Solid-Gas Phase Photo-Catalytic Behaviour of Rutile and TiOn (1 < n < 2) Sub-Oxide Phases for Self-Cleaning Applications
Abstract
1. Introduction
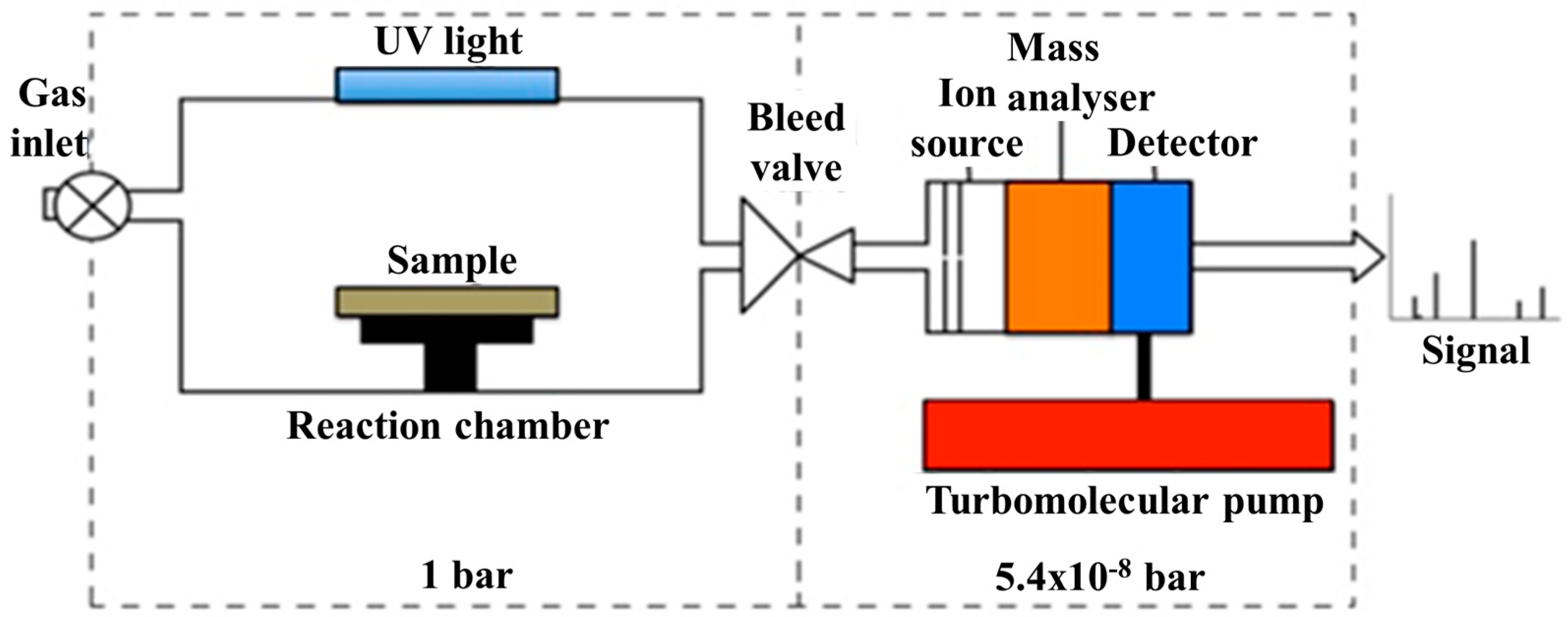
2. Materials and Methods
3. Results and Discussion
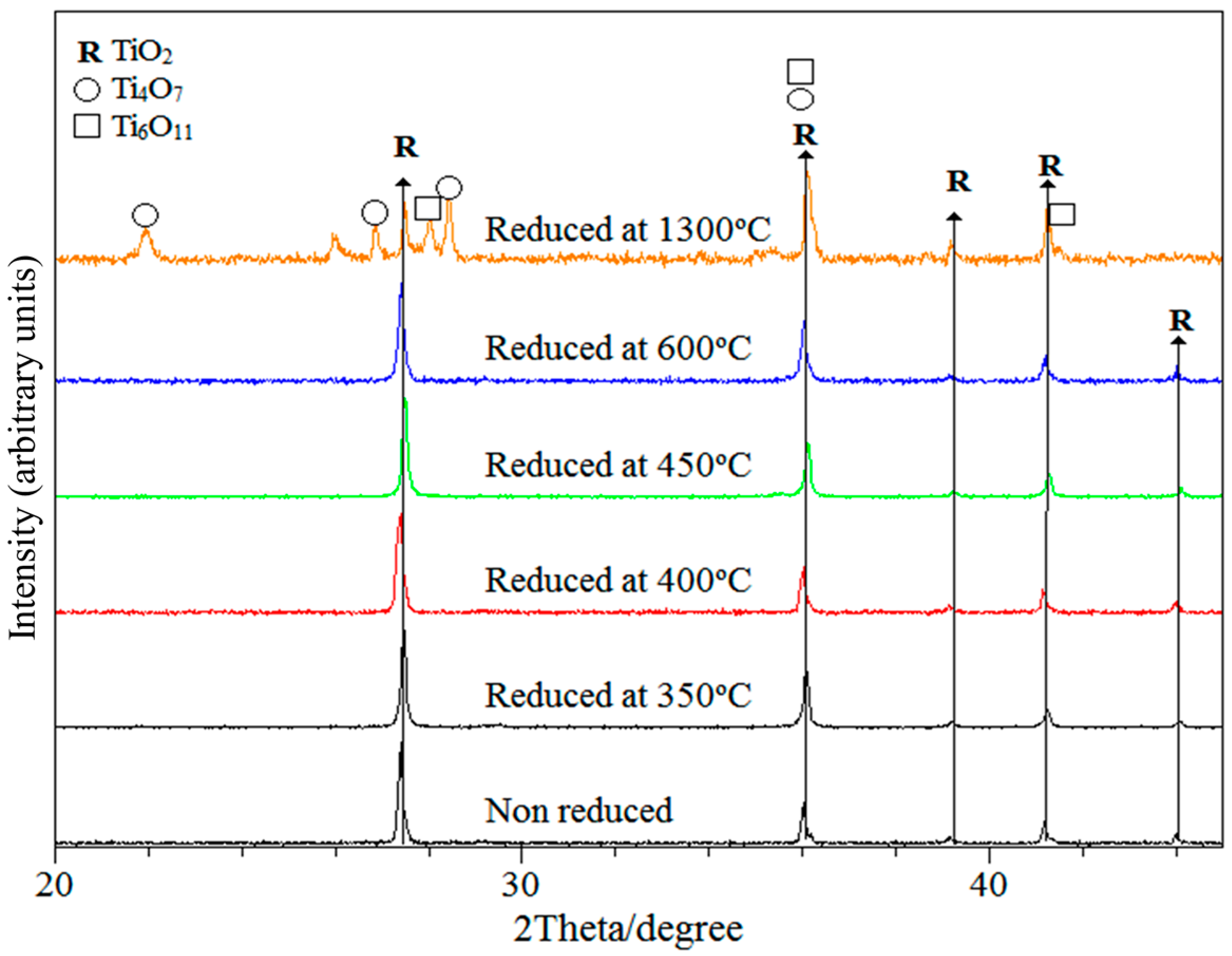
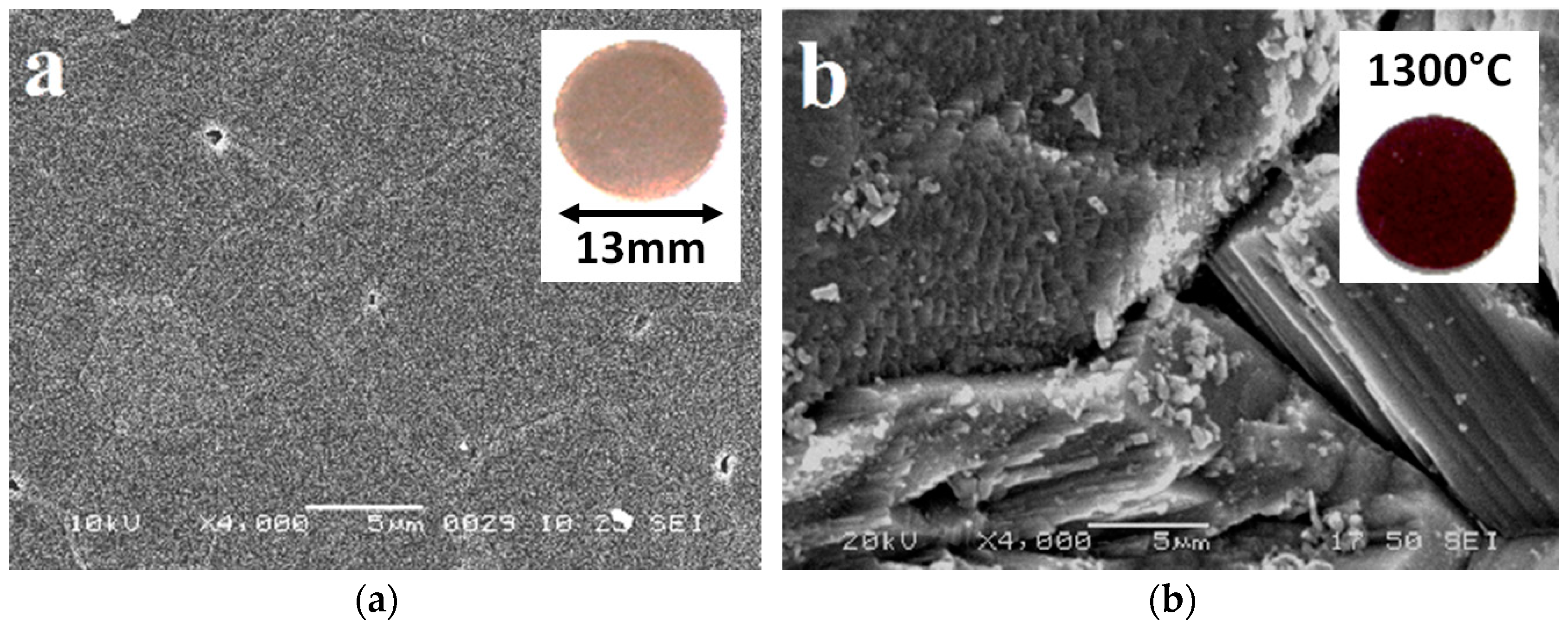
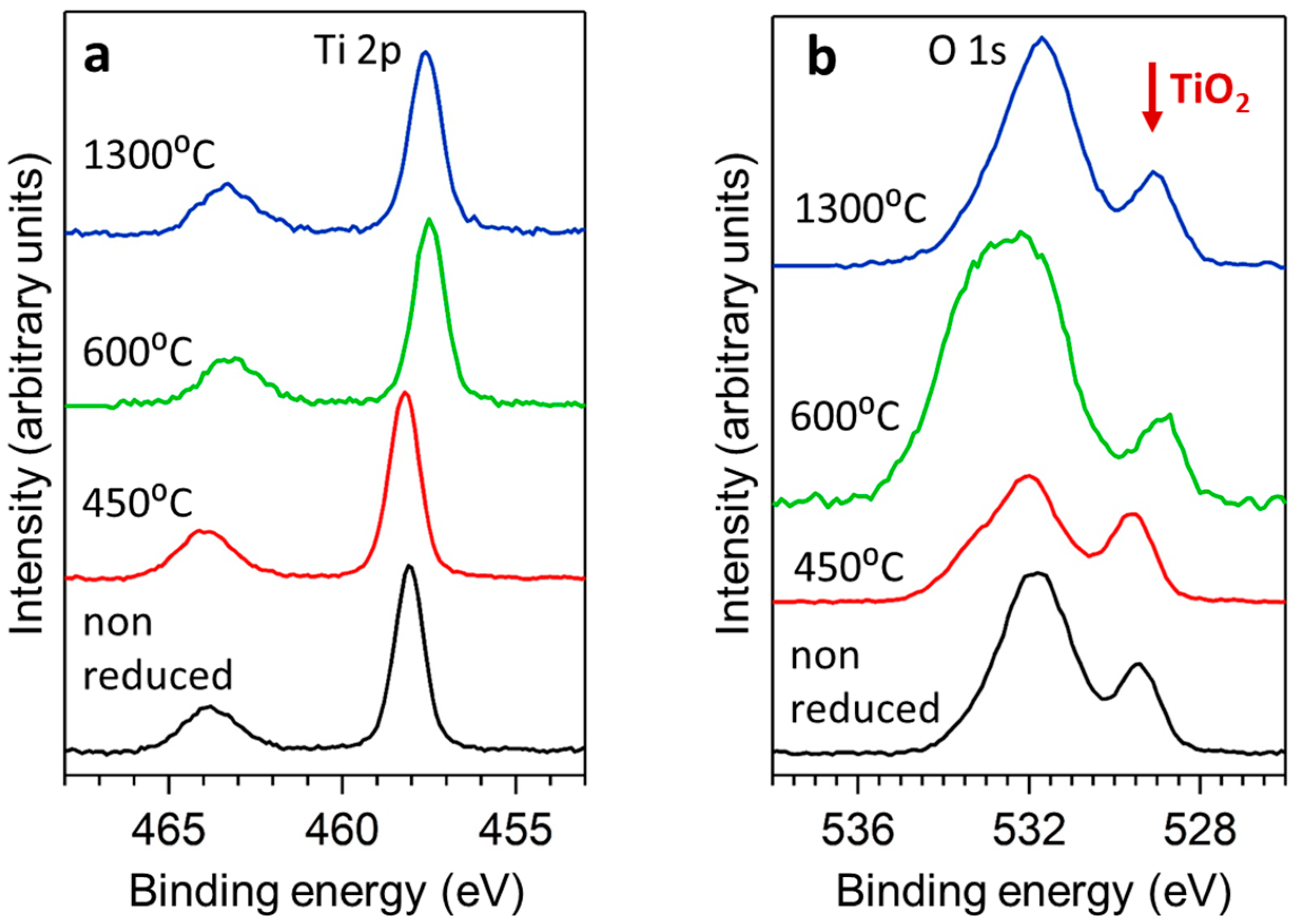
3.1. X-ray Characterisation and Electron Microscopy
3.2. UV-Visible Diffuse Reflectance Spectroscopy
3.3. Electrical Characterization
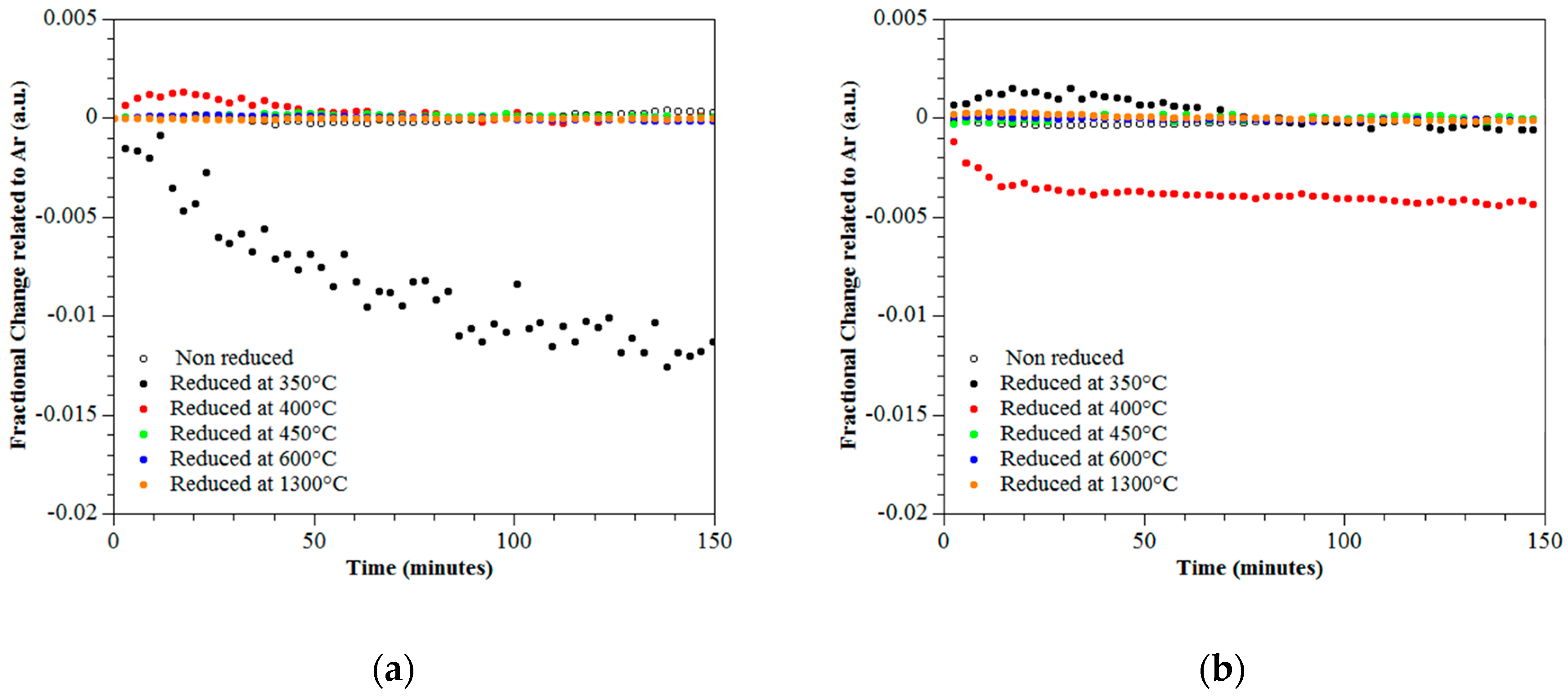
3.4. Photo-Catalytic Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Meinhold, G. Rutile and its applications in earth sciences. Earth-Sci. Rev. 2010, 102, 1–28. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Thermodynamic analysis of phase stability of nanocrystalline titania. J. Mater. Chem. 1998, 8, 2073–2076. [Google Scholar] [CrossRef]
- Giampiccolo, A.; Ansell, M.P.; Tobaldi, D.M.; Ball, R.J. Synthesis of Co–TiO2 nanostructured photocatalytic coatings for MDF substrates. Green Mater. 2016, 4, 140–149. [Google Scholar] [CrossRef]
- Nuño, M.; Ball, R.J.; Bowen, C.R.; Kurchania, R.; Sharma, G.D. Photocatalytic activity of electrophoretically deposited (EPD) TiO2 coatings. J. Mater. Sci. 2015, 50, 4822–4835. [Google Scholar] [CrossRef]
- Giampiccolo, A.; Tobaldi, D.M.; Leonardi, S.G.; Murdoch, B.J.; Seabra, M.P.; Ansell, M.P.; Neri, G.; Ball, R.J. Sol gel graphene/TiO2 nanoparticles for the photocatalytic-assisted sensing and abatement of NO2. Appl. Catal. B Environ. 2019, 243, 83–194. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Seabra, M.P.; Otero-Irurueta, G.; De Miguel, Y.R.; Ball, R.J.; Singh, M.K.; Pullar, R.C.; Labrincha, J.A. Quantitative XRD characterisation and gas-phase photocatalytic activity testing for visible-light (indoor applications) of KRONOClean 7000®. RSC Adv. 2015, 5, 102911–102918. [Google Scholar] [CrossRef]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.E.; Cab, C.; de Coss, R.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Nuño, M.; Ball, R.J.; Bowen, C.R. Study of solid/gas phase photocatalytic reactions by electron ionization mass spectrometry. J. Mass Spectrom. 2014, 49, 716–726. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Liu, C.; Chen, J.; Li, G.; An, T.; Wong, P.K.; Zhao, H. UV and visible light photoelectrocatalytic bactericidal performance of 100% {111} faceted rutile TiO2 photoanode. Catal. Today 2014, 224, 77–82. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X. Titanium dioxide photocatalysis: Present situation and future approaches. Comptes Rendus Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Wei, S.; Peng, B.; Chai, L.; Liu, Y.; Li, Z. Preparation of doping titania antibacterial powder by ultrasonic spray pyrolysis. Trans. Nonferrous Met. Soc. China 2008, 18, 1145–1150. [Google Scholar] [CrossRef]
- Nishikiori, H.; Fukasawa, Y.; Yokosuka, Y.; Fujii, T. Nitrogen doping into titanium dioxide by the sol-gel method using nitric acid. Res. Chem. Intermed. 2011, 37, 869–881. [Google Scholar] [CrossRef]
- MacFarlane, J.W.; Scott, T.B. Reduction of carbon dioxide on jet spray formed titanium dioxide surfaces. J. Hazard. Mater. 2012, 211–212, 247–254. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wen, L.; Wang, D.; Xue, Y.; Lu, Q.; Wu, C.; Chen, J.; Song, S. Photocatalytic reduction of CO2 in aqueous solution on surface-fluorinated anatase TiO2 nanosheets with exposed {001} facets. Energy Fuels 2014, 28, 3982–3993. [Google Scholar] [CrossRef]
- Inoue, H.; Matsuyama, T.; Liu, B.J.; Sakata, T.; Mori, H.; Yoneyama, H. Photocatalytic Activities for Carbon Dioxide Reduction of TiO2 Microcrystals Prepared in SiO2 Matrices Using a Sol–Gel Method. Chem. Lett. 1994, 23, 653–656. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Tripodi, E.B.A.; Villa, A.; Pirola, C.; Prati, L.; Ramis, G.; Rossetti, I. High Pressure Photoreduction of CO2: Effect of Catalyst Formulation, Hole Scavenger Addition and Operating Conditions. Catalysts 2018, 8, 430. [Google Scholar]
- Taniguchi, Y.; Yoneyama, H.; Tamura, H. Photoelectrochemical Reduction of Carbon Dioxide at p-Type Gallium Phosphide Electrodes in the Presence of Crown Ether. Bull. Chem. Soc. Jpn. 1982, 55, 2034–2039. [Google Scholar] [CrossRef]
- Aurian-Blajeni, B.; Halmann, M.; Manassen, J. Photoreduction of carbon dioxide and water into formaldehyde and methanol on semiconductor materials. Sol. Energy 1980, 25, 165–170. [Google Scholar] [CrossRef]
- Jaeger, C.D.; Bard, A.J. Spin trapping and electron spin resonance detection of radical intermediates in the photodecomposition of water at titanium dioxide particulate systems. J. Phys. Chem. 1979, 83, 3146–3152. [Google Scholar] [CrossRef]
- Anpo, M.; Yamashita, H.; Ichihashi, Y.; Ehara, S. Photocatalytic reduction of CO2 with H2O on various titanium oxide catalysts. J. Electroanal. Chem. 1995, 396, 21–26. [Google Scholar] [CrossRef]
- Anpo, M.; Yamashita, H.; Ikeue, K.; Fujii, Y.; Zhang, S.G.; Ichihashi, Y.; Park, D.R.; Suzuki, Y.; Koyano, K.; Tatsumi, T. Photocatalytic reduction of CO2 with H2O on Ti-MCM-41 and Ti-MCM-48 mesoporous zeolite catalysts. Catal. Today 1998, 44, 327–332. [Google Scholar] [CrossRef]
- Ohno, T.; Tsubota, T.; Nishijima, K.; Miyamoto, Z. Degradation of Methylene Blue on Carbonate Species-doped TiO 2 Photocatalysts under Visible Light. Chem. Lett. 2004, 33, 750–751. [Google Scholar] [CrossRef]
- Mizukoshi, Y.; Ohtsu, N.; Semboshi, S.; Masahashi, N. Visible light responses of sulfur-doped rutile titanium dioxide photocatalysts fabricated by anodic oxidation. Appl. Catal. B Environ. 2009, 91, 152–156. [Google Scholar] [CrossRef]
- Ren, Y.; Li, J.; Yu, J. Enhanced electrochemical performance of TiO2 by Ti3+ doping using a facile solvothermal method as anode materials for lithium-ion batteries. Electrochim. Acta 2014, 138, 41–47. [Google Scholar] [CrossRef]
- Saputera, W.H.; Mul, G.; Hamdy, M.S. Ti3+-containing titania: Synthesis tactics and photocatalytic performance. Catal. Today 2015, 246, 60–66. [Google Scholar] [CrossRef]
- Nuño, M.; Pesce, G.L.; Bowen, C.R.; Xenophontos, P.; Ball, R.J. Environmental performance of nano-structured Ca(OH)2/TiO2 photocatalytic coatings for buildings. Build. Environ. 2015, 92, 734–742. [Google Scholar] [CrossRef]
- Xing, M.; Fang, W.; Nasir, M.; Ma, Y.; Zhang, J.; Anpo, M. Self-doped Ti3+-enhanced TiO2 nanoparticles with a high-performance photocatalysis. J. Catal. 2013, 297, 236–243. [Google Scholar] [CrossRef]
- Riyapan, S.; Boonyongmaneerat, Y.; Mekasuwandumrong, O.; Praserthdam, P.; Panpranot, J. Effect of surface Ti3+ on the sol–gel derived TiO2 in the selective acetylene hydrogenation on Pd/TiO2 catalysts. Catal. Today 2015, 245, 134–138. [Google Scholar] [CrossRef]
- Adamaki, V.; Clemens, F.; Ragulis, P.; Pennock, S.R.; Taylor, J.; Bowen, C.R. Manufacturing and characterization of Magnéli phase conductive fibres. J. Mater. Chem. A 2014, 2, 8328–8333. [Google Scholar] [CrossRef]
- Zhu, Q.; Peng, Y.; Lin, L.; Fan, C.M.; Gao, G.Q.; Wang, R.X.; Xu, A.W. Stable blue TiO2−x nanoparticles for efficient visible light photocatalysts. J. Mater. Chem. A 2014, 2, 4429–4437. [Google Scholar] [CrossRef]
- Sangawar, S.R.; Agarwal, J.P.; Sarwade, D.B. A comprehensive study on some binders for piezo-electric ceramics. Indian J. Eng. Mater. Sci. 2001, 8, 26–35. [Google Scholar]
- Bradley, F. The Relation of Ceramics Processing to Materials Properties for Electronic Applications. IEEE Trans. Parts Hybrids Packag. 1972, 8, 15–23. [Google Scholar] [CrossRef]
- Rahaman, M.N. Ceramic Processing; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Kalita, S.J.; Qiu, S.; Verma, S. A quantitative study of the calcination and sintering of nanocrystalline titanium dioxide and its flexural strength properties. Mater. Chem. Phys. 2008, 109, 392–398. [Google Scholar] [CrossRef]
- Walsh, F.C.; Wills, R.G.A. The continuing development of Magnéli phase titanium sub-oxides and Ebonex® electrodes. Electrochim. Acta 2010, 55, 6342–6351. [Google Scholar] [CrossRef]
- Hayfield, P.C.S. Development of a New Material; Royal Society of Chemistry: Cambridge, UK, 2007. [Google Scholar]
- Wang, W.E.; Kim, Y.S. Thermodynamic evaluation of the titanium-oxygen system from O/Ti = 0 to 3/2. J. Nucl. Mater. 1999, 270, 242–247. [Google Scholar] [CrossRef]
- Surendran, K.P.; Bijumon, P.V.; Mohanan, P.; Sebastian, M.T. (1 − x)MgAl2O4−xTiO2 dielectrics for microwave and millimeter wave applications. Appl. Phys. A 2005, 81, 823–826. [Google Scholar] [CrossRef]
- Adamaki, V. Manufacturing and Characterisation of Ti-Suboxides for Sensing and Energy Applications. Ph.D. Thesis, University of Bath, Bath, UK, 2015. [Google Scholar]
- López, R.; Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol–gel and commercial TiO2: A comparative study. J. Sol-Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- McClure, D.S. Mineralogical applications of crystal field theory by R. G. Burns. J. Appl. Crystallogr. 1970, 3, 550. [Google Scholar] [CrossRef]
- Nuño, M. Photocatalytic Coatings in the Built Environment. Ph.D. Thesis, University of Bath, Bath, UK, 2015. [Google Scholar]
- Kang, Q.; Cao, J.; Zhang, Y.; Liu, L.; Xu, H.; Ye, J. Reduced TiO2 nanotube arrays for photoelectrochemical water splitting. J. Mater. Chem. A 2013, 1, 5766–5774. [Google Scholar] [CrossRef]
- Almond, D.P.; Vainas, B. The dielectric properties of random R-C networks as an explanation of the ‘universal’ power law dielectric response of solids. J. Phys. Condens. Matter 1999, 11, 9081–9093. [Google Scholar] [CrossRef]
- Almond, D.P.; Bowen, C.R. Anomalous Power Law Dispersions in ac Conductivity and Permittivity Shown to be Characteristics of Microstructural Electrical Networks. Phys. Rev. Lett. 2004, 92, 157601. [Google Scholar] [CrossRef]
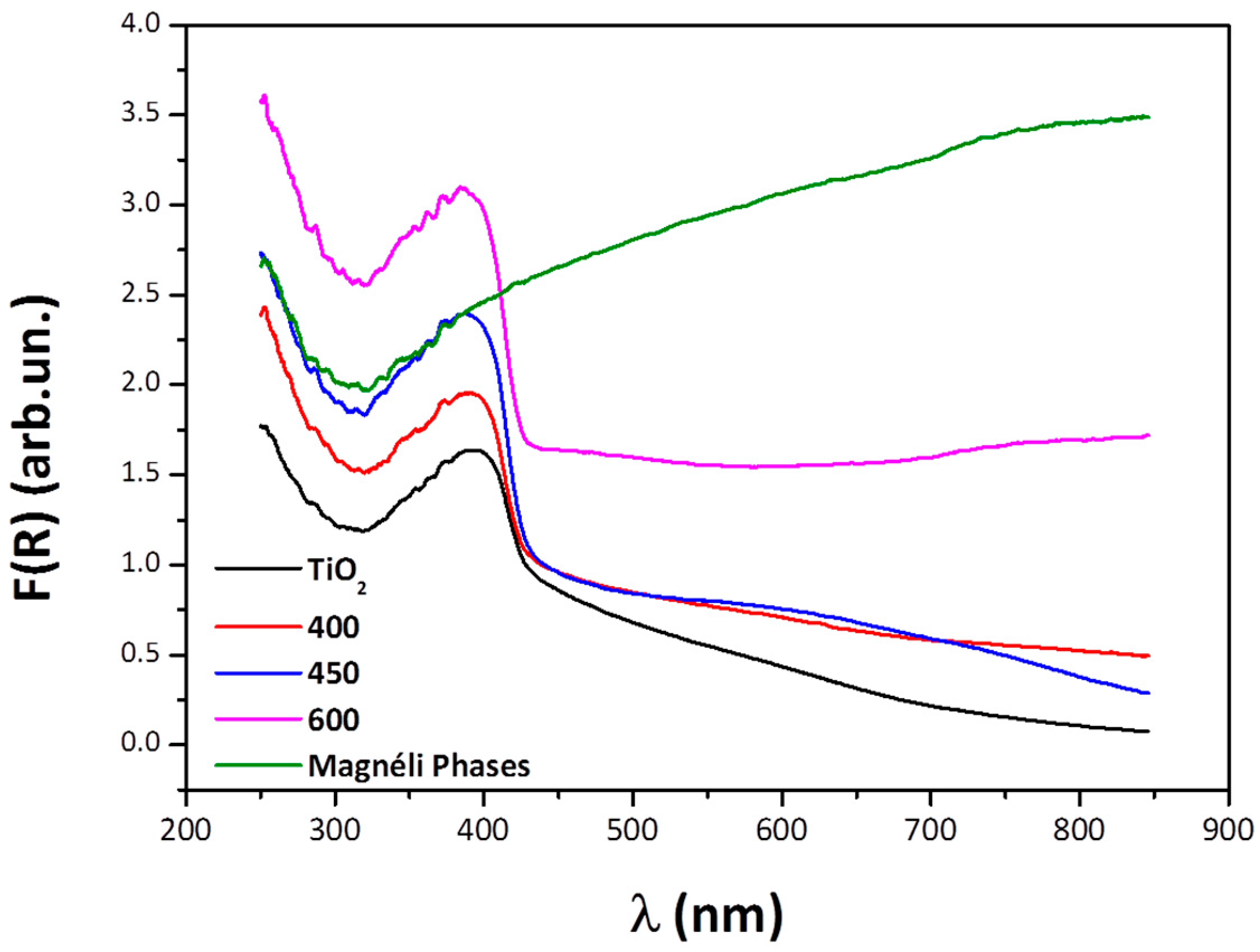
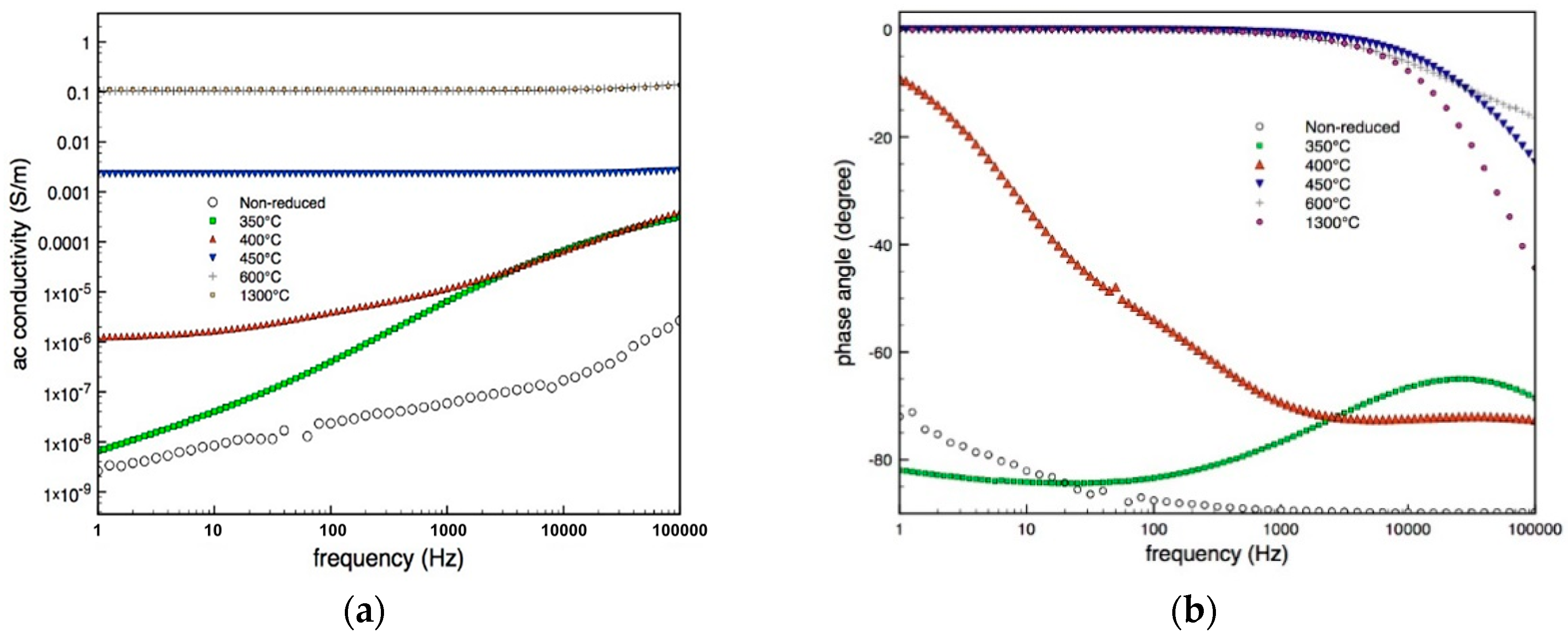
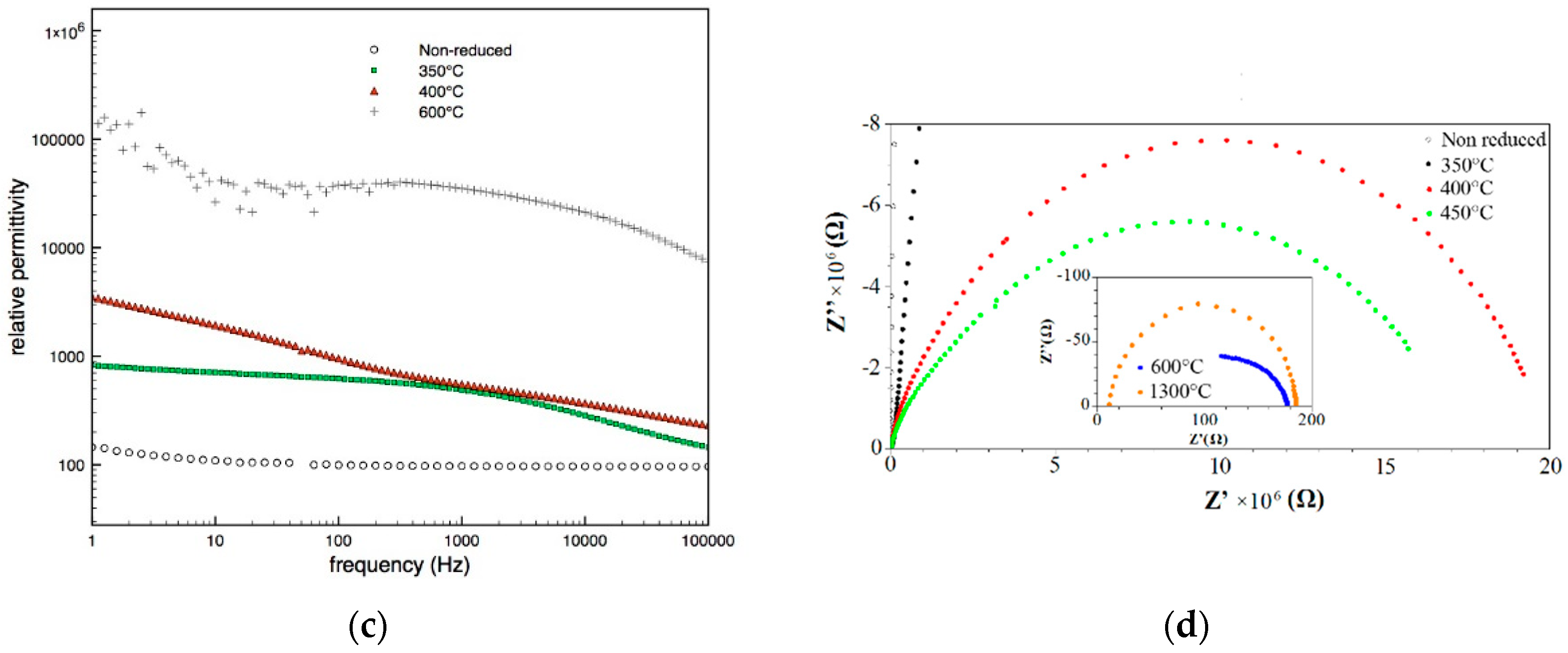
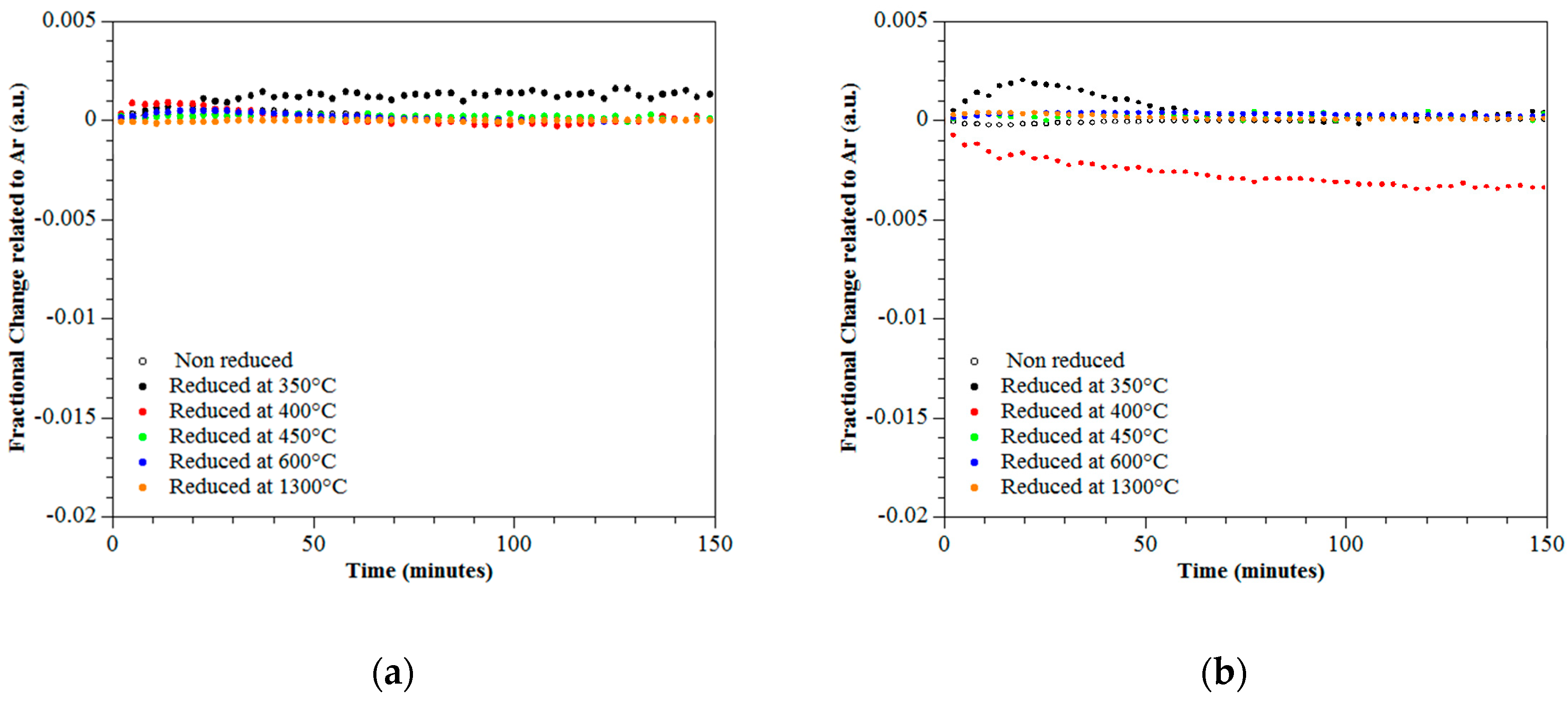
| Specimen-Reduction Temp | Band Gap (eV) | Rietveld Refinement for Oxygen Vacancies |
|---|---|---|
| Rutile TiO2 | 3.03 | 1 |
| TiOn-350 °C | 3.04 | 1 |
| TiOn-400 °C | 3.06 | 1 |
| TiOn-450 °C | 3.04 | 1 |
| TiOn-600 °C | 3.05 | 0.95 |
| TiOn-1300 °C | Overlapped bands | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuño, M.; Adamaki, V.; Tobaldi, D.M.; Hortigüela Gallo, M.J.; Otero-Irurueta, G.; Bowen, C.R.; Ball, R.J. Solid-Gas Phase Photo-Catalytic Behaviour of Rutile and TiOn (1 < n < 2) Sub-Oxide Phases for Self-Cleaning Applications. Materials 2019, 12, 170. https://doi.org/10.3390/ma12010170
Nuño M, Adamaki V, Tobaldi DM, Hortigüela Gallo MJ, Otero-Irurueta G, Bowen CR, Ball RJ. Solid-Gas Phase Photo-Catalytic Behaviour of Rutile and TiOn (1 < n < 2) Sub-Oxide Phases for Self-Cleaning Applications. Materials. 2019; 12(1):170. https://doi.org/10.3390/ma12010170
Chicago/Turabian StyleNuño, Manuel, Vaia Adamaki, David M. Tobaldi, Maria J. Hortigüela Gallo, Gonzalo Otero-Irurueta, Chris R. Bowen, and Richard J. Ball. 2019. "Solid-Gas Phase Photo-Catalytic Behaviour of Rutile and TiOn (1 < n < 2) Sub-Oxide Phases for Self-Cleaning Applications" Materials 12, no. 1: 170. https://doi.org/10.3390/ma12010170
APA StyleNuño, M., Adamaki, V., Tobaldi, D. M., Hortigüela Gallo, M. J., Otero-Irurueta, G., Bowen, C. R., & Ball, R. J. (2019). Solid-Gas Phase Photo-Catalytic Behaviour of Rutile and TiOn (1 < n < 2) Sub-Oxide Phases for Self-Cleaning Applications. Materials, 12(1), 170. https://doi.org/10.3390/ma12010170










