Low Temperature Solution-Processable 3D-Patterned Charge Recombination Layer for Organic Tandem Solar Cells
Abstract
1. Introduction
2. Experimental Section
2.1. Preparation of Solutions
2.2. Electron-Only Device
2.3. Organic Solar Cell Fabrication and Characterization
3. Results and Discussion
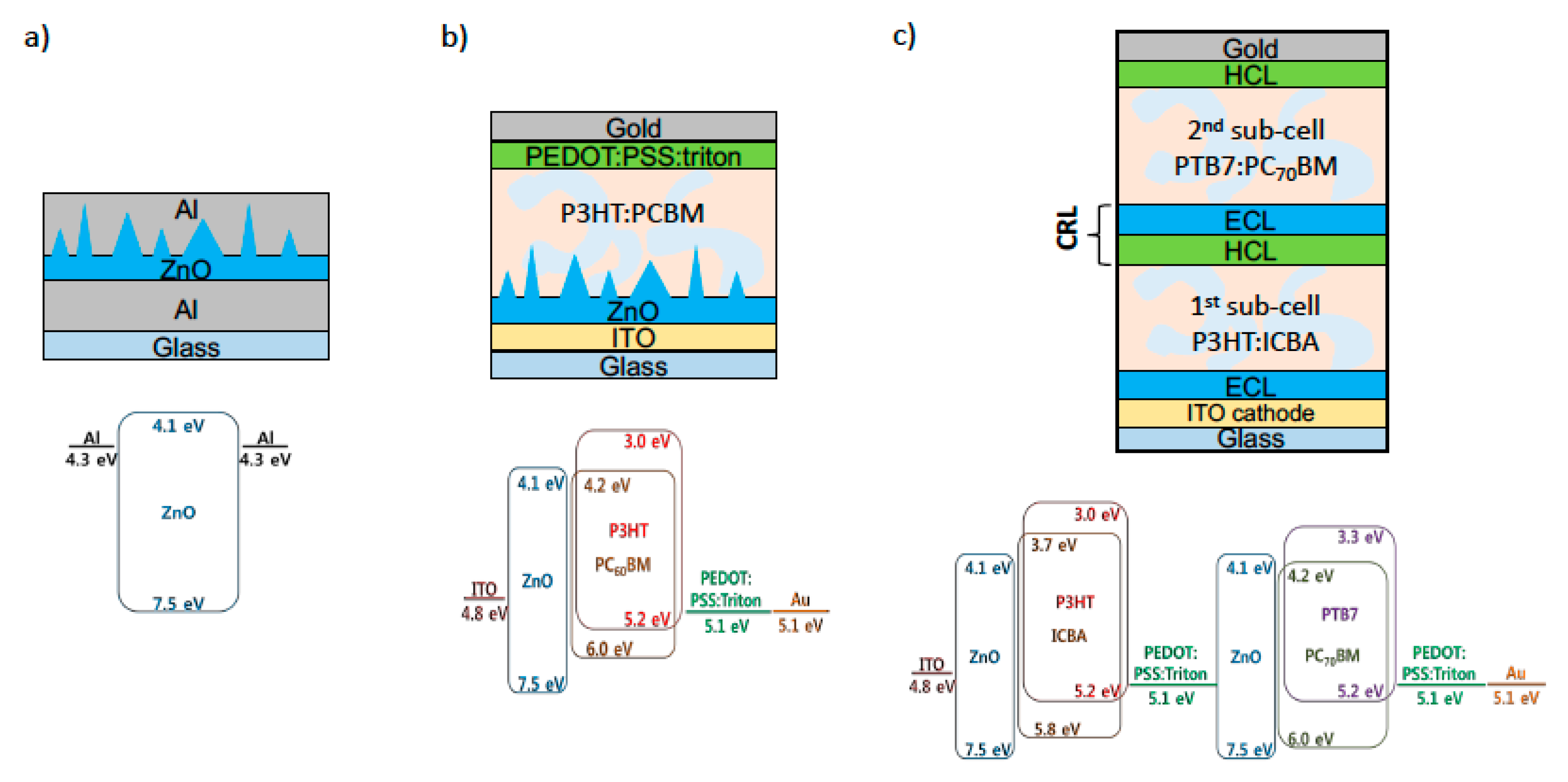
3.1. ZnO Electron Collection Layer
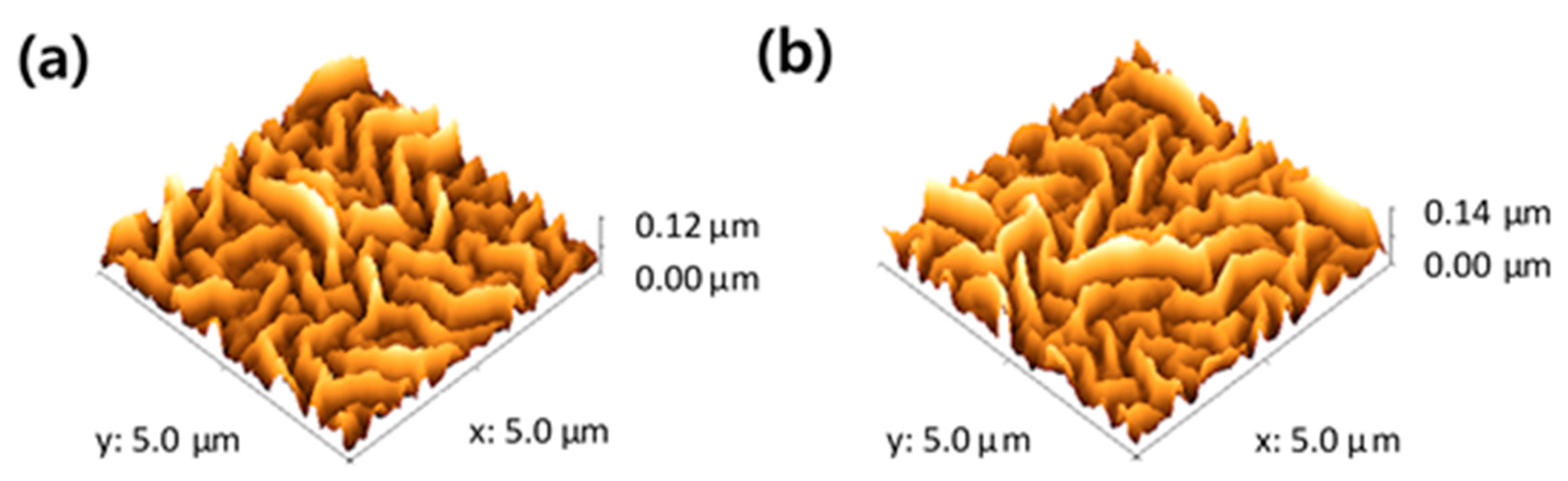
3.2. ZnO Ripple Pattern
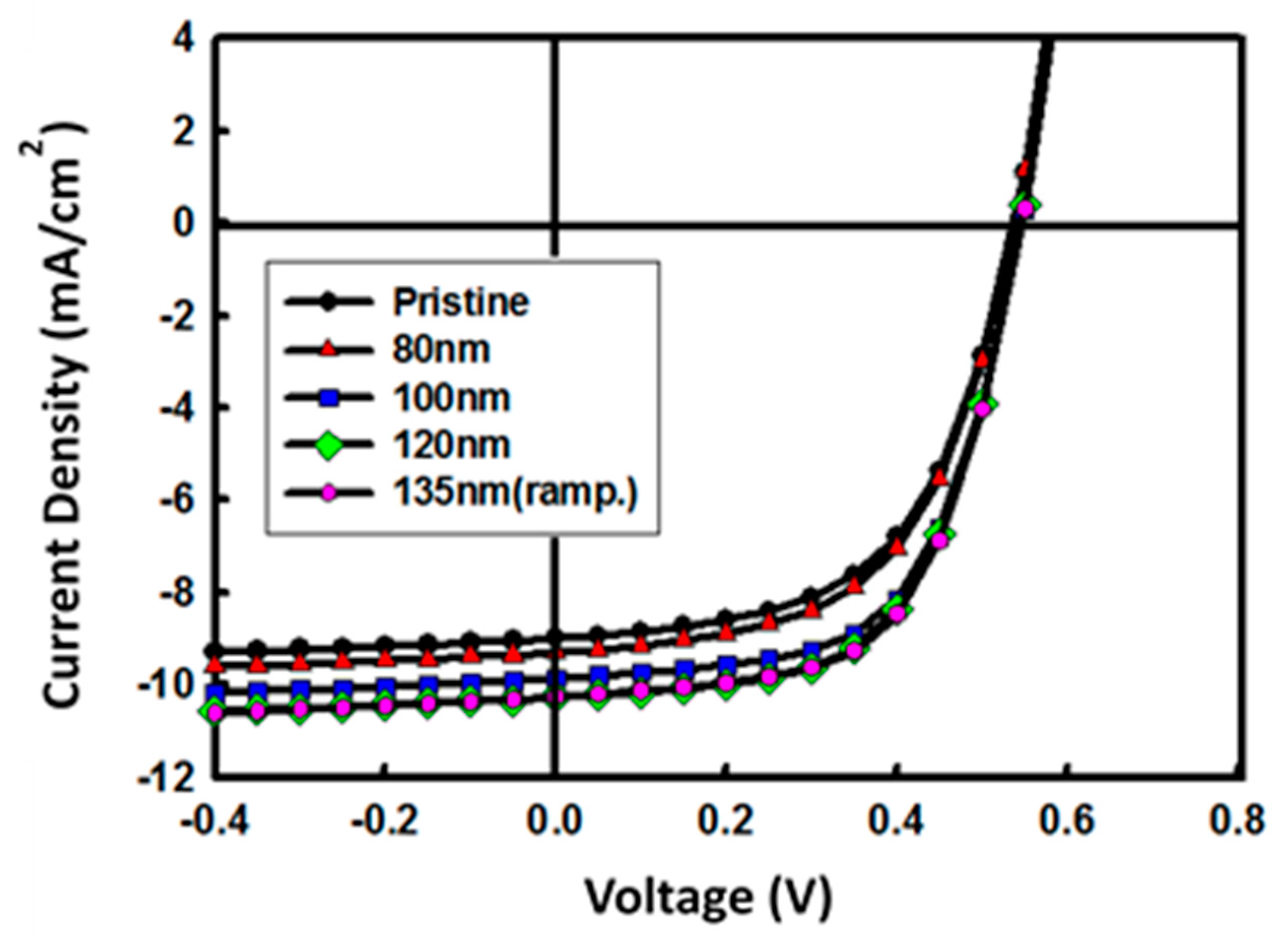
3.3. Single Junction Solar Cell with A 3D Nano-Patterned ZnO Layer
3.4. Tandem Solar Cell with Low-Temperature Processed ZnO
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- BP. BP Statistical Review of World Energy; British Petroleum Company: London, UK, 2014; pp. 1–48. [Google Scholar]
- El Chaar, L.; Lamont, L.A.; El Zein, N. Review of Photovoltaic Technologies. Renew. Sustain. Energy Rev. 2011, 15, 2165–2175. [Google Scholar] [CrossRef]
- Becquerel, E. Mémoire sur les effets électriques produits sous l’influence des rayons solaires. C. R. Acad. Sci. Paris 1839, 9, 561–567. [Google Scholar]
- Chapin, D.M.; Fuller, C.S.; Pearson, G.L. A New Silicon p-n Junction Photocell for Converting Solar Radiation into Electrical Power. J. Appl. Phys. 1954, 25, 676–677. [Google Scholar] [CrossRef]
- Hoppe, H.; Sariciftci, N.S. Organic solar cells: An overview. J. Mater. Res. 2011, 19, 1924–1945. [Google Scholar] [CrossRef]
- Wohrle, D.; Meissner, D. Organic Solar Cells. Adv. Mater. 1991, 3, 129–138. [Google Scholar] [CrossRef]
- Gregg, B.A. Excitonic solar cells. J. Phys. Chem. B 2003, 107, 4688–4698. [Google Scholar] [CrossRef]
- Available online: https://www.nrel.gov/pv/assets/pdfs/pv-efficiencies-07-17-2018.pdf (accessed on 7 December 2018).
- Cheyns, D.; Rand, B.P.; Heremans, P. Organic tandem solar cells with complementary absorbing layers and a high open-circuit voltage. Appl. Phys. Lett. 2010, 97, 033301. [Google Scholar] [CrossRef]
- Dennler, G.; Prall, H.J.; Koeppe, R.; Egginger, M.; Autengruber, R.; Sariciftci, N.S. Enhanced spectral coverage in tandem organic solar cells. Appl. Phys. Lett. 2006, 89, 073502. [Google Scholar] [CrossRef]
- Dou, L.; You, J.; Yang, J.; Chen, C.C.; He, Y.; Murase, S.; Moriarty Emery, K.; Li, G.; Yang, Y. Tandem polymer solar cells featuring a spectrally matched low-bandgap polymer. Nat. Photonics 2012, 6, 180–185. [Google Scholar] [CrossRef]
- Zhou, Y.; Fuentes-Hernandez, C.; Shim, J.W.; Khan, T.M.; Kippelen, B. High performance polymeric charge recombination layer for organic tandem solar cells. Energy Environ. Sci. 2012, 5, 9827–9832. [Google Scholar] [CrossRef]
- Janssen, A.G.F.; Riedl, T.; Hamwi, S.; Johannes, H.H.; Kowalsky, W. Highly efficient organic tandem solar cells using an improved connecting architecture. Appl. Phys. Lett. 2007, 91, 073519. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, K.; Coates, N.E.; Moses, D.; Nguyen, T.Q.; Dante, M.; Heeger, A.J. Efficient tandem polymer solar cells fabricated by all-solution processing. Science 2007, 317, 222–225. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Dou, L.; Yoshimura, K.; Kato, T.; Ohya, K.; Moriarty, T.; Emery, K.; Chen, C.C.; Gao, J.; Li, G.; et al. A polymer tandem solar cell with 10.6% power conversion efficiency. Nat. Commun. 2013, 4, 1446–1456. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, C.H.; Lee, S.K.; Jeong, J.H.; Lee, J.; Jin, S.H.; Shin, W.S.; Song, C.E.; Choi, J.H.; Jeong, J.R. Highly efficient uniform ZnO nanostructures for an electron transport layer of inverted organic solar cells. Chem. Commun. 2013, 49, 6033–6035. [Google Scholar] [CrossRef] [PubMed]
- Bouclé, J.; Snaith, H.J.; Greenham, N.C. Simple approach to hybrid polymer/porous metal oxide solar cells from solution-processed ZnO nanocrystals. J. Phys. Chem. C 2010, 114, 3664–3674. [Google Scholar] [CrossRef]
- Zhao, D.; Peng, T.; Lu, L.; Cai, P.; Jiang, P.; Bian, Z. Effect of Annealing temperature on the photoelectrochemical properties of dye-sensitized solar cells made with mesoporous TiO2 nanoparticles. J. Phys. Chem. C 2008, 112, 8486–8494. [Google Scholar] [CrossRef]
- Takanezawa, K.; Tajima, K.; Hashimoto, K. Efficiency enhancement of polymer photovoltaic devices hybridized with ZnO nanorod arrays by the introduction of a vanadium oxide buffer layer. Appl. Phys. Lett. 2008, 93, 063308. [Google Scholar] [CrossRef]
- Yang, T.; Sun, K.; Liu, X.; Wei, W.; Yu, T.; Gong, X.; Wang, D.; Cao, Y. Zinc oxide nanowire as an electron-extraction layer for broadband polymer photodetectors with an inverted device structure. J. Phys. Chem. C 2012, 116, 13650–13653. [Google Scholar] [CrossRef]
- Baek, W.H.; Seo, I.L.; Yoon, T.S.; Lee, H.H.; Yun, C.M.; Kim, Y.S. Hybrid inverted bulk heterojunction solar cells with nanoimprinted TiO2 nanopores. Sol. Energy Mater. Sol. Cells 2009, 93, 1587–1591. [Google Scholar] [CrossRef]
- Sekine, N.; Chou, C.H.; Kwan, W.L.; Yang, Y. ZnO nano-ridge structure and its application in inverted polymer solar cell. Org. Electron. 2009, 10, 1473–1477. [Google Scholar] [CrossRef]
- Kim, K.D.; Lim, D.C.; Hu, J.; Kwon, J.D.; Jeong, M.G.; Seo, H.O.; Lee, J.Y.; Lim, K.H.; Lee, K.H.; Jeong, Y.; et al. Surface modification of a ZnO electron-collecting layer using atomic layer deposition to fabricate high-performing inverted organic photovoltaics. ACS Appl. Mater. Interfaces 2013, 5, 8718–8723. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.C.; Shim, H.H.; Kim, K.D.; Seo, H.O.; Lim, J.H.; Jeong, Y.; Kim, Y.D.; Lee, K.H. Spontaneous formation of nanoripples on the surface of ZnO thin films as hole-blocking layer of inverted organic solar cells. Sol. Energy Mater. Sol. Cells 2011, 95, 3036–3040. [Google Scholar] [CrossRef]
- Liao, S.H.; Jhuo, H.J.; Cheng, Y.S.; Chen, S.A. Fullerene derivative-doped zinc oxide nanofilm as the cathode of inverted polymer solar cells with low-bandgap polymer (PTB7-Th) for high performance. Adv. Mater. 2013, 25, 4766–4771. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Tai, W.P. Electrical and optical properties of Al-doped ZnO thin films by sol-gel process. Appl. Surf. Sci. 2007, 253, 4911–4916. [Google Scholar] [CrossRef]
- Pita, K.; Baudin, P.; Vu, Q.V.; Aad, R.; Couteau, C.; Lerondel, G. Annealing temperature and environment effects on ZnO nanocrystals embedded in SiO. Nanoscale Res. Lett. 2013, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.C.; You, H.C.; Meena, J.S.; Shieh, S.H.; Shao, C.Y.; Chang, F.C.; Ko, F.H. Facile electroless deposition of zinc oxide ultrathin film for zinc acetate solution-processed transistors. Int. J. Electrochem. Sci. 2012, 7, 5977–5989. [Google Scholar]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Noh, Y.J.; Na, S.L.; Kim, S.S. Inverted polymer solar cells including ZnO electron transport layer fabricated by facile spray pyrolysis. Sol. Energy Mater. Sol. Cells 2013, 117, 139–144. [Google Scholar] [CrossRef]
- Rabani, E.; Reichman, D.R.; Geissler, P.L.; Brus, L.E. Drying-mediated self-assembly of nanoparticles. Nature 2003, 426, 271–274. [Google Scholar] [CrossRef]
- Kim, Y.S.; Tai, W.P.; Shu, S.J. Effect of preheating temperature on structural and optical properties of ZnO thin films by sol-gel process. Thin Solid Films 2005, 491, 153–160. [Google Scholar] [CrossRef]
- Kyaw, A.K.K.; Wang, D.H.; Wynands, D.; Zhang, J.; Nguyen, T.Q.; Bazan, G.C.; Heeger, A.J. Improved light harvesting and improved efficiency by insertion of an optical spacer (ZnO) in solution-processed small-molecule solar cells. Nano Lett. 2013, 13, 3796–3801. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Lan, S.; Sun, J.Y.; Lin, C.F. Influence of mixed solvent on the morphology of the P3HT:Indene-C60 bisadduct (ICBA) blend film and the performance of inverted polymer solar cells. Org. Electron. 2013, 14, 26–31. [Google Scholar] [CrossRef]
- Cheng, F.; Fang, G.; Fan, X.; Huang, H.; Zheng, Q.; Qin, P.; Lei, H.; Li, Y. Enhancing the performance of P3HTICBA based polymer solar cells using LiF as electron collecting buffer layer and UV-ozone treated MoO3 as hole collecting buffer layer. Sol. Energy Mater. Sol. Cells 2013, 110, 63–68. [Google Scholar] [CrossRef]
- Yeh, P.N.; Jen, T.H.; Cheng, Y.S.; Chen, S.A. Large active area inverted tandem polymer solar cell with high performance via insertion of subnano-scale silver layer. Sol. Energy Mater. Sol. Cells 2014, 120, 728–734. [Google Scholar] [CrossRef]
- Li, G.; Yao, Y.; Yang, H.; Shrotriya, V.; Yang, G.; Yang, Y. “Solvent Annealing” effect in polymer solar cells based on poly(3-hexylthiophene) and methanofullerenes. Adv. Funct. Mater. 2007, 17, 1636–1644. [Google Scholar] [CrossRef]
- Wang, J.C.; Ren, X.C.; Shi, S.Q.; Leung, C.W.; Chan, P.K.L. Charge accumulation induced S-shape J-V curves in bilayer heterojunction organic solar cells. Org. Electron. 2011, 12, 880–885. [Google Scholar] [CrossRef]
- Liu, F.; Nunzi, J.M. Air stable hybrid inverted tandem solar cell design. Appl. Phys. Lett. 2011, 99, 063301. [Google Scholar] [CrossRef]
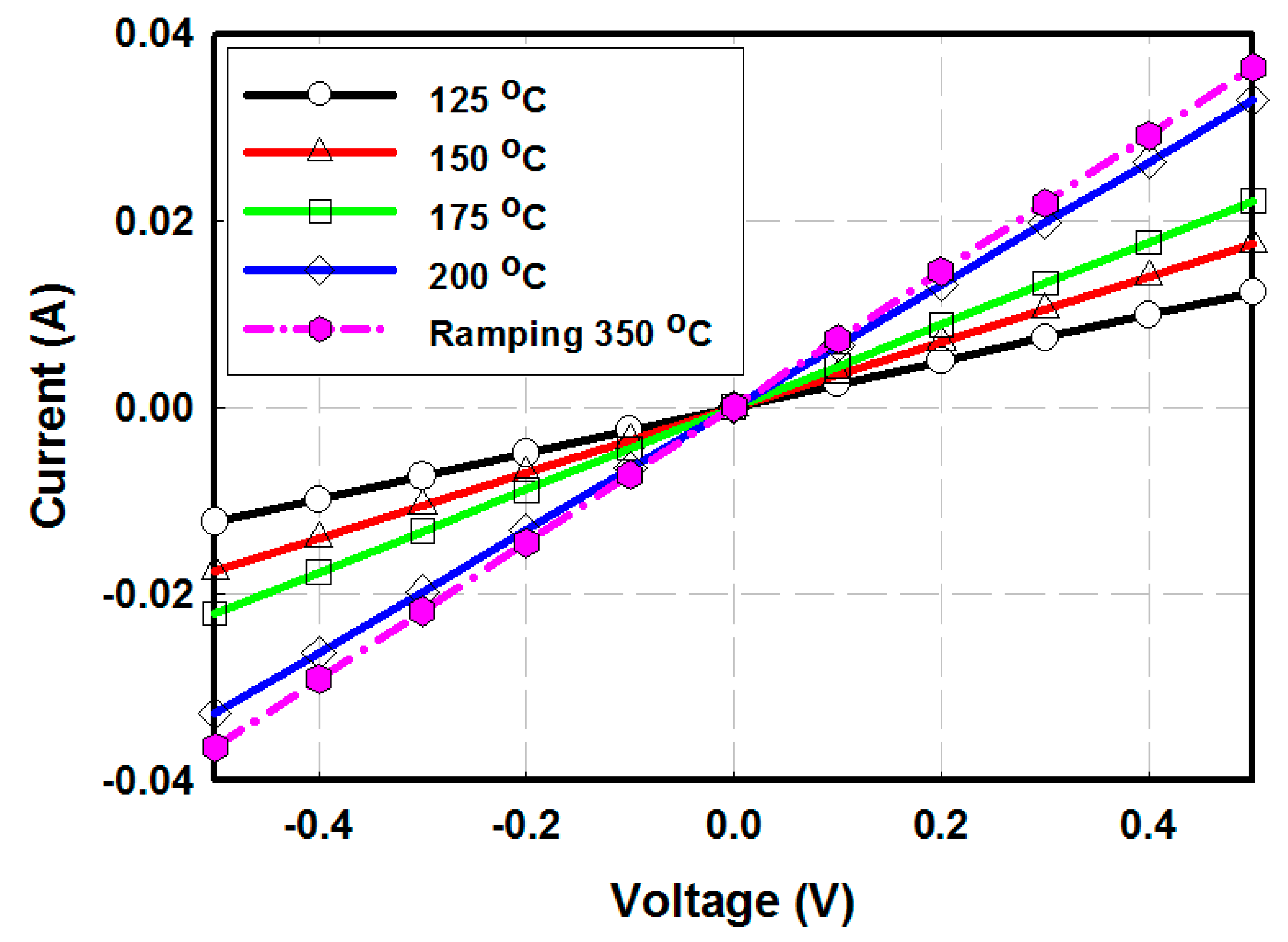

| Temperature | 125 °C | 150 °C | 175 °C | 200 °C | Ramping 350 °C |
|---|---|---|---|---|---|
| Conductivity (S/m) | 2.5 × 10−3 | 3.5 × 10−3 | 4.4 × 10−3 | 6.7 × 10−3 | 7.3 × 10−3 |
| Drying Time | Annealing Temperature | |||
|---|---|---|---|---|
| 125 °C | 150 °C | 175 °C | 200 °C | |
| 0 min | 78 | 109 | 93 | 21 |
| 5 min | 84 | 111 | 113 | 22 |
| 10 min | 96 | 122 | 110 | 19 |
| 30 min | 102 | 106 | 99 | 21 |
| 60 min | 110 | 98 | 93 | 22 |
| 3 h | 43 | 87 | 83 | 21 |
| Device X | Pattern Size (nm) | Voc (V) | JSC (mA/cm2) | FF (%) | PCE (%) | Rs (Ω∙cm2) |
|---|---|---|---|---|---|---|
| A | 0 | 0.54 | −9.0 | 56 | 2.7 | 27 |
| B | 80 | 0.54 | −9.3 | 56 | 2.8 | 25 |
| C | 100 | 0.55 | −9.9 | 61 | 3.3 | 21 |
| D | 120 | 0.55 | −10.3 | 60 | 3.4 | 21 |
| E | 135 | 0.55 | −10.3 | 61 | 3.4 | 21 |
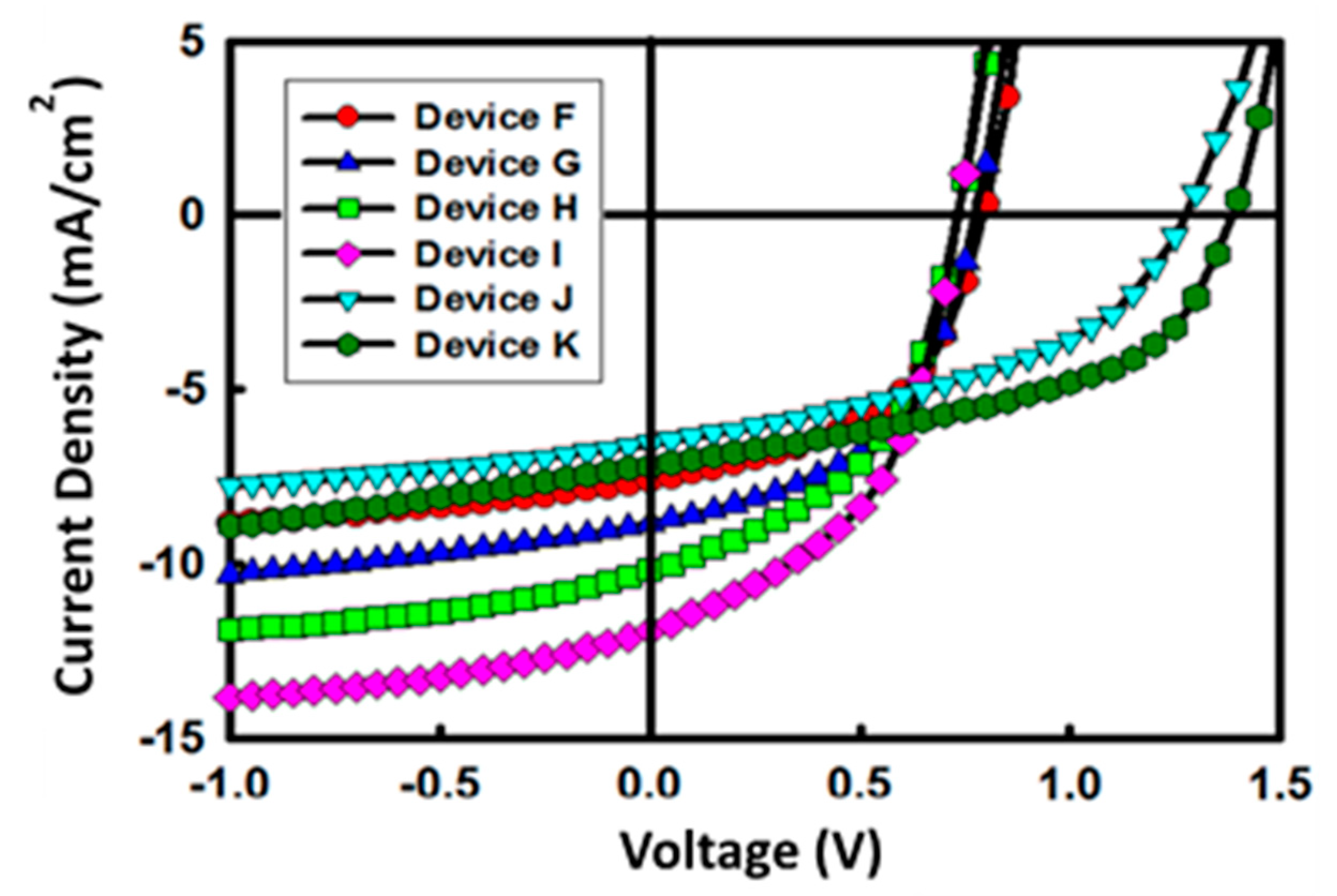
| Device | Structure | ECL (ZnO Layer) | VOC (V) | Jsc (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|---|---|
| F | First sub-cell | Non-patterned | 0.79 | −7.6 | 51 | 3.1 |
| G | Nano-ripple | 0.78 | −8.8 | 50 | 3.5 | |
| H | Second sub-cell | Non-patterned | 0.73 | −10.2 | 48 | 3.6 |
| I | Nano-ripple | 0.73 | −11.9 | 48 | 4.2 | |
| J | Tandem cell | Non-patterned | 1.28 | −6.5 | 44 | 3.7 |
| K | Nano-ripple | 1.38 | −7.2 | 49 | 4.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.W.; Jin, J.W.; Tondelier, D.; Bonnassieux, Y.; Geffroy, B. Low Temperature Solution-Processable 3D-Patterned Charge Recombination Layer for Organic Tandem Solar Cells. Materials 2019, 12, 162. https://doi.org/10.3390/ma12010162
Choi JW, Jin JW, Tondelier D, Bonnassieux Y, Geffroy B. Low Temperature Solution-Processable 3D-Patterned Charge Recombination Layer for Organic Tandem Solar Cells. Materials. 2019; 12(1):162. https://doi.org/10.3390/ma12010162
Chicago/Turabian StyleChoi, Jin Woo, Jong Woo Jin, Denis Tondelier, Yvan Bonnassieux, and Bernard Geffroy. 2019. "Low Temperature Solution-Processable 3D-Patterned Charge Recombination Layer for Organic Tandem Solar Cells" Materials 12, no. 1: 162. https://doi.org/10.3390/ma12010162
APA StyleChoi, J. W., Jin, J. W., Tondelier, D., Bonnassieux, Y., & Geffroy, B. (2019). Low Temperature Solution-Processable 3D-Patterned Charge Recombination Layer for Organic Tandem Solar Cells. Materials, 12(1), 162. https://doi.org/10.3390/ma12010162





