Photosystem II Is More Sensitive than Photosystem I to Al3+ Induced Phytotoxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Al Treatment
2.3. Lipid Peroxidation Measurements
2.4. Measurements of Chlorophyll a Fluorescence
2.5. Measurements of Leaf Absorbance Changes at 820 nm
2.6. Statistical Analysis
3. Results
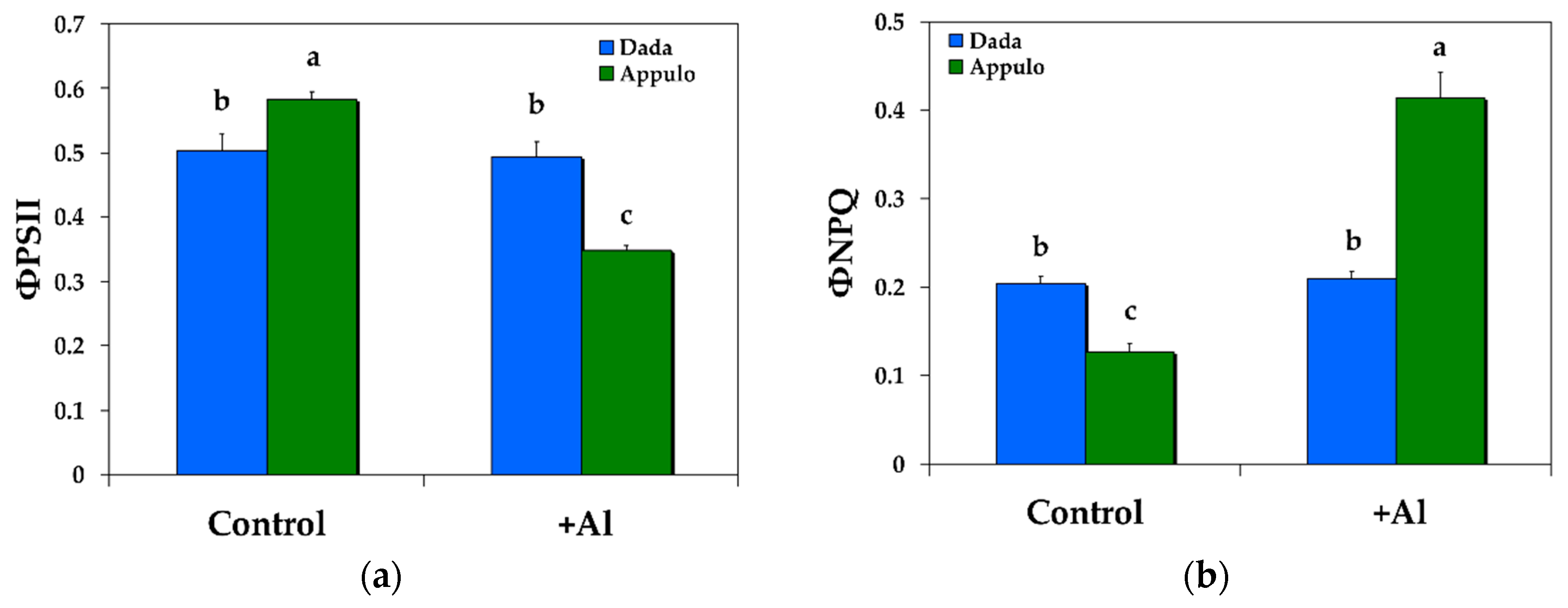
3.1. Allocation of the Absorbed Light Energy in PSII under Normal Growth and Al3+ Exposure
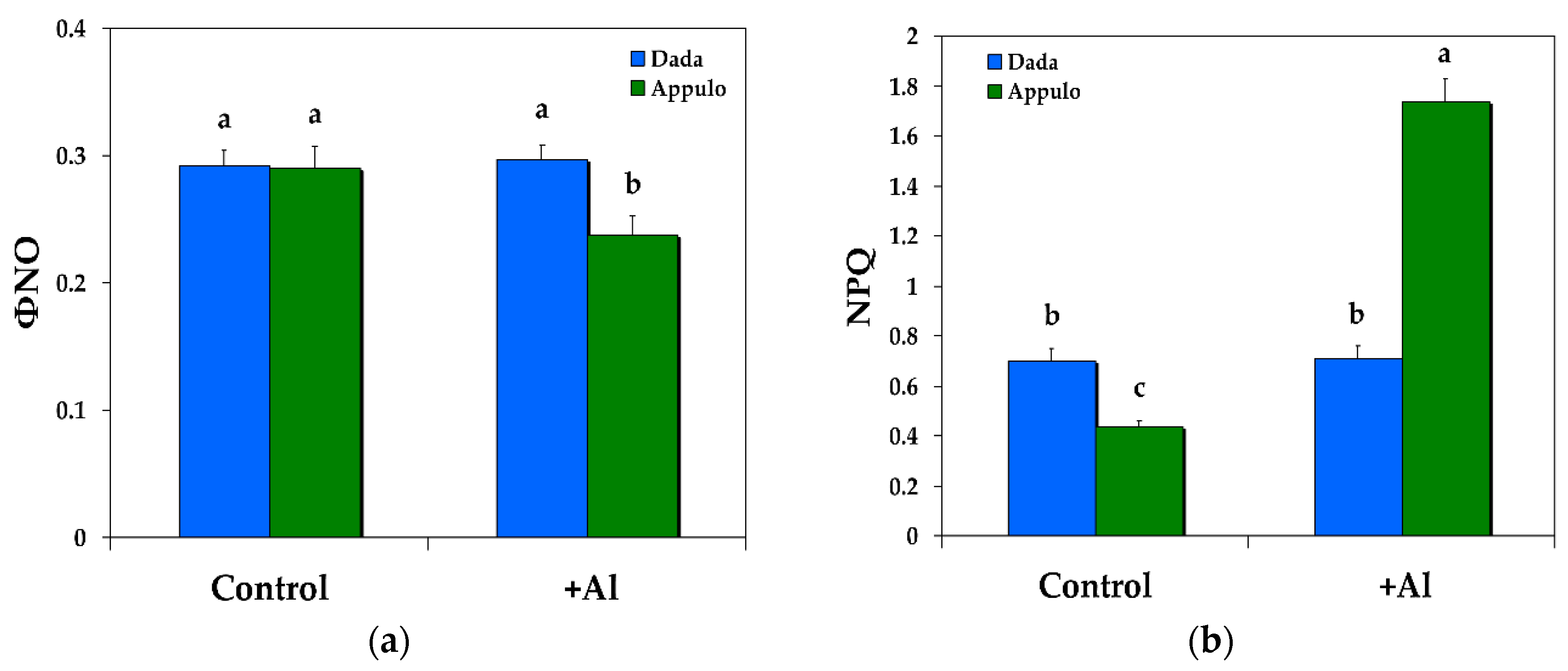
3.2. Non-Photochemical Quenching under Normal Growth and Al3+ Exposure
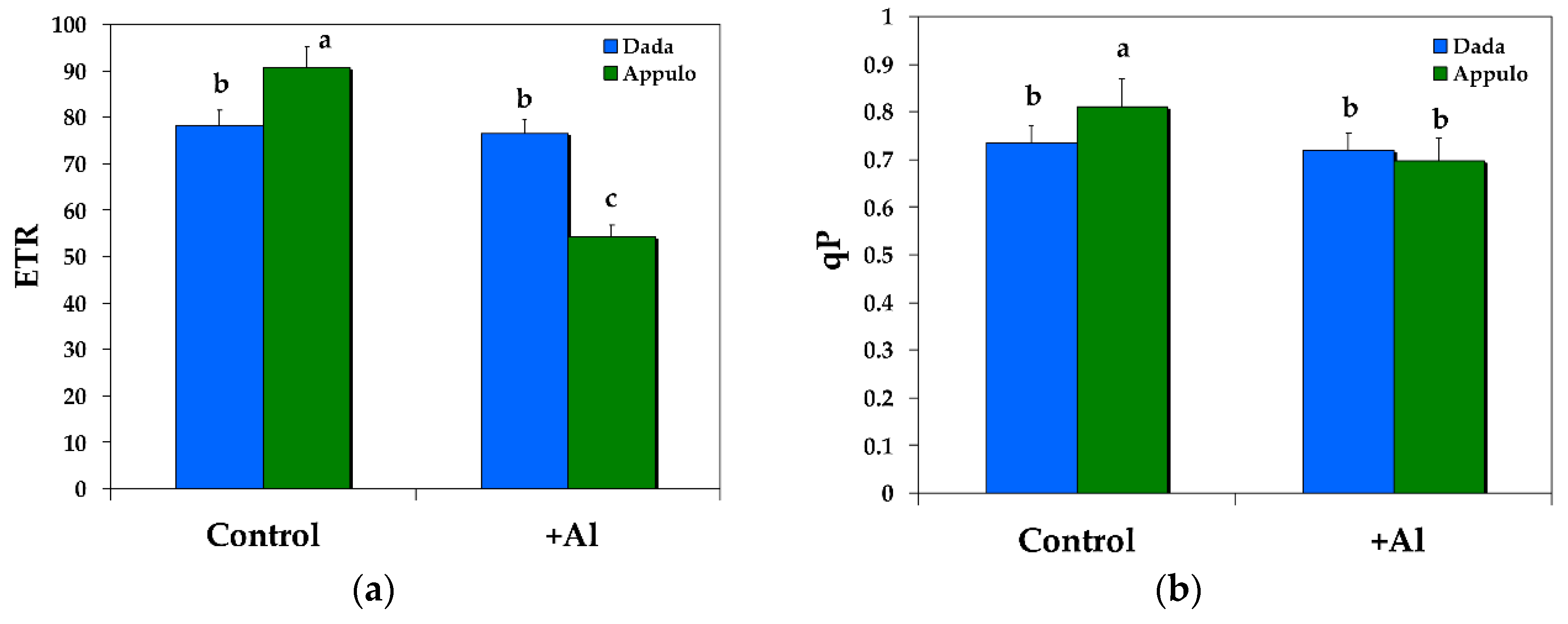
3.3. Electron Transport Rate and the Redox State of PSII under Normal Growth and Al3+ Exposure
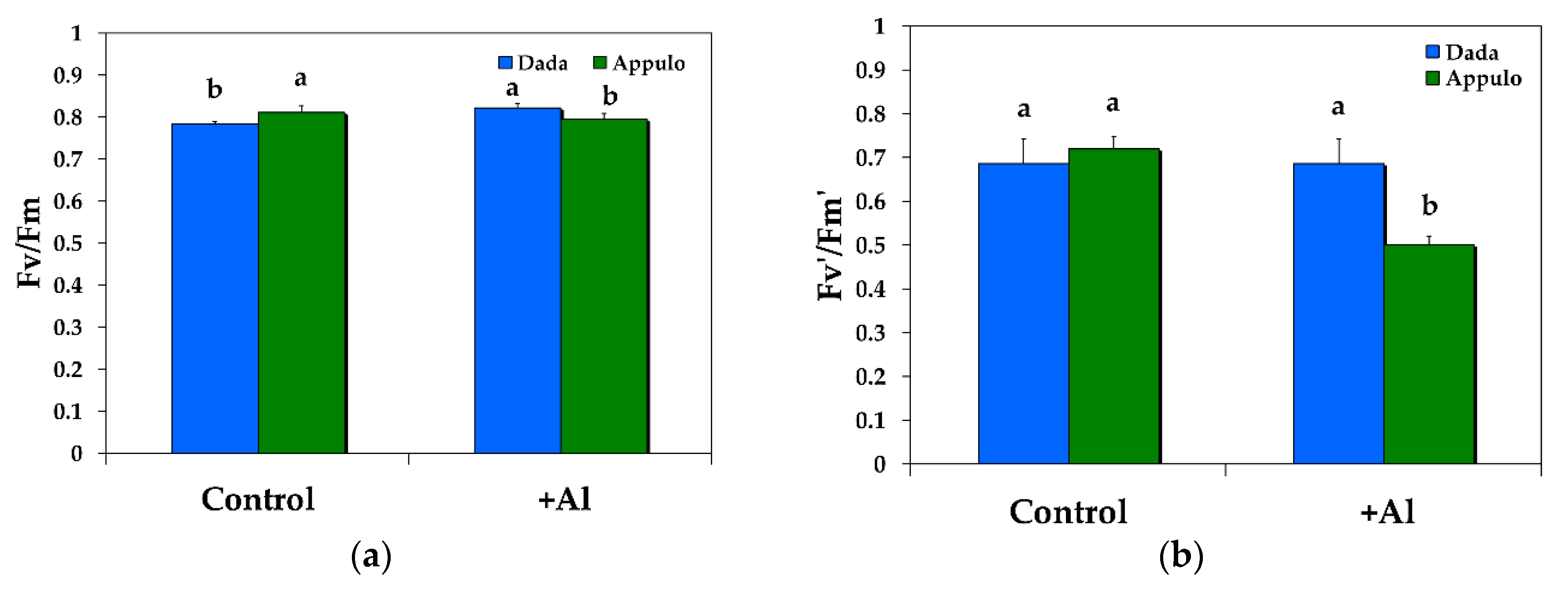
3.4. The Maximum PSII Quantum Efficiency (Fv/Fm) and PSII Maximum Efficiency in Light (Fv’/Fm’) under Normal Growth and Al3+ Exposure
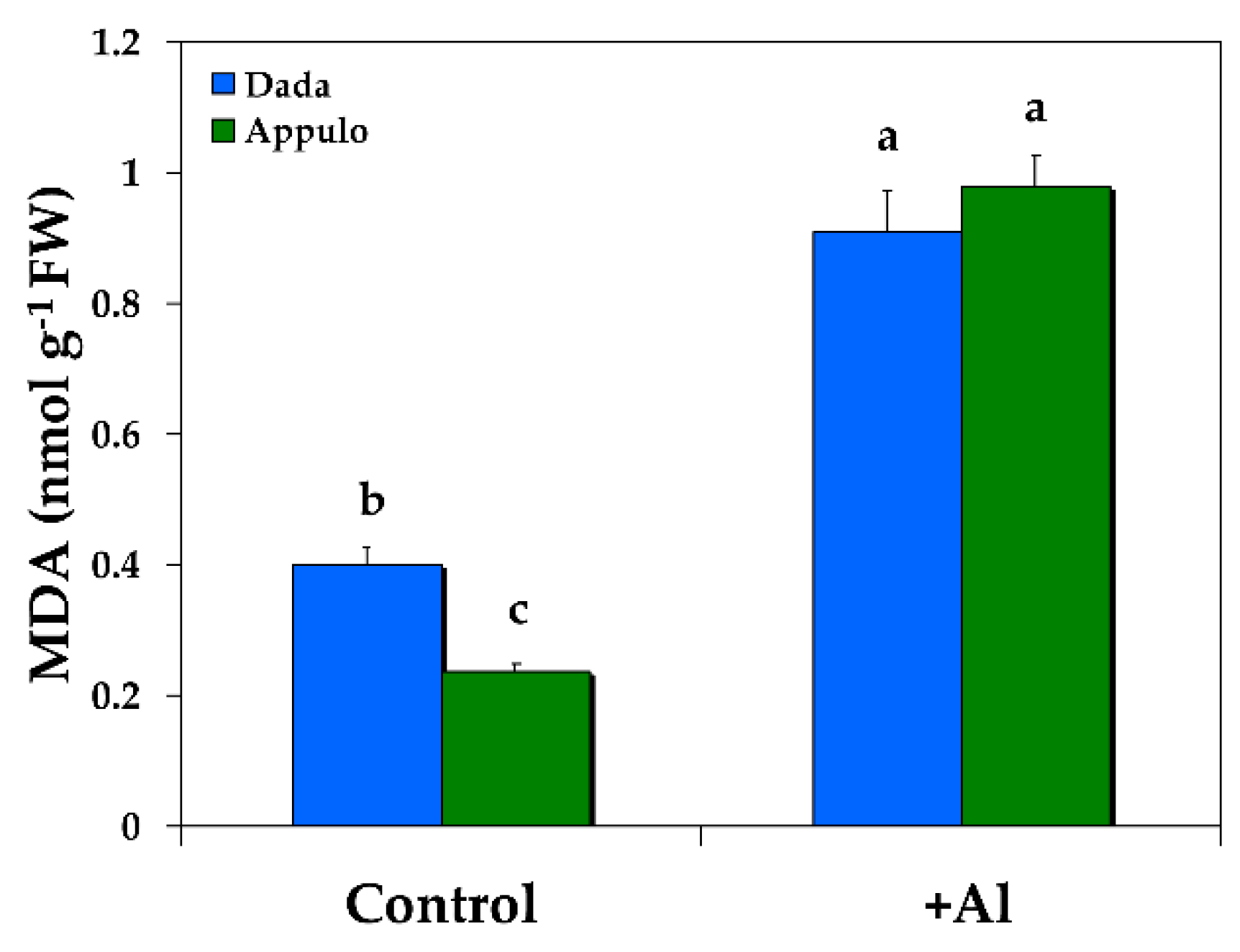
3.5. Oxidative Damage under Normal Growth and Al3+ Exposure
3.6. Excitation Pressure in PSI and PSII under Normal Growth and Al3+ Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wolt, J. Applications to Environmental Science and Agriculture. In Soil Solution Chemistry; John Wiley & Sons: New York, NY, USA, 1994. [Google Scholar]
- Famoso, A.N.; Clark, R.T.; Shaff, J.E.; Craft, E.; McCouch, S.R.; Kochian, L.V. Development of a novel aluminum tolerance phenotyping platform used for comparisons of cereal aluminum tolerance and investigations into rice aluminum tolerance mechanisms. Plant Physiol. 2010, 153, 1678–1691. [Google Scholar] [CrossRef] [PubMed]
- Kinraide, T.B. Identity of the rhizotoxic aluminium species. Plant Soil 1991, 134, 167–178. [Google Scholar] [CrossRef]
- Eleftheriou, E.P.; Moustakas, M.; Fragiskos, N. Aluminate-induced changes in morphology and ultrastructure of Thinopyrum roots. J. Exp. Bot. 1993, 44, 427–436. [Google Scholar] [CrossRef]
- Rengel, Z. Role of calcium in aluminium toxicity. New Phytol. 1992, 121, 499–513. [Google Scholar] [CrossRef]
- Moustakas, M.; Ouzounidou, G.; Lannoye, R. Aluminum effects on photosynthesis and elemental uptake in an aluminum-tolerant and non-tolerant wheat cultivar. J. Plant Nutr. 1995, 18, 669–683. [Google Scholar] [CrossRef]
- Barceló, J.; Poschenrieder, C. Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: A review. Environ. Exp. Bot. 2002, 48, 75–92. [Google Scholar] [CrossRef]
- Tamás, L.; Simonovicová, M.; Huttová, J.; Mistrík, I. Aluminium stimulated hydrogen peroxide production of germinating barley seeds. Environ. Exp. Bot. 2004, 51, 281–288. [Google Scholar] [CrossRef]
- Doncheva, S.; Amenos, M.; Poschenrieder, C.; Barcelo, J. Root cell patterning: A primary target for aluminium toxicity in maize. J. Exp. Bot. 2005, 56, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Poschenrieder, C.; Gunsé, B.; Corrales, I.; Barceló, J. A glance into aluminum toxicity and resistance. Sci. Total Environ. 2008, 40, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.S.; Qi, Y.P.; Jiang, H.X.; Yang, L.T.; Yang, G.H. Photosynthesis and photoprotective systems of plants in response to aluminum toxicity. Afr. J. Biotechnol. 2010, 9, 9237–9247. [Google Scholar]
- Giannakoula, A.; Moustakas, M.; Mylona, P.; Papadakis, I.; Yupsanis, T. Aluminium tolerance in maize is correlated with increased levels of mineral nutrients, carbohydrates and proline and decreased levels of lipid peroxidation and Al accumulation. J. Plant Physiol. 2008, 165, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Giannakoula, A.; Moustakas, M.; Syros, T.; Yupsanis, T. Aluminium stress induces up-regulation of an efficient antioxidant system in the Al-tolerant maize line but not in the Al-sensitive line. Environ. Exp. Bot. 2010, 67, 487–494. [Google Scholar] [CrossRef]
- Zelinová, V.; Halušková, L.; Huttová, J.; Illéš, P.; Mistrík, I.; Valentovičová, K.; Tamás, L. Short-term aluminium-induced changes in barley root tips. Protoplasma 2011, 248, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xing, F.; Xing, D. Characterization of target site of aluminum phytotoxicity in photosynthetic electron transport by fluorescence techniques in tobacco leaves. Plant Cell Physiol. 2012, 53, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Kochian, L.V.; Piñeros, M.A.; Jiping, L.; Magalhaes, J.V. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Ann. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, H.; Zhu, Y.; Zou, J.; Zhao, F.J.; Huang, C.F. Genome-wide transcriptomic and phylogenetic analyses reveal distinct aluminum-tolerance mechanisms in the aluminum-accumulating species buckwheat (Fagopyrum tataricum). BMC Plant Biol. 2015, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Xu, J.M.; Lou, H.Q.; Xiao, C.; Chen, W.W.; Yang, J.L. Physiological and molecular analysis of aluminium-induced organic acid anion secretion from grain amaranth (Amaranthus hypochondriacus L.) roots. Int. J. Mol. Sci. 2016, 17, 608. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, L.T.; Qi, Y.P.; Guo, P.; Lu, Y.B.; Chen, L.S. Aluminum toxicity-induced alterations of leaf proteome in two citrus species differing in aluminum tolerance. Int. J. Mol. Sci. 2016, 17, 1180. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Tripathi, D.K.; Singh, S.; Sharma, S.; Dubey, N.K.; Chauhan, D.K.; Vaculik, M. Toxicity of aluminium on various levels of plant cells and organism: A review. Environ. Exp. Bot. 2017, 137, 177–193. [Google Scholar] [CrossRef]
- Foy, C.D. Plant adaptation to acid, aluminium-toxic soils. Commun. Soil Sci. Plant Anal. 1988, 19, 959–987. [Google Scholar] [CrossRef]
- Garcia-Oliveira, A.L.; Martins-Lopes, P.; Tolrà, R.; Poschenrieder, C.; Guedes-Pinto, H.; Benito, C. Differential physiological responses of Portuguese bread wheat (Triticum aestivum L.) genotypes under aluminium stress. Diversity 2016, 8, 26. [Google Scholar] [CrossRef]
- Moustakas, M.; Yupsanis, T.; Symeonidis, L.; Karataglis, S. Aluminum toxicity effects on durum wheat cultivars. J. Plant Nutr. 1992, 15, 627–638. [Google Scholar] [CrossRef]
- Bona, L.; Wright, R.J.; Baligar, V.C.; Matuz, J. Screening wheat and other small grains for acid soil tolerance. Landsc. Urban Plan. 1993, 27, 175–178. [Google Scholar] [CrossRef]
- Moustakas, M.; Ouzounidou, G.; Lannoye, R. Rapid screening for aluminum tolerance in cereals by use of the chlorophyll fluorescence test. Plant Breed. 1993, 111, 343–346. [Google Scholar] [CrossRef]
- Von Uexkül, H.R.; Mutert, E. Global extent, development and economic impact of acid soils. Plant Soil 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Ma, J.F.; Chen, Z.C.; Shen, R.F. Molecular mechanisms of Al tolerance in gramineous plants. Plant Soil 2014, 381, 1–12. [Google Scholar] [CrossRef]
- Allen, A.M.; Barker, G.L.A.; Berry, S.T.; Coghill, J.A.; Gwilliam, R.; Kirby, S.; Robinson, P.; Brenchley, R.C.; D’Amore, R.; McKenzie, N.; et al. Transcript-specific, single-nucleotide polymorphism discovery and linkage analysis in hexaploid bread wheat (Triticum aestivum L.). Plant Biotechnol. J. 2011, 9, 1086–1099. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M. Changing concepts about the distribution of photosystems I and II between grana-appressed and stroma-exposed thylakoid membranes. Photosynth. Res. 2002, 73, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, E.L.; Dobrikova, A.G.; Ivanova, P.I.; Petkanchin, I.B.; Taneva, S.G. Relationship between the organization of the PSII supercomplex and the functions of the photosynthetic apparatus. J. Photochem. Photobiol. B. 2006, 83, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Mustárdy, L.; Garab, G. Granum revisited. A three-dimensional model-where things fall into place. Trends Plant Sci. 2003, 8, 117–122. [Google Scholar] [CrossRef]
- Trissl, H.W.; Wilhelm, C. Why do thylakoid membranes from higher plants form grana stacks? Trends Biochem. Sci. 1993, 18, 415–419. [Google Scholar] [CrossRef]
- Edwards, G.E.; Walker, D.A. C3, C4: Mechanisms, and Cellular and Environmental Regulation, of Photosynthesis; Blackwell Scientific: London/Oxford, UK, 1983. [Google Scholar]
- Huang, W.; Zhang, S.B.; Xu, J.C.; Liu, T. Plasticity in roles of cyclic electron flow around photosystem I at contrasting temperatures in the chilling-sensitive plant Calotropis gigantea. Environ. Exp. Bot. 2017, 141, 145–153. [Google Scholar] [CrossRef]
- Moustakas, M.; Ouzounidou, G.; Eleftheriou, E.P.; Lannoye, R. Indirect effects of aluminium stress on the function of the photosynthetic apparatus. Plant Physiol. Biochem. 1996, 34, 553–560. [Google Scholar]
- Moustakas, M.; Eleftheriou, E.P.; Ouzounidou, G. Short-term effects of aluminium at alkaline pH on the structure and function of the photosynthetic apparatus. Photosynthetica 1997, 34, 169–177. [Google Scholar] [CrossRef]
- Chen, L.S.; Qi, Y.P.; Liu, X.H. Effects of aluminum on light energy utilization and photoprotective systems in citrus leaves. Ann. Bot. 2005, 96, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.X.; Chen, L.S.; Zheng, J.G.; Han, S.; Tang, N.; Smith, B.R. Aluminum-induced effects on photosystem II photochemistry in citrus leaves assessed by the chlorophyll a fluorescence transient. Tree Physiol. 2008, 28, 1863–1871. [Google Scholar] [CrossRef] [PubMed]
- Inostroza-Blancheteau, C.; Reyes-Díaz, M.; Aquea, F.; Nunes-Nesi, A.; Alberdi, M.; Arce-Johnson, P. Biochemical and molecular changes in response to aluminium-stress in highbush blueberry (Vaccinium corymbosum L.). Plant Physiol. Biochem. 2011, 49, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Hasni, I.; Hamdani, S.; Carpentier, R. Destabilization of the Oxygen Evolving Complex of Photosystem II by Al3+. Photochem. Photobiol. 2013, 89, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Ouzounidou, G. Increased non-photochemical quenching in leaves of aluminium-stressed wheat plants is due to Al3+-induced elemental loss. Plant Physiol. Biochem. 1994, 32, 527–532. [Google Scholar]
- Hasni, I.; Yaakoubi, H.; Hamdani, S.; Tajmir-Riahi, H.A.; Carpentier, R. Mechanism of interaction of Al3+ with the proteins composition of photosystem II. PLoS ONE 2015, 10, e0120876. [Google Scholar] [CrossRef] [PubMed]
- Moustaka, J.; Ouzounidou, G.; Bayçu, G.; Moustakas, M. Aluminum resistance in wheat involves maintenance of leaf Ca2+ and Mg2+ content, decreased lipid peroxidation and Al accumulation, and low photosystem II excitation pressure. BioMetals 2016, 29, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Hasni, I.; Msilini, N.; Hamdani, S.; Tajmir-Riahi, H.A.; Carpentier, R. Characterization of the structural changes and photochemical activity of photosystem I under Al3+ effect. J. Photochem. Photobiol. B 2015, 149, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Stewart, J.J.; Adams, W.W., III. Multiple feedbacks between chloroplast and whole plant in the context of plant adaptation and acclimation to the environment. Philos. Trans. R. Soc. B 2014, 369, 20130244. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, C.K.A.; Yamori, W.; Takahashi, S.; Terashima, I.; Noguchi, K. Mitochondrial alternative pathway-associated photoprotection of photosystem II is related to the photorespiratory pathway. Plant Cell Physiol. 2016, 57, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, K.K. Photoprotection revisited: Genetic and molecular approaches. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 333–359. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams, W.W., III. Photoprotection in an ecological context: The remarkable complexity of thermal energy dissipation. New Phytol. 2006, 172, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Moustaka, J.; Panteris, E.; Adamakis, I.D.S.; Tanou, G.; Giannakoula, A.; Eleftheriou, E.P.; Moustakas, M. High anthocyanin accumulation in poinsettia leaves is accompanied by thylakoid membrane unstacking, acting as a photoprotective mechanism, to prevent ROS formation. Environ. Exp. Bot. 2018, 154, 44–55. [Google Scholar] [CrossRef]
- Muller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V. Non photochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Geng, Q.; Du, Y.; Yang, X.; Zhai, H. Induction of cyclic electron flow around photosystem I during heatstress in grape leaves. Plant Sci. 2017, 256, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Guidi, L.; Calatayud, A. Non-invasive tools to estimate stress-induced changes in photosynthetic performance in plants inhabiting Mediterranean areas. Environ. Exp. Bot. 2014, 103, 42–52. [Google Scholar] [CrossRef]
- Gorbe, E.; Calatayud, A. Applications of chlorophyll fluorescence imaging technique in horticultural research: A review. Sci. Hortic. 2012, 138, 24–35. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Oukarroum, A.A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M.; Greppin, H.; Strasser, R.J. Functioning of photosystems I and II in pea leaves exposed to heat stress in the presence or absence of light. Analysis using in vivo fluorescence, absorbance, oxygen and photoacoustic measurements. Planta 1991, 186, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Ouzounidou, G.; Moustakas, M.; Strasser, R.J. Sites of action of copper in the photosynthetic apparatus of maize leaves. Aust. J. Plant Physiol. 1997, 24, 81–90. [Google Scholar] [CrossRef]
- Shaff, J.E.; Schultz, B.A.; Craft, E.J.; Clark, R.T.; Kochian, L.V. GEOCHEM-EZ: A chemical speciation program with greater power and flexibility. Plant Soil 2010, 330, 207–214. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Kouna, T.; Antonopoulou, C.I.; Therios, I. Exogenous proline induces soluble sugar accumulation and alleviates drought stress effects on photosystem II functioning of Arabidopsis thaliana leaves. Plant Growth Regul. 2011, 65, 315–325. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Parker, D.R.; Kinraide, T.B.; Zelazny, L.W. Aluminum speciation and phytotoxicity in dilute hydroxy-aluminum solutions. Soil Sci. Soc. Am. J. 1988, 52, 438–444. [Google Scholar] [CrossRef]
- Kochian, L.V.; Hoekenga, O.A.; Piñeros, M.A. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Ann. Rev. Plant Biol. 2004, 55, 459–493. [Google Scholar] [CrossRef] [PubMed]
- Zlobin, I.E.; Ivanov, Y.V.; Kartashov, A.V.; Sarvin, B.A.; Stavrianidi, A.N.; Kreslavski, V.D.; Kuznetsov, V.V. Impact of weak water deficit on growth, photosynthetic primary processes and storage processes in pine and spruce seedlings. Photosynth. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Suorsa, M.; Jarvi, S.; Grieco, M.; Nurmi, M.; Pietrzykowska, M.; Rantala, M.; Kangasjarvi, S.; Paakkarinen, V.; Tikkanen, M.; Jansson, S.; et al. PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 2012, 24, 2934–2948. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, M.; Mekala, N.R.; Aro, E.M. Photosystem II photoinhibition-repair cycle protects photosystem I from irreversible damage. Biochim. Biophys. Acta 2014, 1837, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Takagi, D.; Takumi, S.; Hashiguchi, M.; Sejima, T.; Miyake, C. Superoxide and singlet oxygen produced within the thylakoid membranes both cause photosystem I photoinhibition. Plant Physiol. 2016, 171, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yang, Y.J.; Hu, H.; Zhang, S.B. Moderate photoinhibition of photosystem II protects photosystem I from photodamage at chilling stress in tobacco leaves. Front. Plant Sci. 2016, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M.; Kunderlikova, K.; Sytar, O.; Shao, H.; Kalaji, H.M.; Allakhverdiev, S.I. Low PSI content limits the photoprotection of PSI and PSII in early growth stages of chlorophyll b-deficient wheat mutant lines. Photosynth. Res. 2015, 125, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Brestic, M.; Zivcak, M.; Kunderlikova, K.; Allakhverdiev, S.I. High temperature specifically affects the photoprotective responses of chlorophyll b-deficient wheat mutant lines. Photosynth. Res. 2016, 130, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yang, Y.J.; Hu, H.; Zhang, S.B. Responses of photosystem I compared with photosystem II to fluctuating light in the shade-establishing tropical tree species Psychotria henryi. Front. Plant Sci. 2016, 7, 1549. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Badger, M.R. Photoprotection in plants: A new light on photosystem II damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Moustaka, J.; Tanou, G.; Adamakis, I.D.; Eleftheriou, E.P.; Moustakas, M. Leaf age dependent photoprotective and antioxidative mechanisms to paraquat-induced oxidative stress in Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 13989–14006. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress and signal transduction. Ann. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Gawroński, P.; Witoń, D.; Vashutina, K.; Bederska, M.; Betliński, B.; Rusaczonek, A.; Karpiński, S. Mitogen-activated protein kinase 4 is a salicylic acid-independent regulator of growth but not of photosynthesis in Arabidopsis. Mol. Plant 2014, 7, 1151–1166. [Google Scholar] [CrossRef] [PubMed]
- Bayçu, G.; Gevrek-Kürüm, N.; Moustaka, J.; Csatári, I.; Rognes, S.E.; Moustakas, M. Cadmium-zinc accumulation and photosystem II responses of Noccaea caerulescens to Cd and Zn exposure. Environ. Sci. Pollut. Res. 2017, 24, 2840–2850. [Google Scholar] [CrossRef] [PubMed]
- Moustaka, J.; Moustakas, M. Photoprotective mechanism of the non-target organism Arabidopsis thaliana to paraquat exposure. Pest. Biochem. Physiol. 2014, 111, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E. Environmental hormesis, a fundamental non-monotonic biological phenomenon with implications in ecotoxicology and environmental safety. Ecotoxicol. Environ. Saf. 2018, 148, 1042–1053. [Google Scholar] [CrossRef]
- Georgieva, K.; Doncheva, S.; Mihailova, G.; Petkova, S. Response of sun- and shade-adapted plants of Haberlea rhodopensis to desiccation. Plant Growth Regul. 2012, 67, 121–132. [Google Scholar] [CrossRef]
- Jusovic, M.; Velitchkova, M.Y.; Misheva, S.P.; Börner, A.; Apostolova, E.L.; Dobrikova, A.G. Photosynthetic responses of a wheat mutant (Rht-B1c) with altered DELLA proteins to salt stress. J. Plant Growth Regul. 2018, 37, 645–656. [Google Scholar] [CrossRef]
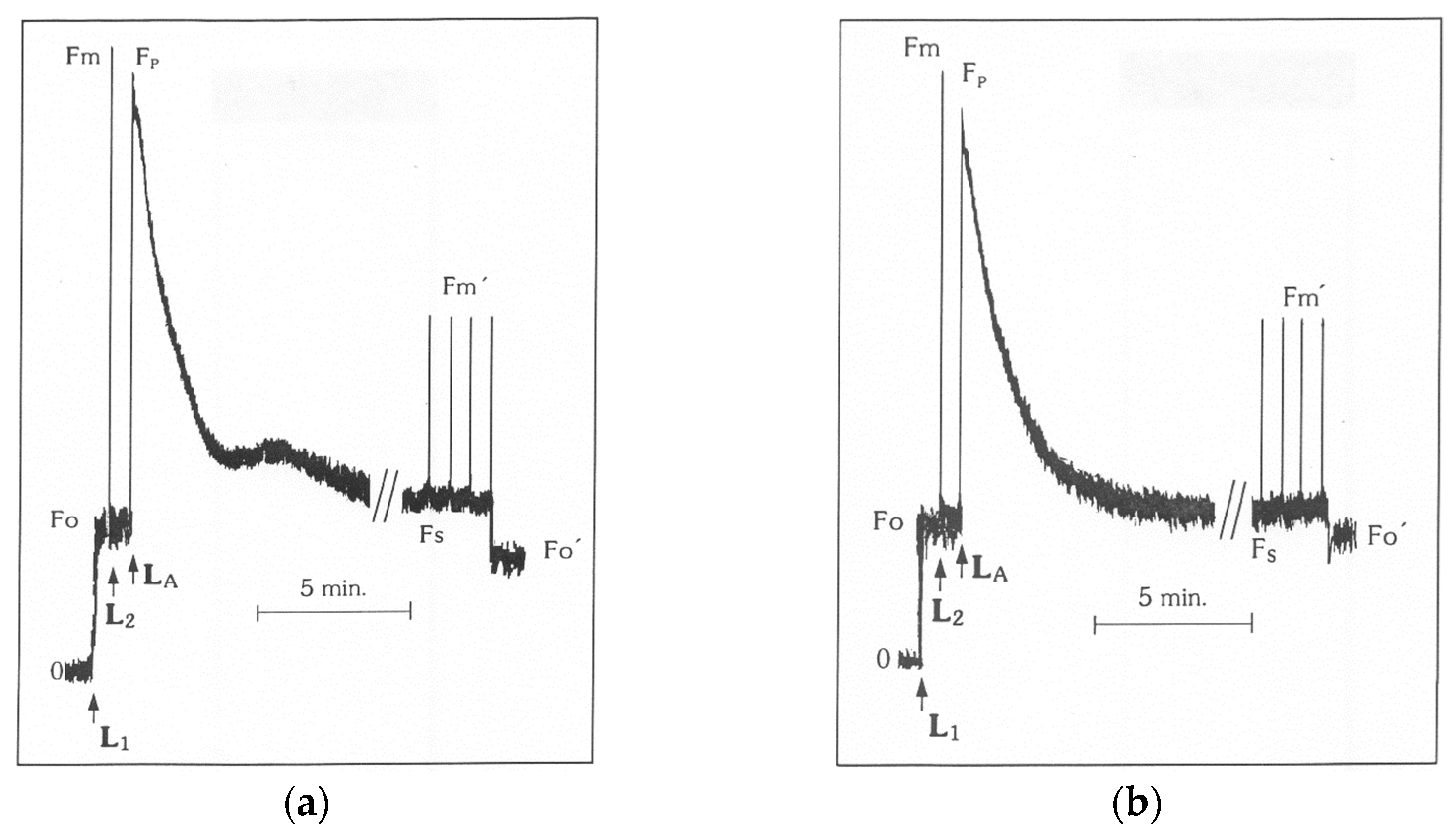
| Chlorophyll Fluorescence Parameter | Definition | Calculation |
|---|---|---|
| Fv/Fm | The maximum quantum efficiency of photosystem II (PSII) photochemistry | Calculated as (Fm − Fo)/Fm |
| Fv’/Fm’ | The PSII maximum efficiency is an estimate of the maximum efficiency of PSII photochemistry at a given PPFD (photosynthetic photon flux density) | Calculated as (Fm’ − Fo’)/Fm’ |
| ΦPSII | The effective quantum yield of photochemical energy conversion in PSII estimating the efficiency at which light absorbed by PSII is used for photochemistry, that means is used for reduction of the primary acceptor of PSII quinone A (QA) | Calculated as (Fm’ − Fs)/Fm’ |
| qp | The photochemical quenching is a measure of the fraction of open PSII reaction centers, that is the redox state of QA | Calculated as (Fm’ − Fs)/(Fm’ − Fo’) |
| NPQ | The non-photochemical quenching that reflects heat dissipation of excitation energy | Calculated as (Fm − Fm’)/Fm’ |
| ETR | The relative PSII electron transport rate | Calculated as ΦPSII × PPFD × c × abs, where c is 0.5 since the absorbed light energy is assumed to be equally distributed between PSII and PSI, and abs is the total light absorption of the leaf taken as 0.84. |
| ΦNPQ | The quantum yield of regulated non-photochemical energy loss in PSII, that is the quantum yield for dissipation by down regulation in PSII | Calculated as Fs/Fm’ − Fs/Fm |
| ΦNO | The quantum yield of non-regulated energy loss in PSII, a loss process due to PSII inactivity | Calculated as Fs/Fm |
| 1 − qp | Excitation pressure of PSII, or the fraction of closed PSII reaction centers | Calculated as 1 − qp |
| Chlorophyll Fluorescence Parameter | Control Growth | +148 μM Al | Change % |
|---|---|---|---|
| B1 (excitation pressure in PSI) ‘Dada’ | 0.259 | 0.265 | +2.3 |
| B1 (excitation pressure in PSI) ‘Appulo E’ | 0.280 | 0.339 | +21.0 |
| 1-qp (excitation pressure in PSII) ‘Dada’ | 0.266 | 0.281 | +5.6 |
| 1-qp (excitation pressure in PSII) ‘Appulo E’ | 0.188 | 0.303 | +61.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moustaka, J.; Ouzounidou, G.; Sperdouli, I.; Moustakas, M. Photosystem II Is More Sensitive than Photosystem I to Al3+ Induced Phytotoxicity. Materials 2018, 11, 1772. https://doi.org/10.3390/ma11091772
Moustaka J, Ouzounidou G, Sperdouli I, Moustakas M. Photosystem II Is More Sensitive than Photosystem I to Al3+ Induced Phytotoxicity. Materials. 2018; 11(9):1772. https://doi.org/10.3390/ma11091772
Chicago/Turabian StyleMoustaka, Julietta, Georgia Ouzounidou, Ilektra Sperdouli, and Michael Moustakas. 2018. "Photosystem II Is More Sensitive than Photosystem I to Al3+ Induced Phytotoxicity" Materials 11, no. 9: 1772. https://doi.org/10.3390/ma11091772
APA StyleMoustaka, J., Ouzounidou, G., Sperdouli, I., & Moustakas, M. (2018). Photosystem II Is More Sensitive than Photosystem I to Al3+ Induced Phytotoxicity. Materials, 11(9), 1772. https://doi.org/10.3390/ma11091772








