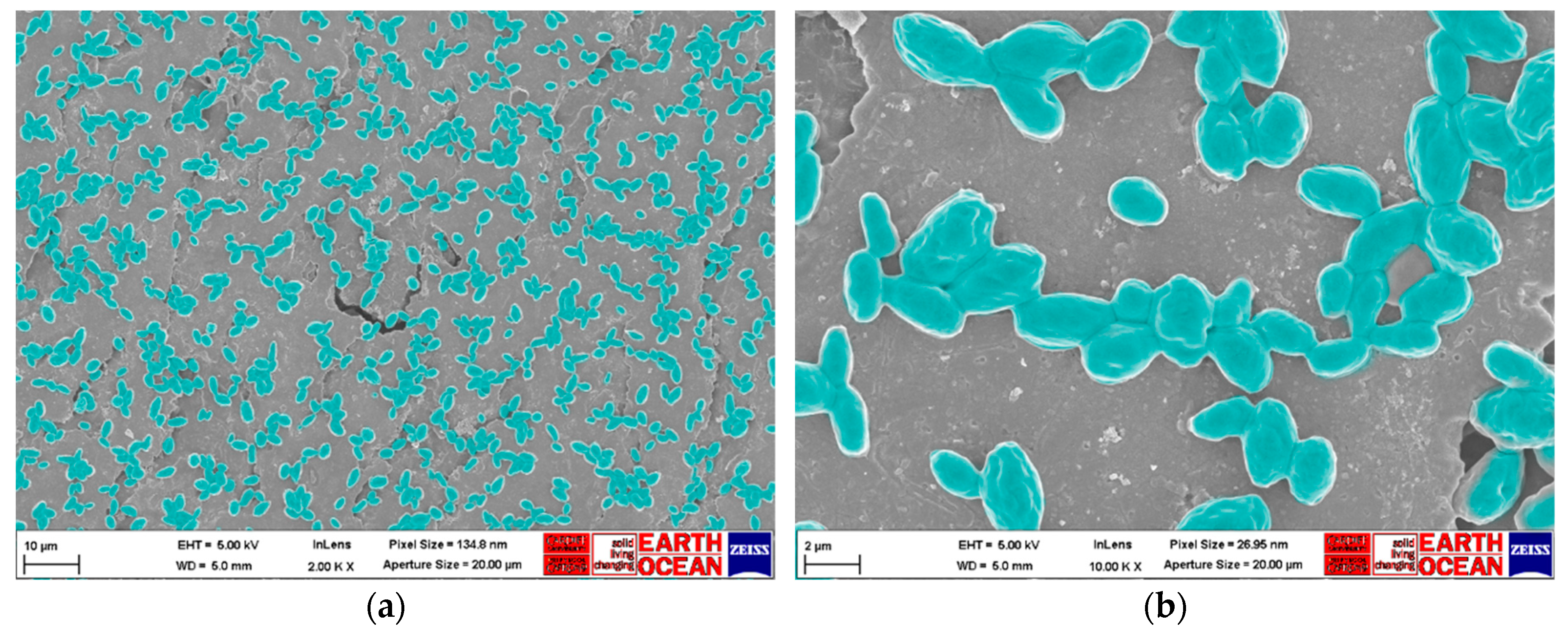
Scanning Electron Microscopy (SEM) Imaging
Product Tested
ASTM E2967-15 Test
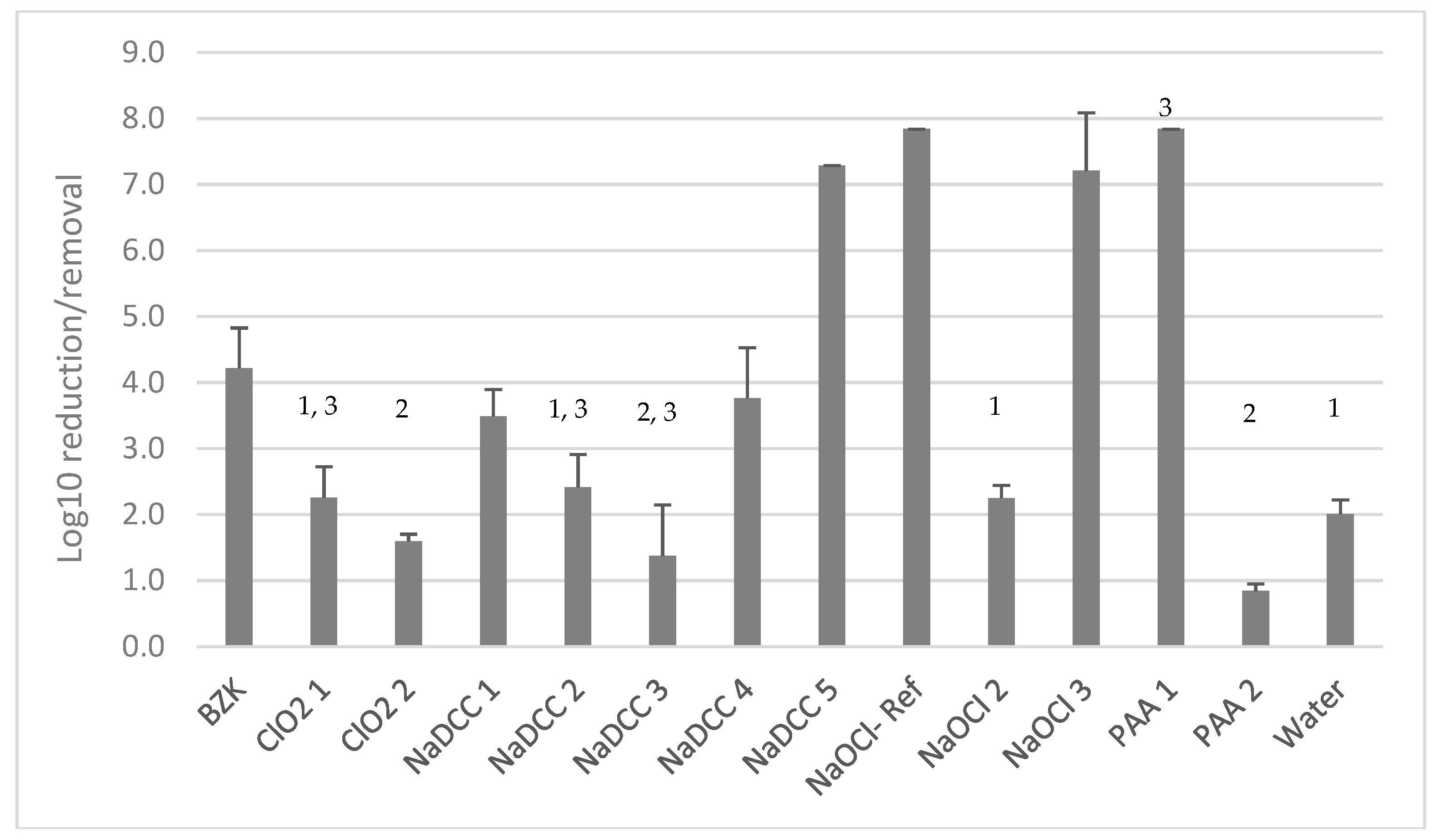
Reduction in Viability for Yeasts Embedded in Dry Biofilms
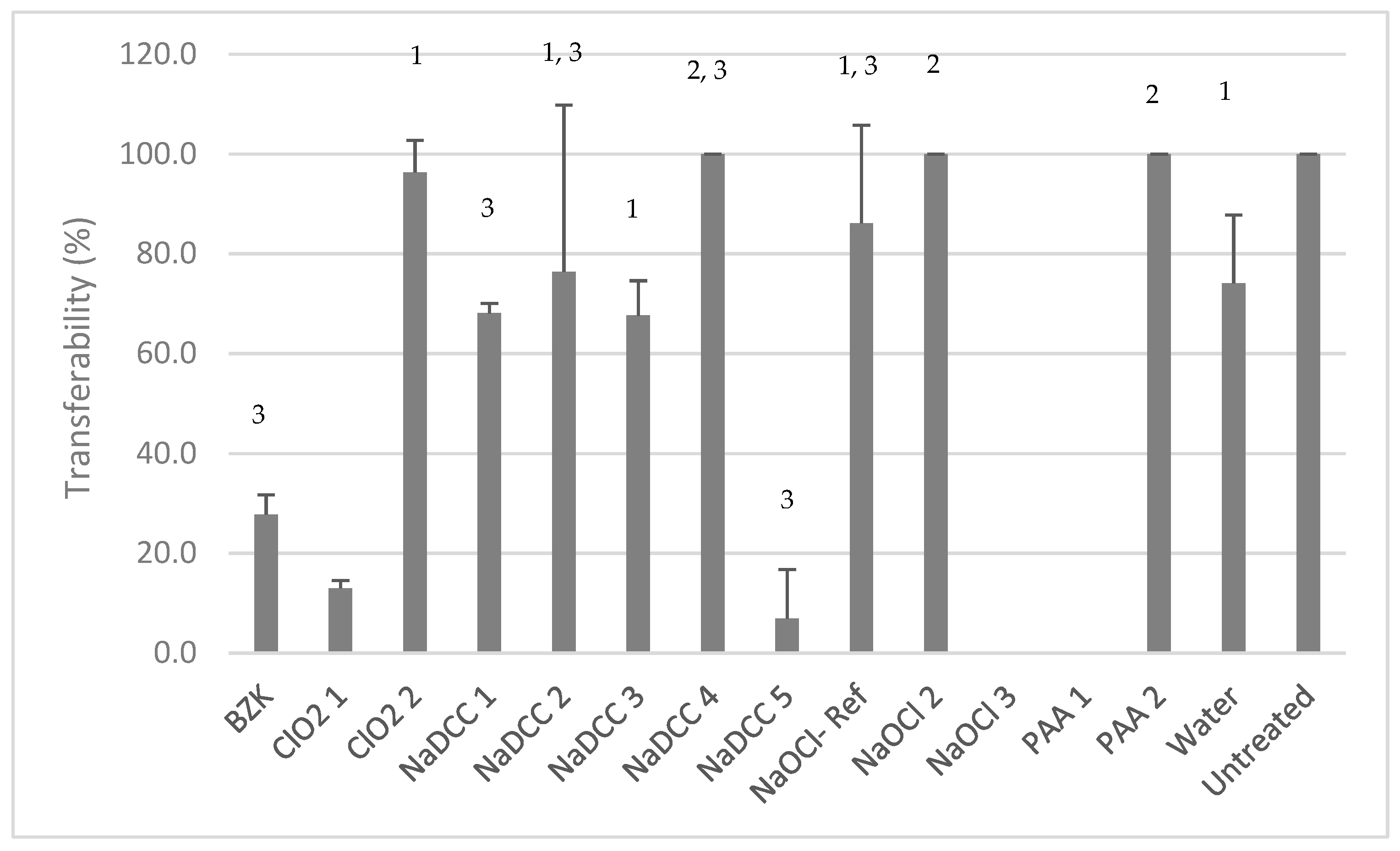
Transferability Test
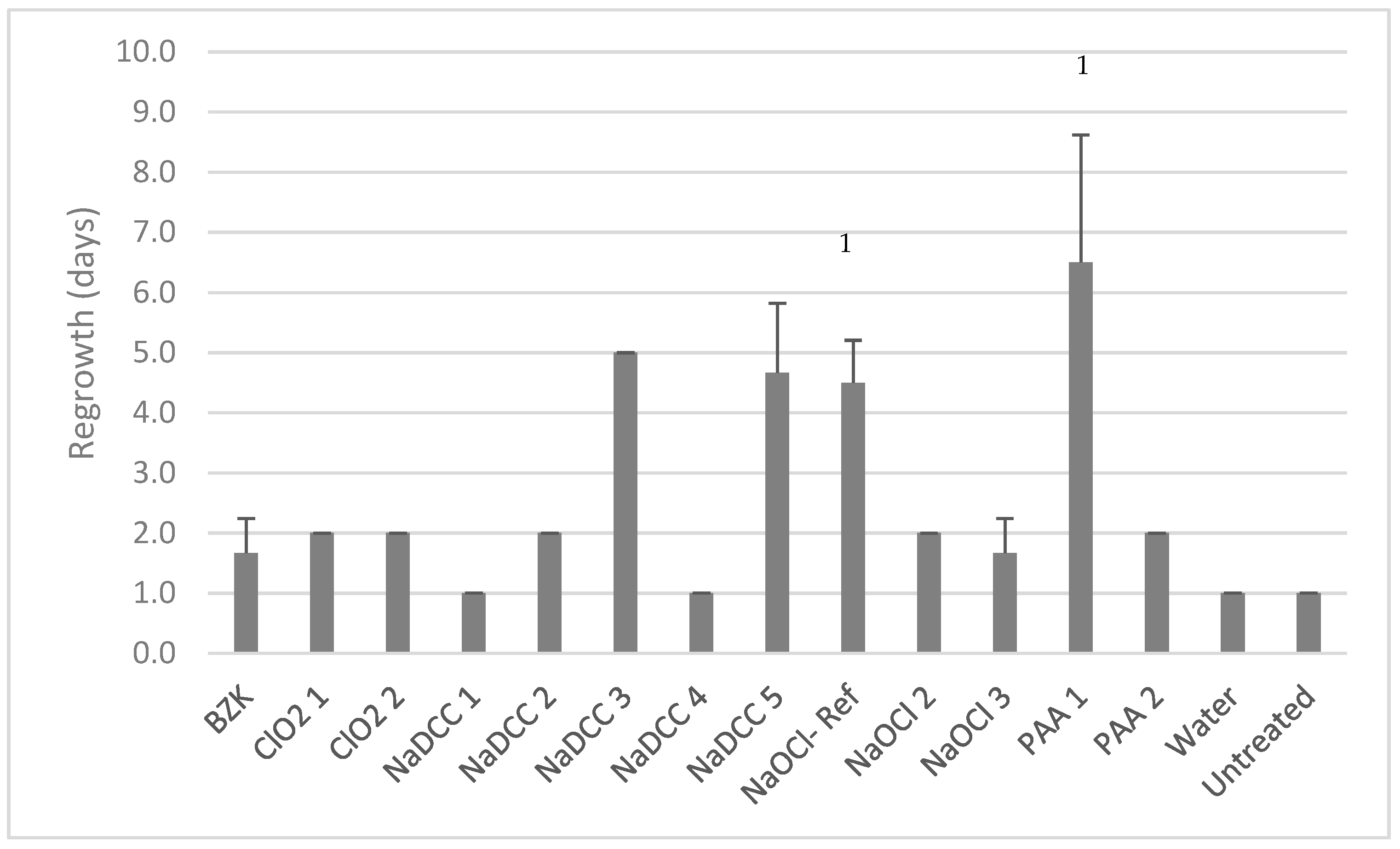
Dry-Biofilm Regrowth
Statistical Analysis
3. Results
SEM Analysis of C. auris Dry Surface Biofilm
Product Efficacy
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef]
- Araúz, A.B.; Caceres, D.H.; Santiago, E.; Armstrong, P.; Arosemena, S.; Ramos, C.; Espinosa-Bode, A.; Borace, J.; Hayer, L.; Cedeño, I.; et al. Isolation of Candida auris from 9 patients in Central America: Importance of accurate diagnosis and susceptibility testing. Mycoses 2018, 61, 44–47. [Google Scholar] [CrossRef]
- Morales-López, S.E.; Parra-Giraldo, C.M.; Ceballos-Garzón, A.; Martínez, H.P.; Rodríguez, G.J.; Álvarez-Moreno, C.A.; Rodríguez, J.Y. Invasive Infections with Multidrug-Resistant Yeast Candida auris, Colombia. Emerg. Infect. Dis. 2017, 23, 162. [Google Scholar] [CrossRef]
- Calvo, B.; Melo, A.S.A.; Perozo-Mena, A.; Hernandez, M.; Francisco, E.C.; Hagen, F.; Meis, J.F.; Colombo, A.L. First report of Candida auris in America: Clinical and microbiological aspects of 18 episodes of candidemia. J. Infect. 2016, 73, 369–374. [Google Scholar] [CrossRef]
- Weber, D.J.; Rutala, W.A.; Miller, M.B.; Huslage, K.; Sickbert-Bennett, E. Role of hospital surfaces in the transmission of emerging healthcare associated pathogens: Norovirus, Clostridium difficile, and Acinetobacter species. Am. J. Infect. Control 2010, 38, S25–S33. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Anderson, D.; Rutala, W.A. The role of the surface environment in healthcare-associated infections. Curr. Opin. Infect. Dis. 2013, 26, 338–344. [Google Scholar] [CrossRef]
- Otter, J.A.; Yezli, S.; Salkeld, J.A.G.; French, G.L. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am. J. Infect. Control 2013, 41, S6–S11. [Google Scholar] [CrossRef] [PubMed]
- Vickery, K.; Deva, A.; Jacombs, A.; Allan, J.; Valente, P.; Gosbell, I.B. Presence of biofilm containing viable multiresistant organisms despite terminal cleaning on clinical surfaces in an intensive care unit. J. Hosp. Infect. 2012, 80, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Johani, K.; Gosbell, I.B.; Jacombs, A.S.W.; Almatroudi, A.; Whiteley, G.S.; Deva, A.K.; Jensen, S.; Vickery, K. Intensive care unit environmental surfaces are contaminated by multidrug-resistant bacteria in biofilms: Combined results of conventional culture, pyrosequencing, scanning electron microscopy, and confocal laser microscopy. J. Hosp. Infect. 2015, 91, 35–44. [Google Scholar] [CrossRef]
- Ledwoch, K.; Dancer, S.J.; Otter, J.A.; Kerr, K.; Roposte, D.; Rushton, L.; Weiser, R.; Mahenthiralingam, E.; Muir, D.D.; Maillard, J.-Y. Beware biofilm! Dry biofilms containing bacterial pathogens on multiple healthcare surfaces; a multi-centre study. J. Hosp. Infect. 2018, 100, 47–56. [Google Scholar] [CrossRef]
- Piedrahita, C.T.; Cadnum, J.L.; Jencson, A.L.; Shaikh, A.A.; Ghannoum, M.A.; Donskey, C.J. Environmental surfaces in healthcare facilities are a potential source for transmission of Candida auris and other Candida species. Infect. Control Hosp. Epidemiol. 2017, 38, 1107–1109. [Google Scholar] [CrossRef] [PubMed]
- Welsh, R.M.; Bentz, M.L.; Shams, A.; Houston, H.; Lyons, A.; Rose, L.; Litvintseva, A.P. Survival, persistence, and isolation of the emerging multidrug-resistant pathogenic yeast Candida auris on a plastic health care surface. J. Clin. Microbiol. 2017, 55, 2996–3005. [Google Scholar] [CrossRef] [PubMed]
- Vallabhaneni, S.; Kallen, A.; Tsay, S.; Chow, N.; Welsh, R.; Kerins, J.; Kemble, S.K.; Pacilli, M.; Black, S.R.; Landon, E.; et al. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus-United States, May 2013–August 2016. Am. J. Transplant. 2017, 17, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Biswal, M.; Rudramurthy, S.M.; Jain, N.; Shamanth, A.S.; Sharma, D.; Jain, K.; Yaddanapudi, L.N.; Chakrabarti, A. Controlling a possible outbreak of Candida auris infection: Lessons learnt from multiple interventions. J. Hosp. Infect. 2017, 97, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Ku, T.S.N.; Walraven, C.J.; Lee, S.A. Candida auris: Disinfectants and implications for infection control. Front. Microbiol. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Guidance for the Laboratory Investigation, Management and Infection Prevention and Control for Cases of Candida auris (August 2017 v2.0); Public Health England: London, UK, 2016; PHE publications gateway number: 2016122.
- Abdolrasouli, A.; Armstrong-James, D.; Ryan, L.; Schelenz, S. In vitro efficacy of disinfectants utilised for skin decolonization and environmental decontamination during a hospital outbreak with Candida auris. Mycoses 2017, 60, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Cadnum, J.L.; Shaikh, A.A.; Piedrahita, C.T.; Sankar, T.; Jencson, A.L.; Larkin, E.L.; Ghannoum, M.A.; Donskey, C.J. Effectiveness of disinfectants against Candida auris and other Candida species. Infect. Control Hosp. Epidemiol. 2017, 38, 1240–1243. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.; Schelenz, S.; Borman, A.M.; Johnson, E.M.; Brown, C.S. Yeasticidal activity of chemical disinfectants and antiseptics against Candida auris. J. Hosp. Infect. 2017, 97, 371–375. [Google Scholar] [CrossRef]
- Kean, R.; Sherry, L.; Townsend, E.; McKloud, E.; Short, B.; Akinbobola, A.; Mackay, W.G.; Williams, C.; Jones, B.L.; Ramage, G. Surface disinfection challenges for Candida auris: An in vitro study. J. Hosp. Infect. 2018, 98, 433–436. [Google Scholar] [CrossRef]
- ASTM E2967-15 Standard Test Method for Assessing the Ability of Pre-wetted Towelettes to Remove and Transfer Bacterial Contamination on Hard, Non-Porous Environmental Surfaces Using the Wiperator; ASTM: West Conshohocken, PA, USA, 2015.
- Ledwoch, K.; Said, J.; Maillard, J.-Y. Artificial dry-biofilm models for disinfectant efficacy testing. Lett. Appl. Microbiol. 2018. under review. [Google Scholar]
- Maris, P. Modes of action of disinfectants. Rev. Sci. Tech. 1995, 14, 47–55. [Google Scholar] [CrossRef]
- Virto, R.; Mañas, P.; Álvarez, I.; Condon, S.; Raso, J. Membrane damage and microbial inactivation by chlorine in the absence and presence of a chlorine-demanding substrate. Appl. Environ. Microbiol. 2005, 71, 5022–5028. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D. Microbial sensitivity and resistance to chemical and physical agents. In Topley and Wilson’s Microbiology and Microbial Infections, 9th ed.; Edward Arnold: London, UK, 1998; pp. 149–184. [Google Scholar]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [PubMed]
- Estrela, C.; Estrela, C.R.A.; Barbin, E.L.; Spanó, J.C.E.; Marchesan, M.A.; Pécora, J.D. Mechanism of action of sodium hypochlorite. Braz. Dent. J. 2002, 13, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Block, S.S. Disinfection, Sterilization and Preservation, 4th ed.; Lea & Febiger Press: Philadelphia, PA, USA, 1991. [Google Scholar]
- Kitis, M. Disinfection of wastewater with peracetic acid: A review. Environ. Int. 2004, 30, 47–54. [Google Scholar] [CrossRef]
- Tutumi, M.; Imamura, K.; Hatano, S.; Watanabe, T. Antimicrobial action of peracetic acid. J. Food Hyg. Soc. Jpn. 1973, 14, 443–447. [Google Scholar] [CrossRef]
- Sattar, S.A.; Maillard, J.Y. The crucial role of wiping in decontamination of high-touch environmental surfaces: Review of current status and directions for the future. Am. J. Infect. Control 2013, 41, S97–S104. [Google Scholar] [CrossRef]
- Chemical Disinfectants and Antiseptics-Quantitative Test Method for The Evaluation of Bactericidal and Yeasticidal Activity on Non-Porous Surfaces with Mechanical Action Employing Wipes in the Medical Area (4-Field Test); British Standards Institute: London, UK, 2015.
- Wesgate, R.; Robertson, A.; Barrell, M.; Tesca, P.; Maillard, J.-Y. Impact of test protocols and material binding on antimicrobial wipes efficacy. J. Hosp. Infect. 2018, in press. [Google Scholar] [CrossRef]
- Pajkos, A.; Vickery, K.; Cossart, Y. Is biofilm accumulation on endoscope tubing a contributor to the failure of cleaning and decontamination? J. Hosp. Infect. 2004, 58, 224–229. [Google Scholar] [CrossRef]
- Forbes, S.; Cowley, N.; Humphreys, G.; Mistry, H.; Amézquita, A.; McBain, A.J. Formulation of biocides increases antimicrobial potency and mitigates the enrichment of nonsusceptible bacteria in multispecies biofilms. Appl. Environ. Microbiol. 2017, 83, AEM.03054-16. [Google Scholar] [CrossRef]
| Abbreviation | Main Active Ingredient 1 | Excipients (from MSDS) 1 | Concentration of the Main Active Ingredient 4 | pH 5 | Mechanism of Disinfectant Action 6 | Wipe Material |
|---|---|---|---|---|---|---|
| BZK | Benzalkonium chloride, polyhexamethylene biguanide (PHMB) | Didecyl dimethyl ammonium chloride | < 0.5% (<5000 ppm) | 5.41 | Membrane active agents; damage cytoplasmic membrane and increase permeability [23] | Non-Woven Wipe 7 |
| ClO2-1 | Chlorine dioxide | Sodium chlorite, sodium dodecyl sulphate, sodium carbonate, citric acid, sodium dichloroisocyanurate | 300 ppm | 5.05 | Affect membrane permeability of the membrane and inhibits cellular respiration [23] | Microfiber cloth 8 |
| ClO2-2 | Chlorine dioxide | Not mentioned | 1000 ppm | 4.31 | Microfiber cloth 8 | |
| NaDCC-1 | Sodium dichloroisocyanurate | Adipic acid, arylsulfonates, sodium fatty acid sarcosides | 1000 ppm | 6.31 | Permeabilization of the cytoplasmic membrane [24], progressive oxidation of thiol groups to disulphides [25] and deleterious effects on DNA synthesis [26] | Microfiber cloth 8 |
| NaDCC-2 | Sodium dichloroisocyanurate | Adipic acid, sodium toluene sulphonate, sodium n-lauroylsarcosinate | 1000 ppm | 5.93 | Microfiber cloth 8 | |
| NaDCC-3 | Sodium dichloroisocyanurate | Sulfonic acid | 10,000 ppm | 5.77 | Non-woven wipe 9 | |
| NaDCC-4 | Sodium dichloroisocyanurate | Adipic acid, sodium carbonate | 1000 ppm | 5.86 | Microfiber cloth 8 | |
| NaDCC-5 | Sodium dichloroisocyanurate | Adipic acid, sodium toluenesulphonate, sodium N-lauroyl sarcosinate | 1000 ppm | 5.64 | Microfiber cloth 8 | |
| NaOCl-Ref 2 | Sodium hypochlorite | N/A | 1000 ppm | 11.31 | Biosynthetic alterations in cellular metabolism [27], phospholipid degradation, irreversible enzymatic inactivation in bacteria, lipid and fatty acid degradation [28] | Microfiber cloth 8 |
| NaOCl-2 | Sodium hypochlorite | Sodium hydroxide, sodium chloride | 500 ppm | 8.68 | Non-woven wipe 7 | |
| NaOCl-3 | Sodium hypochlorite | phosphoric acid (trisodium salt, dodecahydrate), sodium hydroxide, phosphoric acid | 1000 ppm | 13.13 | Non-woven wipe 7 | |
| PAA-1 | Peracetic acid | sodium percarbonate, citric acid | 3500 ppm | 8.82 | Rupture or dislocation of cell wall, disruption of biochemical processes intercellularly [29] and impairment of DNA replication [30] | Non-woven wipe 9 |
| PAA-2 | Peracetic acid | Not mentioned | 250 ppm | 7.74 | Microfibre cloth 8 | |
| Water 3 | N/A | N/A | N/A | 6.99 | N/A | Microfibre cloth 8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ledwoch, K.; Maillard, J.-Y. Candida auris Dry Surface Biofilm (DSB) for Disinfectant Efficacy Testing. Materials 2019, 12, 18. https://doi.org/10.3390/ma12010018
Ledwoch K, Maillard J-Y. Candida auris Dry Surface Biofilm (DSB) for Disinfectant Efficacy Testing. Materials. 2019; 12(1):18. https://doi.org/10.3390/ma12010018
Chicago/Turabian StyleLedwoch, Katarzyna, and Jean-Yves Maillard. 2019. "Candida auris Dry Surface Biofilm (DSB) for Disinfectant Efficacy Testing" Materials 12, no. 1: 18. https://doi.org/10.3390/ma12010018
APA StyleLedwoch, K., & Maillard, J.-Y. (2019). Candida auris Dry Surface Biofilm (DSB) for Disinfectant Efficacy Testing. Materials, 12(1), 18. https://doi.org/10.3390/ma12010018




